Synthesis of Network Polymers by Means of Addition Reactions of Multifunctional-Amine and Poly(ethylene glycol) Diglycidyl Ether or Diacrylate Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Network Polymers
Synthesis of Gels
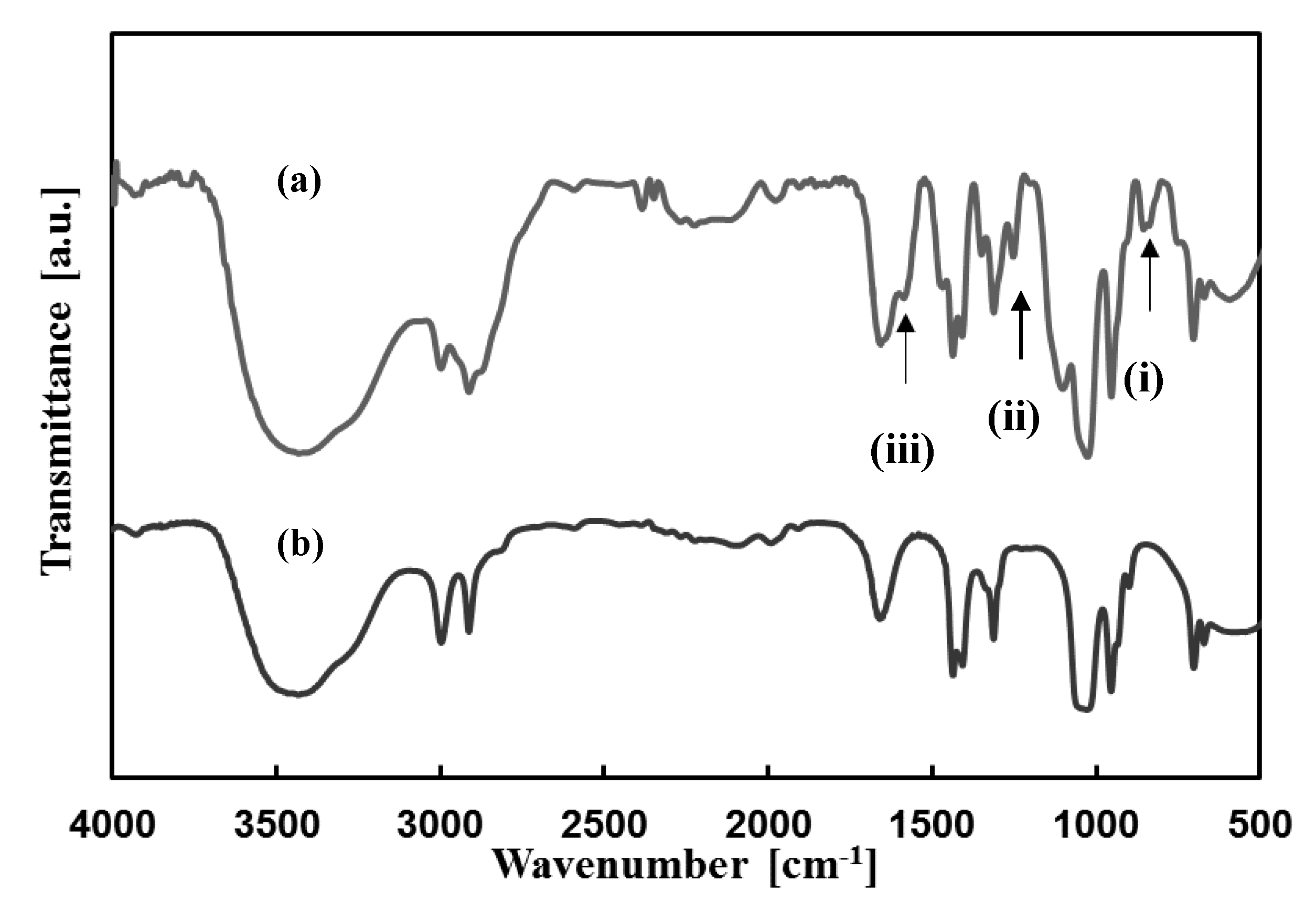
2.3. Analytical Procedures
3. Results and Discussion
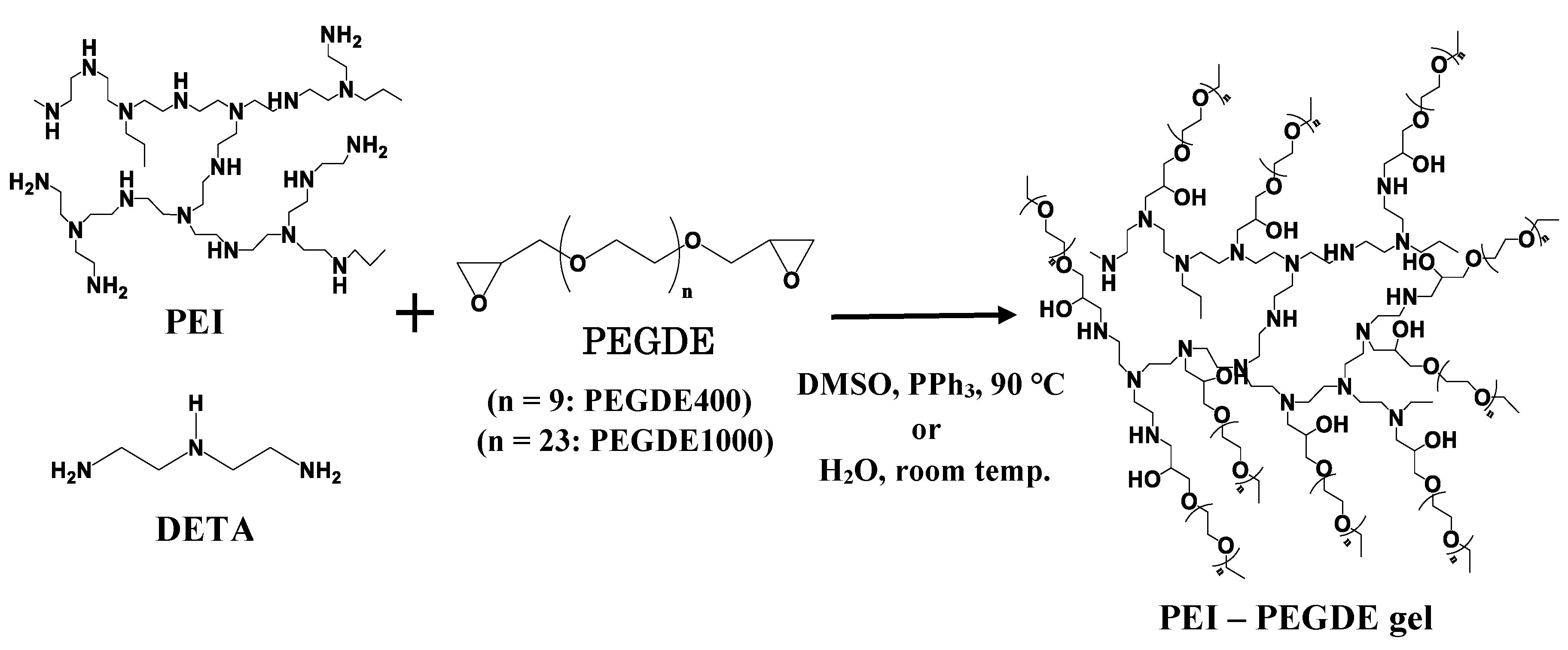
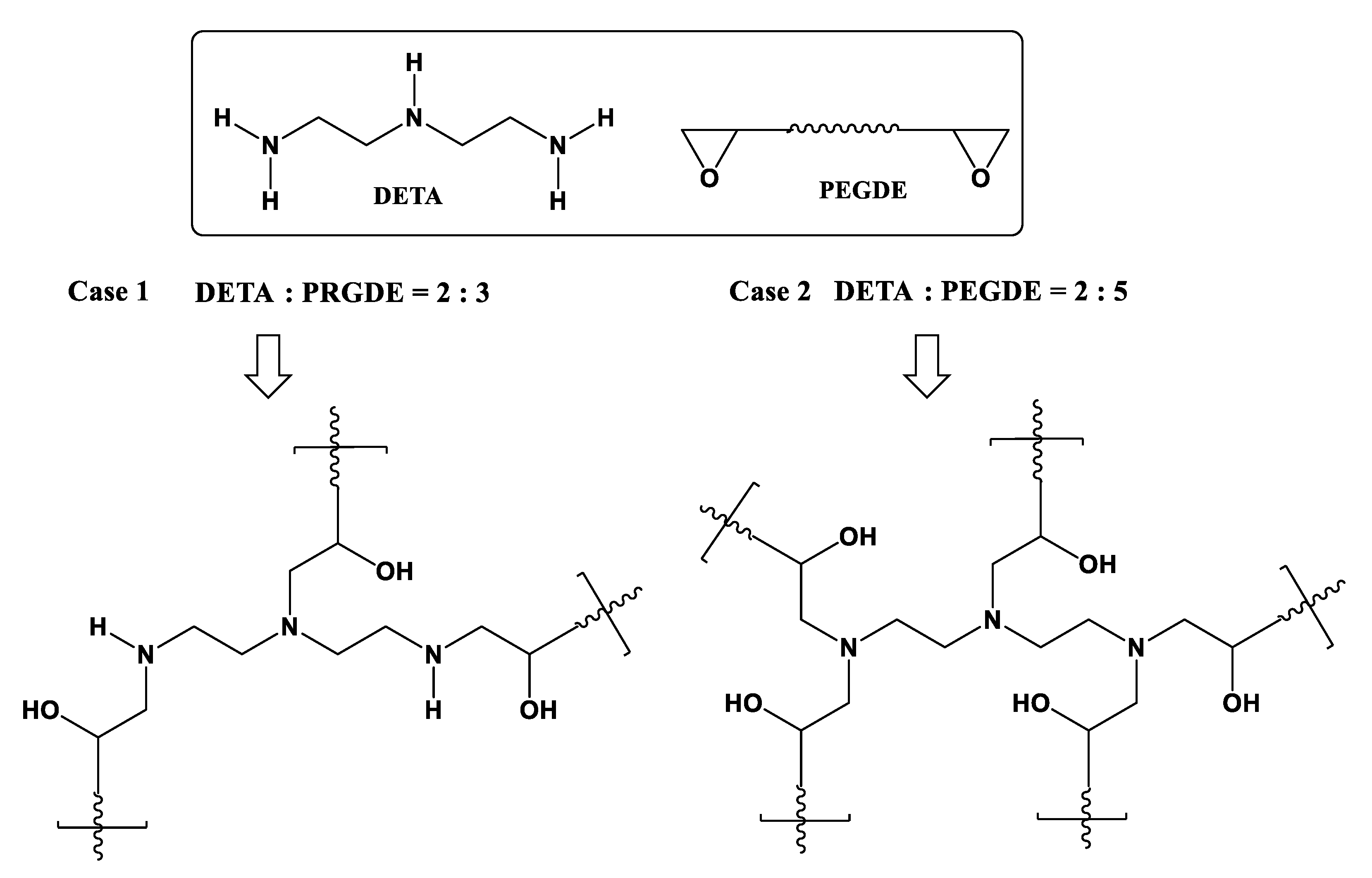
3.1. Sythessis of Network Polymers by Means of Ring Opening Addition Reaction of PEI or DETA and PEGDE
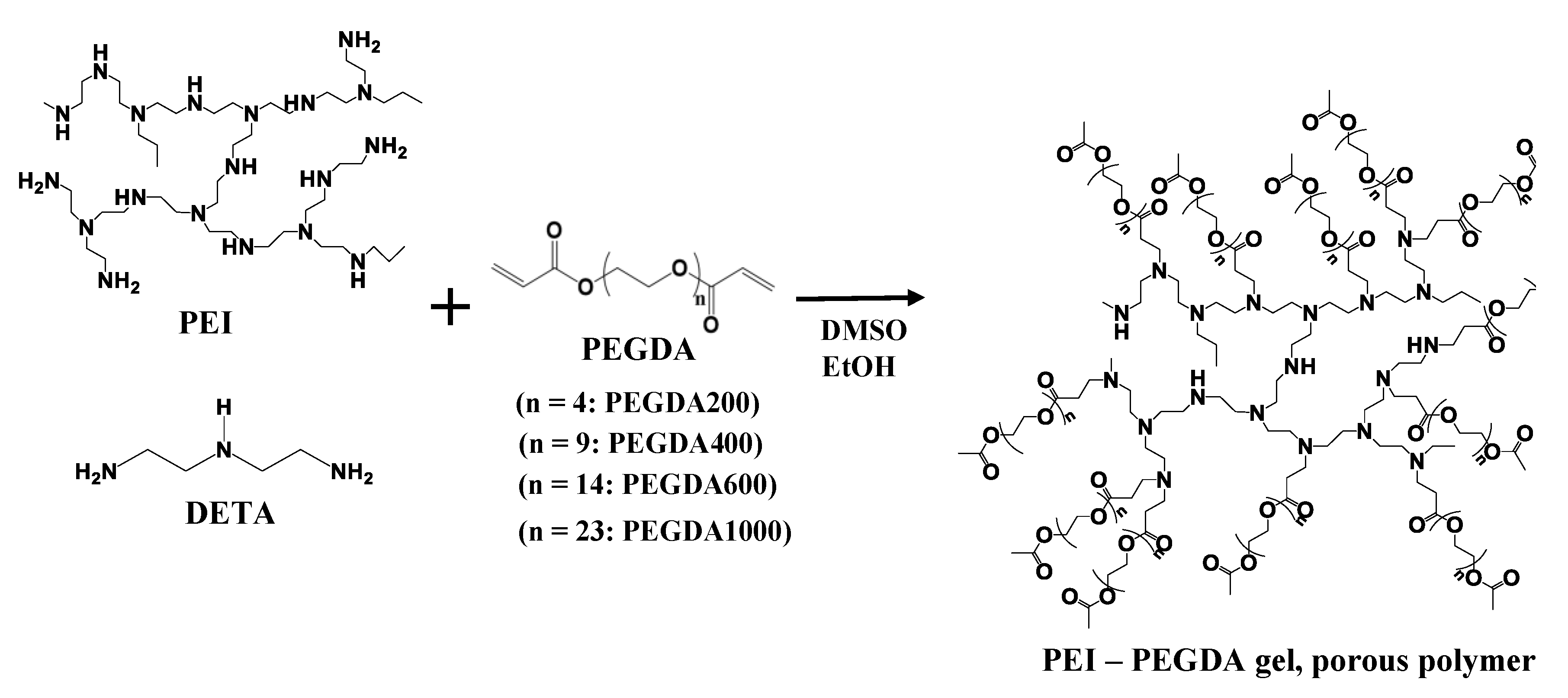
3.2. Synthesis of Network Polymers by Means of Aza-Michael Addition Reaction of DETA or PEI and PEGDA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Naga, N.; Oda, E.; Toyota, A.; Horie, K.; Furukawa, H. Tailored synthesis and fundamental characterization of organic-inorganic hybrid gels by means of hydrosilylation reaction. Macromol. Chem. Phys. 2006, 207, 627–635. [Google Scholar] [CrossRef]
- Naga, N.; Kihara, Y.; Miyanaga, T.; Furukawa, H. Synthesis of organic-inorganic hybrid gels from siloxane or silsesquioxane and α,ω -nonconjugated dienes by means of a photo hydrosilylation reaction. Macromolecules 2009, 42, 3454–3462. [Google Scholar] [CrossRef]
- Naga, N.; Fujioka, S.; Inose, D.; Ahmed, K.; Nageh, H.; Nakano, T. Synthesis and properties of porous polymers synthesized by Michael addition reactions of multi-functional acrylate, diamine, and dithiol compounds. RSC Adv. 2020, 20, 60–69. [Google Scholar] [CrossRef]
