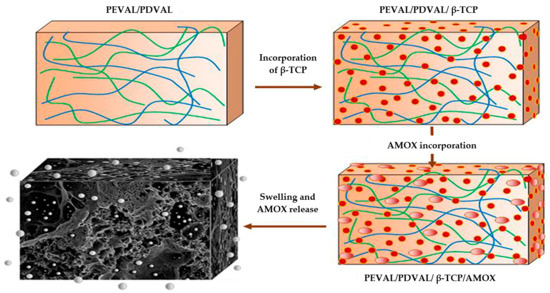
Poly(δ-valerolactone)/Poly(ethylene-co-vinylalcohol)/β-Tricalcium Phosphate Composite as Scaffolds: Preparation, Properties, and In Vitro Amoxicillin Release
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of PDVAL/PEVAL/β-TCP Composite
2.3. Pores Interconnection
2.4. Characterization of PEVAL/PDVAL/β-TCP Composite
2.5. In Vitro Biocompatibility
2.5.1. Cell Culture
2.5.2. Cell Seeding and Viability Assessment
2.6. In Vitro Drug Release Studies
3. Results and Discussion
3.1. Characterization
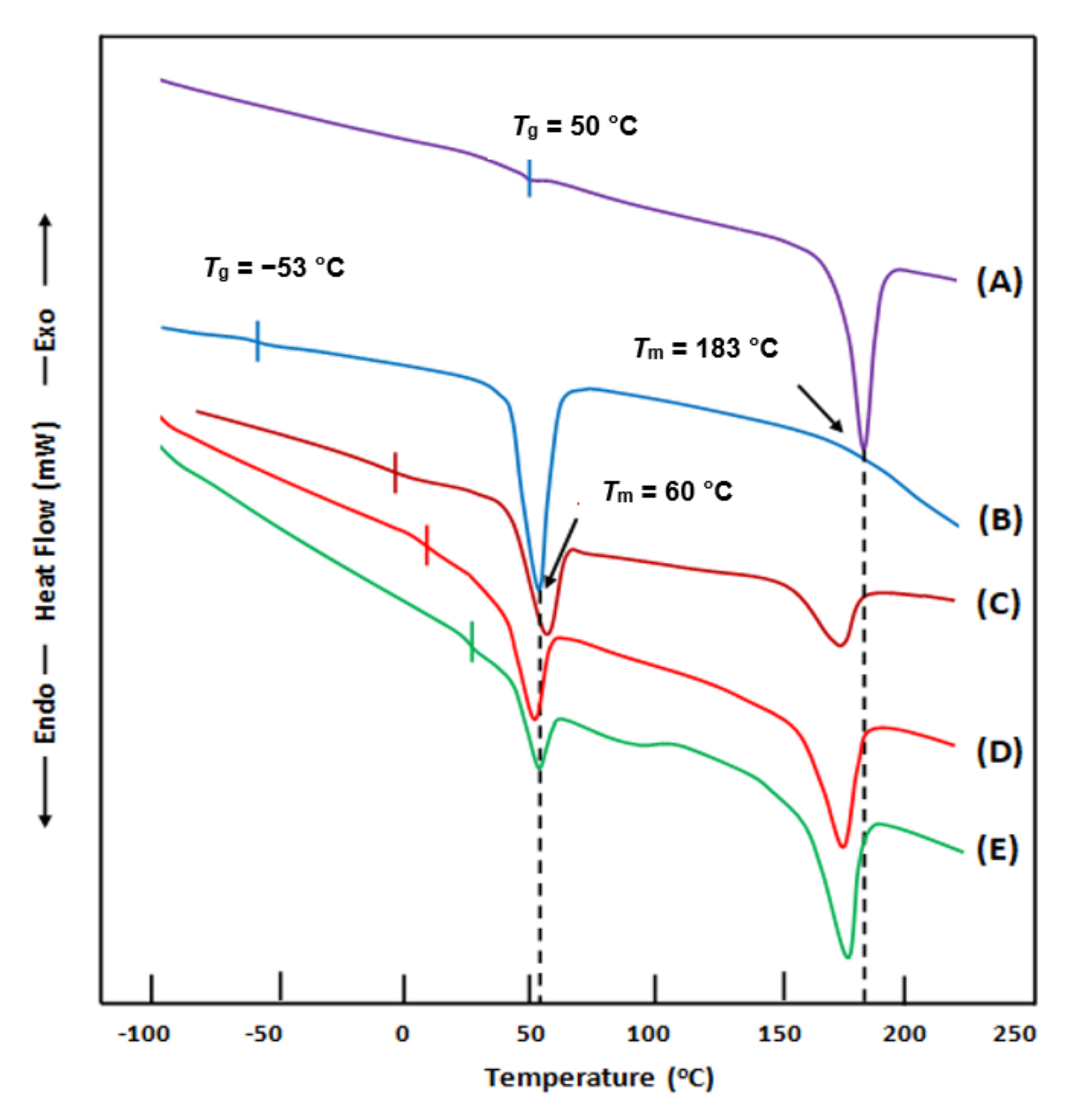
3.1.1. Differential Scanning Calorimetry (DSC) Analysis
3.1.2. X-ray Diffraction (XRD) Analysis
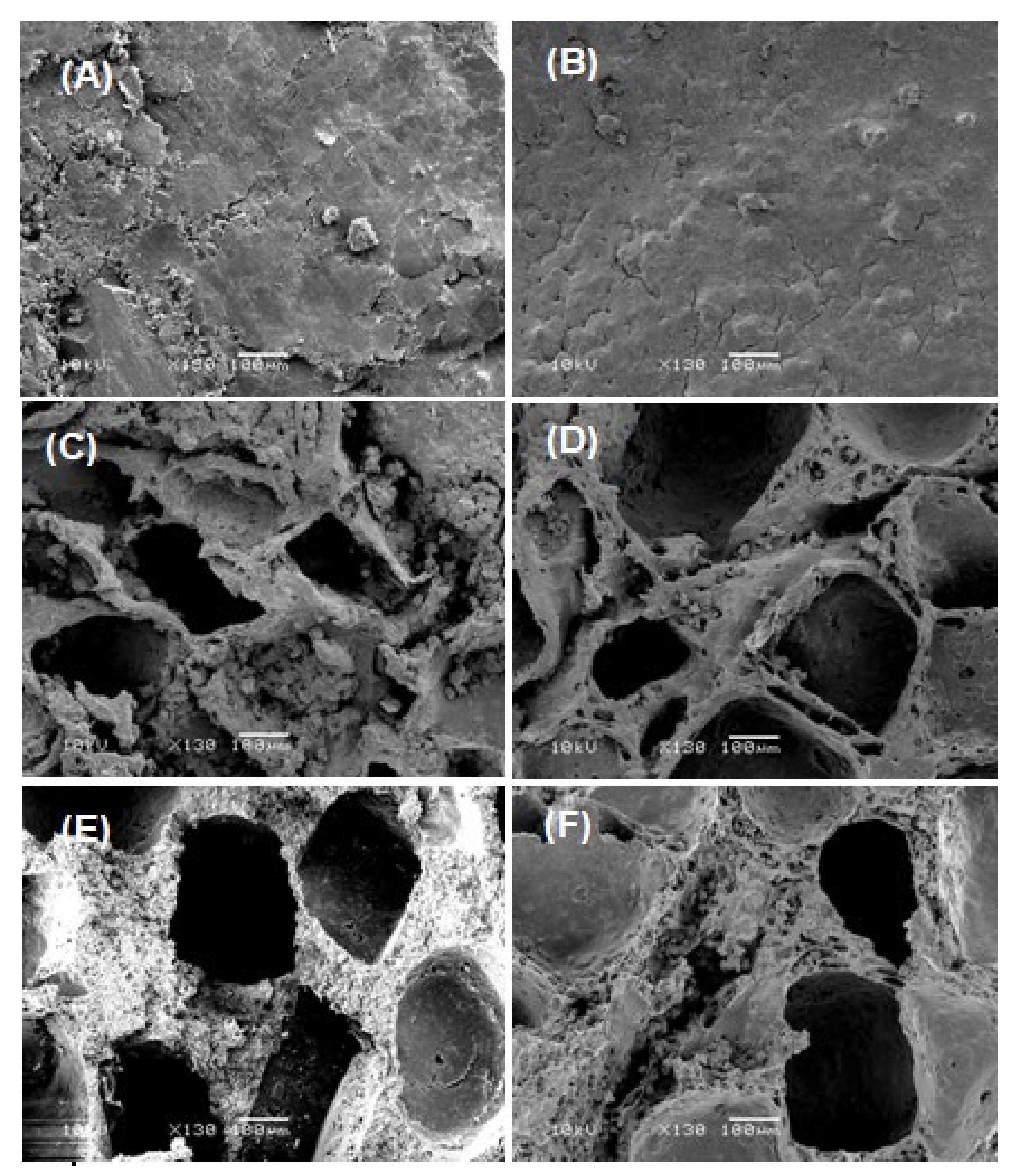
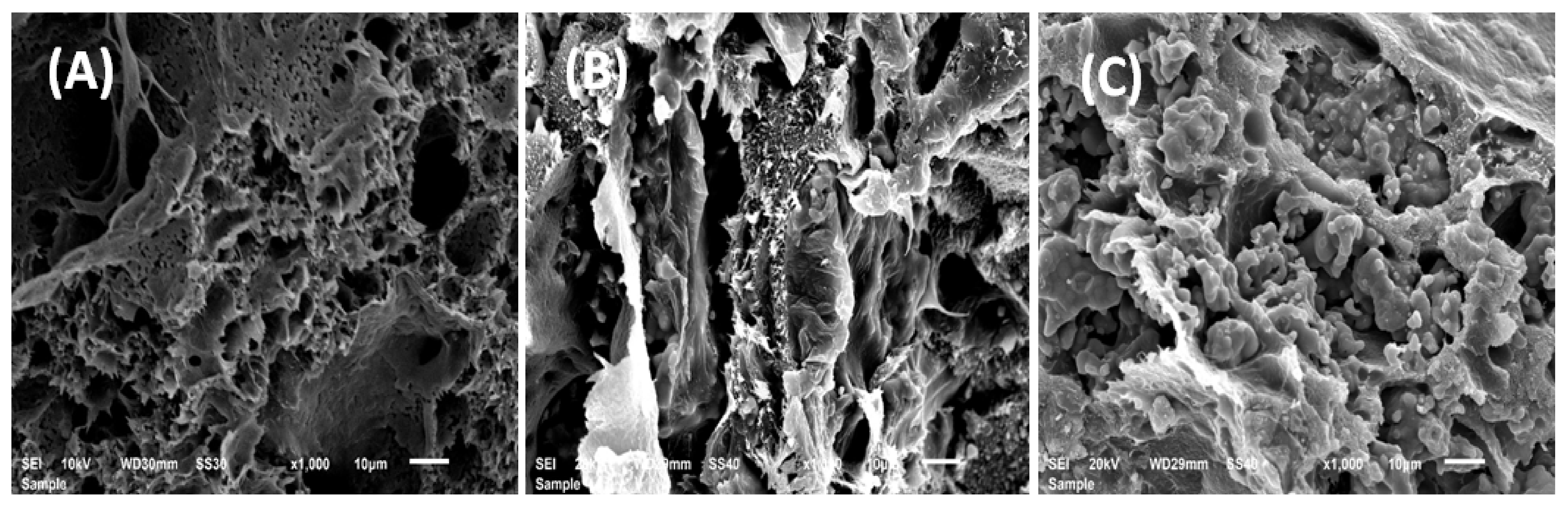
3.1.3. Scanning Electron Microscopy (SEM) Analysis
3.1.4. Porosity
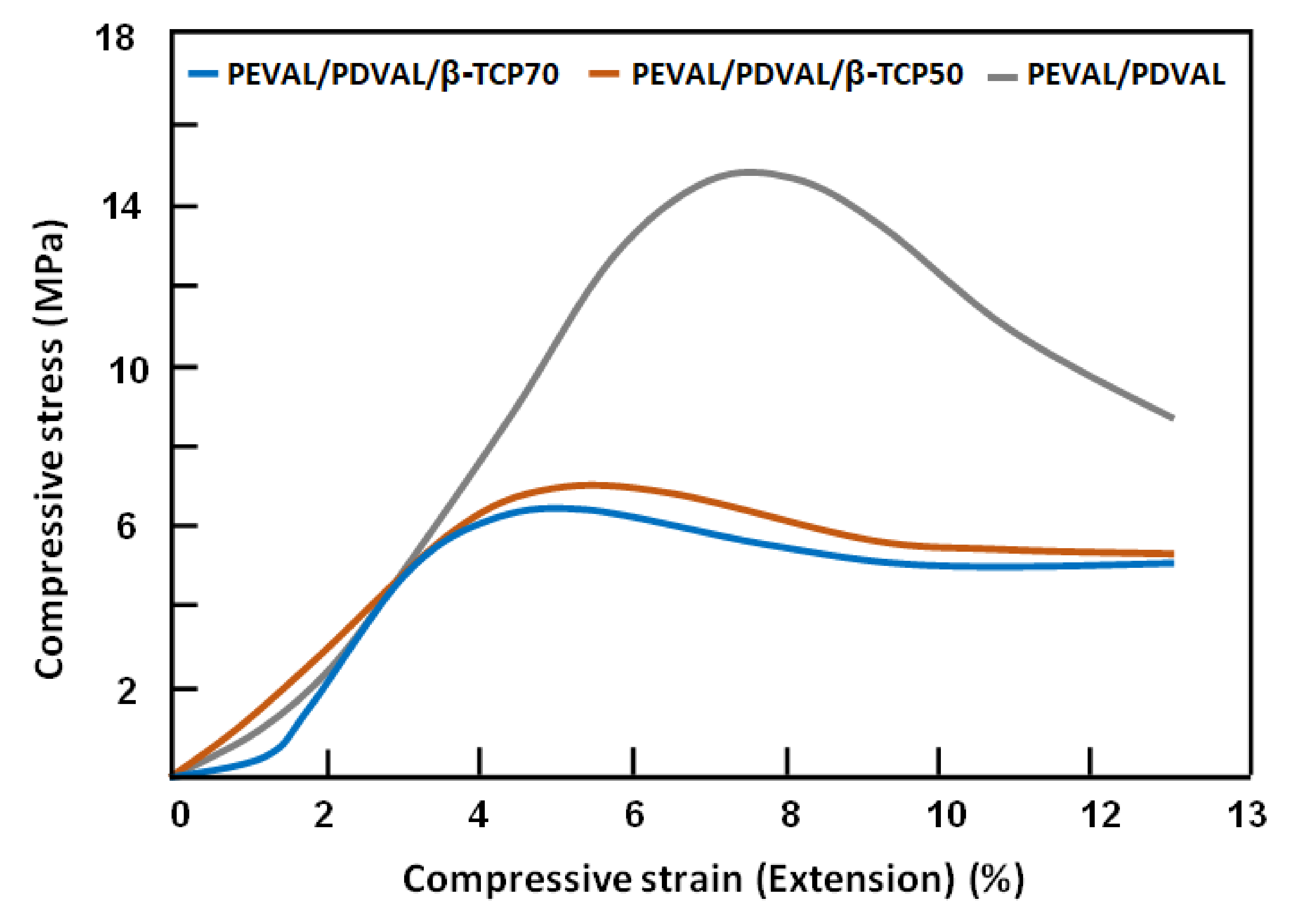
3.1.5. Mechanical Testing
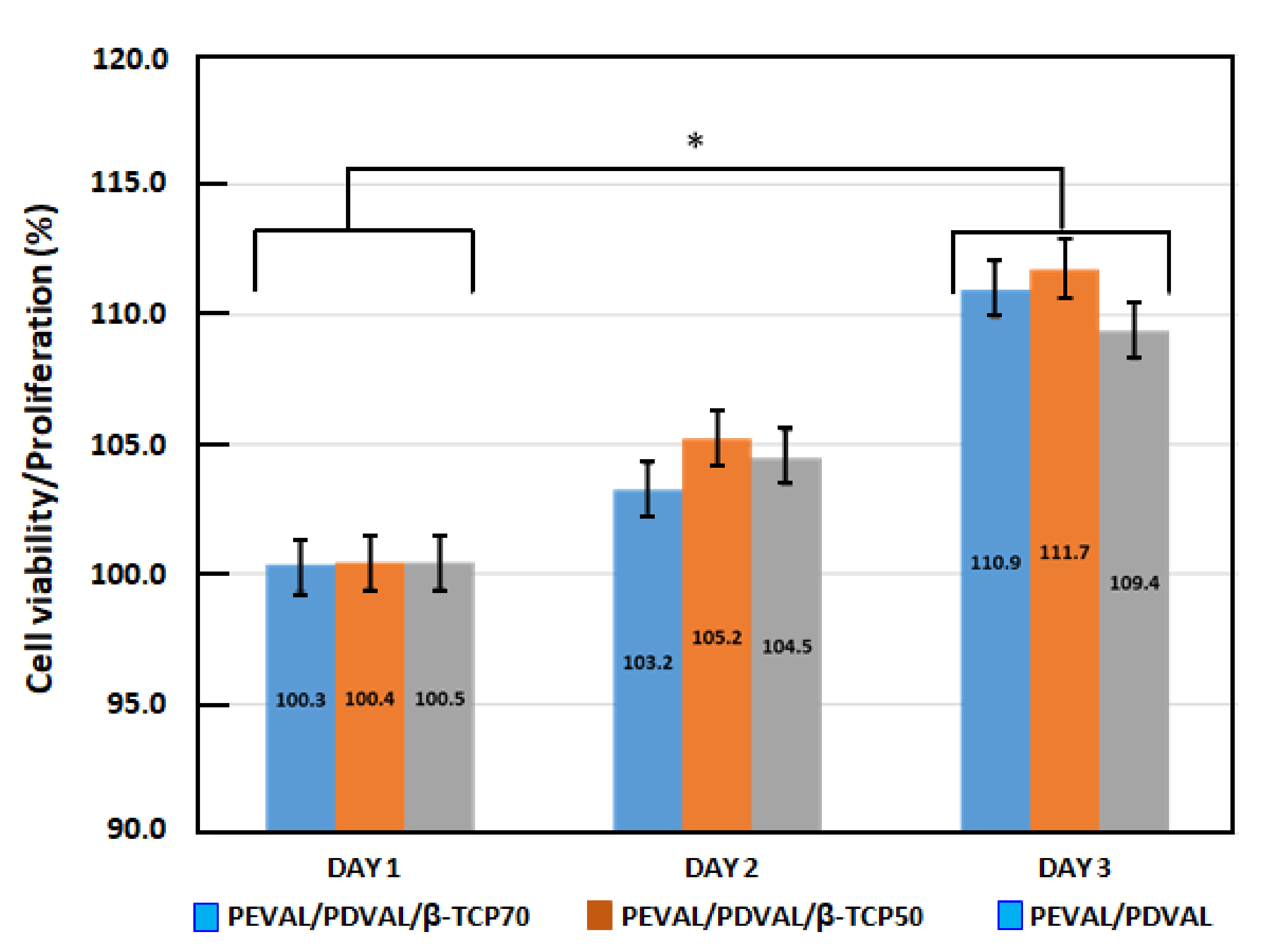
3.1.6. Cellular Viability and Proliferation
3.1.7. Antibacterial Activity
3.1.8. Statistical Analysis
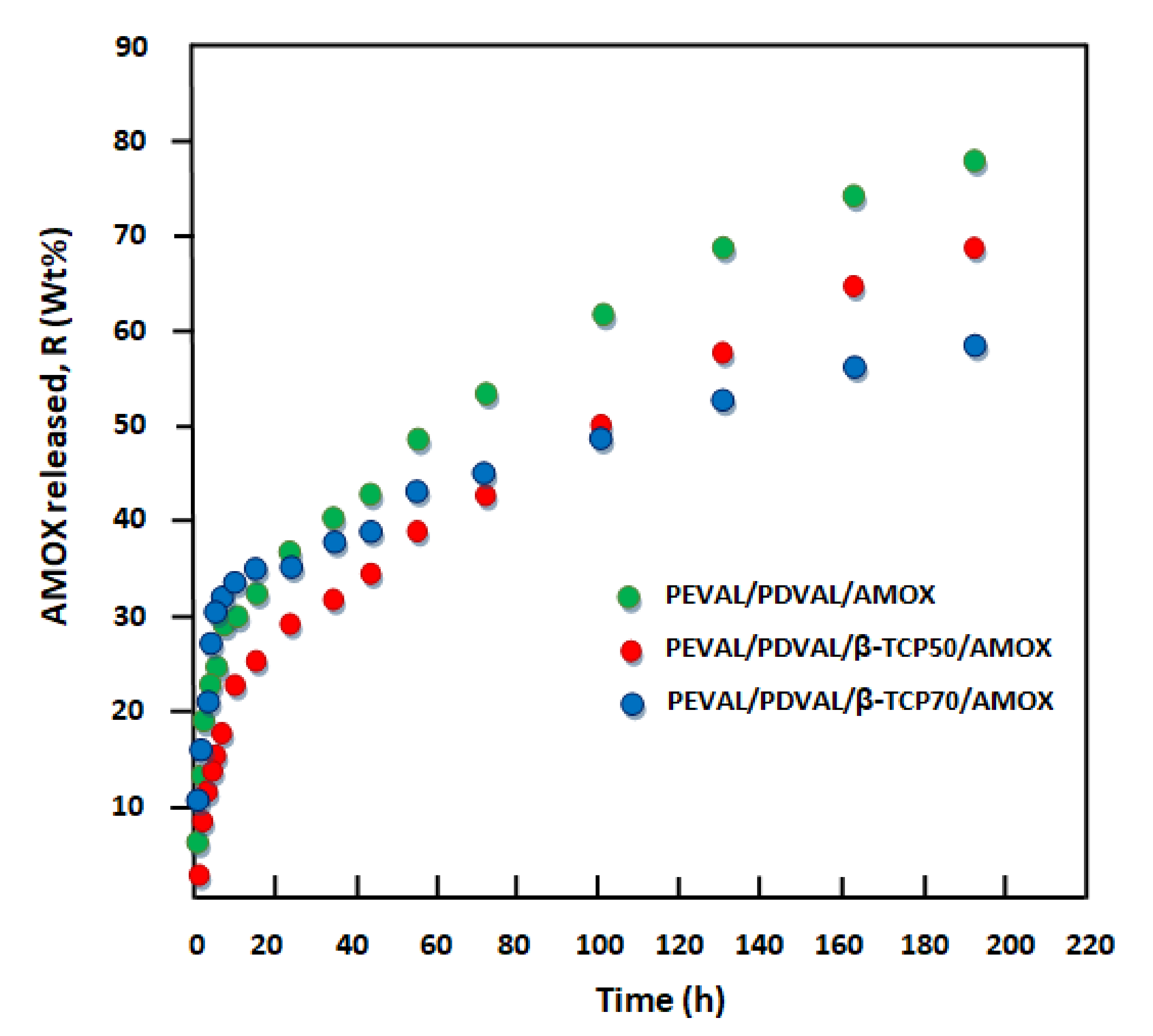
3.2. Kinetics Release of Amoxicillin
Diffusion Behavior of Amoxicillin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, X.; Li, Y.; Huang, Q.; Yang, J.; Shen, B.; Pei, F. Evaluation of a biodegradable graft substitute in rabbit bone defect model. Indian J. Orthop. 2012, 46, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Bruder, S.P.; Fox, B.S. Tissue engineering of bone. Cell based strategies. Clin. Orthop. Relat. Res. 1999, 367, S68–S83. [Google Scholar] [CrossRef] [PubMed]
- Pina, S.; Oliveira, J.M.; Reis, R.L. Natural-based nanocomposites for bone tissue engineering and regenerative medicine: A review. Adv. Mater. 2015, 27, 1143–1169. [Google Scholar] [CrossRef] [PubMed]
- Mano, J.; Silva, G.; Azevedo, H.S.; Malafaya, P.; Sousa, R.; Silva, S.S.; Boesel, L.; Oliveira, J.M.; Santos, T.; Marques, A. Natural origin biodegradable systems in tissue engineering and regenerative medicine: Present status and some moving trends. J. R. Soc. Interface 2007, 4, 999–1030. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.H.; Alves, A.; Popa, E.G.; Reys, L.L.; Gomes, M.E.; Sousa, R.A.; Silva, S.S.; Mano, J.F.; Reis, R.L. Marine algae sulfated polysaccharides for tissue engineering and drug delivery approaches. Biomatter 2012, 2, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Barbani, N.; Guerra, G.D.; Cristallini, C.; Urciuoli, P.; Avvisati, R.; Sala, A.; Rosellini, E. Hydroxyapatite/gelatin/gellan sponges as nanocomposite scaffolds for bone reconstruction. J. Mater. Sci. Mater. Med. 2012, 23, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Thavornyutikarn, B.; Chantarapanich, N.; Sitthiseripratip, K.; Thouas, G.A.; Chen, Q. Bone tissue engineering scaffolding: Computer-aided scaffolding techniques. Prog. Biomater. 2014, 3, 61–102. [Google Scholar] [CrossRef]
