A Review of Recent Advancements in Electrospun Anode Materials to Improve Rechargeable Lithium Battery Performance
Abstract
1. Introduction
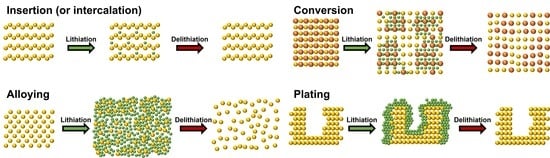
2. Basic Lithium Storage Principles
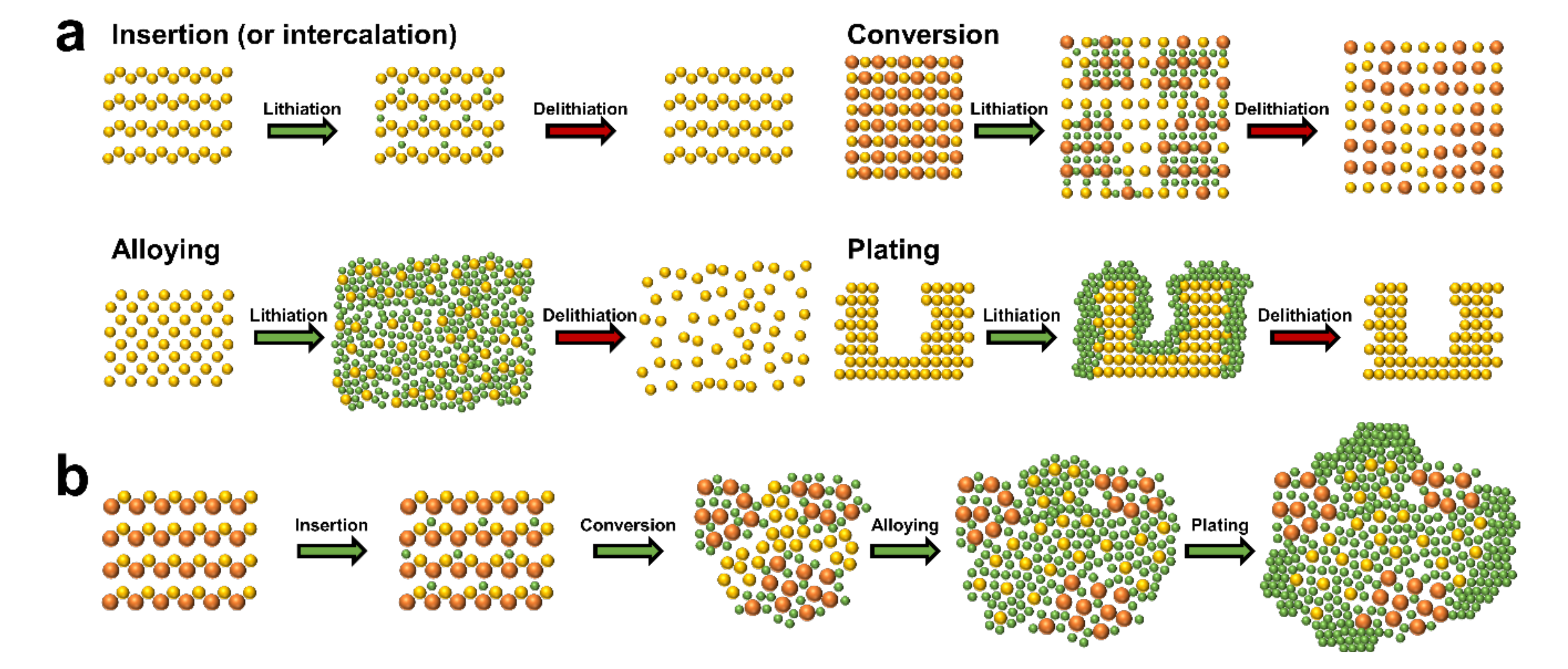
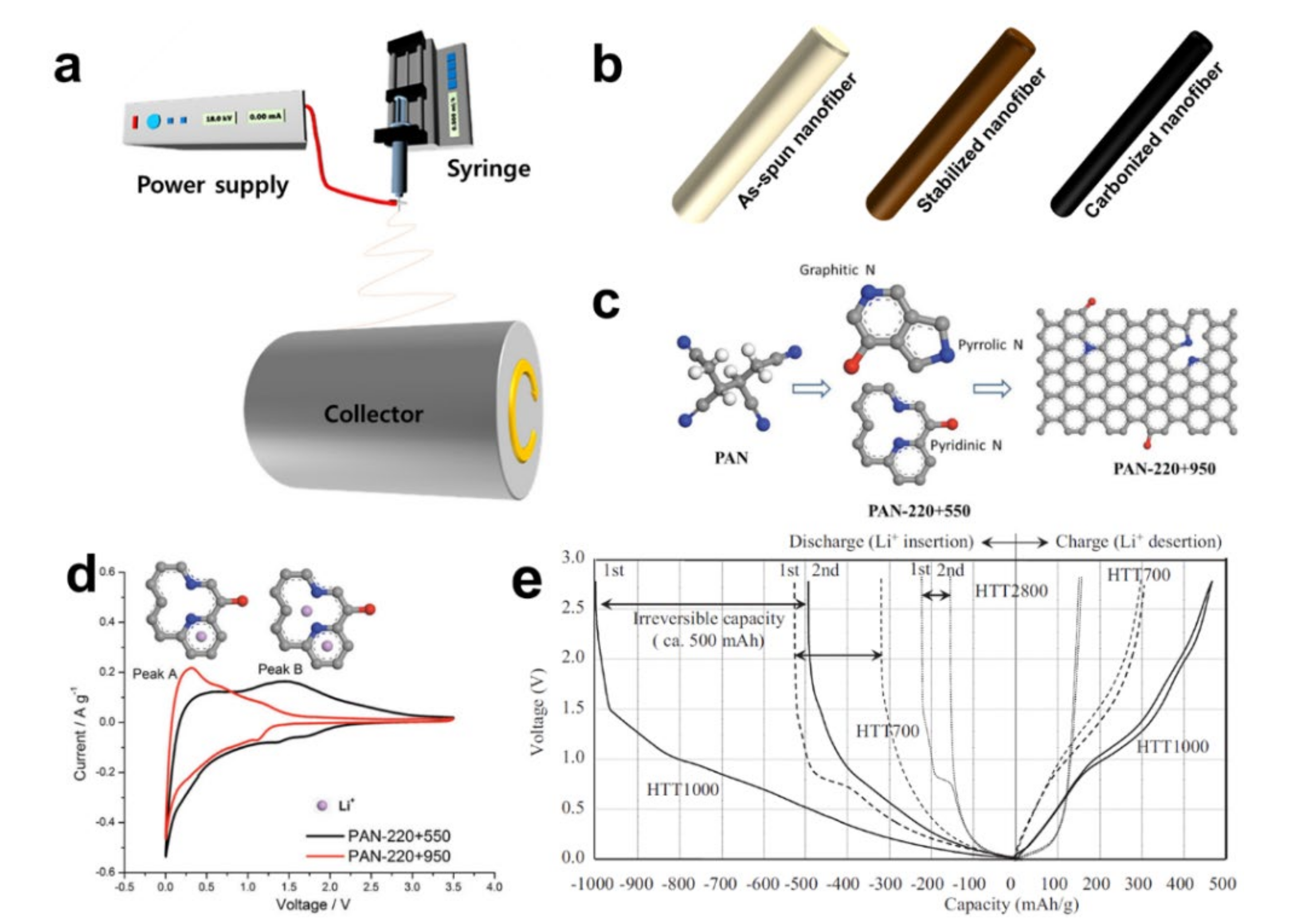
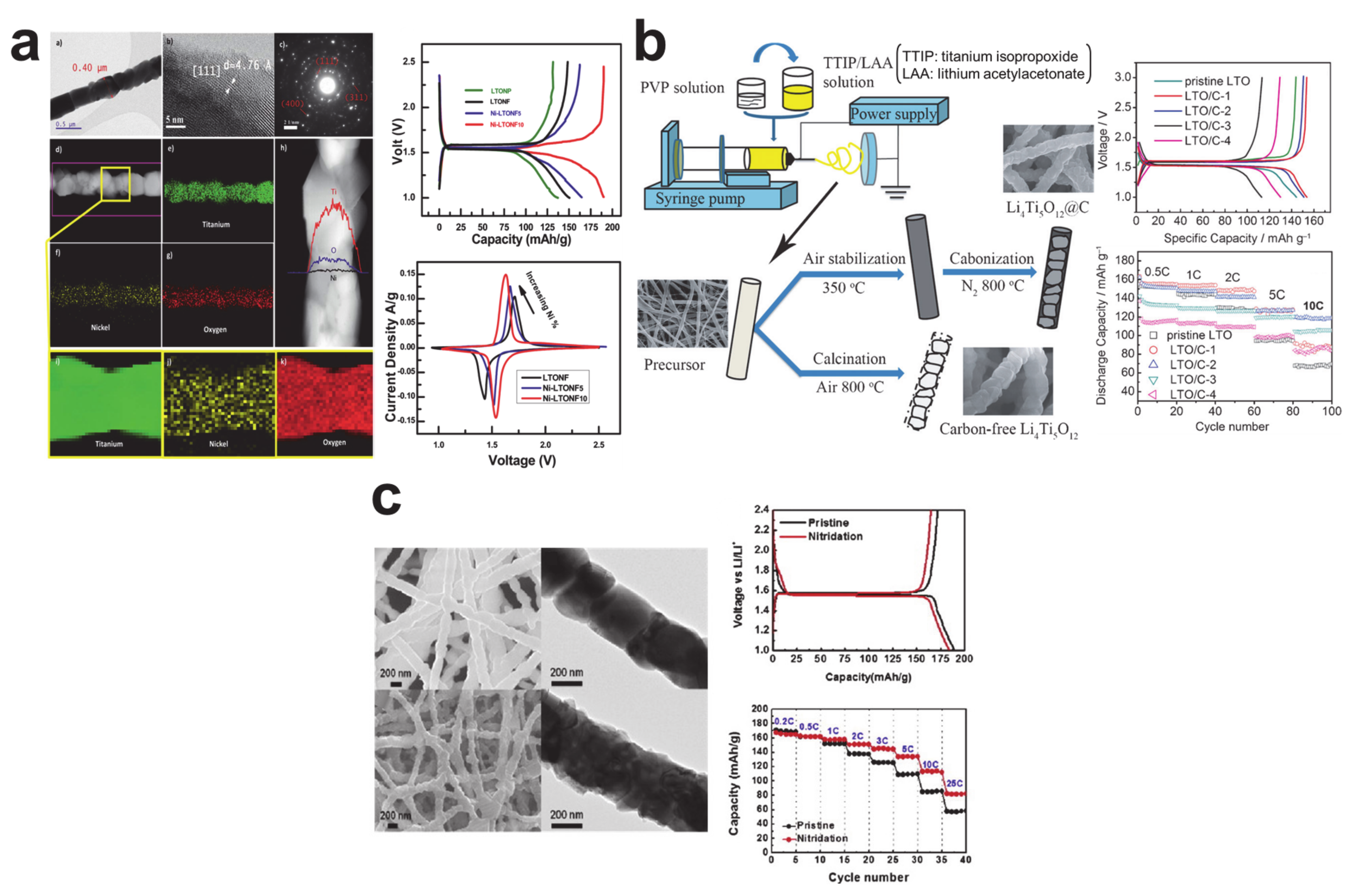
3. Insertion/Extraction (or Intercalation/Deintercalation)-Based Storage Materials
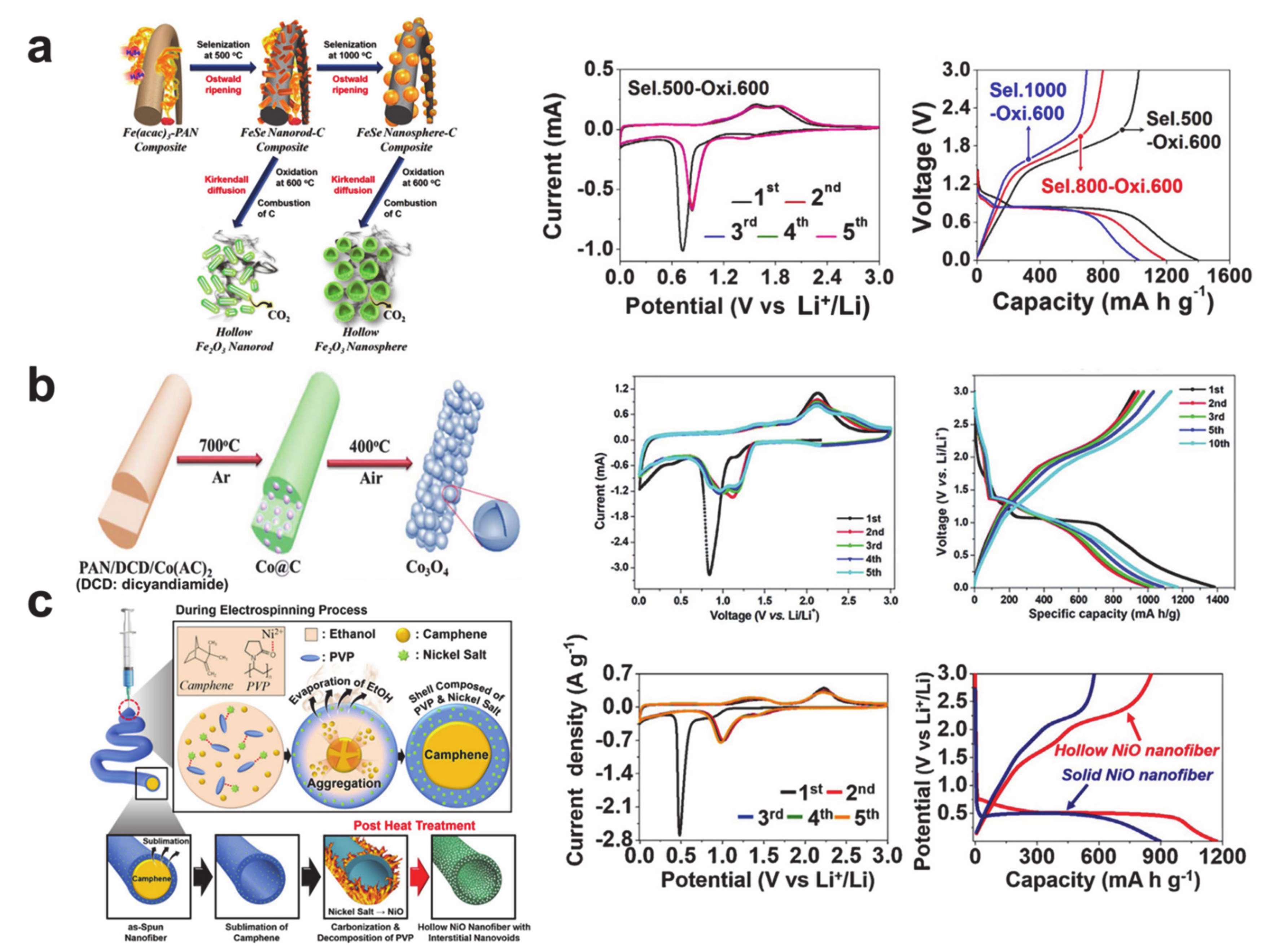
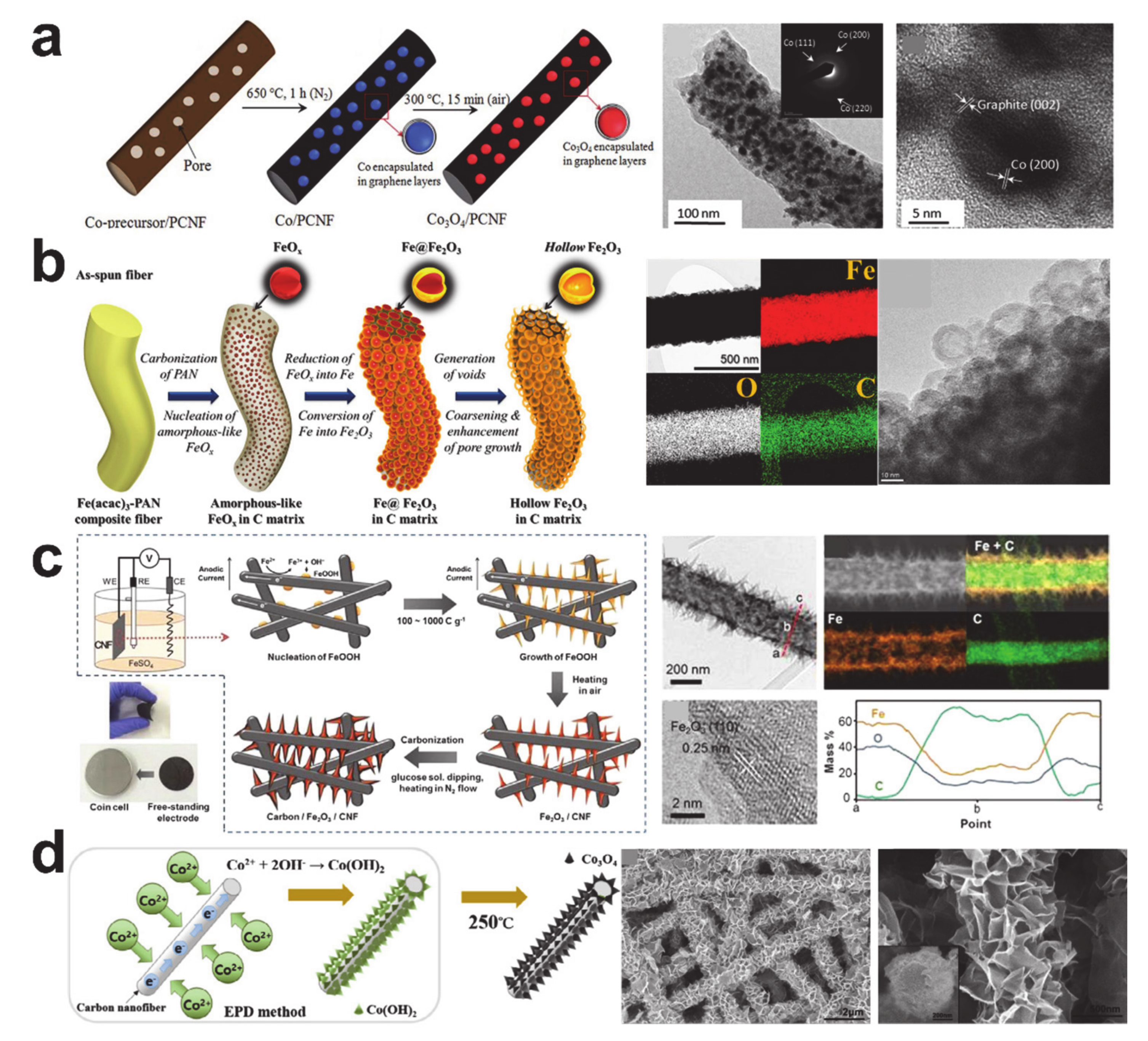
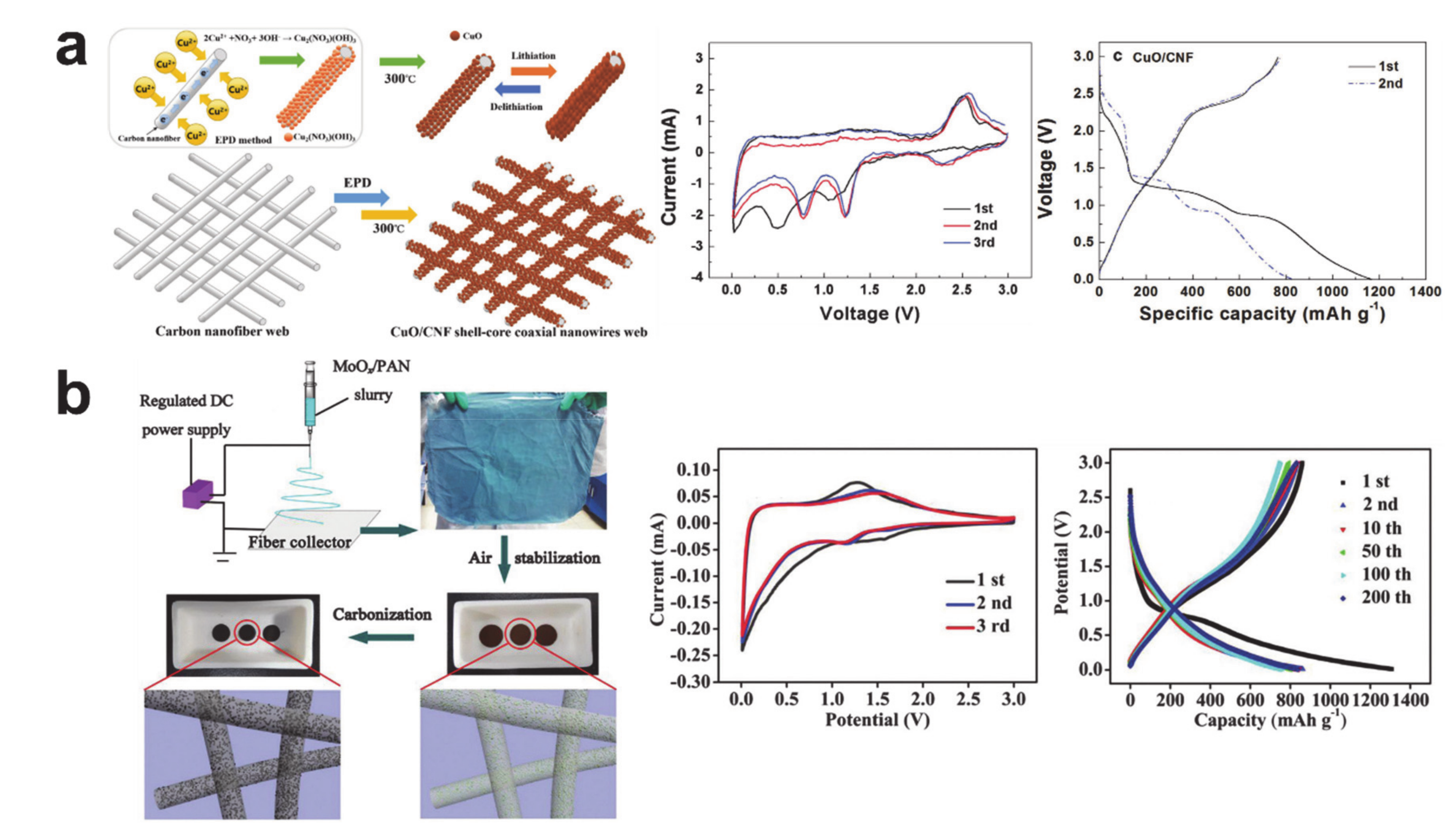
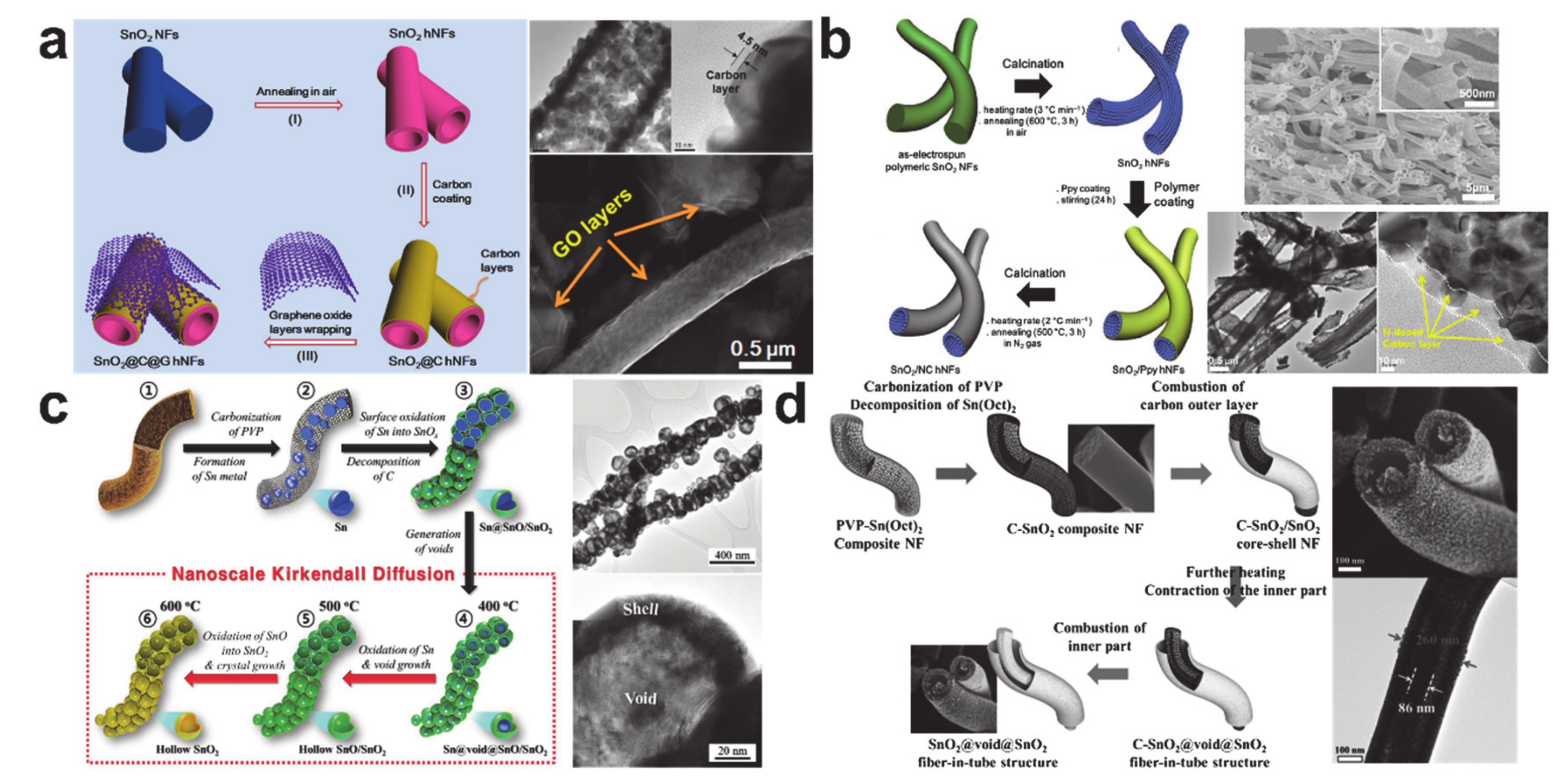
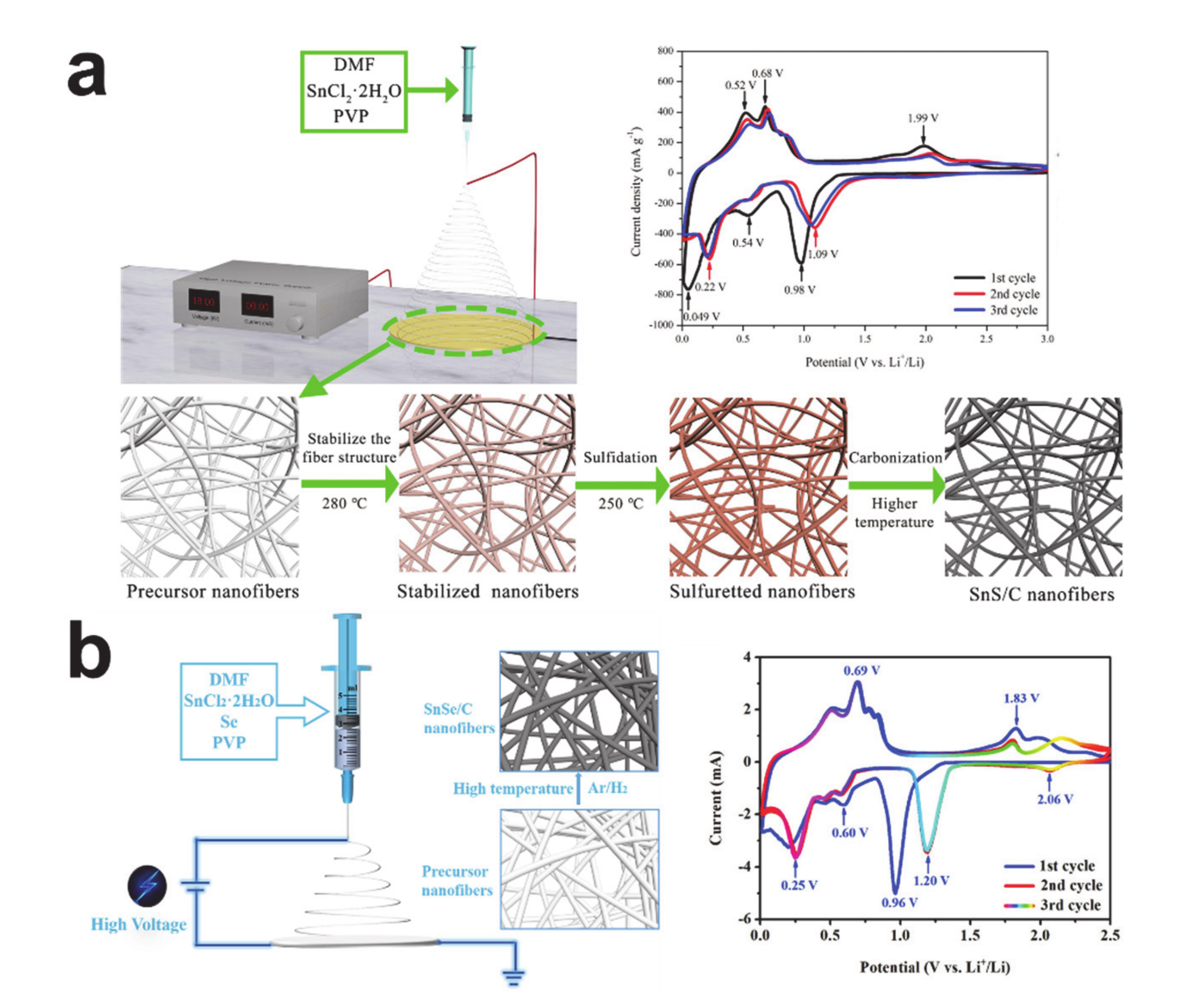
4. Conversion Reaction-Based Storage Materials
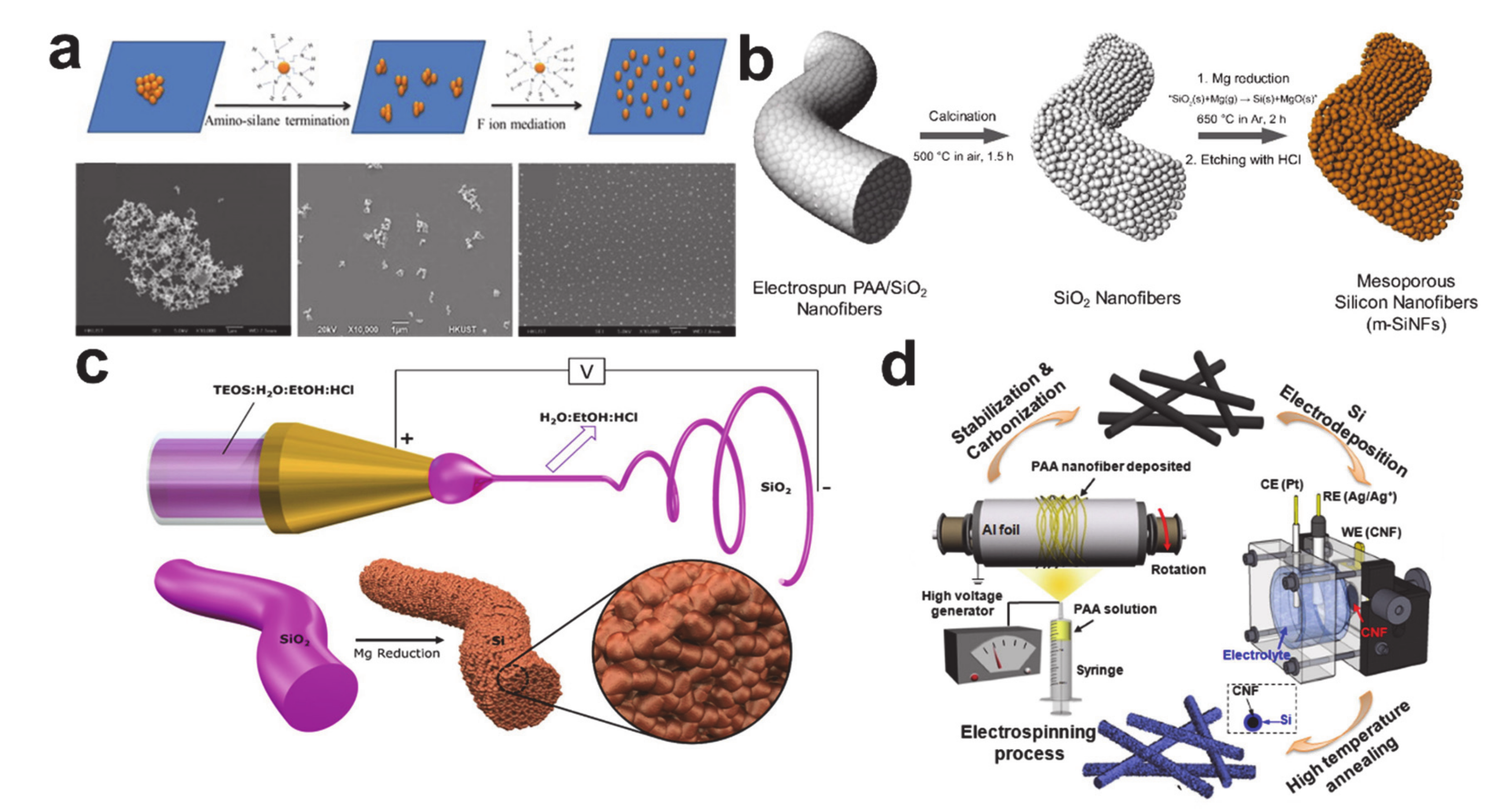
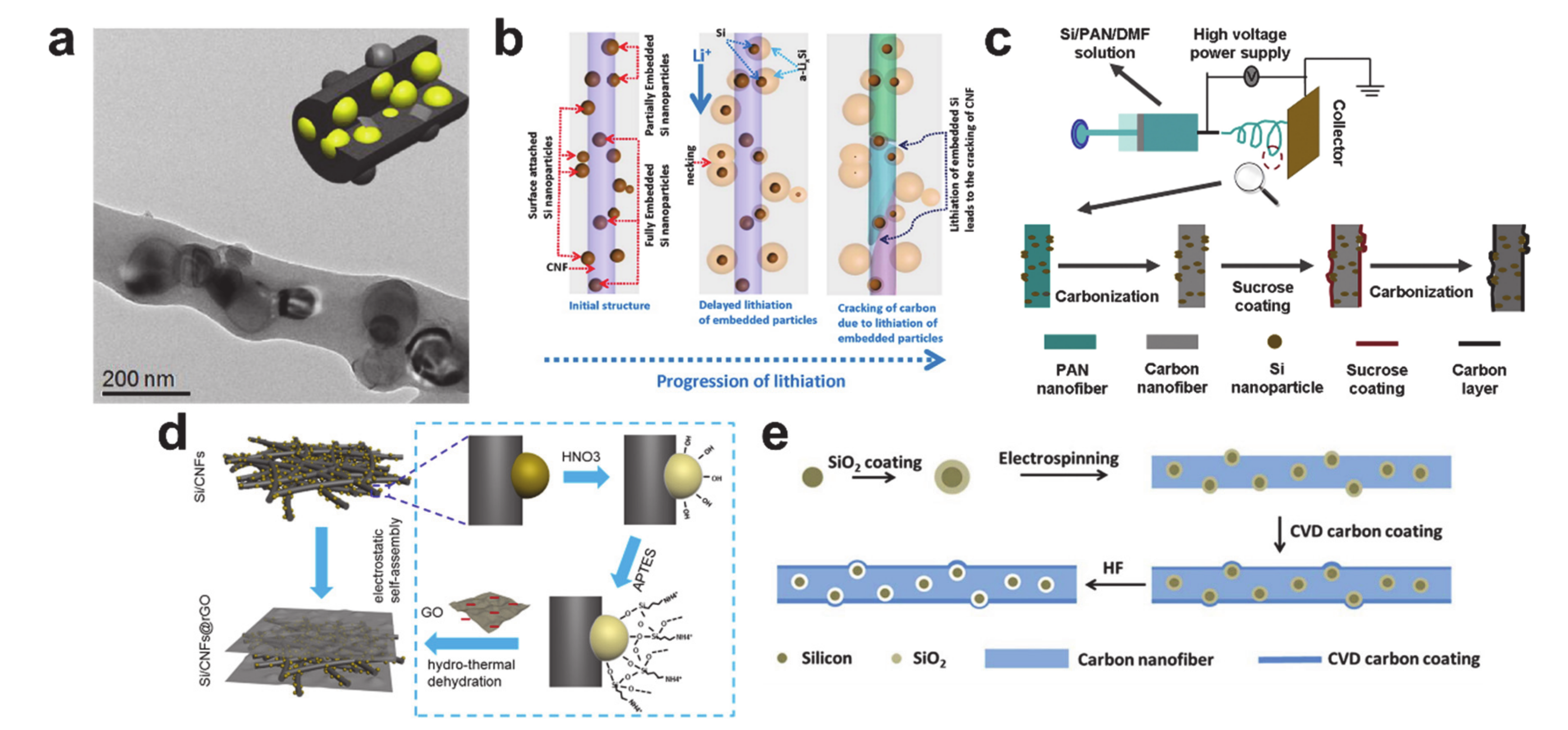
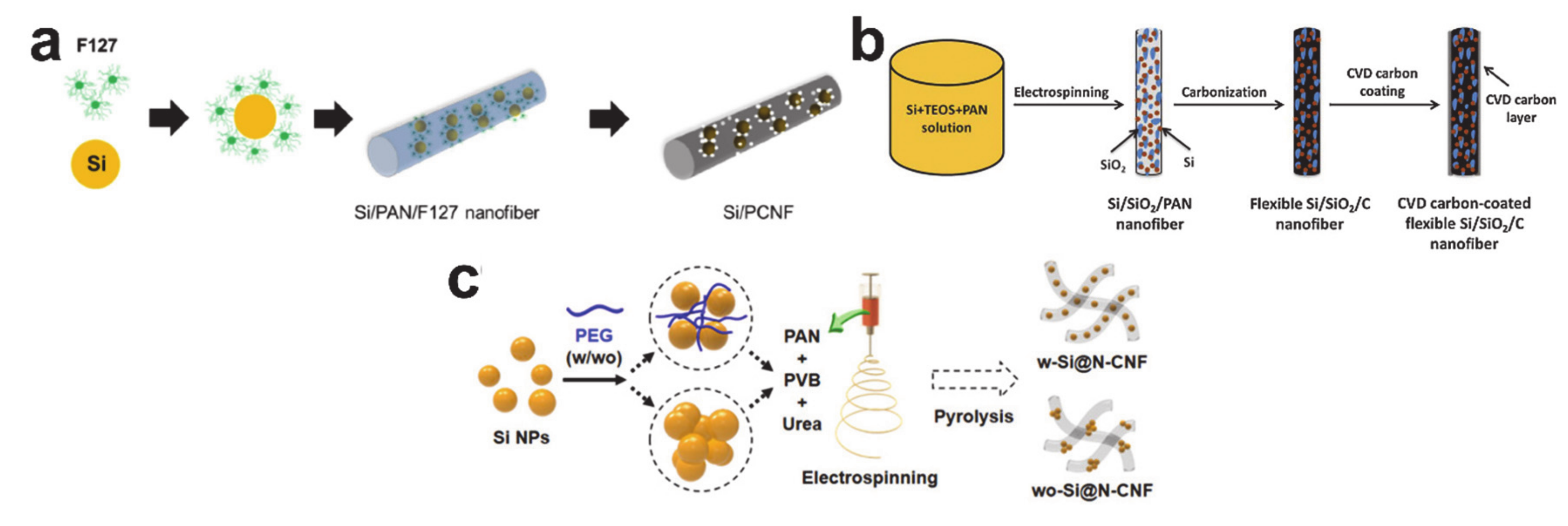
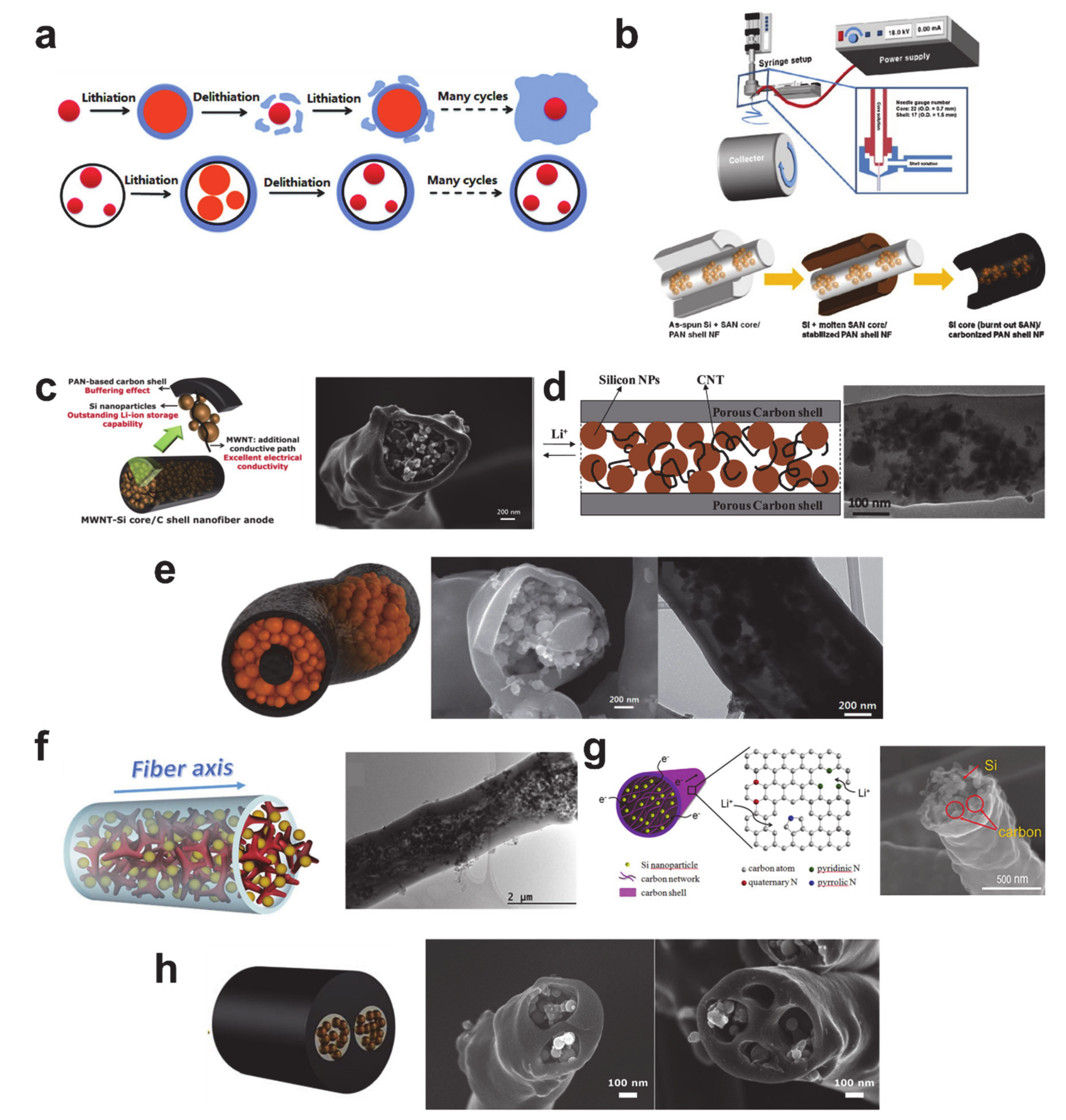
5. Alloying/Dealloying Reaction-Based Storage Materials
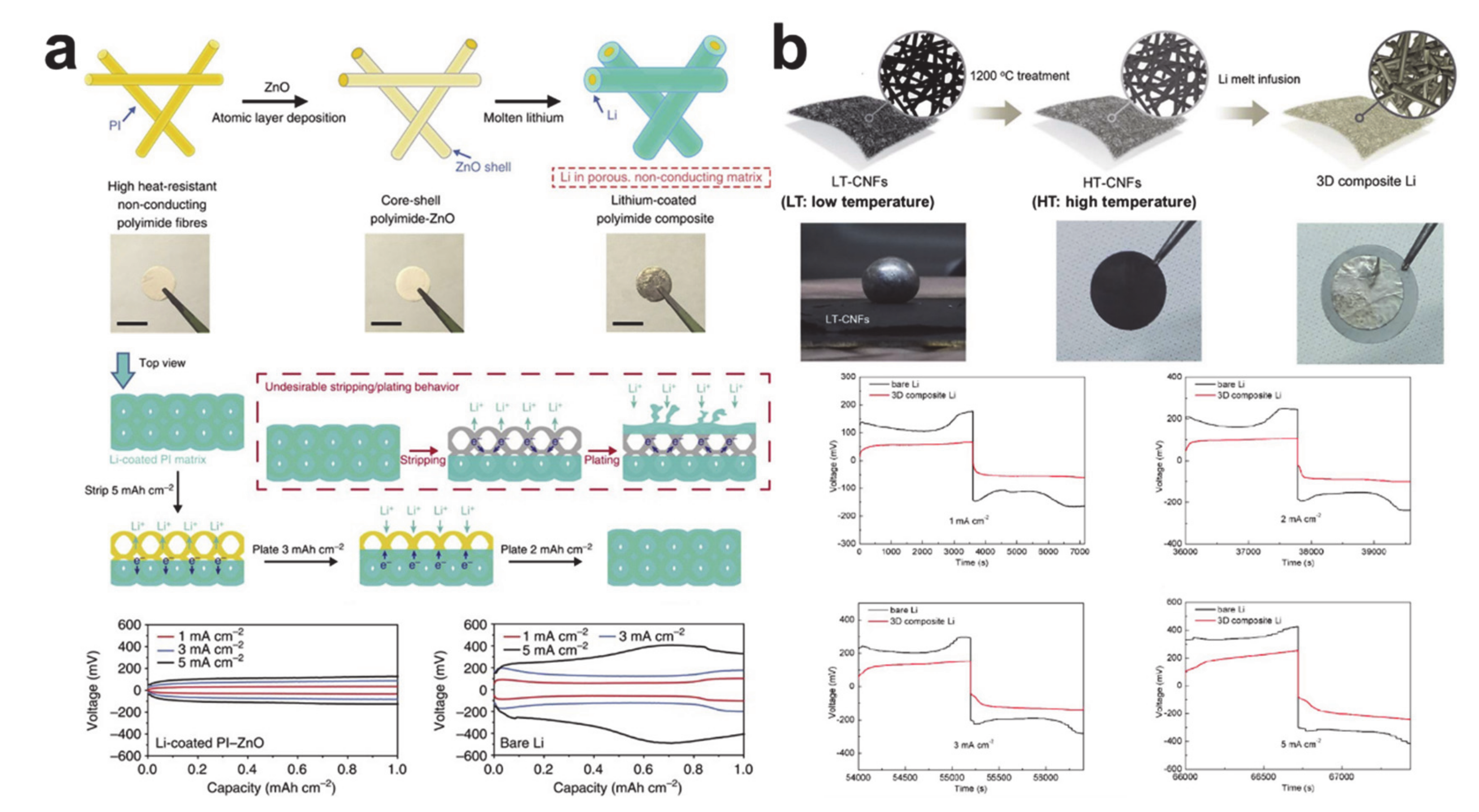
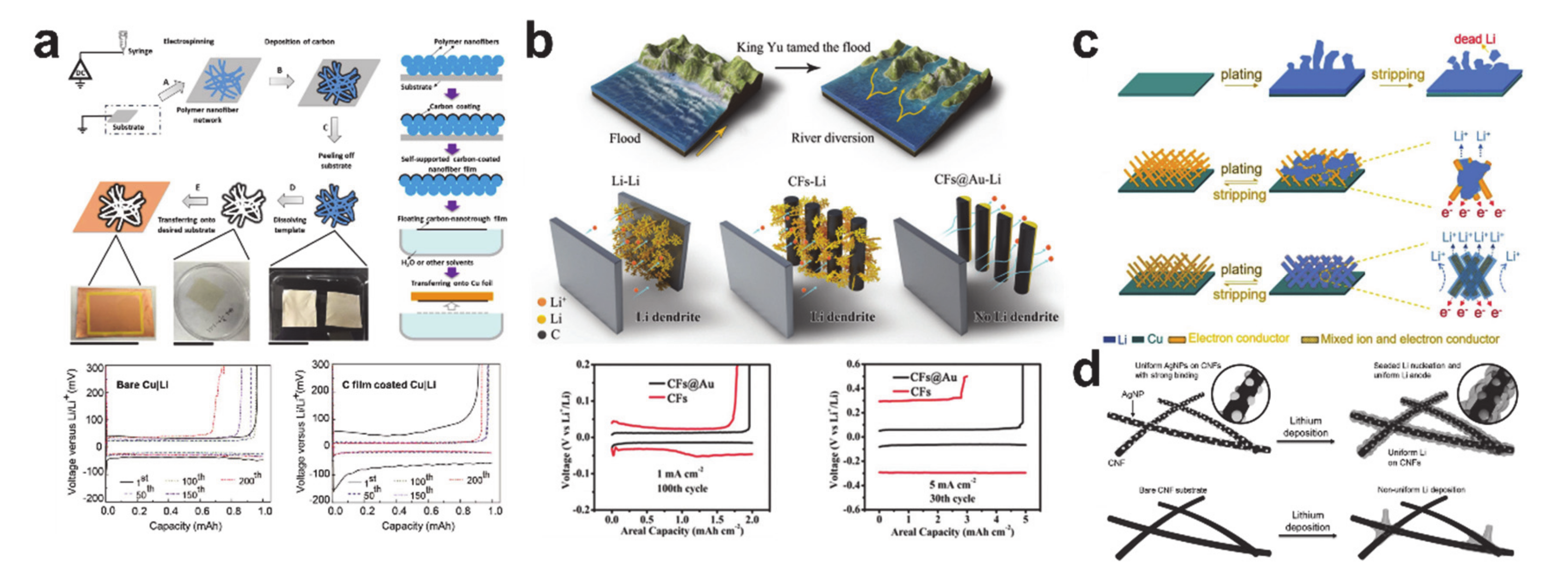
6. Plating/Stripping-Based Storage Materials
7. Current Limitations and Prospects
8. Concluding Remarks
Funding
Conflicts of Interest
References
- Wang, Z.; Yang, H.; Li, Y.; Wang, G.; Wang, J. Thermal runaway and fire behaviors of large-scale lithium ion batteries with different heating methods. J. Hazard. Mater. 2019, 379, 120730. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, W.; Ming, J.; Li, M.; Xie, L.; He, X.; Wang, J.; Liang, S.; Wu, Y. An Exploration of New Energy Storage System: High Energy Density, High Safety, and Fast Charging Lithium Ion Battery. Adv. Funct. Mater. 2019, 29, 1805978. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, C.; Wan, C.; Tsuchida, E. Effects of catalytic oxidation on the electrochemical performance of common natural graphite as an anode material for lithium ion batteries. Electrochem. Commun. 2000, 2, 272–275. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L. The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef]
- Zaghib, K.; Brochu, F.; Guerfi, A.; Kinoshita, K. Effect of particle size on lithium intercalation rates in natural graphite. J. Power Sources 2001, 103, 140–146. [Google Scholar] [CrossRef]
- Shen, C.; Hu, G.; Cheong, L.-Z.; Huang, S.; Zhang, J.-G.; Wang, D. Direct Observation of the Growth of Lithium Dendrites on Graphite Anodes by Operando EC-AFM. Small Methods 2018, 2, 1700298. [Google Scholar] [CrossRef]
- Wu, H.; Hu, L.; Rowell, M.W.; Kong, D.; Cha, J.J.; McDonough, J.R.; Zhu, J.; Yang, Y.; McGehee, M.D.; Cui, Y. Electrospun Metal Nanofiber Webs as High-Performance Transparent Electrode. Nano Lett. 2010, 10, 4242–4248. [Google Scholar] [CrossRef]
- Kim, S.J.; Naguib, M.; Zhao, M.; Zhang, C.; Jung, H.-T.; Barsoum, M.W.; Gogotsi, Y. High mass loading, binder-free MXene anodes for high areal capacity Li-ion batteries. Electrochim. Acta 2015, 163, 246–251. [Google Scholar] [CrossRef]
- Son, Y.; Kim, N.; Lee, T.; Lee, Y.; Ma, J.; Chae, S.; Sung, J.; Cha, H.; Yoo, Y.; Cho, J. Calendering-Compatible Macroporous Architecture for Silicon–Graphite Composite toward High-Energy Lithium-Ion Batteries. Adv. Mater. 2020, 2003286. [Google Scholar] [CrossRef]
- Chae, S.; Kim, N.; Ma, J.; Cho, J.; Ko, M. One-to-One Comparison of Graphite-Blended Negative Electrodes Using Silicon Nanolayer-Embedded Graphite versus Commercial Benchmarking Materials for High-Energy Lithium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1700071. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, G.; Lv, G.; Mu, Y.; Zhao, Y.; Wang, Y.; Li, X.; Yao, P.; Deng, Y.; Cui, Y.; et al. Minimized lithium trapping by isovalent isomorphism for high initial Coulombic efficiency of silicon anodes. Sci. Adv. 2019, 5, eaax0651. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Sun, R.; Liu, C.; Xiong, J.; Qin, X. Mass production of nanofibers from needleless electrospinning by a novel annular spinneret. Mater. Des. 2019, 179, 107885. [Google Scholar] [CrossRef]
- Vysloužilová, L.; Buzgo, M.; Pokorný, P.; Chvojka, J.; Míčková, A.; Rampichová, M.; Kula, J.; Pejchar, K.; Bílek, M.; Lukáš, D.; et al. Needleless coaxial electrospinning: A novel approach to mass production of coaxial nanofibers. Int. J. Pharm. 2017, 516, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.G.; Lee, J.-H.; Unnithan, A.R.; Park, C.-H.; Kim, C.S. A comprehensive electric field analysis of cylinder-type multi-nozzle electrospinning system for mass production of nanofibers. J. Ind. Eng. Chem. 2015, 31, 251–256. [Google Scholar] [CrossRef]
- Nan, D.; Huang, Z.-H.; Lv, R.; Yang, L.; Wang, J.-G.; Shen, W.; Lin, Y.; Yu, X.; Ye, L.; Sun, H.; et al. Nitrogen-enriched electrospun porous carbon nanofiber networks as high-performance free-standing electrode materials. J. Mater. Chem. A 2014, 2, 19678–19684. [Google Scholar] [CrossRef]
- Zhu, S.; Sun, J.; Wu, T.; Su, X.; Su, H.; Qu, S.; Xie, Y.; Chen, M.; Diao, G. Graphitized porous carbon nanofibers prepared by electrospinning as anode materials for lithium ion batteries. RSC Adv. 2016, 6, 83185–83195. [Google Scholar] [CrossRef]
- Kim, J.H.; Hong, Y.J.; Kang, Y.C.; Choi, Y.J.; Kim, Y.S. Superior electrochemical properties of α-Fe2O3 nanofibers with a porous core/dense shell structure formed from iron acetylacetonate-polyvinylpyrrolidone composite fibers. Electrochim. Acta 2015, 154, 211–218. [Google Scholar] [CrossRef]
- Fan, X.; Zou, L.; Zheng, Y.-P.; Kang, F.-Y.; Shen, W.-C. Electrospinning Preparation of Nanosilicon/Disordered Carbon Composite as Anode Materials in Li-Ion Battery. Electrochem. Solid-State Lett. 2009, 12, A199. [Google Scholar] [CrossRef]
