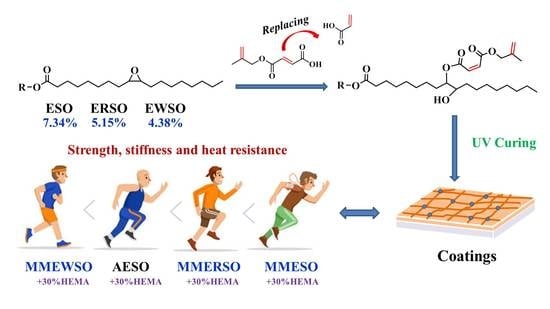
Preparation and Properties of Plant-Oil-Based Epoxy Acrylate-Like Resins for UV-Curable Coatings
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of MAAMA
2.3. Epoxidation of Plant Oils
2.4. Synthesis of MMESO, MMERSO, and MMEWSO
2.5. Curing of MMESO, MMERSO, and MMEWSO Resins
2.6. Characterization
2.6.1. Acid Value (Av)
2.6.2. Epoxy Values (E)
2.6.3. Fourier Transform Infrared Spectroscopy Analysis (FT-IR)
2.6.4. Nuclear Magnetic Resonance Analysis (NMR)
2.6.5. Gel Content (Cgel)
2.6.6. Dynamic Mechanical Analysis (DMA)
2.6.7. Thermogravimetric Analysis (TGA)
2.6.8. Mechanical Properties
2.6.9. Coating Properties
2.6.10. UV-Curing Kinetics
3. Results and Discussion
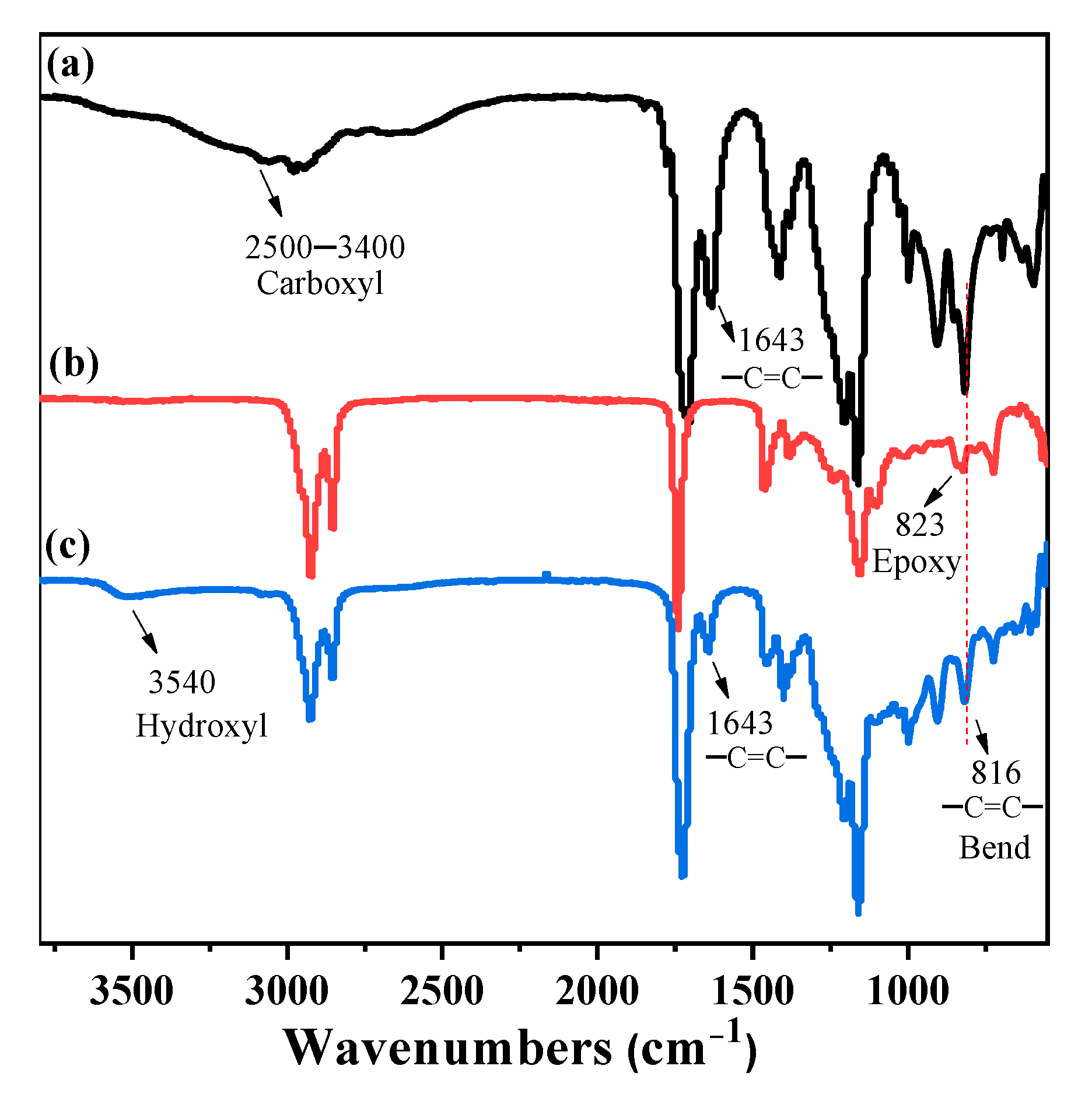
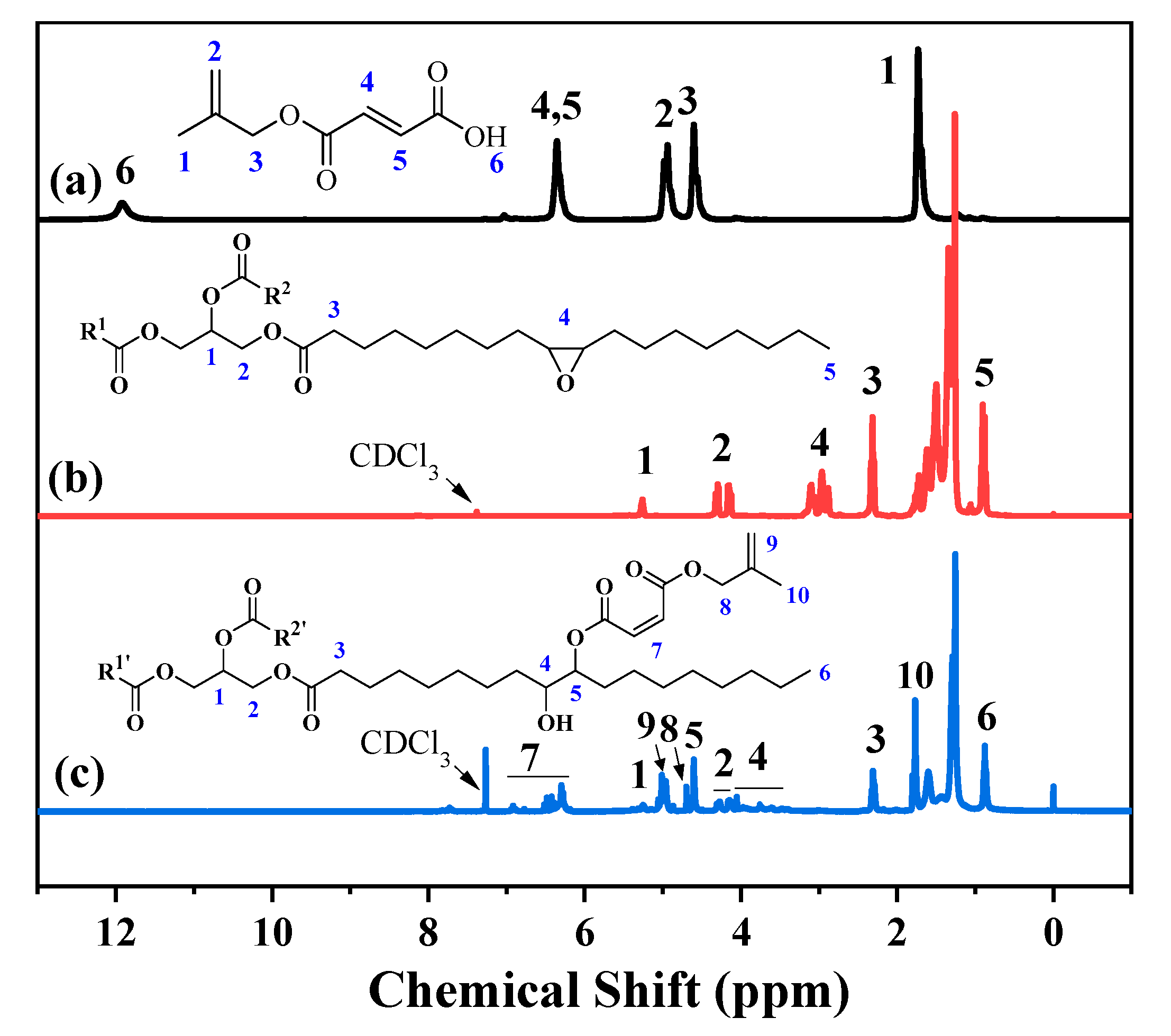
3.1. Synthesis and Characterization of Oil-Based EA-Like Prepolymers
3.2. Bio-Based Content of the UV-Cured EA-Like Materials
3.3. Gel Contents of the UV-Cured EA-Like Materials
3.4. Properties of the UV-Cured EA-Like Materials
3.4.1. Dynamic Mechanical Analysis
3.4.2. Thermogravimetric Analysis
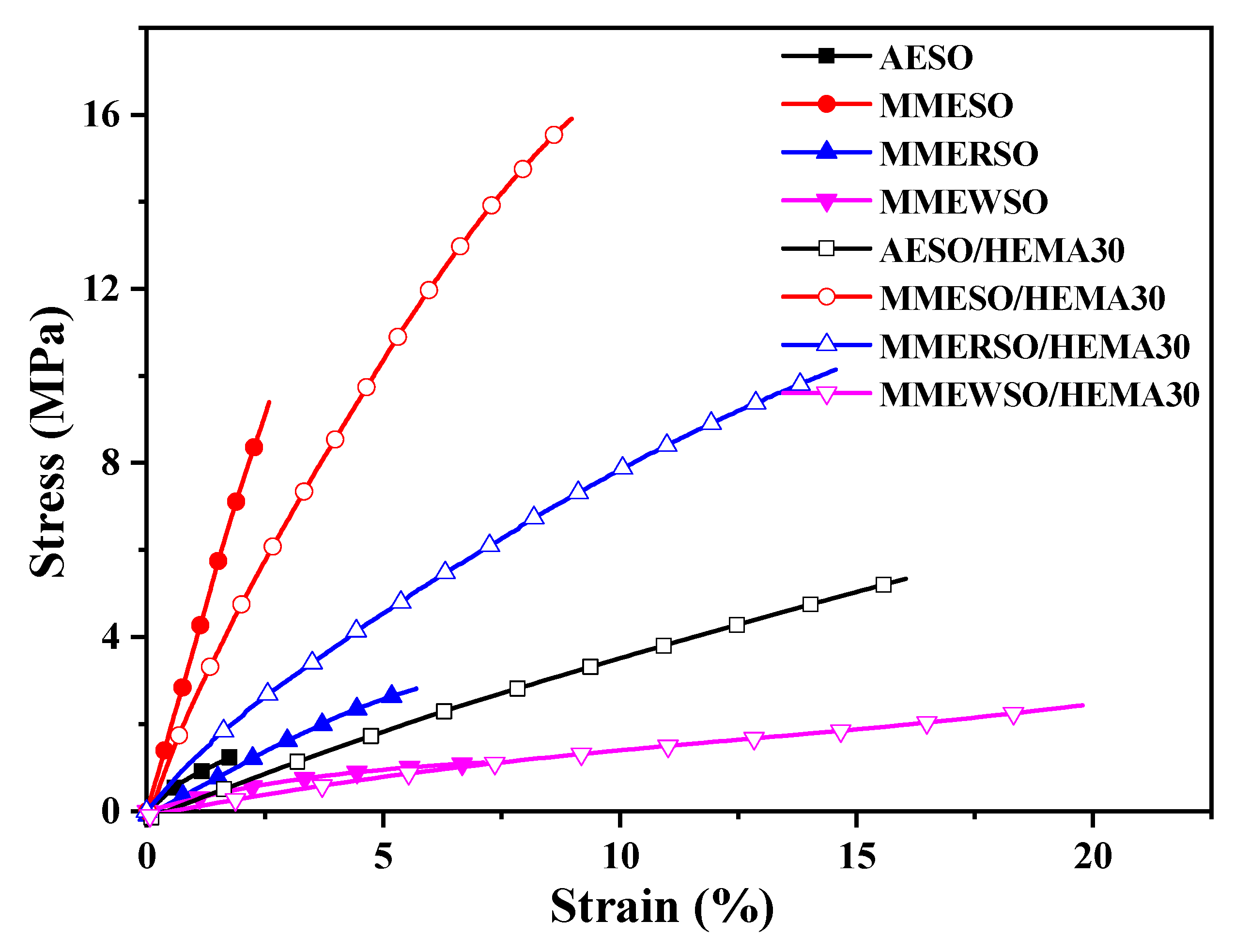
3.4.3. Mechanical Properties
3.4.4. Coating Properties
3.5. UV-Curing Kinetics of the EA-Like Resins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zovi, O.; Lecamp, L.; Loutelier-Bourhis, C.; Lange, C.M.; Bunel, C. A solventless synthesis process of new UV-curable materials based on linseed oil. Green Chem. 2001, 13, 1014–1022. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, Y.; Liu, X.; Fan, L.; Zhu, J. Bio-based tetrafunctional crosslink agent from gallic acid and its enhanced soybean oil-based UV-cured coatings with high performance. RSC Adv. 2014, 4, 23036–23042. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, G.; Cui, Y.; Tian, J.; He, M.; Yang, J.-W. Castor Oil Based Biothiol as a Highly Stable and Self-Initiated Oligomer for Photoinitiator-Free UV Coatings. ACS Sustain. Chem. Eng. 2016, 5, 376–381. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, H.; Han, X.; Zhou, Z.; Xu, W.; Ren, F. Synthesis and properties of unsaturated modified linoleate for fast UV-curable coatings. Energ. Source Part A 2019, 1–10. [Google Scholar] [CrossRef]