- Wojtowicz, A.M.; Shekaran, A.; Oest, M.E.; Dupont, K.M.; Templeman, K.L.; Hutmacher, D.W.; Guldberg, R.E.; García, A.J. Coating of biomaterial scaffolds with the collagen-mimetic peptide GFOGER for bone defect repair. Biomaterials 2010, 31, 2574–2582. [Google Scholar] [CrossRef]
- Garg, T.; Bilandi, A.; Kapoor, B.; Kumar, S.; Joshi, R. Scaffold: Tissue engineering and regenerative medicine. Int Res. J. Pharm. 2011, 2, 37–42. [Google Scholar]
- Kim, J.Y.; Cho, D.-W. Blended PCL/PLGA scaffold fabrication using multi-head deposition system. Microelectron. Eng. 2009, 86, 1447–1450. [Google Scholar] [CrossRef]
- Shan, X.; Yuan, Y.; Liu, C.; Tao, X.; Sheng, Y.; Xu, F. Influence of PEG chain on the complement activation suppression and longevity in vivo prolongation of the PCL biomedical nanoparticles. Biomed. Microdevices 2009, 11, 1187. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, G. Biocomposites electrospun with poly (ε-caprolactone) and silk fibroin powder for biomedical applications. J. Biomater. Sci. Polym. Ed. 2010, 21, 1687–1699. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-W.; Su, J.-C.; Ma, Y.-H.; Zhang, X.; Cao, L.-H.; Li, M. Preparation and Properties of Nano Calcium Deficient Apatite/Poly (epsilon-caprolactone) Composite Scaffold. J. Inorg. Mater. 2010, 25, 500–506. [Google Scholar] [CrossRef]
- Palumbo, F.S.; Bongiovì, F.; Carfì Pavia, F.; Vitrano, I.; La Carrubba, V.; Pitarresi, G.; Brucato, V.; Giammona, G. Blend scaffolds with polyaspartamide/polyester structure fabricated via TIPS and their RGDC functionalization to promote osteoblast adhesion and proliferation. J. Biomed. Mater. Res. Part A 2019, 107, 2726–2735. [Google Scholar] [CrossRef]
- Shahmoradi, S.; Ahmadalipour, A.; Salehi, M. Evaluation of ACE gene I/D polymorphism in Iranian elite athletes. Adv. Biomed. Res. 2014, 3, 207. [Google Scholar] [PubMed]
- Miao, X.; Lim, W.-K.; Huang, X.; Chen, Y. Preparation and characterization of interpenetrating phased TCP/HA/PLGA composites. Mater. Lett. 2005, 59, 4000–4005. [Google Scholar] [CrossRef]