- Cavallo, A.; Madaghiele, M.; Masullo, U.; Lionetto, M.G.; Sannino, A. Photo-crosslinked poly(ethylene glycol) diacrylate (PEGDA) hydrogels from low molecular weight prepolymer: Swelling and permeation studies. J. Appl. Polym. Sci. 2017, 134, 44380. [Google Scholar] [CrossRef]
- Valentin, T.M.; DuBois, E.M.; Machnicki, C.E.; Bhaskar, D.; Cui, F.R.; Wonng, I.Y. 3D printed self-adhesive PEGDA-PAA hydrogels as modular components for soft actuator and microfluidics. Polym. Chem. 2019, 10, 2015–2028. [Google Scholar] [CrossRef]
- Liang, J.L.; Zhang, X.; Chen, Z.; Li, S.; Yan, C. Thiol-ene click reaction initiated rapid gelation of GEGDA/silk fibroin hydrogels. Polymers 2019, 11, 2102. [Google Scholar] [CrossRef]
- Lee, S.; Tong, X.; Yang, F. The effects of varying poly(ethylene glycol) hydrogel crosslinking density and the crosslinking mechanism of protein accumulation in three-dimensional hydrogels. Acta Biomater. 2014, 10, 4167–4174. [Google Scholar] [CrossRef]
- Stocke, N.A.; Zhang, X.; Hilt, J.Z.; DeRouchey, J.E. Transport in PEG-based hydrogels: Role of water content at synthesis and crosslinker molecular weight. Macromol. Chem. Phys. 2017, 218, 1600340. [Google Scholar] [CrossRef]
- Cui, J.; Lackey, M.A.; Tew, G.N.; Crosby, A.J. Mechanical properties of end-linked PEG/PDMS hydrogels. Macromolecules 2012, 45, 6104–6110. [Google Scholar] [CrossRef]
- Sakai, T.; Matsunaga, T.; Yamamoto, Y.; Ito, C.; Yoshida, R.; Suzuki, S.; Sasaki, N.; Shibayama, M.; Chung, U.I. Design and fabrication of a high-strength hydrogel with ideally homogeneous network structure from tetrahedron-like macromonomers. Macromolecules 2008, 41, 5379–5384. [Google Scholar] [CrossRef]