- Shen, L.; Pan, Y.; Fu, H. Fabrication of UV curable coating for super hydrophobic cotton fabrics. Polym. Eng. Sci. 2019, 59. [Google Scholar] [CrossRef]
- Ang, D.T.C.; Gan, S.N. Novel approach to convert non-self drying palm stearin alkyds into environmental friendly UV curable resins. Prog. Org. Coat. 2012, 73, 409–414. [Google Scholar] [CrossRef]
- Dai, J.; Liu, X.; Ma, S.; Wang, J.; Shen, X.; You, S.; Zhu, J. Soybean oil-based UV-curable coatings strengthened by crosslink agent derived from itaconic acid together with 2-hydroxyethyl methacrylate phosphate. Prog. Org. Coat. 2016, 97, 210–215. [Google Scholar] [CrossRef]
- Huang, Y.; Ye, G.; Yang, J. Synthesis and properties of UV-curable acrylate functionalized tung oil based resins via Diels–Alder reaction. Prog. Org. Coat. 2015, 78, 28–34. [Google Scholar] [CrossRef]
- Patil, D.M.; Phalak, G.A.; Mhakse, S.T. Boron-containing UV-curable oligomer-based linseed oil as flame-retardant coatings: Synthesis and characterization. Iran. Polym. J. 2018, 27, 795–806. [Google Scholar] [CrossRef]
- Biermann, U.; Bornscheuer, U.; Meier, M.A.; Metzger, J.O.; Schafer, H.J. Oils and fats as renewable raw materials in chemistry. Angew Chem. Int. Ed. Engl. 2011, 50, 3854–3871. [Google Scholar] [CrossRef]
- Ahn, B.K.; Sung, J.; Kim, N.; Kraft, S.; Sun, X.S. UV-curable pressure-sensitive adhesives derived from functionalized soybean oils and rosin ester. Polym. Int. 2013, 62, 1293–1301. [Google Scholar] [CrossRef]
- Sharmin, E.; Zafar, F.; Akram, D.; Alam, M.; Ahmad, S. Recent advances in vegetable oils based environment friendly coatings: A review. Ind. Crop. Prod. 2015, 76, 215–229. [Google Scholar] [CrossRef]
- Fertier, L.; Koleilat, H.; Stemmelen, M.; Giani, O.; Joly-Duhamel, C.; Lapinte, V.; Robin, J.-J. The use of renewable feedstock in UV-curable materials—A new age for polymers and green chemistry. Prog. Polym. Sci. 2013, 38, 932–962. [Google Scholar] [CrossRef]