- Miao, X.; Tan, D.M.; Li, J.; Xiao, Y.; Crawford, R. Mechanical and biological properties of hydroxyapatite/tricalcium phosphate scaffolds coated with poly (lactic-co-glycolic acid). Acta Biomater. 2008, 4, 638–645. [Google Scholar] [CrossRef]
- Kucharska, M.; Walenko, K.; Lewandowska-Szumieł, M.; Brynk, T.; Jaroszewicz, J.; Ciach, T. Chitosan and composite microsphere-based scaffold for bone tissue engineering: Evaluation of tricalcium phosphate content influence on physical and biological properties. J. Mater. Sci. Mater. Med. 2015, 26, 143. [Google Scholar] [CrossRef]
- Albertsson, A.-C.; Varma, I.K. Recent developments in ring opening polymerization of lactones for biomedical applications. Biomacromolecules 2003, 4, 1466–1486. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, Y.; Zhang, Y.; Liu, C.; Shi, W.; Li, Q. Lipase/esterase-catalyzed ring-opening polymerization: A green polyester synthesis technique. Process. Biochem. 2011, 46, 1900–1908. [Google Scholar] [CrossRef]
- Khalil, M.; Al-Shamary, D.; Al-Deyab, S. Synthesis of poly (δ-valerolactone) by activated monomer polymerization, its characterization and potential medical application. Asian J. Biochem. Pharm. Res. 2015, 5, 137–147. [Google Scholar]
- Hu, Q.; Jie, S.-Y.; Braunstein, P.; Li, B.-G. Ring-opening Copolymerization of ε-Caprolactone and δ-Valerolactone Catalyzed by a 2, 6-Bis (amino) phenol Zinc Complex. Chin. J. Polym. Sci. 2020, 38, 240–247. [Google Scholar] [CrossRef]
- Keskin, S.; Elliott, J.R. Binary interactions of poly (ethylene covinyl alcohol) with poly (4-vinyl pyridine) and poly (n-butyl methacrylate). Ind. Eng. Chem. Res. 2003, 42, 6331–6337. [Google Scholar] [CrossRef]
- Wu, H.; Jiang, T.; Zhu, J.; Cui, K.; Zhao, Q.; Ma, Z. Synthesis of poly (ethylene-co-vinyl alcohol)-g-polystyrene graft copolymer and their applications for ordered porous film and compatibilizer. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 516–524. [Google Scholar] [CrossRef]
- Nakano, A. Ethylene vinyl alcohol co-polymer as a high-performance membrane: An EVOH membrane with excellent biocompatibility. In High-Performance Membrane Dialyzers; Karger Publishers: Basel, Switzerland, 2011; Volume 173, pp. 164–171. [Google Scholar]
- Arboleda, E.C.; Segura, S.F.; Mejía, G.A.I. Enzymatic transformation of crystalline structure of copolymer poly (ethylene-co-vinyl alcohol)(EVOH). Vitae 2007, 14, 25–30. [Google Scholar]