- Yang, T.; Maikoch, M.; Hult, A. Sequential interpenetrating poly(ethylene glycol) hydrogels prepared by UV-initiated thiol-ene coupling chemistry. J. Polym. Sci. Part. A Polym. Chem. 2013, 51, 363–371. [Google Scholar] [CrossRef]
- Yang, T.; Long, H.; Malkoch, M.; Gamstedt, E.K.; Berglund, L.; Hult, A. Characterization of well-defined poly(ethylene glycol) hydrogels prepared by thiol-ene chemistry. J. Polym. Sci. Part. A Polym. Chem. 2011, 49, 4044–4054. [Google Scholar] [CrossRef]
- Tan, G.; Wang, Y.; Li, J.; Zhang, S. Synthesis and characterization of injectable photocrosslinking poly(ethylene glycol) diacrylate hydrogels. Polym. Bull. 2008, 61, 91–98. [Google Scholar] [CrossRef]
- Teng, D.Y.; Wu, Z.M.; Zhang, X.G.; Wang, Y.X.; Zheng, C.; Wang, Z.; Li, C.X. Synthesis and characterization of situ cross-linked hydrogels based on self-assembly of thiol modified chitosan with PEG diacrylate using Michael type addition. Polymer 2010, 51, 639–646. [Google Scholar] [CrossRef]
- Chang, C.W.; Spreeuwel, A.V.; Zhang, C.; Varghese, S. PEG/clay nanocomposite hydrogel: A mechanically robust tissue engineering scaffold. Soft Matter 2012, 6, 5157–5164. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Hwang, Y.; Chen, A.C.; Varghese, S.; Sah, R.L. Cartilage-like mechanical properties of poly(ethylene glycol)-diacrylate hydrogels. Biomaterials 2012, 33, 6682–6690. [Google Scholar] [CrossRef]