- Luo, L.; Li, J.; Yaghoobnejad Asl, H.; Manthiram, A. A 3D Lithiophilic Mo2N-Modified Carbon Nanofiber Architecture for Dendrite-Free Lithium-Metal Anodes in a Full Cell. Adv. Mater. 2019, 31, 1904537. [Google Scholar] [CrossRef]
- Sitinamaluwa, H.; Zhang, S.; Senadeera, W.; Will, G.; Yan, C. Carbon-based silicon nanohybrid anode materials for rechargeable lithium ion batteries. Mater. Technol. 2016, 31, 872–883. [Google Scholar] [CrossRef]
- Rahman, M.A.; Song, G.; Bhatt, A.I.; Wong, Y.C.; Wen, C. Nanostructured Silicon Anodes for High-Performance Lithium-Ion Batteries. Adv. Funct. Mater. 2016, 26, 647–678. [Google Scholar] [CrossRef]
- Peng, S.; Li, L.; Kong Yoong Lee, J.; Tian, L.; Srinivasan, M.; Adams, S.; Ramakrishna, S. Electrospun carbon nanofibers and their hybrid composites as advanced materials for energy conversion and storage. Nano Energy 2016, 22, 361–395. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Huang, H.; Mai, Y.-W.; Zhou, L. Electrospun carbon-based nanostructured electrodes for advanced energy storage—A review. Energy Storage Mater. 2016, 5, 58–92. [Google Scholar] [CrossRef]
- Liu, L.; Xie, F.; Lyu, J.; Zhao, T.; Li, T.; Choi, B.G. Tin-based anode materials with well-designed architectures for next-generation lithium-ion batteries. J. Power Sources 2016, 321, 11–35. [Google Scholar] [CrossRef]
- Li, L.; Peng, S.; Lee, J.K.Y.; Ji, D.; Srinivasan, M.; Ramakrishna, S. Electrospun hollow nanofibers for advanced secondary batteries. Nano Energy 2017, 39, 111–139. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.-W.; Yu, L.-G.; Zhang, J.-W. Electrospun porous nanofibers for electrochemical energy storage. J. Mater. Sci. 2017, 52, 6173–6195. [Google Scholar] [CrossRef]
- Li, S.; Cui, Z.; Li, D.; Yue, G.; Liu, J.; Ding, H.; Gao, S.; Zhao, Y.; Wang, N.; Zhao, Y. Hierarchically structured electrospinning nanofibers for catalysis and energy storage. Compos. Commun. 2019, 13, 1–11. [Google Scholar] [CrossRef]
- Jung, J.-W.; Lee, C.-L.; Yu, S.; Kim, I.-D. Electrospun nanofibers as a platform for advanced secondary batteries: A comprehensive review. J. Mater. Chem. A 2016, 4, 703–750. [Google Scholar] [CrossRef]
- Pampal, E.S.; Stojanovska, E.; Simon, B.; Kilic, A. A review of nanofibrous structures in lithium ion batteries. J. Power Sources 2015, 300, 199–215. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, J.; Zhang, L.; Qiu, Y. Recent advances in energy materials by electrospinning. Renew. Sustain. Energy Rev. 2018, 81, 1825–1858. [Google Scholar] [CrossRef]
- Santangelo, S. Electrospun nanomaterials for energy applications: Recent advances. Appl. Sci. 2019, 9, 1049. [Google Scholar] [CrossRef]
- Sawai, K.; Iwakoshi, Y.; Ohzuku, T. Carbon materials for lithium-ion (shuttlecock) cells. Solid State Ion. 1994, 69, 273–283. [Google Scholar] [CrossRef]
- Ge, H.; Li, N.; Li, D.; Dai, C.; Wang, D. Study on the Theoretical Capacity of Spinel Lithium Titanate Induced by Low-Potential Intercalation. J. Phys. Chem. C 2009, 113, 6324–6326. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, G.; Seh, Z.W.; Liu, N.; Wang, S.; Sun, J.; Lee, H.R.; Cui, Y. Graphite-Encapsulated Li-Metal Hybrid Anodes for High-Capacity Li Batteries. Chem 2016, 1, 287–297. [Google Scholar] [CrossRef]
- Nie, A.; Gan, L.-Y.; Cheng, Y.; Asayesh-Ardakani, H.; Li, Q.; Dong, C.; Tao, R.; Mashayek, F.; Wang, H.-T.; Schwingenschlögl, U.; et al. Atomic-Scale Observation of Lithiation Reaction Front in Nanoscale SnO2 Materials. ACS Nano 2013, 7, 6203–6211. [Google Scholar] [CrossRef] [PubMed]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material—fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Z.; Zhou, L.; Mai, Y.-W.; Huang, H. In situ formation of hollow graphitic carbon nanospheres in electrospun amorphous carbon nanofibers for high-performance Li-based batteries. Nanoscale 2012, 4, 6800–6805. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, Z.; Zhou, L.; Mai, Y.-W.; Huang, H. Triple-coaxial electrospun amorphous carbon nanotubes with hollow graphitic carbon nanospheres for high-performance Li ion batteries. Energy Environ. Sci. 2012, 5, 7898–7902. [Google Scholar] [CrossRef]
- Kim, C.; Yang, K.S.; Kojima, M.; Yoshida, K.; Kim, Y.J.; Kim, Y.A.; Endo, M. Fabrication of Electrospinning-Derived Carbon Nanofiber Webs for the Anode Material of Lithium-Ion Secondary Batteries. Adv. Funct. Mater. 2006, 16, 2393–2397. [Google Scholar] [CrossRef]
- Lee, B.-S.; Son, S.-B.; Park, K.-M.; Yu, W.-R.; Oh, K.-H.; Lee, S.-H. Anodic properties of hollow carbon nanofibers for Li-ion battery. J. Power Sources 2012, 199, 53–60. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, Y.; Xu, Z.-L.; Abouali, S.; Akbari, M.; He, Y.-B.; Kang, F.; Kim, J.-K. Correlation Between Atomic Structure and Electrochemical Performance of Anodes Made from Electrospun Carbon Nanofiber Films. Adv. Energy Mater. 2014, 4, 1301448. [Google Scholar] [CrossRef]
- Wang, T.; Shi, S.; Li, Y.; Zhao, M.; Chang, X.; Wu, D.; Wang, H.; Peng, L.; Wang, P.; Yang, G. Study of Microstructure Change of Carbon Nanofibers as Binder-Free Anode for High-Performance Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 33091–33101. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xiao, N.; Wang, Y.; Zhou, Y.; Wang, G.; Li, H.; Ji, Y.; Qiu, J. Electrospun nitrogen-doped carbon nanofibers with tuned microstructure and enhanced lithium storage properties. Carbon 2018, 139, 716–724. [Google Scholar] [CrossRef]