- De Lima, J.A.; Felisberti, M.I. Poly (ethylene-co-vinyl alcohol) and poly (methyl methacrylate) blends: Phase behavior and morphology. Eur. Polym. J. 2008, 44, 1140–1148. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Layman, J.M.; Watkins, J.R.; Bowlin, G.L.; Matthews, J.A.; Simpson, D.G.; Wnek, G.E. Electrospinning of poly (ethylene-co-vinyl alcohol) fibers. Biomaterials 2003, 24, 907–913. [Google Scholar] [CrossRef]
- Alghamdi, A.A.; Saeed, W.S.; Al-Odayni, A.-B.; Alharthi, F.A.; Semlali, A.; Aouak, T. Poly (ethylene-co-vinylalcohol)/Poly (δ-valerolactone)/Aspirin Composite: Model for a New Drug-Carrier System. Polymers 2019, 11, 439. [Google Scholar] [CrossRef]
- López, O.B.L.; Sierra, G.L.; Mejía, G.A.I. Biodegradability of poly (vinyl alcohol). Polym. Eng. Sci. 1999, 39, 1346–1352. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates. J. Mater. Sci. 2007, 42, 1061–1095. [Google Scholar] [CrossRef]
- Fernandez-Yague, M.A.; Abbah, S.A.; McNamara, L.; Zeugolis, D.I.; Pandit, A.; Biggs, M.J. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Adv. Drug Deliv. Rev. 2015, 84, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Kanungo, B.P.; Silva, E.; Van Vliet, K.; Gibson, L.J. Characterization of mineralized collagen-glycosaminoglycan scaffolds for bone regeneration. Acta Biomater. 2008, 4, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and Synthetic Polymers for Bone Scaffolds Optimization. Polymers 2020, 12, 905. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.; Al-Rasheed, A.; ArRejaie, A.; Nooh, N.; Al-Kindi, M.; Al-Hezaimi, K. Guided bone regeneration in standardized calvarial defects using beta-tricalcium phosphate and collagen membrane: A real-time in vivo micro-computed tomographic experiment in rats. Odontology 2016, 104, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Brkovic, B.; Prasad, H.S.; Konandreas, G.; Milan, R.; Antunovic, D.; Sándor, G.; Rohrer, M.D. Simple preservation of a maxillary extraction socket using beta-tricalcium phosphate with type I collagen: Preliminary clinical and histomorphometric observations. J. Can. Dent. Assoc. 2008, 74, 523–528. [Google Scholar]
- Franceschetti, G.; Farina, R.; Minenna, L.; Riccardi, O.; Stacchi, C.; Di Raimondo, R.; Maietti, E.; Trombelli, L. The impact of graft remodeling on peri-implant bone support at implants placed concomitantly with transcrestal sinus floor elevation: A multicenter, retrospective case series. Clin. Oral Implant. Res. 2020, 31, 105–120. [Google Scholar] [CrossRef]
- Siqueira, L.D.; Passador, F.R.; Lobo, A.O.; Trichês, E.D.S. Morphological, thermal and bioactivity evaluation of electrospun PCL/β-TCP fibers for tissue regeneration. Polímeros 2019, 29, e2019005. [Google Scholar] [CrossRef]