- Yesilyurt, V.; Webber, J.W.; Appel, E.A.; Godwin, C.; Langer, R.; Anderson, D.G. Injectable self-healing glucose-responsive hydrogels with pH-regulated mechanical properties. Adv. Mater. 2016, 28, 86–91. [Google Scholar] [CrossRef]
- Rahman, C.V.; Kuhn, G.; White, L.J.; Kirby, G.T.S.; Varghese, O.P.; McLaren, J.S.; Cox, H.C.; Rose, F.R.A.; Muller, R.; Hilborn, J.; et al. PLGA/PEG-hydrogel composite scaffolds with controllable mechanical properties. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2013, 101, 648–655. [Google Scholar] [CrossRef]
- Malkoch, M.; Vestberg, R.; Gupta, N.; Mespouille, L.; Dubois, P.; Mason, A.F.; Hendrick, J.L.; Liao, Q.; Frank, C.W.; Kingsbury, K.; et al. Synthesis of well-defined hydrogel networks using Click chemistry. Chem. Commun. 2006, 2774–2776. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, N.; Liu, W.; Zhao, X.; Lu, W. Intermolecular hydrogen bonding strategy to fabricate mechanically strong hydrogels with high elasticity and fatigue resistance. Soft Matter 2013, 9, 6331–6337. [Google Scholar] [CrossRef]
- Lin-Gibson, S.; Bencherif, S.; Cooper, J.A.; Wetzel, S.J.; Antonucci, J.M.; Vogel, B.M.; Horkay, F.; Washburn, N.R. Synthesis and characterization of PEG dimethacrylates and their hydrogels. Biomacromolecules 2004, 5, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Clapper, J.D.; Guymon, C.A. Physical behavior of cross-linked PEG hydrogels photopolymerized within nanostructured lyotropic liquid crystalline templates. Macromolecules 2007, 40, 1101–1107. [Google Scholar] [CrossRef]