- Huang, Y.; Pang, L.; Wang, H.; Zhong, R.; Zeng, Z.; Yang, J. Synthesis and properties of UV-curable tung oil based resins via modification of Diels—Alder reaction, nonisocyanate polyurethane and acrylates. Prog. Org. Coat. 2013, 76, 654–661. [Google Scholar] [CrossRef]
- Rengasamy, S.; Mannari, V. Development of soy-based UV-curable acrylate oligomers and study of their film properties. Prog. Org. Coat. 2013, 76, 78–85. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, T.; Ma, G.; Li, P.; Ling, L.; Wang, B. Synthesis of a tung oil-rosin adduct via the diels-alder reaction: Its reaction mechanism and properties in an ultraviolet-curable adhesive. J. Appl. Polym. Sci. 2013, 130. [Google Scholar] [CrossRef]
- Li, K.; Shen, Y.; Fei, G.; Wang, H.; Li, J. Preparation and properties of castor oil/pentaerythritol triacrylate-based UV curable waterborne polyurethane acrylate. Prog. Org. Coat. 2015, 78, 146–154. [Google Scholar] [CrossRef]
- Liu, J.; Liu, R.; Zhang, X.; Li, Z.; Tang, H.; Liu, X. Preparation and properties of UV-curable multi-arms cardanol-based acrylates. Prog. Org. Coat. 2016, 90, 126–131. [Google Scholar] [CrossRef]
- Liang, B.; Li, R.; Zhang, C.; Yang, Z.; Yuan, T. Synthesis and characterization of a novel tri-functional bio-based methacrylate prepolymer from castor oil and its application in UV-curable coatings. Ind. Crop. Prod. 2019, 135, 170–178. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, J.F.; Fernando, S.; Jagodzinski, K. Soy-based, high biorenewable content UV curable coatings. Prog. Org. Coat. 2011, 71, 98–109. [Google Scholar] [CrossRef]
- Wu, J.F.; Fernando, S.; Jagodzinski, K.; Weerasinghe, D.; Chen, Z. Effect of hyperbranched acrylates on UV-curable soy-based biorenewable coatings. Polym. Int. 2011, 60, 571–577. [Google Scholar] [CrossRef]