- Li, D.; Wang, D.; Rui, K.; Ma, Z.; Xie, L.; Liu, J.; Zhang, Y.; Chen, R.; Yan, Y.; Lin, H.; et al. Flexible phosphorus doped carbon nanosheets/nanofibers: Electrospun preparation and enhanced Li-storage properties as free-standing anodes for lithium ion batteries. J. Power Sources 2018, 384, 27–33. [Google Scholar] [CrossRef]
- Eom, K.; Joshi, T.; Bordes, A.; Do, I.; Fuller, T.F. The design of a Li-ion full cell battery using a nano silicon and nano multi-layer graphene composite anode. J. Power Sources 2014, 249, 118–124. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Bareño, J.; Spila, T.; Trask, S.E.; Miller, D.J.; Polzin, B.J.; Jansen, A.N.; Abraham, D.P. Cycling Behavior of NCM523/Graphite Lithium-Ion Cells in the 3–4.4 V Range: Diagnostic Studies of Full Cells and Harvested Electrodes. J. Electrochem. Soc. 2016, 164, A6054–A6065. [Google Scholar] [CrossRef]
- Mabuchi, A. A Survey on the Carbon Anode Materials for Rechargeable Lithium Batteries. Tanso 1994, 1994, 298–306. [Google Scholar] [CrossRef][Green Version]
- Ji, L.; Lin, Z.; Medford, A.J.; Zhang, X. Porous carbon nanofibers from electrospun polyacrylonitrile/SiO2 composites as an energy storage material. Carbon 2009, 47, 3346–3354. [Google Scholar] [CrossRef]
- Nan, D.; Wang, J.-G.; Huang, Z.-H.; Wang, L.; Shen, W.; Kang, F. Highly porous carbon nanofibers from electrospun polyimide/SiO2 hybrids as an improved anode for lithium-ion batteries. Electrochem. Commun. 2013, 34, 52–55. [Google Scholar] [CrossRef]
- Chen, R.; Hu, Y.; Shen, Z.; Pan, P.; He, X.; Wu, K.; Zhang, X.; Cheng, Z. Facile fabrication of foldable electrospun polyacrylonitrile-based carbon nanofibers for flexible lithium-ion batteries. J. Mater. Chem. A 2017, 5, 12914–12921. [Google Scholar] [CrossRef]
- Shi, Z.; Jin, G.; Wang, J.; Zhang, J. Free-standing, welded mesoporous carbon nanofibers as anode for high-rate performance Li-ion batteries. J. Electroanal. Chem. 2017, 795, 26–31. [Google Scholar] [CrossRef]
- Chen, C.; Agrawal, R.; Hao, Y.; Wang, C. Activated Carbon Nanofibers as High Capacity Anodes for Lithium-Ion Batteries. ECS J. Solid State Sci. Technol. 2013, 2, M3074–M3077. [Google Scholar] [CrossRef]
- Li, W.; Li, M.; Wang, M.; Zeng, L.; Yu, Y. Electrospinning with partially carbonization in air: Highly porous carbon nanofibers optimized for high-performance flexible lithium-ion batteries. Nano Energy 2015, 13, 693–701. [Google Scholar] [CrossRef]
- Lee, B.-S.; Son, S.-B.; Park, K.-M.; Lee, G.; Oh, K.H.; Lee, S.-H.; Yu, W.-R. Effect of Pores in Hollow Carbon Nanofibers on Their Negative Electrode Properties for a Lithium Rechargeable Battery. ACS Appl. Mater. Interfaces 2012, 4, 6702–6710. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-S.; Seo, J.-H.; Son, S.-B.; Kim, S.C.; Choi, I.-S.; Ahn, J.-P.; Oh, K.H.; Lee, S.-H.; Yu, W.-R. Face-Centered-Cubic Lithium Crystals Formed in Mesopores of Carbon Nanofiber Electrodes. ACS Nano 2013, 7, 5801–5807. [Google Scholar] [CrossRef]
- Lee, B.-S.; Park, K.-M.; Yu, W.-R.; Youk, J.H. An effective method for manufacturing hollow carbon nanofibers and microstructural analysis. Macromol. Res. 2012, 20, 605–613. [Google Scholar] [CrossRef]
- Lee, B.-S.; Jeon, S.-Y.; Park, H.; Lee, G.; Yang, H.-S.; Yu, W.-R. New Electrospinning Nozzle to Reduce Jet Instability and Its Application to Manufacture of Multi-layered Nanofibers. Sci. Rep. 2014, 4, 6758. [Google Scholar] [CrossRef]
- Lee, B.-S.; Yang, H.-S.; Yu, W.-R. Fabrication of double-tubular carbon nanofibers using quadruple coaxial electrospinning. Nanotechnology 2014, 25, 465602. [Google Scholar] [CrossRef]
- Liu, B.; Yu, Y.; Chang, J.; Yang, X.; Wu, D.; Yang, X. An enhanced stable-structure core-shell coaxial carbon nanofiber web as a direct anode material for lithium-based batteries. Electrochem. Commun. 2011, 13, 558–561. [Google Scholar] [CrossRef]
- Lee, B.-S.; Yang, H.-S.; Jung, H.; Mah, S.K.; Kwon, S.; Park, J.-H.; Lee, K.H.; Yu, W.-R.; Doo, S.-G. Facile method to improve initial reversible capacity of hollow carbon nanofiber anodes. Eur. Polym. J. 2015, 70, 392–399. [Google Scholar] [CrossRef]
- Jayaraman, S.; Aravindan, V.; Suresh Kumar, P.; Chui Ling, W.; Ramakrishna, S.; Madhavi, S. Exceptional Performance of TiNb2O7 Anode in All One-Dimensional Architecture by Electrospinning. ACS Appl. Mater. Interfaces 2014, 6, 8660–8666. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Mu, X.; van Aken, P.A.; Yu, Y.; Maier, J. “Nano-Pearl-String” TiNb2O7 as Anodes for Rechargeable Lithium Batteries. Adv. Energy Mater. 2013, 3, 49–53. [Google Scholar] [CrossRef]
- Reddy, M.V.; Jose, R.; Le Viet, A.; Ozoemena, K.I.; Chowdari, B.V.R.; Ramakrishna, S. Studies on the lithium ion diffusion coefficients of electrospun Nb2O5 nanostructures using galvanostatic intermittent titration and electrochemical impedance spectroscopy. Electrochim. Acta 2014, 128, 198–202. [Google Scholar] [CrossRef]
- Wang, L.; Wu, L.; Li, Z.; Lei, G.; xiao, Q.; Zhang, P. Synthesis and electrochemical properties of Li2ZnTi3O8 fibers as an anode material for lithium-ion batteries. Electrochim. Acta 2011, 56, 5343–5346. [Google Scholar] [CrossRef]
- Wu, X.; Li, X.; Zhu, C.; Li, P.; Yu, H.; Guo, Z.; Shu, J. Electrospun one-dimensional BaLi2Ti6O14 nanofibers for high rate performing lithium-ion battery. Mater. Today Energy 2016, 1–2, 17–23. [Google Scholar] [CrossRef]
- Zhang, X.; Suresh Kumar, P.; Aravindan, V.; Liu, H.H.; Sundaramurthy, J.; Mhaisalkar, S.G.; Duong, H.M.; Ramakrishna, S.; Madhavi, S. Electrospun TiO2–Graphene Composite Nanofibers as a Highly Durable Insertion Anode for Lithium Ion Batteries. J. Phys. Chem. C 2012, 116, 14780–14788. [Google Scholar] [CrossRef]