- Kloxin, A.M.; Kasko, A.M.; Salinas, C.N.; Anseth, K.S. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 2009, 324, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Naga, N.; Inose, D.; Ishida, T.; Kubota, K.; Nageh, H.; Nakano, T. Synthesis of polymer networks by means of addition reactions of tri-amine and poly(ethylene glycol) diacrylate or diglycidyl ether compounds. Polym. Bull. (in press). [CrossRef]
- Ehlers, J.E.; Rondan, N.G.; Huynh, L.K.; Pham, H.; Marks, M.; Truong, T.N. Theoretical study on mechanisms of the epoxy-amine curing reaction. Macromolecules 2007, 40, 4370–4377. [Google Scholar] [CrossRef]
- Del Rector, F.; Blount, W.W.; Leonard, D.R. Applications for acetoacetyl chemistry in thermoset coatings. J. Coat. Technol. 1989, 61, 31–37. [Google Scholar]
- Yamasaki, H.; Morita, S. Epoxy curing reaction studied by using two-dimensional correlation infrared and near-infrared spectroscopy. J. Appl. Polym. Sci. 2011, 119, 871–881. [Google Scholar] [CrossRef]
- Desmet, G.B.; D’hooge, D.R.; Omurtag, P.S.; Espeel, P.; Marin, G.B.; Du Prez, F.E.; Reyniers, M.F. Quantitative first-principles kinetic modeling of the Aza-Michael addition to acrylates in polar aprotic solvents. J. Org. Chem. 2016, 81, 12291–12302. [Google Scholar] [CrossRef]
- Inoue, T. Reaction-Induced Phase Decomposition in Polymer Blends. Prog. Polym. Sci. 1995, 20, 119–153. [Google Scholar] [CrossRef]