- Backes, E.H.; de Nóbile Pires, L.; Selistre-de-Araujo, H.S.; Costa, L.C.; Passador, F.R.; Pessan, L.A. Development and characterization of printable PLA/β-TCP bioactive composites for bone tissue applications. J. Appl. Polym. Sci. 2020, 138, 49759. [Google Scholar] [CrossRef]
- Dong, X.; Cheng, Q.; Long, Y.; Xu, C.; Fang, H.; Chen, Y.; Dai, H. A Chitosan Based Scaffold with Enhanced Mechanical and Biocompatible Performance for Biomedical Applications. Polym. Degrad. Stab. 2020, 181, 109322. [Google Scholar] [CrossRef]
- Pihlman, H.; Keränen, P.; Paakinaho, K.; Linden, J.; Hannula, M.; Manninen, I.-K.; Hyttinen, J.; Manninen, M.; Laitinen-Vapaavuori, O. Novel osteoconductive β-tricalcium phosphate/poly (L-lactide-co-e-caprolactone) scaffold for bone regeneration: A study in a rabbit calvarial defect. J. Mater. Sci. Mater. Med. 2018, 29, 156. [Google Scholar] [CrossRef]
- Aouak, T.; Ouladsmane, M.; Alghamdi, A.A.; Al-Owais, A.A.; Al-Turki, T.M.; Alothman, Z.A.; Saeed, W.S. Fabrication of tissue engineering scaffold from poly (vinylalcohol-co-ethylene)/poly (D, L-lactic-co-glycolic acid) blend: Miscibility, thermomechanical properties, and morphology. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 526–536. [Google Scholar] [CrossRef]
- Lino, A.B.; McCarthy, A.D.; Fernández, J.M. Evaluation of strontium-containing PCL-PDIPF scaffolds for bone tissue engineering: In vitro and in vivo studies. Ann. Biomed. Eng. 2019, 47, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Castillo, S.; Bernal-Ballén, A.; Bernal-López, C.; Segura-Puello, H.; Nieto-Mosquera, D.; Villamil-Ballesteros, A.; Muñoz-Forero, D.; Munster, L. Synthesis and characterization of poly (vinyl alcohol)-chitosan-hydroxyapatite scaffolds: A promising alternative for bone tissue regeneration. Molecules 2018, 23, 2414. [Google Scholar] [CrossRef]
- Xu, T.; Liang, Z.; Ding, B.; Feng, Q.; Fong, H. Polymer blend nanofibers containing polycaprolactone as biocompatible and biodegradable binding agent to fabricate electrospun three-dimensional scaffolds/structures. Polymer 2018, 151, 299–306. [Google Scholar] [CrossRef]
- Saeed, W.S.; Al-Odayni, A.-B.; Ali Alghamdi, A.; Abdulaziz Al-Owais, A.; Semlali, A.; Aouak, T. Miscibility of Poly (Ethylene-co-Vinylalcohol)/Poly (δ-Valerolactone) Blend and Tissue Engineering Scaffold Fabrication Using Naphthalene as Porogen. Polym. Plast. Technol. Mater. 2019, 58, 1–23. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- Saeed, W.S.; Al-Odayni, A.-B.; Alrahlah, A.; Alghamdi, A.A.; Aouak, T. Preparation and Characterization of Poly (δ-Valerolactone)/TiO2 Nanohybrid Material with Pores Interconnected for Potential Use in Tissue Engineering. Materials 2019, 12, 528. [Google Scholar] [CrossRef]