- Zhu, P.; Wu, Y.; Reddy, M.V.; Sreekumaran Nair, A.; Chowdari, B.V.R.; Ramakrishna, S. Long term cycling studies of electrospun TiO2 nanostructures and their composites with MWCNTs for rechargeable Li-ion batteries. RSC Adv. 2012, 2, 531–537. [Google Scholar] [CrossRef]
- Aravindan, V.; Sundaramurthy, J.; Kumar, P.S.; Shubha, N.; Ling, W.C.; Ramakrishna, S.; Madhavi, S. A novel strategy to construct high performance lithium-ion cells using one dimensional electrospun nanofibers, electrodes and separators. Nanoscale 2013, 5, 10636–10645. [Google Scholar] [CrossRef]
- Li, S.; Guo, J.; Ma, Q.; Yang, Y.; Dong, X.; Yang, M.; Yu, W.; Wang, J.; Liu, G. Electrospun Li4Ti5O12/Li2TiO3 composite nanofibers for enhanced high-rate lithium ion batteries. J. Solid State Electrochem. 2017, 21, 2779–2790. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, Q.; Wu, L.; Lei, G.; Li, Z. Spinel LiCrTiO4 fibers as an advanced anode material in high performance lithium ion batteries. Solid State Ion. 2013, 236, 43–47. [Google Scholar] [CrossRef]
- Abureden, S.; Hassan, F.M.; Lui, G.; Ahn, W.; Sy, S.; Yu, A.; Chen, Z. Multigrain electrospun nickel doped lithium titanate nanofibers with high power lithium ion storage. J. Mater. Chem. A 2016, 4, 12638–12647. [Google Scholar] [CrossRef]
- Sandhya, C.P.; John, B.; Gouri, C. Synthesis and electrochemical characterisation of electrospun lithium titanate ultrafine fibres. J. Mater. Sci. 2013, 48, 5827–5832. [Google Scholar] [CrossRef]
- Zhu, N.; Liu, W.; Xue, M.; Xie, Z.; Zhao, D.; Zhang, M.; Chen, J.; Cao, T. Graphene as a conductive additive to enhance the high-rate capabilities of electrospun Li4Ti5O12 for lithium-ion batteries. Electrochim. Acta 2010, 55, 5813–5818. [Google Scholar] [CrossRef]
- Qing, R.; Liu, L.; Bohling, C.; Sigmund, W. Conductivity dependence of lithium diffusivity and electrochemical performance for electrospun TiO2 fibers. J. Power Sources 2015, 274, 667–675. [Google Scholar] [CrossRef]
- Liu, C.; Wang, S.; Zhang, C.; Fu, H.; Nan, X.; Yang, Y.; Cao, G. High power high safety battery with electrospun Li3V2(PO4)3 cathode and Li4Ti5O12 anode with 95% energy efficiency. Energy Storage Mater. 2016, 5, 93–102. [Google Scholar] [CrossRef]
- Arun, N.; Aravindan, V.; Jayaraman, S.; Shubha, N.; Ling, W.C.; Ramakrishna, S.; Madhavi, S. Exceptional performance of a high voltage spinel LiNi0.5Mn1.5O4 cathode in all one dimensional architectures with an anatase TiO2 anode by electrospinning. Nanoscale 2014, 6, 8926–8934. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium Dioxide-Based Nanomaterials for Photocatalytic Fuel Generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef]
- Lafont, U.; Carta, D.; Mountjoy, G.; Chadwick, A.V.; Kelder, E.M. In Situ Structural Changes upon Electrochemical Lithium Insertion in Nanosized Anatase TiO2. J. Phys. Chem. C 2010, 114, 1372–1378. [Google Scholar] [CrossRef]
- Schmidt, W.; Bottke, P.; Sternad, M.; Gollob, P.; Hennige, V.; Wilkening, M. Small Change—Great Effect: Steep Increase of Li Ion Dynamics in Li4Ti5O12 at the Early Stages of Chemical Li Insertion. Chem. Mater. 2015, 27, 1740–1750. [Google Scholar] [CrossRef]
- Sutrisno, H.; Sunarto, S. Polymorphic transformation of titanium dioxide caused by heat treatment of protonic lepidocrocite titanate. Indones. J. Chem. 2010, 10, 143–148. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Zhou, Q.; Kong, M.; Ye, H.; Yang, G. Improved lithium storage properties of electrospun TiO2 with tunable morphology: From porous anatase to necklace rutile. Nanoscale 2013, 5, 10267–10274. [Google Scholar] [CrossRef]
- Fang, H.-T.; Liu, M.; Wang, D.-W.; Sun, T.; Guan, D.-S.; Li, F.; Zhou, J.; Sham, T.-K.; Cheng, H.-M. Comparison of the rate capability of nanostructured amorphous and anatase TiO2for lithium insertion using anodic TiO2nanotube arrays. Nanotechnology 2009, 20, 225701. [Google Scholar] [CrossRef]
- Zhang, G.; Duan, H.; Lu, B.; Xu, Z. Electrospinning directly synthesized metal nanoparticles decorated on both sidewalls of TiO2 nanotubes and their applications. Nanoscale 2013, 5, 5801–5808. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Maiti, S.; Das, I.; Mahanty, S.; De, G. Electrospun TiO2–rGO Composite Nanofibers with Ordered Mesopores by Molecular Level Assembly: A High Performance Anode Material for Lithium-Ion Batteries. Adv. Mater. Interfaces 2016, 3, 1600761. [Google Scholar] [CrossRef]
- Yang, Z.; Du, G.; Meng, Q.; Guo, Z.; Yu, X.; Chen, Z.; Guo, T.; Zeng, R. Synthesis of uniform TiO2@carbon composite nanofibers as anode for lithium ion batteries with enhanced electrochemical performance. J. Mater. Chem. 2012, 22, 5848–5854. [Google Scholar] [CrossRef]
- Fehse, M.; Cavaliere, S.; Lippens, P.E.; Savych, I.; Iadecola, A.; Monconduit, L.; Jones, D.J.; Rozière, J.; Fischer, F.; Tessier, C.; et al. Nb-Doped TiO2 Nanofibers for Lithium Ion Batteries. J. Phys. Chem. C 2013, 117, 13827–13835. [Google Scholar] [CrossRef]
- Deepa, T.D.; Mohapatra, S.; Nair, S.V.; Nair, A.S.; Rai, A.K. Surfactant-assisted synthesis of porous TiO2 nanofibers as an anode material for secondary lithium ion batteries. Sustain. Energy Fuels 2017, 1, 138–144. [Google Scholar] [CrossRef]
- Cho, J.S.; Hong, Y.J.; Kang, Y.C. Electrochemical Properties of Fiber-in-Tube- and Filled-Structured TiO2 Nanofiber Anode Materials for Lithium-Ion Batteries. Chem. Eur. J. 2015, 21, 11082–11087. [Google Scholar] [CrossRef]