- Li, P.; Ma, S.; Dai, J.; Liu, X.; Jiang, Y.; Wang, S.; Wei, J.; Chen, J.; Zhu, J. Itaconic Acid as a Green Alternative to Acrylic Acid for Producing a Soybean Oil-Based Thermoset: Synthesis and Properties. ACS Sustain. Chem. Eng. 2016, 5, 1228–1236. [Google Scholar] [CrossRef]
- Kahraman, M.V.; Bayramoğlu, G.; Boztoprak, Y.; Güngör, A.; Kayaman-Apohan, N. Synthesis of fluorinated/methacrylated epoxy based oligomers and investigation of its performance in the UV curable hybrid coatings. Prog. Org. Coat. 2009, 66, 52–58. [Google Scholar] [CrossRef]
- Xiao, M.; He, Y.; Nie, J. Novel Bisphenol A Epoxide–Acrylate Hybrid Oligomer and Its Photopolymerization. Des. Monomers Polym. 2012, 11, 383–394. [Google Scholar] [CrossRef]
- Öztürk, C.; Küsefoğlu, S.H. Polymerization of epoxidized soybean oil with maleinized soybean oil and maleic anhydride grafted polypropylene mixtures. J. Appl. Polym. Sci. 2010, 118, 3311–3317. [Google Scholar] [CrossRef]
- Li, Y.; Sun, X.S. Di-Hydroxylated Soybean Oil Polyols with Varied Hydroxyl Values and Their Influence on UV-Curable Pressure-Sensitive Adhesives. J. Am. Oil Chem. Soc. 2014, 91, 1425–1432. [Google Scholar] [CrossRef]
- Wu, Y.; Li, K. Acrylated epoxidized soybean oil as a styrene replacement in a dicyclopentadiene-modified unsaturated polyester resin. J. Appl. Polym. Sci. 2018, 135. [Google Scholar] [CrossRef]
- Liu, H.; Lu, W.; Liu, S. Development of acrylated soybean oil-based UV-curable coatings with high impact strength from low viscosity oligomer. J. Appl. Polym. Sci. 2018, 135. [Google Scholar] [CrossRef]
- Wu, Y.; Li, K. Replacement of styrene with acrylated epoxidized soybean oil in an unsaturated polyester resin from propylene glycol and maleic anhydride. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Wu, Q.; Hu, Y.; Tang, J.; Zhang, J.; Wang, C.; Shang, Q.; Feng, G.; Liu, C.; Zhou, Y.; Lei, W. High-Performance Soybean-Oil-Based Epoxy Acrylate Resins: “Green” Synthesis and Application in UV-Curable Coatings. ACS Sustain. Chem. Eng. 2018, 6, 8340–8349. [Google Scholar] [CrossRef]