- Kumar, P.T.S.; Abhilash, S.; Manzoor, K.; Nair, S.V.; Tamura, H.; Jayakumar, R. Preparation and characterization of novel β-chitin/nanosilver composite scaffolds for wound dressing applications. Carbohydr. Polym. 2010, 80, 761–767. [Google Scholar] [CrossRef]
- Manikandan, M.; Abuelreich, S.; Elsafadi, M.; Alsalman, H.; Almalak, H.; Siyal, A.; Hashmi, J.A.; Aldahmash, A.; Kassem, M.; Alfayez, M.; et al. NR2F1 mediated down-regulation of osteoblast differentiation was rescued by bone morphogenetic protein-2 (BMP-2) in human MSC. Differentiation 2018, 104, 36–41. [Google Scholar] [CrossRef]
- Paris, J.L.; Lafuente-Gómez, N.; Cabañas, M.V.; Román, J.; Peña, J.; Vallet-Regí, M. Fabrication of a nanoparticle-containing 3D porous bone scaffold with proangiogenic and antibacterial properties. Acta Biomater. 2019, 86, 441–449. [Google Scholar] [CrossRef]
- Tsuji, A.; Nakashima, E.; Hamano, S.; Yamana, T. Physicochemical properties of amphoteric β-lactam antibiotics I: Stability, solubility, and dissolution behavior of amino penicillins as a function of pH. J. Pharm. Sci. 1978, 67, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Beale, J.M.; Block, J.; Hill, R. Organic Medicinal and Pharmaceutical Chemistry; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011. [Google Scholar]
- Sariibrahimoglu, K.; Wolke, J.G.; Leeuwenburgh, S.C.; Yubao, L.; Jansen, J.A. Injectable biphasic calcium phosphate cements as a potential bone substitute. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Yang, Y.; Fu, Z.; Li, Y.; Shi, J.; Ma, D.; Liu, S.; Luo, D. Fe/Zn-modified tricalcium phosphate (TCP) biomaterials: Preparation and biological properties. RSC Adv. 2019, 9, 781–789. [Google Scholar] [CrossRef]
- Takahashi, M.; Tashiro, K.; Amiya, S. Crystal Structure of Ethylene—Vinyl Alcohol Copolymers. Macromolecules 1999, 32, 5860–5871. [Google Scholar] [CrossRef]
- Ren, Y.; Wei, Z.; Wu, T.; Bian, Y.; Leng, X.; Zhou, C.; Li, Y. Synthesis of highly branched poly (δ-valerolactone)s: A comparative study between comb and linear analogues. RSC Adv. 2016, 6, 45791–45801. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Penk, A.; Förster, Y.; Scheidt, H.A.; Nimptsch, A.; Hacker, M.C.; Schulz-Siegmund, M.; Ahnert, P.; Schiller, J.; Rammelt, S.; Huster, D. The pore size of PLGA bone implants determines the de novo formation of bone tissue in tibial head defects in rats. Magn. Reson. Med. 2013, 70, 925–935. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes—Different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef]