- Zhang, X.; Aravindan, V.; Kumar, P.S.; Liu, H.; Sundaramurthy, J.; Ramakrishna, S.; Madhavi, S. Synthesis of TiO2 hollow nanofibers by co-axial electrospinning and its superior lithium storage capability in full-cell assembly with olivine phosphate. Nanoscale 2013, 5, 5973–5980. [Google Scholar] [CrossRef]
- Nam, S.H.; Shim, H.-S.; Kim, Y.-S.; Dar, M.A.; Kim, J.G.; Kim, W.B. Ag or Au Nanoparticle-Embedded One-Dimensional Composite TiO2 Nanofibers Prepared via Electrospinning for Use in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2010, 2, 2046–2052. [Google Scholar] [CrossRef]
- Yuan, T.; Zhao, B.; Cai, R.; Zhou, Y.; Shao, Z. Electrospinning based fabrication and performance improvement of film electrodes for lithium-ion batteries composed of TiO2 hollow fibers†. J. Mater. Chem. 2011, 21, 15041–15048. [Google Scholar] [CrossRef]
- Han, H.; Song, T.; Bae, J.-Y.; Nazar, L.F.; Kim, H.; Paik, U. Nitridated TiO2 hollow nanofibers as an anode material for high power lithium ion batteries. Energy Environ. Sci. 2011, 4, 4532–4536. [Google Scholar] [CrossRef]
- Wu, Y.; Reddy, M.V.; Chowdari, B.V.R.; Ramakrishna, S. Electrochemical studies on electrospun Li(Li1/3Ti5/3)O4 grains as an anode for Li-ion batteries. Electrochim. Acta 2012, 67, 33–40. [Google Scholar] [CrossRef]
- Park, H.; Song, T.; Han, H.; Paik, U. Electrospun Li4Ti5O12 nanofibers sheathed with conductive TiN/TiOxNy layer as an anode material for high power Li-ion batteries. J. Power Sources 2013, 244, 726–730. [Google Scholar] [CrossRef]
- Xu, H.; Hu, X.; Luo, W.; Sun, Y.; Yang, Z.; Hu, C.; Huang, Y. Electrospun Conformal Li4Ti5O12/C Fibers for High-Rate Lithium-Ion Batteries. ChemElectroChem 2014, 1, 611–616. [Google Scholar] [CrossRef]
- Kim, J.-G.; Shi, D.; Park, M.-S.; Jeong, G.; Heo, Y.-U.; Seo, M.; Kim, Y.-J.; Kim, J.H.; Dou, S.X. Controlled Ag-driven superior rate-capability of Li4Ti5O12 anodes for lithium rechargeable batteries. Nano Res. 2013, 6, 365–372. [Google Scholar] [CrossRef]
- Li, H.; Shen, L.; Zhang, X.; Nie, P.; Chen, L.; Xu, K. Electrospun Hierarchical Li4Ti4.95Nb0.05O12/Carbon Composite Nanofibers for High Rate Lithium Ion Batteries. J. Electrochem. Soc. 2012, 159, A426–A430. [Google Scholar] [CrossRef]
- Kim, J.-G.; Park, M.-S.; Hwang, S.M.; Heo, Y.-U.; Liao, T.; Sun, Z.; Park, J.H.; Kim, K.J.; Jeong, G.; Kim, Y.-J.; et al. Zr4+ Doping in Li4Ti5O12 Anode for Lithium-Ion Batteries: Open Li+ Diffusion Paths through Structural Imperfection. ChemSusChem 2014, 7, 1451–1457. [Google Scholar] [CrossRef]
- Zou, H.L.; Xiang, H.F.; Liang, X.; Feng, X.Y.; Cheng, S.; Jin, Y.; Chen, C.H. Electrospun Li3.9Cr0.3Ti4.8O12 nanofibers as anode material for high-rate and low-temperature lithium-ion batteries. J. Alloy. Compd. 2017, 701, 99–106. [Google Scholar] [CrossRef]
- Liu, B.; Hu, X.; Xu, H.; Luo, W.; Sun, Y.; Huang, Y. Encapsulation of MnO Nanocrystals in Electrospun Carbon Nanofibers as High-Performance Anode Materials for Lithium-Ion Batteries. Sci. Rep. 2014, 4, 4229. [Google Scholar] [CrossRef]
- Zeng, S.; Zhao, R.; Li, A.; Xue, S.; Lv, D.; Luo, Q.; Shu, D.; Chen, H. MnO/Carbon fibers prepared by an electrospinning method and their properties used as anodes for lithium ion batteries. Appl. Surf. Sci. 2019, 463, 211–216. [Google Scholar] [CrossRef]
- Joshi, B.; Samuel, E.; Jo, H.S.; Kim, Y.-I.; Park, S.; Swihart, M.T.; Yoon, W.Y.; Yoon, S.S. Carbon Nanofibers Loaded with Carbon Nanotubes and Iron Oxide as Flexible Freestanding Lithium-Ion Battery Anodes. Electrochim. Acta 2017, 253, 479–488. [Google Scholar] [CrossRef]
- Aravindan, V.; Suresh Kumar, P.; Sundaramurthy, J.; Ling, W.C.; Ramakrishna, S.; Madhavi, S. Electrospun NiO nanofibers as high performance anode material for Li-ion batteries. J. Power Sources 2013, 227, 284–290. [Google Scholar] [CrossRef]
- Xiang Wei, K.; Rong Liang, Z.; Sheng Kui, Z.; Ling, W. Electrospinning synthesis of 3D porous NiO nanorods as anode material for lithium-ion batteries. Mater. Sci.-Pol. 2016, 34, 227–232. [Google Scholar] [CrossRef]
- Park, S.-K.; Seong, C.-Y.; Yoo, S.; Piao, Y. Porous Mn3O4 nanorod/reduced graphene oxide hybrid paper as a flexible and binder-free anode material for lithium ion battery. Energy 2016, 99, 266–273. [Google Scholar] [CrossRef]
- Wang, J.; Du, N.; Wu, H.; Zhang, H.; Yu, J.; Yang, D. Order-aligned Mn3O4 nanostructures as super high-rate electrodes for rechargeable lithium-ion batteries. J. Power Sources 2013, 222, 32–37. [Google Scholar] [CrossRef]
- He, J.; Zhao, S.; Lian, Y.; Zhou, M.; Wang, L.; Ding, B.; Cui, S. Graphene-doped carbon/Fe3O4 porous nanofibers with hierarchical band construction as high-performance anodes for lithium-ion batteries. Electrochim. Acta 2017, 229, 306–315. [Google Scholar] [CrossRef]
- Wang, L.; Yu, Y.; Chen, P.C.; Zhang, D.W.; Chen, C.H. Electrospinning synthesis of C/Fe3O4 composite nanofibers and their application for high performance lithium-ion batteries. J. Power Sources 2008, 183, 717–723. [Google Scholar] [CrossRef]
- Abouali, S.; Akbari Garakani, M.; Zhang, B.; Luo, H.; Xu, Z.-L.; Huang, J.-Q.; Huang, J.; Kim, J.-K. Co3O4/porous electrospun carbon nanofibers as anodes for high performance Li-ion batteries. J. Mater. Chem. A 2014, 2, 16939–16944. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, W.; Zhu, S.; Lu, Y. Enhanced Lithium Storage Capability in Li-Ion Batteries Using Porous 3D Co3O4 Nanofiber Anodes. Ind. Eng. Chem. Res. 2017, 56, 2046–2053. [Google Scholar] [CrossRef]