- Bakare, I.O.; Pavithran, C.; Okieimen, F.E.; Pillai, C.K.S. Polyesters from renewable resources: Preparation and characterization. J. Appl. Polym. Sci. 2006, 100, 3748–3755. [Google Scholar] [CrossRef]
- Satyanarayana, M.; Muraleedharan, C. Comparative Studies of Biodiesel Production from Rubber Seed Oil, Coconut Oil, and Palm Oil Including Thermogravimetric Analysis. Energ. Source Part A 2011, 33, 925–937. [Google Scholar] [CrossRef]
- Liu, Q.; Lei, X.; Cao, Z.; Zhang, J.; Kuang, T.; Liu, G.; Fang, Y.; Qian, K.; Fu, J.; Du, H.; et al. Protective effect of oil from Cornus wilsoniana fruits against carbon tetrachloride-induced hepatic fibrosis in mice. Food Nutr. Res. 2020, 64. [Google Scholar] [CrossRef]
- Onoji, S.E.; Iyuke, S.E.; Igbafe, A.I.; Nkazi, D.B. Rubber seed oil: A potential renewable source of biodiesel for sustainable development in sub-Saharan Africa. Energ. Convers. Manag. 2016, 110, 125–134. [Google Scholar] [CrossRef]
- Aigbodion, A.I.; Okieimen, F.E.; Obazee, E.O.; Bakare, I.O. Utilisation of maleinized rubber seed oil and its alkyd resin as binders in water-borne coatings. Prog. Org. Coat. 2003, 46, 28–31. [Google Scholar] [CrossRef]
- Aigbodion, A.I.; Pillai, C.K.S. Preparation, analysis and applications of rubber seed oil and its derivatives in surface coatings. Prog. Org. Coat. 2000, 38, 187–192. [Google Scholar] [CrossRef]
- Ikhuoria, E.U.; Maliki, M.; Okieimen, F.E.; Aigbodion, A.I.; Obaze, E.O.; Bakare, I.O. Synthesis and characterisation of chlorinated rubber seed oil alkyd resins. Prog. Org. Coat. 2007, 52, 134–137. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Wang, Y.; Huang, J.; Li, K.; Nie, X.; Jiang, J. Epoxidized dimeric acid methyl ester derived from rubber seed oil and its application as secondary plasticizer. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Chaikul, P.; Lourith, N.; Kanlayavattanakul, M. Antimelanogenesis and cellular antioxidant activities of rubber (Hevea brasiliensis) seed oil for cosmetics. Ind. Crop. Prod. 2017, 108, 56–62. [Google Scholar] [CrossRef]