- Schwarz, K.; Epple, M. Hierarchically structured polyglycolide—A biomaterial mimicking natural bone. Macromol. Rapid Commun. 1998, 19, 613–617. [Google Scholar]
- Holmes, R.E. Bone regeneration within a coralline hydroxyapatite implant. Plast. Reconstr. Surg. 1979, 63, 626–633. [Google Scholar] [CrossRef]
- Mao, D.; Li, Q.; Bai, N.; Dong, H.; Li, D. Porous stable poly(lactic acid)/ethyl cellulose/hydroxyapatite composite scaffolds prepared by a combined method for bone regeneration. Carbohydr. Polym. 2018, 180, 104–111. [Google Scholar] [CrossRef]
- Shi, X.; Sitharaman, B.; Pham, Q.P.; Liang, F.; Wu, K.; Edward Billups, W.; Wilson, L.J.; Mikos, A.G. Fabrication of porous ultra-short single-walled carbon nanotube nanocomposite scaffolds for bone tissue engineering. Biomaterials 2007, 28, 4078–4090. [Google Scholar] [CrossRef]
- Arvidson, K.; Abdallah, B.; Applegate, L.; Baldini, N.; Cenni, E.; Gomez-Barrena, E.; Granchi, D.; Kassem, M.; Konttinen, Y.; Mustafa, K. Bone regeneration and stem cells. J. Cell. Mol. Med. 2011, 15, 718–746. [Google Scholar] [CrossRef]
- Kang, Y.; Scully, A.; Young, D.A.; Kim, S.; Tsao, H.; Sen, M.; Yang, Y. Enhanced mechanical performance and biological evaluation of a PLGA coated β-TCP composite scaffold for load-bearing applications. Eur. Polym. J. 2011, 47, 1569–1577. [Google Scholar] [CrossRef]
- Kim, H.-W.; Knowles, J.C.; Kim, H.-E. Hydroxyapatite/poly(ε-caprolactone) composite coatings on hydroxyapatite porous bone scaffold for drug delivery. Biomaterials 2004, 25, 1279–1287. [Google Scholar] [CrossRef]
- Gibson, L. The mechanical behaviour of cancellous bone. J. Biomech. 1985, 18, 317–328. [Google Scholar] [CrossRef]
- Alghamdi, A.A.; Alattas, H.; Saeed, W.S.; Al-Odayni, A.-B.; Alrahlah, A.; Aouak, T. Preparation and Characterization of Poly (ethylene-co-vinyl alcohol)/poly (ε-caprolactone) Blend for Bioscaffolding Applications. Int. J. Mol. Sci. 2020, 21, 5881. [Google Scholar] [CrossRef]
- Güncüm, E.; Işıklan, N.; Anlaş, C.; Ünal, N.; Bulut, E.; Bakırel, T. Development and characterization of polymeric-based nanoparticles for sustained release of amoxicillin—An antimicrobial drug. Artif. Cells Nanomed. Biotechnol. 2018, 46, 964–973. [Google Scholar] [CrossRef]