- Ji, L.; Toprakci, O.; Alcoutlabi, M.; Yao, Y.; Li, Y.; Zhang, S.; Guo, B.; Lin, Z.; Zhang, X. α-Fe2O3 Nanoparticle-Loaded Carbon Nanofibers as Stable and High-Capacity Anodes for Rechargeable Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2012, 4, 2672–2679. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Oh, M.; Park, J.S.; Baek, S.-H.; Kim, M.; Kim, S.; Kim, J.H. Electrochemically deposited Fe2O3 nanorods on carbon nanofibers for free-standing anodes of lithium-ion batteries. Carbon 2015, 94, 9–17. [Google Scholar] [CrossRef]
- Yin, L.; Gao, Y.J.; Jeon, I.; Yang, H.; Kim, J.-P.; Jeong, S.Y.; Cho, C.R. Rice-panicle-like γ-Fe2O3@C nanofibers as high-rate anodes for superior lithium-ion batteries. Chem. Eng. J. 2019, 356, 60–68. [Google Scholar] [CrossRef]
- Zhang, L.; Song, J.; Liu, Y.; Yuan, X.; Guo, S. Tailoring nanostructured MnO2 as anodes for lithium ion batteries with high reversible capacity and initial Coulombic efficiency. J. Power Sources 2018, 379, 68–73. [Google Scholar] [CrossRef]
- Deng, J.; Chen, L.; Sun, Y.; Ma, M.; Fu, L. Interconnected MnO2 nanoflakes assembled on graphene foam as a binder-free and long-cycle life lithium battery anode. Carbon 2015, 92, 177–184. [Google Scholar] [CrossRef]
- Cho, J.S.; Hong, Y.J.; Kang, Y.C. Design and Synthesis of Bubble-Nanorod-Structured Fe2O3–Carbon Nanofibers as Advanced Anode Material for Li-Ion Batteries. ACS Nano 2015, 9, 4026–4035. [Google Scholar] [CrossRef]
- Narsimulu, D.; Rao, B.N.; Satyanarayana, N.; Srinadhu, E.S. High Capacity Electrospun MgFe2O4–C Composite Nanofibers as an Anode Material for Lithium Ion Batteries. ChemistrySelect 2018, 3, 8010–8017. [Google Scholar] [CrossRef]
- Pantò, F.; Fan, Y.; Frontera, P.; Stelitano, S.; Fazio, E.; Patanè, S.; Marelli, M.; Antonucci, P.; Neri, F.; Pinna, N.; et al. Are Electrospun Carbon/Metal Oxide Composite Fibers Relevant Electrode Materials for Li-Ion Batteries? J. Electrochem. Soc. 2016, 163, A2930–A2937. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, P.; Reddy, M.V.; Chowdari, B.V.R.; Ramakrishna, S. Maghemite Nanoparticles on Electrospun CNFs Template as Prospective Lithium-Ion Battery Anode. ACS Appl. Mater. Interfaces 2014, 6, 1951–1958. [Google Scholar] [CrossRef]
- Chaudhari, S.; Srinivasan, M. 1D hollow α-Fe2O3 electrospun nanofibers as high performance anode material for lithium ion batteries. J. Mater. Chem. 2012, 22, 23049–23056. [Google Scholar] [CrossRef]
- Yan, X.; Tong, X.; Wang, J.; Gong, C.; Zhang, M.; Liang, L. Synthesis of hollow nickel oxide nanotubes by electrospinning with structurally enhanced lithium storage properties. Mater. Lett. 2014, 136, 74–77. [Google Scholar] [CrossRef]
- Du, D.; Cao, M. Ligand-Assisted Hydrothermal Synthesis of Hollow Fe2O3 Urchin-like Microstructures and Their Magnetic Properties. J. Phys. Chem. C 2008, 112, 10754–10758. [Google Scholar] [CrossRef]
- Cho, J.S.; Hong, Y.J.; Lee, J.-H.; Kang, Y.C. Design and synthesis of micron-sized spherical aggregates composed of hollow Fe2O3 nanospheres for use in lithium-ion batteries. Nanoscale 2015, 7, 8361–8367. [Google Scholar] [CrossRef]
- Yu, L.; Han, R.; Sang, X.; Liu, J.; Thomas, M.P.; Hudak, B.M.; Patel, A.; Page, K.; Guiton, B.S. Shell-Induced Ostwald Ripening: Simultaneous Structure, Composition, and Morphology Transformations during the Creation of Hollow Iron Oxide Nanocapsules. ACS Nano 2018, 12, 9051–9059. [Google Scholar] [CrossRef]
- Cho, J.S.; Park, J.-S.; Kang, Y.C. Preparation of Hollow Fe2O3 Nanorods and Nanospheres by Nanoscale Kirkendall Diffusion, and Their Electrochemical Properties for Use in Lithium-Ion Batteries. Sci. Rep. 2016, 6, 38933. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, H.; Petnikota, S.; Ramakrishna, S.; Fan, H.J. Electrospun Fe2O3–carbon composite nanofibers as durable anode materials for lithium ion batteries. J. Mater. Chem. A 2014, 2, 10835–10841. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Li, F.; Xie, S.; Xu, G.; She, Y.; Leung, M.K.H.; Liu, T. Oxidizing solid Co into hollow Co3O4 within electrospun (carbon) nanofibers towards enhanced lithium storage performance. J. Mater. Chem. A 2019, 7, 3024–3030. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Lu, B.-R.; Cao, F.-H.; Yu, Z.-L.; Cong, H.-P.; Yu, S.-H. Hierarchically structured Co3O4@carbon porous fibers derived from electrospun ZIF-67/PAN nanofibers as anodes for lithium ion batteries. J. Mater. Chem. A 2018, 6, 12962–12968. [Google Scholar] [CrossRef]
- Qiu, H.; Wang, Y.; Liu, Y.; Li, D.; Zhu, X.; Ji, Q.; Quan, F.; Xia, Y. Synthesis of Co/Co3O4 nanoparticles embedded in porous carbon nanofibers for high performance lithium-ion battery anodes. J. Porous Mater. 2017, 24, 551–557. [Google Scholar] [CrossRef]
- Oh, J.H.; Su Jo, M.; Jeong, S.M.; Cho, C.; Kang, Y.C.; Cho, J.S. New synthesis strategy for hollow NiO nanofibers with interstitial nanovoids prepared via electrospinning using camphene for anodes of lithium-ion batteries. J. Ind. Eng. Chem. 2019, 77, 76–82. [Google Scholar] [CrossRef]
- Wang, B.; Cheng, J.L.; Wu, Y.P.; Wang, D.; He, D.N. Porous NiO fibers prepared by electrospinning as high performance anode materials for lithium ion batteries. Electrochem. Commun. 2012, 23, 5–8. [Google Scholar] [CrossRef]
- Bell, J.; Ye, R.; Ahmed, K.; Liu, C.; Ozkan, M.; Ozkan, C.S. Free-standing Ni–NiO nanofiber cloth anode for high capacity and high rate Li-ion batteries. Nano Energy 2015, 18, 47–56. [Google Scholar] [CrossRef]