- Liu, C.; Dai, Y.; Hu, Y.; Shang, Q.; Feng, G.; Zhou, J.; Zhou, Y. Highly Functional Unsaturated Ester Macromonomer Derived from Soybean Oil: Synthesis and Copolymerization with Styrene. ACS Sustain. Chem. Eng. 2016, 4, 4208–4216. [Google Scholar] [CrossRef]
- Liang, B.; Zhao, J.; Li, G.; Huang, Y.; Yang, Z.; Yuan, T. Facile synthesis and characterization of novel multi-functional bio-based acrylate prepolymers derived from tung oil and its application in UV-curable coatings. Ind. Crop. Prod. 2019, 138. [Google Scholar] [CrossRef]
- Liu, C.; Wang, C.; Hu, Y.; Zhang, F.; Shang, Q.; Lei, W.; Zhou, Y.; Cai, Z. Castor oil-based polyfunctional acrylate monomers: Synthesis and utilization in UV-curable materials. Prog. Org. Coat. 2018, 121, 236–246. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, X.; Zhu, J.; Liu, X.; Wang, Z.; Yan, J. UV-Curable Coatings from Multiarmed Cardanol-Based Acrylate Oligomers. ACS Sustain. Chem. Eng. 2015, 3, 1313–1320. [Google Scholar] [CrossRef]
- Liu, R.; Zhu, G.; Li, Z.; Liu, X.; Chen, Z.; Ariyasivam, S. Cardanol-based oligomers with “hard core, flexible shell” structures: From synthesis to UV curing applications. Green Chem. 2015, 17, 3319–3325. [Google Scholar] [CrossRef]
- Liang, B.; Kuang, S.; Huang, J.; Man, L.; Yang, Z.; Yuan, T. Synthesis and characterization of novel renewable tung oil-based UV-curable active monomers and bio-based copolymers. Prog. Org. Coat. 2019, 129, 116–124. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, T.; Ye, X.; Man, L.; Zhou, C.; Hu, Y.; Zhang, C.; Yang, Z. Study on the UV curing behavior of tung oil: Mechanism, curing activity and film-forming property. Ind. Crop. Prod. 2018, 112, 61–69. [Google Scholar] [CrossRef]
- Phalak, G.; Patil, D.; Vignesh, V.; Mhaske, S. Development of tri-functional biobased reactive diluent from ricinoleic acid for UV curable coating application. Ind. Crop. Prod. 2018, 119, 9–21. [Google Scholar] [CrossRef]