- Yamanaka, K.; Inoue, T. Structure development in epoxy resin modified with poly(ether sulphone). Polymer 1989, 30, 662–667. [Google Scholar] [CrossRef]
- Ohnaga, T.; Inoue, T. Growth and decay of concentration fluctuations in polymer-polymer mixtures. J. Polym. Sci. Part. B Polym. Phys. 1989, 27, 1675–1689. [Google Scholar] [CrossRef]
- Inoue, T.; Ichihara, S. Polymer Alloy; Society of Polymer Science, Ed.; Kyoritsu Shuppan: Tokyo, Japan, 1988. [Google Scholar]
- Hansen, C. The Three Dimensional Solubility Parameter and Solvent Diffusion Coefficient and Their Importance in Surface Coating Formulation; Danish Technical Press: Copenhagen, Denmark, 1967. [Google Scholar]


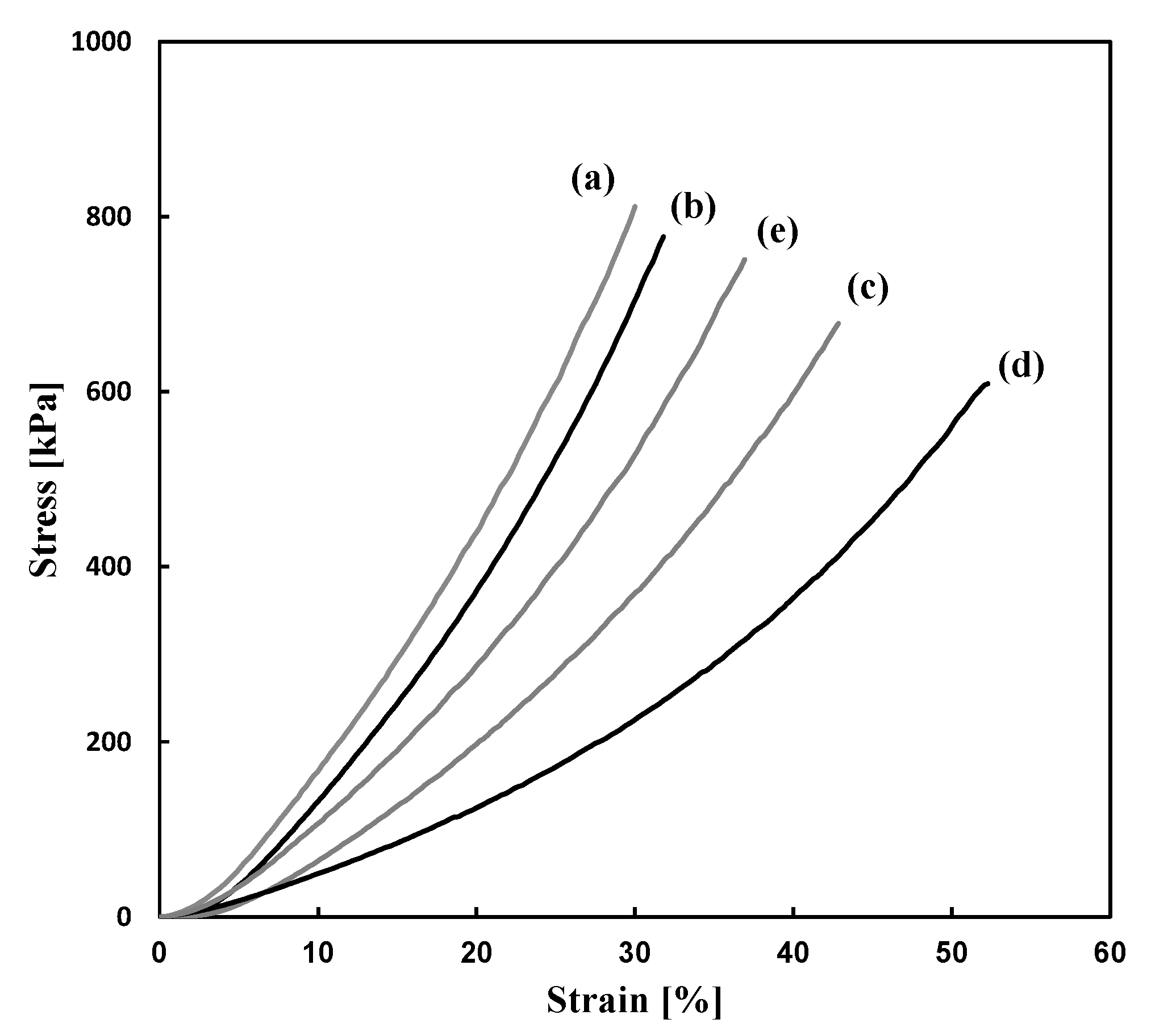
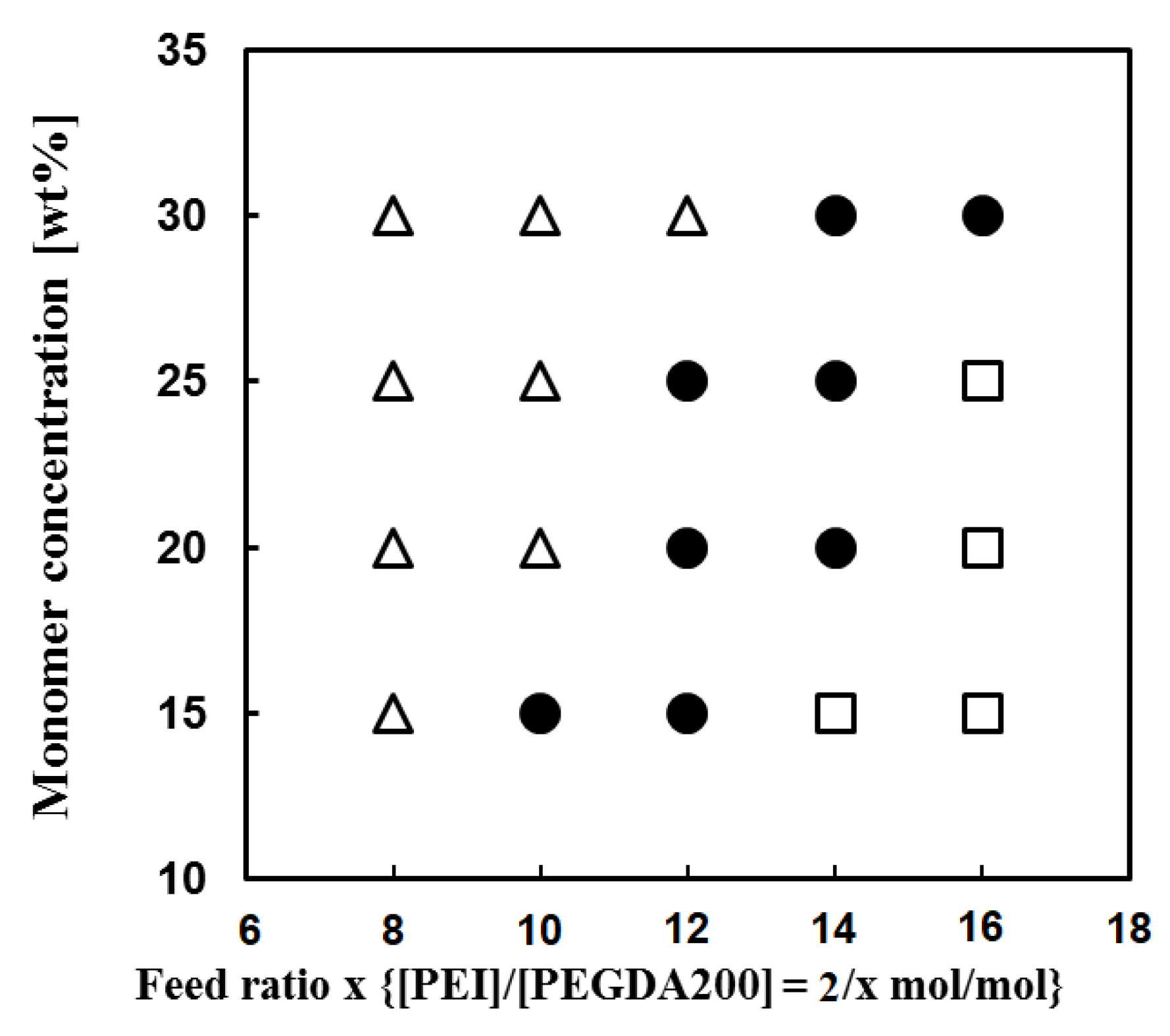

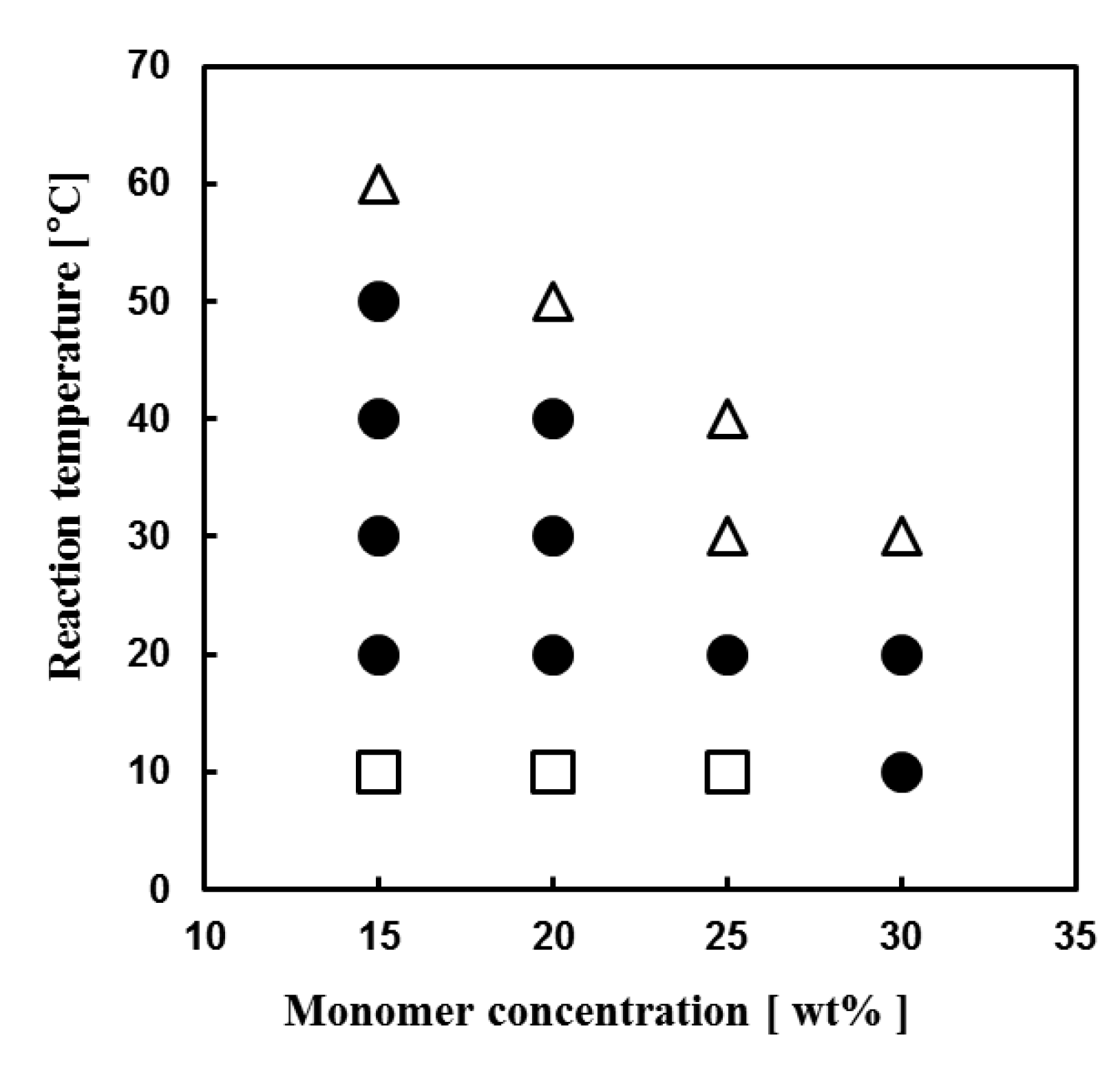
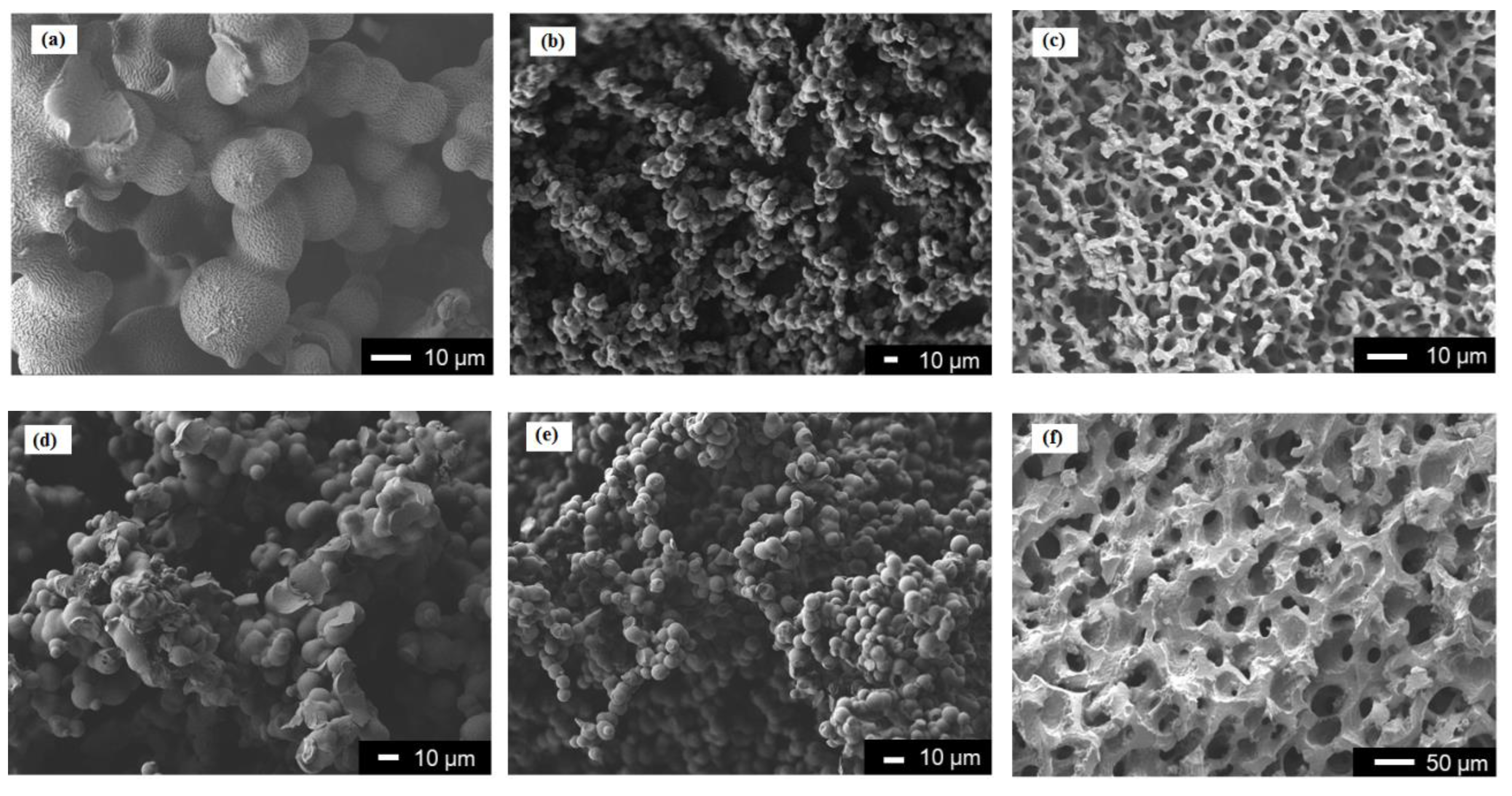
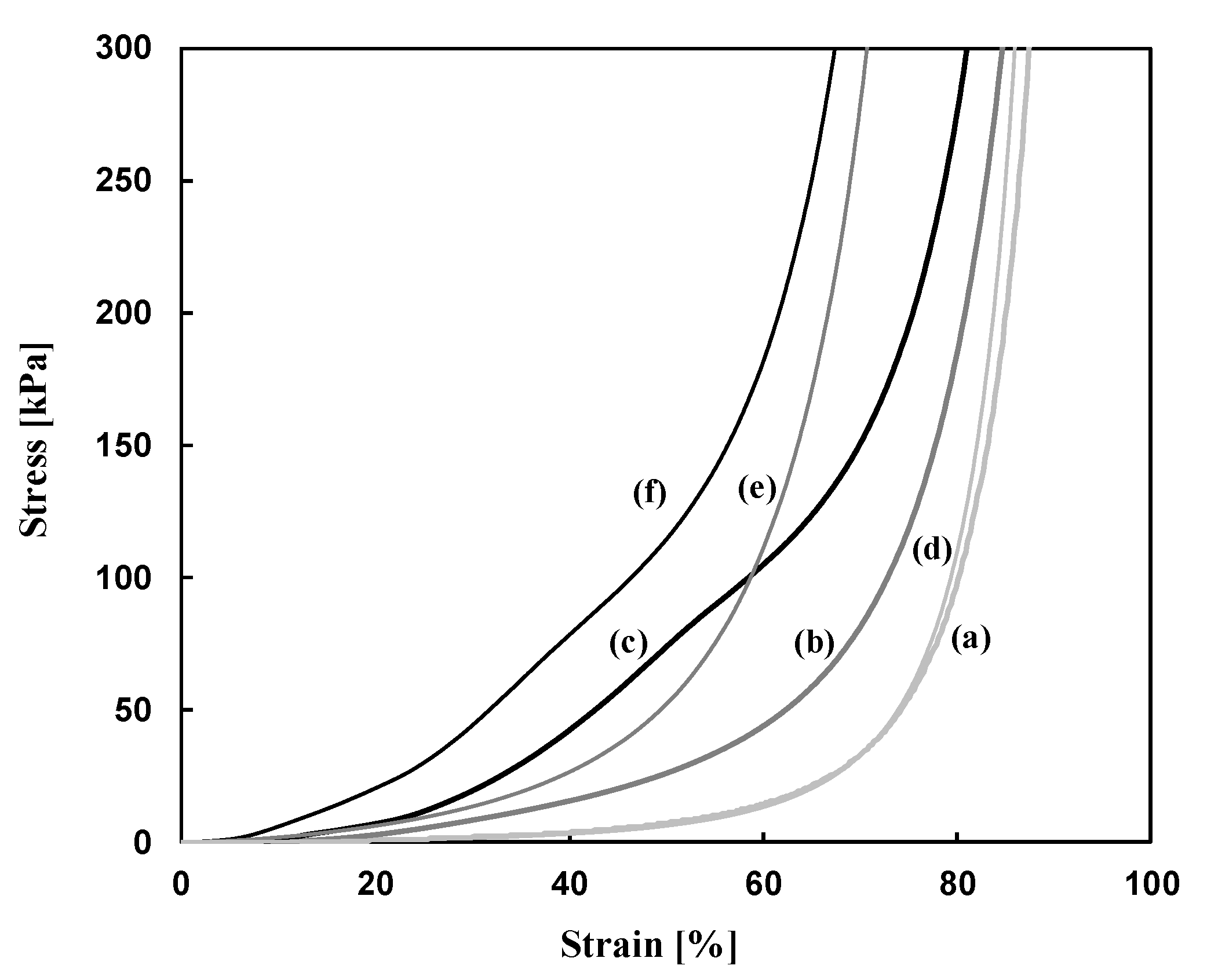
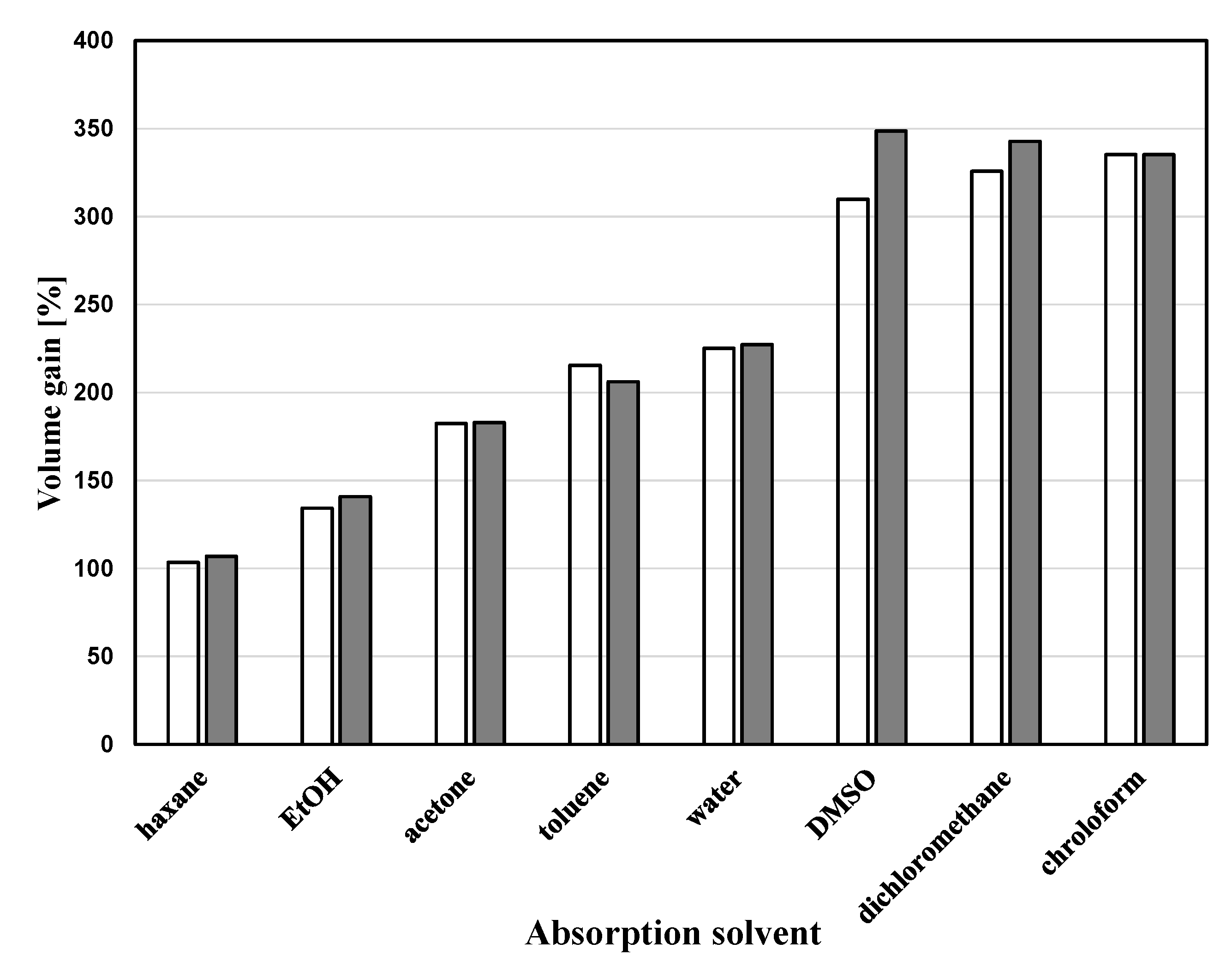
| Run | Amine | PEGDE | Amine/ PEGDE Feed | Solvent | Epoxy a (mol/L) | Gel Formation Time (min) | Young’s Modulus (kPa) | Stress at Break (kPa) | Strain at Break (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | PEI | 400 | Case 1 | H2O | 0.96 | 35 | 426 | 400 | 26.5 |
| 2 | PEI | 400 | Case 2 | H2O | 1.03 | 78 | 540 | 530 | 24.4 |
| 3 | DETA | 400 | Case 1 | H2O | 1.02 | 128 | 241 | 228 | 44.4 |
| 4 | DETA | 400 | Case 2 | H2O | 1.09 | 328 | 344 | 39.2 | |
| 5 | PEI | 1000 | Case 1 | H2O | 0.49 | 132 | 210 | 317 | 41.0 |
| 6 | PEI | 1000 | Case 2 | H2O | 0.51 | 228 | 340 | 36.1 | |
| 7 | PEI | 400 | Case 1 | DMSO | 1.03 | 94 | 210 | 466 | 37.3 |