- Lin, M.; Wang, H.; Meng, S.; Zhong, W.; Li, Z.; Cai, R.; Chen, Z.; Zhou, X.; Du, Q. Structure and release behavior of PMMA/silica composite drug delivery system. J. Pharm. Sci. 2007, 96, 1518–1526. [Google Scholar] [CrossRef]
- Reinhard, C.S.; Radomsky, M.L.; Saltzman, W.M.; Hilton, J.; Brem, H. Polymeric controlled release of dexamethasone in normal rat brain. J. Control. Release 1991, 16, 331–339. [Google Scholar] [CrossRef]







| System | PEVAL (g) | PDVAL (g) | β-TCP (g) | β-TCP (wt%) |
|---|---|---|---|---|
| PEVAL/PDVAL0 | 0.50 | 0.50 | 0 | 0 |
| PEVAL/PDVAL/β-TCP50 | 0.25 | 0.25 | 0.5 | 50 |
| PEVAL/PDVAL/β-TCP70 | 0.15 | 0.15 | 0.7 | 70 |
| Drug Carrier System | Stable Zone (h) | Amoxicillin Released (wt%) | Release Rate (wt%∙h−1) |
|---|---|---|---|
| PEVAL/PDVAL/AMOX | 0–5 | 30.10 ± 0.34 | 6.02 ± 0.02 |
| 5–158 | 48.02 ± 3.03 | 0.31 ± 0.06 | |
| PEVAL/PDVAL/β-TCP50/AMOX | 0–5 | 22.65 ± 0.35 | 4.53 ± 0.03 |
| 5–156 | 42.0 ± 2.50 | 0.27 ± 0.05 | |
| PEVAL/PDVAL/β-TCP70/AMOX | 0–5 | 33.70 ± 2.50 | 6.73 ± 0.02 |
| 5–156 | 25.0 ± 0.42 | 0.16 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badwelan, M.; Alkindi, M.; Alghamdi, O.; Saeed, W.S.; Al-Odayni, A.-B.; Alrahlah, A.; Aouak, T. Poly(δ-valerolactone)/Poly(ethylene-co-vinylalcohol)/β-Tricalcium Phosphate Composite as Scaffolds: Preparation, Properties, and In Vitro Amoxicillin Release. Polymers 2021, 13, 46. https://doi.org/10.3390/polym13010046
Badwelan M, Alkindi M, Alghamdi O, Saeed WS, Al-Odayni A-B, Alrahlah A, Aouak T. Poly(δ-valerolactone)/Poly(ethylene-co-vinylalcohol)/β-Tricalcium Phosphate Composite as Scaffolds: Preparation, Properties, and In Vitro Amoxicillin Release. Polymers. 2021; 13(1):46. https://doi.org/10.3390/polym13010046
Chicago/Turabian StyleBadwelan, Mohammed, Mohammed Alkindi, Osama Alghamdi, Waseem Sharaf Saeed, Abdel-Basit Al-Odayni, Ali Alrahlah, and Taieb Aouak. 2021. "Poly(δ-valerolactone)/Poly(ethylene-co-vinylalcohol)/β-Tricalcium Phosphate Composite as Scaffolds: Preparation, Properties, and In Vitro Amoxicillin Release" Polymers 13, no. 1: 46. https://doi.org/10.3390/polym13010046
APA StyleBadwelan, M., Alkindi, M., Alghamdi, O., Saeed, W. S., Al-Odayni, A.-B., Alrahlah, A., & Aouak, T. (2021). Poly(δ-valerolactone)/Poly(ethylene-co-vinylalcohol)/β-Tricalcium Phosphate Composite as Scaffolds: Preparation, Properties, and In Vitro Amoxicillin Release. Polymers, 13(1), 46. https://doi.org/10.3390/polym13010046