- Kolanthai, E.; Sarkar, K.; Meka, S.R.K.; Madras, G.; Chatterjee, K. Copolyesters from Soybean Oil for Use as Resorbable Biomaterials. ACS Sustain. Chem. Eng. 2015, 3, 880–891. [Google Scholar] [CrossRef]
- Caillol, S.; Desroches, M.; Boutevin, G.; Loubat, C.; Auvergne, R.; Boutevin, B. Synthesis of new polyester polyols from epoxidized vegetable oils and biobased acids. Eur. J. Lipid Sci. Tech. 2012, 114, 1447–1459. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Xia, J.; Song, J.; Huang, K.; Li, M. Novel renewable resource-based UV-curable copolymers derived from myrcene and tung oil: Preparation, characterization and properties. Ind. Crop. Prod. 2015, 63, 17–25. [Google Scholar] [CrossRef]
- Duan, H.; Dong, W.; Wang, X.; Tao, X.; Ma, H. UV-curable polyurethane acrylate resin containing multiple active terminal groups for enhanced mechanical properties. J. Appl. Polym. Sci. 2019, 136. [Google Scholar] [CrossRef]




| Samples * | EA (wt %) | HEMA (wt %) | Darocur 1173 (wt %) |
|---|---|---|---|
| MMESO | 100 | 0 | 1.5 |
| MMERSO | 100 | 0 | 1.5 |
| MMEWSO | 100 | 0 | 1.5 |
| MMESO/HEMA30 | 70 | 30 | 1.5 |
| MMERSO/HEMA30 | 70 | 30 | 1.5 |
| MMEWSO/HEMA30 | 70 | 30 | 1.5 |
| Samples | Ava mgKOH/g | NC=Cb | Cbioc (%) | Cgeld (%) |
|---|---|---|---|---|
| AESO | 18.2 | 2.25 | 84.1 | 93.1 |
| MMESO | 20.2 | 7.81 | 59.0 | 95.9 |
| MMERSO | 23.2 | 5.92 | 65.1 | 93.3 |
| MMEWSO | 22.4 | 4.40 | 71.3 | 87.1 |
| AESO/HEMA30 | - | - | 58.8 | 98.0 |
| MMESO/HEMA30 | - | - | 41.3 | 99.9 |
| MMERSO/HEMA30 | - | - | 45.6 | 99.5 |
| MMEWSO/HEMA30 | - | - | 50.0 | 98.5 |
| Samples | E’25a (MPa) | Tgb (°C) | E’Tg + 60c (MPa) | νed (×103 mol/m3) | T5e (°C ) | Tpf (°C) | Wcharg (%) |
|---|---|---|---|---|---|---|---|
| AESO | 99.2 | 15.7 | 31.6 | 3.63 | 331.2 | 401.0 | 1.54 |
| MMESO | 697.5 | 69.4 | 57.6 | 5.74 | 271.1 | 399.8 | 3.01 |
| MMERSO | 343.5 | 55.6 | 28.8 | 2.97 | 265.9 | 401.6 | 1.96 |
| MMEWSO | 42.6 | 17.2 | 7.5 | 0.86 | 251.0 | 399.8 | 3.08 |
| AESO/HEMA30 | 550.9 | 56.2 | 18.6 | 1.91 | 292.2 | 432.2 | 2.14 |