- Gu, S.; Liu, Y.; Zhang, G.; Shi, W.; Liu, Y.; Zhu, J. Fe3O4/carbon composites obtained by electrospinning as an anode material with high rate capability for lithium ion batteries. RSC Adv. 2014, 4, 41179–41184. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, H.; Wu, J.; Chu, X.; He, Y.-B.; Han, C.; Miao, C.; Wang, S.; Li, B.; Kang, F. Fe3O4 nanoparticles encapsulated in electrospun porous carbon fibers with a compact shell as high-performance anode for lithium ion batteries. Carbon 2015, 87, 347–356. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, M.; Sun, J.; Liu, J.; Wu, T.; Su, H.; Qu, S.; Xie, Y.; Wang, S.; Su, X.; et al. Novel highly conductive ferroferric oxide/porous carbon nanofiber composites prepared by electrospinning as anode materials for high performance Li-ion batteries. RSC Adv. 2016, 6, 58529–58540. [Google Scholar] [CrossRef]
- Zhang, M.; Uchaker, E.; Hu, S.; Zhang, Q.; Wang, T.; Cao, G.; Li, J. CoO–carbon nanofiber networks prepared by electrospinning as binder-free anode materials for lithium-ion batteries with enhanced properties. Nanoscale 2013, 5, 12342–12349. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Abe, J.; Kawase, K.; Takahashi, K.; Vogt, B.D.; Shiratori, S. Enhanced stability of smoothly electrodeposited amorphous Fe2O3@electrospun carbon nanofibers as self-standing anodes for lithium ion batteries. New J. Chem. 2018, 42, 1867–1878. [Google Scholar] [CrossRef]
- Park, S.-H.; Lee, W.-J. Hierarchically mesoporous flower-like cobalt oxide/carbon nanofiber composites with shell–core structure as anodes for lithium ion batteries. Carbon 2015, 89, 197–207. [Google Scholar] [CrossRef]
- Sahay, R.; Suresh Kumar, P.; Aravindan, V.; Sundaramurthy, J.; Chui Ling, W.; Mhaisalkar, S.G.; Ramakrishna, S.; Madhavi, S. High Aspect Ratio Electrospun CuO Nanofibers as Anode Material for Lithium-Ion Batteries with Superior Cycleability. J. Phys. Chem. C 2012, 116, 18087–18092. [Google Scholar] [CrossRef]
- Park, S.-H.; Lee, W.-J. Hierarchically mesoporous CuO/carbon nanofiber coaxial shell-core nanowires for lithium ion batteries. Sci. Rep. 2015, 5, 9754. [Google Scholar] [CrossRef]
- Wang, W.; Shi, G.; Cai, H.; Zhao, C.; Wu, J.; Yu, Y.; Hu, J.; Fang, Z.; Yan, J.; Liu, B. Yolk-shell structured Mo/MoO2 composite microspheres function as high-performance anode materials for lithium-ion batteries. J. Alloy. Compd. 2019, 792, 191–202. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, T.; Shi, H.; Wang, T.; Zhang, M.; Cao, G. Single Nozzle Electrospinning Synthesized MoO2@C Core Shell Nanofibers with High Capacity and Long-Term Stability for Lithium-Ion Storage. Adv. Mater. Interfaces 2017, 4, 1600816. [Google Scholar] [CrossRef]
- Petnikota, S.; Teo, K.W.; Chen, L.; Sim, A.; Marka, S.K.; Reddy, M.V.; Srikanth, V.V.S.S.; Adams, S.; Chowdari, B.V.R. Exfoliated Graphene Oxide/MoO2 Composites as Anode Materials in Lithium-Ion Batteries: An Insight into Intercalation of Li and Conversion Mechanism of MoO2. ACS Appl. Mater. Interfaces 2016, 8, 10884–10896. [Google Scholar] [CrossRef]
- Xiang, J.; Wu, Z.; Zhang, X.; Yao, S. Enhanced electrochemical performance of an electrospun carbon/MoO2 composite nanofiber membrane as self-standing anodes for lithium-ion batteries. Mater. Res. Bull. 2018, 100, 254–258. [Google Scholar] [CrossRef]
- Liu, X.; Xu, H.; Huang, Y.; Hu, X. Direct planting of ultrafine MoO2+δ nanoparticles in carbon nanofibers by electrospinning: Self-supported mats as binder-free and long-life anodes for lithium-ion batteries. Phys. Chem. Chem. Phys. 2016, 18, 19832–19837. [Google Scholar] [CrossRef]
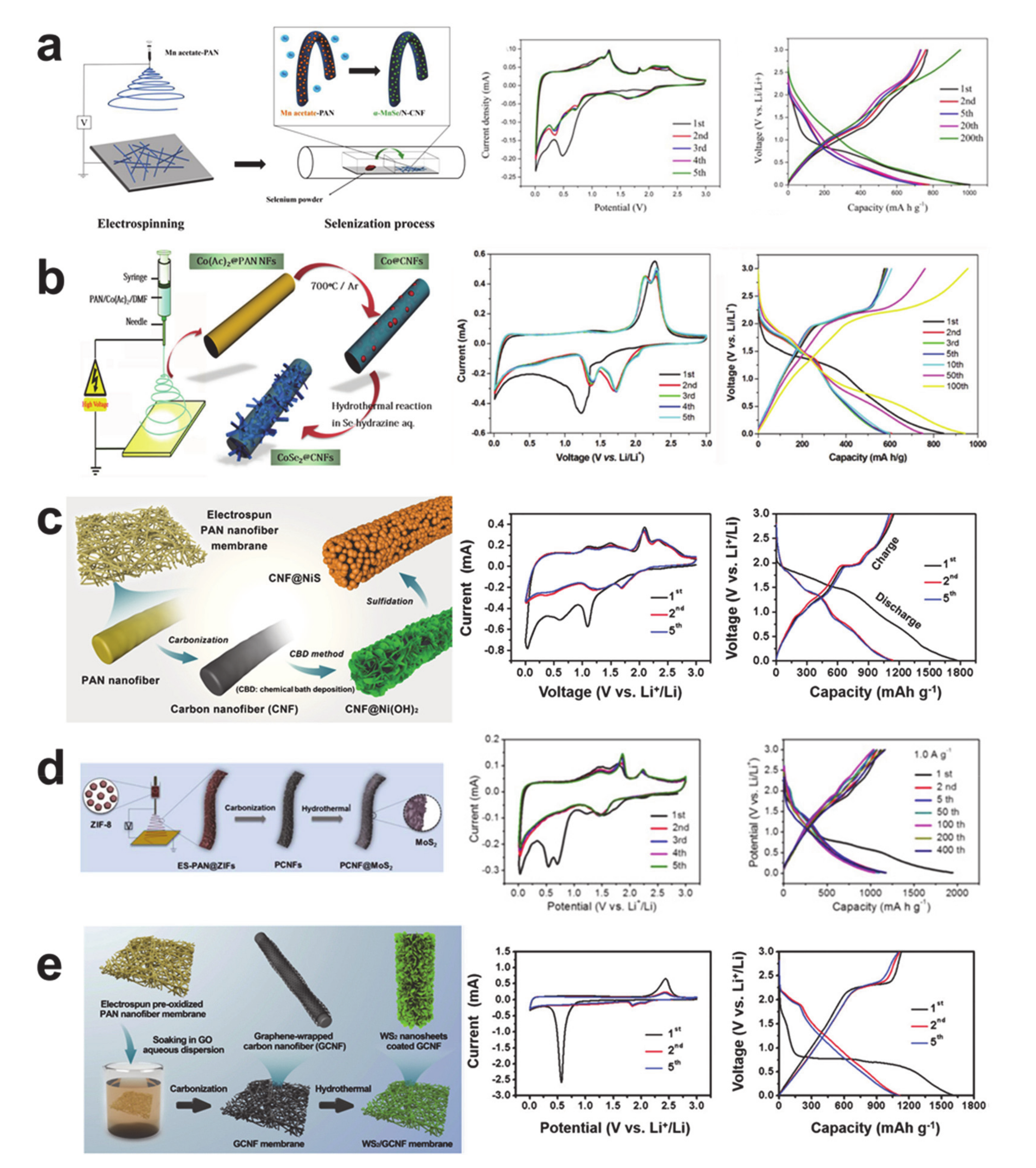
- Zhou, P.; Chen, L.; Zhang, M.; Huang, Q.; Cui, C.; Li, X.; Wang, L.; Li, L.; Yang, C.; Li, Y. Embedding α-MnSe nanodots in nitrogen-doped electrospinning carbon nanofibers to enhanced storage properties of lithium-ion batteries. J. Alloy. Compd. 2019, 797, 826–833. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Cao, D.; Lu, X.; Han, X.; Niu, C. Epitaxial Growth of Urchin-Like CoSe2 Nanorods from Electrospun Co-Embedded Porous Carbon Nanofibers and Their Superior Lithium Storage Properties. Part. Part. Syst. Charact. 2017, 34, 1700185. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y.; Zhang, Y.; Gu, H.; Fan, W.; Liu, T. Flexible Electrospun Carbon Nanofiber@NiS Core/Sheath Hybrid Membranes as Binder-Free Anodes for Highly Reversible Lithium Storage. Adv. Mater. Interfaces 2016, 3, 1500467. [Google Scholar] [CrossRef]
- Liu, A.; Liang, X.; Ren, X.; Guan, W.; Gao, M.; Yang, Y.; Yang, Q.; Gao, L.; Li, Y.; Ma, T. Recent Progress in MXene-Based Materials: Potential High-Performance Electrocatalysts. Adv. Funct. Mater. 2020, 2003437. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Jiang, Z.-H.; Lu, B.-R.; Liu, J.-T.; Cao, F.-H.; Li, H.; Yu, Z.-L.; Yu, S.-H. MoS2 nanoplates assembled on electrospun polyacrylonitrile-metal organic framework-derived carbon fibers for lithium storage. Nano Energy 2019, 61, 104–110. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, W.; Liu, T. Flexible hierarchical membranes of WS2 nanosheets grown on graphene-wrapped electrospun carbon nanofibers as advanced anodes for highly reversible lithium storage. Nanoscale 2016, 8, 16387–16394. [Google Scholar] [CrossRef]
- Zhao, C.; Kong, J.; Yao, X.; Tang, X.; Dong, Y.; Phua, S.L.; Lu, X. Thin MoS2 Nanoflakes Encapsulated in Carbon Nanofibers as High-Performance Anodes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2014, 6, 6392–6398. [Google Scholar] [CrossRef]
- Miao, Y.-E.; Huang, Y.; Zhang, L.; Fan, W.; Lai, F.; Liu, T. Electrospun porous carbon nanofiber@MoS2 core/sheath fiber membranes as highly flexible and binder-free anodes for lithium-ion batteries. Nanoscale 2015, 7, 11093–11101. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, J.; Gan, L.; Zhang, Q.; Zheng, Z.; Li, H.; Zhai, T. Scalable production of self-supported WS2/CNFs by electrospinning as the anode for high-performance lithium-ion batteries. Sci. Bull. 2016, 61, 227–235. [Google Scholar] [CrossRef][Green Version]