| 8 | PEI | 400 | Case 2 | DMSO | 1.10 | 135 | 265 | 388 | 37.7 |
| Run | Amine | PEGDA | Amine/ PEGDA Feed | Acrylate a (mol/L) | Gel Formation Time (min) | Young’s Modulus (kPa) | Stress at Break (kPa) | Strain at Break (%) |
|---|---|---|---|---|---|---|---|---|
| 9 | PEI | 200 | Case 1 | 1.53 | 3 | 540 | 871 | 24.4 |
| 10 | PEI | 400 | Case 1 | 1.05 | 5 | 850 | 811 | 29.6 |
| 11 | PEI | 400 | Case 2 | 1.12 | 135 | 740 | 777 | 31.5 |
| 12 | PEI | 600 | Case 1 | 0.80 | 11 | 265 | 784 | 32.6 |
| 13 | PEI | 1000 | Case 1 | 0.53 | 123 | 552 | 751 | 37.1 |
| 14 | PEI | 1000 | Case 2 | 0.55 | 471 | 664 | 50.0 | |
| 15 | DETA | 400 | Case 1 | 1.14 | 16 | 552 | 751 | 37.2 |
| 16 | DETA | 400 | Case 2 | 1.09 | 52 | 471 | 664 | 50.4 |
| Run | Amine | PEGDA | Amine/ PEGDA Feed | Acrylate a (mol/L) | Gel Formation Time (min) | Young’s Modulus (kPa) | Stress at Break (kPa) | Strain at Break (%) |
|---|---|---|---|---|---|---|---|---|
| 17 | PEI | 400 | Case 1 | 0.79 | 13 | 92.1 | 223 | 27.1 |
| 18 | PEI | 400 | Case 2 | 0.64 | 41 | 67.2 | 226 | 28.1 |
| 19 | PEI | 600 | Case 1 | 0.60 | 17 | 62.7 | 231 | 30.5 |
| 20 | PEI | 1000 | Case 1 | 0.39 | 1000 | 37.4 | 233 | 37.1 |
| 21 | PEI | 1000 | Case 2 | 0.28 | 32.4 | 195 | 42.6 | |
| 22 | DETA | 400 | Case 1 | 0.86 | 63 | 81.3 | 86 | 37.0 |
| 23 | DETA | 400 | Case 2 | 0.66 | 134 | 73.5 | 191 | 50.6 |
| Run | Amine | Amine/ PEGDA200 mol/mol | Monomer Concentration a (wt%) | Porosity (%) | Specific Surface Area (m2/g) | Young’s Modulus (kPa) |