| MMESO/HEMA30 | 1017.2 | 84.0 | 19.8 | 1.92 | 281.3 | 426.3 | 3.37 |
| MMERSO/HEMA30 | 695.7 | 73.9 | 12.2 | 1.20 | 271.4 | 411.3 | 4.09 |
| MMEWSO/HEMA30 | 43.7 | 17.5, 107.8 | 2.7 | 0.25 | 263.8 | 408.9 | 4.05 |
| Samples | σ a (MPa) | E b (MPa) | ε c (%) | Ad. d (Grade) | P.H. e | Fl f (mm) |
|---|---|---|---|---|---|---|
| AESO | 1.02 ± 0.17 | 59.5 ± 8.2 | 1.75 ± 0.91 | 4 | 6B | 2 |
| MMESO | 9.42 ± 0.79 | 200.3 ± 29.0 | 2.49 ± 0.86 | 3 | 5B | 2 |
| MMERSO | 2.90 ± 0.32 | 76.6 ± 5.4 | 5.55 ± 0.79 | 4 | 6B | 2 |
| MMEWSO | 1.17 ± 0.26 | 19.4 ± 2.4 | 6.26 ± 1.33 | 4 | 6B | 2 |
| AESO/HEMA30 | 5.30 ± 0.12 | 83.3 ± 5.0 | 15.61 ± 0.56 | 2 | 2H | 2 |
| MMESO/HEMA30 | 15.81 ± 0.13 | 259.2 ± 7.3 | 8.99 ± 0.60 | 3 | H | 2 |
| MMERSO/HEMA30 | 10.12 ± 0.55 | 186.7 ± 6.9 | 14.56 ± 1.11 | 2 | 2B | 2 |
| MMEWSO/HEMA30 | 2.41 ± 0.29 | 11.8 ± 0.6 | 19.58 ± 1.36 | 2 | 4B | 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Zhang, J.; Lu, J.; Huang, J.; Zhang, F.; Hu, Y.; Liu, C.; An, R.; Miao, H.; Chen, Y.; et al. Preparation and Properties of Plant-Oil-Based Epoxy Acrylate-Like Resins for UV-Curable Coatings. Polymers 2020, 12, 2165. https://doi.org/10.3390/polym12092165
Tang J, Zhang J, Lu J, Huang J, Zhang F, Hu Y, Liu C, An R, Miao H, Chen Y, et al. Preparation and Properties of Plant-Oil-Based Epoxy Acrylate-Like Resins for UV-Curable Coatings. Polymers. 2020; 12(9):2165. https://doi.org/10.3390/polym12092165
Chicago/Turabian StyleTang, Jijun, Jinshuai Zhang, Jianyu Lu, Jia Huang, Fei Zhang, Yun Hu, Chengguo Liu, Rongrong An, Hongcheng Miao, Yuanyuan Chen, and et al. 2020. "Preparation and Properties of Plant-Oil-Based Epoxy Acrylate-Like Resins for UV-Curable Coatings" Polymers 12, no. 9: 2165. https://doi.org/10.3390/polym12092165
APA StyleTang, J., Zhang, J., Lu, J., Huang, J., Zhang, F., Hu, Y., Liu, C., An, R., Miao, H., Chen, Y., Huang, T., & Zhou, Y. (2020). Preparation and Properties of Plant-Oil-Based Epoxy Acrylate-Like Resins for UV-Curable Coatings. Polymers, 12(9), 2165. https://doi.org/10.3390/polym12092165