|---|---|---|---|---|---|---|
| 24 | PEI | 2/14 | 20 | 71.4 | 2.2 | 4.5 |
| 25 | PEI | 2/14 | 25 | 65.7 | 1.8 | 19.3 |
| 26 | PEI | 2/14 | 30 | 64.0 | 1.2 | 22.2 |
| 27 | DETA | 2/5 Case 2 | 20 | 72.9 | 4.7 | 37.4 |
| 28 | DETA | 2/5 Case 2 | 25 | 67.0 | 3.8 | 62.7 |
| 29 | DETA | 2/5 Case 2 | 30 | 65.3 | 2.6 | 67.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naga, N.; Sato, M.; Mori, K.; Nageh, H.; Nakano, T. Synthesis of Network Polymers by Means of Addition Reactions of Multifunctional-Amine and Poly(ethylene glycol) Diglycidyl Ether or Diacrylate Compounds. Polymers 2020, 12, 2047. https://doi.org/10.3390/polym12092047
Naga N, Sato M, Mori K, Nageh H, Nakano T. Synthesis of Network Polymers by Means of Addition Reactions of Multifunctional-Amine and Poly(ethylene glycol) Diglycidyl Ether or Diacrylate Compounds. Polymers. 2020; 12(9):2047. https://doi.org/10.3390/polym12092047
Chicago/Turabian StyleNaga, Naofumi, Mitsusuke Sato, Kensuke Mori, Hassan Nageh, and Tamaki Nakano. 2020. "Synthesis of Network Polymers by Means of Addition Reactions of Multifunctional-Amine and Poly(ethylene glycol) Diglycidyl Ether or Diacrylate Compounds" Polymers 12, no. 9: 2047. https://doi.org/10.3390/polym12092047
APA StyleNaga, N., Sato, M., Mori, K., Nageh, H., & Nakano, T. (2020). Synthesis of Network Polymers by Means of Addition Reactions of Multifunctional-Amine and Poly(ethylene glycol) Diglycidyl Ether or Diacrylate Compounds. Polymers, 12(9), 2047. https://doi.org/10.3390/polym12092047