- Yu, X.; Pei, C.; Chen, W.; Feng, L. 2 dimensional WS2 tailored nitrogen-doped carbon nanofiber as a highly pseudocapacitive anode material for lithium-ion battery. Electrochim. Acta 2018, 272, 119–126. [Google Scholar] [CrossRef]
- Szczech, J.R.; Jin, S. Nanostructured silicon for high capacity lithium battery anodes. Energy Environ. Sci. 2011, 4, 56–72. [Google Scholar] [CrossRef]
- Lee, B.-S.; Yoon, J.; Jung, C.; Kim, D.Y.; Jeon, S.-Y.; Kim, K.-H.; Park, J.-H.; Park, H.; Lee, K.H.; Kang, Y.-S.; et al. Silicon/Carbon Nanotube/BaTiO3 Nanocomposite Anode: Evidence for Enhanced Lithium-Ion Mobility Induced by the Local Piezoelectric Potential. ACS Nano 2016, 10, 2617–2627. [Google Scholar] [CrossRef]
- Liang, S.; Zhu, X.; Lian, P.; Yang, W.; Wang, H. Superior cycle performance of Sn@C/graphene nanocomposite as an anode material for lithium-ion batteries. J. Solid State Chem. 2011, 184, 1400–1404. [Google Scholar] [CrossRef]
- Li, H.; Huang, X.; Chen, L. Anodes based on oxide materials for lithium rechargeable batteries11Paper presented at the 1997 Hawaii Solid State Ionics meeting (A30). Solid State Ion. 1999, 123, 189–197. [Google Scholar] [CrossRef]
- Mo, R.; Rooney, D.; Sun, K.; Yang, H.Y. 3D nitrogen-doped graphene foam with encapsulated germanium/nitrogen-doped graphene yolk-shell nanoarchitecture for high-performance flexible Li-ion battery. Nat. Commun. 2017, 8, 13949. [Google Scholar] [CrossRef]
- Mo, R.; Rooney, D.; Sun, K. Yolk-Shell Germanium@Polypyrrole Architecture with Precision Expansion Void Control for Lithium Ion Batteries. iScience 2018, 9, 521–531. [Google Scholar] [CrossRef]
- Hassoun, J.; Derrien, G.; Panero, S.; Scrosati, B. The role of the morphology in the response of Sb–C nanocomposite electrodes in lithium cells. J. Power Sources 2008, 183, 339–343. [Google Scholar] [CrossRef]
- Caballero, Á.; Morales, J.; Sánchez, L. A simple route to high performance nanometric metallic materials for Li-ion batteries involving the use of cellulose: The case of Sb. J. Power Sources 2008, 175, 553–557. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Y.; Li, K.; Xu, F.; Zhang, P.; Ding, Y. Electrospun free-standing N-doped C@SnO2 anode paper for flexible Li-ion batteries. Mater. Res. Bull. 2019, 109, 41–48. [Google Scholar] [CrossRef]
- Zhang, G.; Zhu, J.; Zeng, W.; Hou, S.; Gong, F.; Li, F.; Li, C.C.; Duan, H. Tin quantum dots embedded in nitrogen-doped carbon nanofibers as excellent anode for lithium-ion batteries. Nano Energy 2014, 9, 61–70. [Google Scholar] [CrossRef]
- Ji, H.; Ma, C.; Ding, J.; Yang, J.; Yang, G.; Chao, Y.; Yang, Y. Complementary stabilization by core/sheath carbon nanofibers/spongy carbon on submicron tin oxide particles as anode for lithium-ion batteries. J. Power Sources 2019, 413, 42–49. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, Q.; Teng, D.; Yang, X.; Ryu, S. Reticular Sn nanoparticle-dispersed PAN-based carbon nanofibers for anode material in rechargeable lithium-ion batteries. Electrochem. Commun. 2010, 12, 1187–1190. [Google Scholar] [CrossRef]
- Huang, Z.; Gao, H.; Wang, Q.; Zhao, Y.; Li, G. Fabrication of amorphous SnO2@C nanofibers as anode for lithium-ion batteries. Mater. Lett. 2017, 186, 231–234. [Google Scholar] [CrossRef]
- Zhou, H.; Naeem, M.A.; Lv, P.; Zhang, J.; Pang, Z.; Luo, L.; Cai, Y.; Xia, X.; Wei, Q. Effect of pore distribution on the lithium storage properties of porous C/SnO2 nanofibers. J. Alloy. Compd. 2017, 711, 414–423. [Google Scholar] [CrossRef]
- Yang, Z.; Du, G.; Feng, C.; Li, S.; Chen, Z.; Zhang, P.; Guo, Z.; Yu, X.; Chen, G.; Huang, S.; et al. Synthesis of uniform polycrystalline tin dioxide nanofibers and electrochemical application in lithium-ion batteries. Electrochim. Acta 2010, 55, 5485–5491. [Google Scholar] [CrossRef]
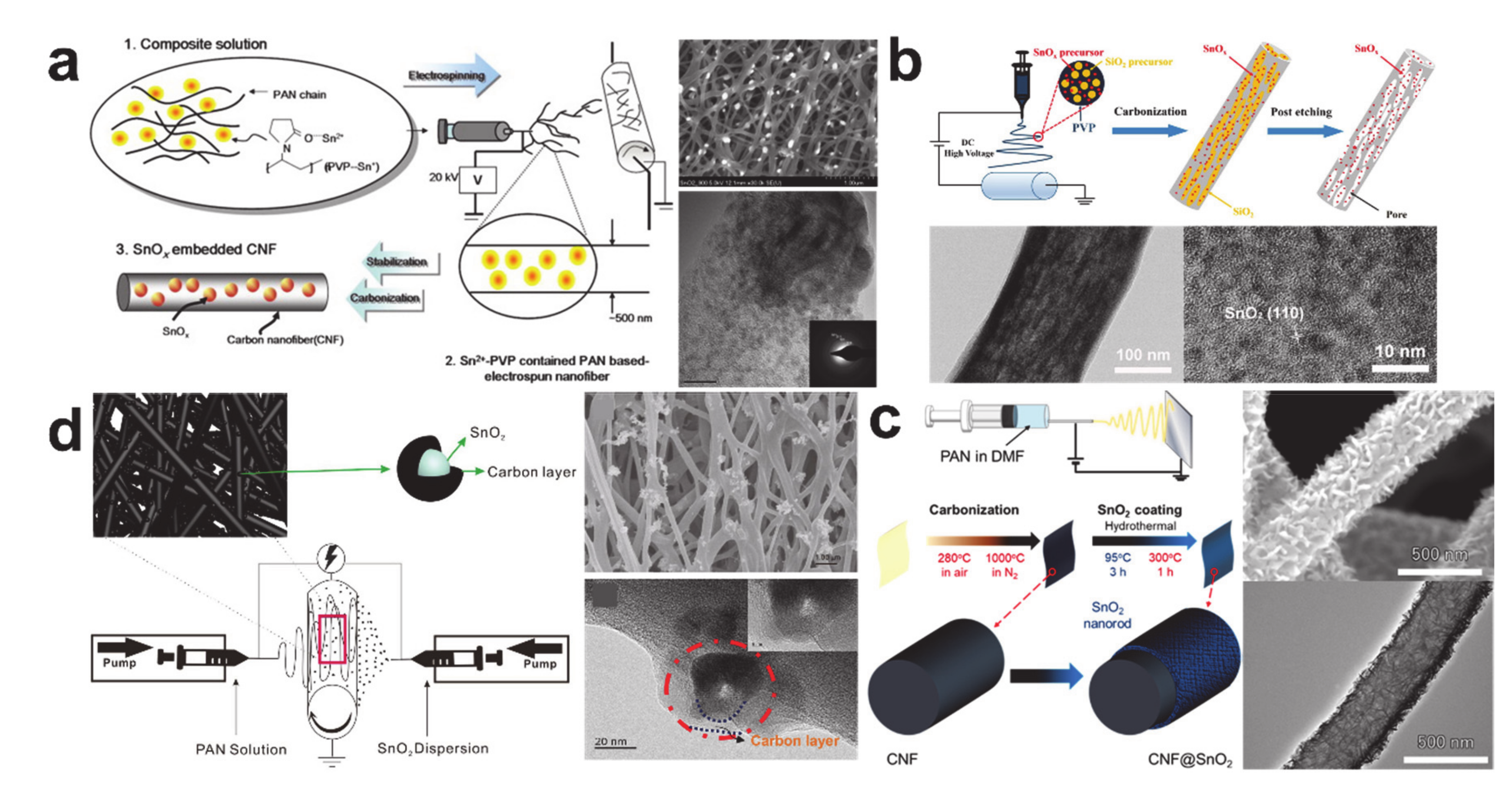
- Zhang, B.; Huang, J.; Kim, J.-K. Ultrafine Amorphous SnOx Embedded in Carbon Nanofiber/Carbon Nanotube Composites for Li-Ion and Na-Ion Batteries. Adv. Funct. Mater. 2015, 25, 5222–5228. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, Y.; Huang, Z.; He, Y.-B.; Jang, D.; Yoon, W.-S.; Mai, Y.-W.; Kang, F.; Kim, J.-K. Exceptional electrochemical performance of freestanding electrospun carbon nanofiber anodes containing ultrafine SnOx particles. Energy Environ. Sci. 2012, 5, 9895–9902. [Google Scholar] [CrossRef]
- Jung, H.-R.; Lee, W.-J. Electrochemical characterization of electrospun SnOx-embedded carbon nanofibers anode for lithium ion battery with EXAFS analysis. J. Electroanal. Chem. 2011, 662, 334–342. [Google Scholar] [CrossRef]
- Wang, H.; Gao, P.; Lu, S.; Liu, H.; Yang, G.; Pinto, J.; Jiang, X. The effect of tin content to the morphology of Sn/carbon nanofiber and the electrochemical performance as anode material for lithium batteries. Electrochim. Acta 2011, 58, 44–51. [Google Scholar] [CrossRef]
- Yang, Z.; Du, G.; Guo, Z.; Yu, X.; Chen, Z.; Zhang, P.; Chen, G.; Liu, H. Easy preparation of SnO2@carbon composite nanofibers with improved lithium ion storage properties. J. Mater. Res. 2011, 25, 1516–1524. [Google Scholar] [CrossRef]
- Wang, J.; Song, W.-L.; Wang, Z.; Fan, L.-Z.; Zhang, Y. Facile Fabrication of Binder-free Metallic Tin Nanoparticle/Carbon Nanofiber Hybrid Electrodes for Lithium-ion Batteries. Electrochim. Acta 2015, 153, 468–475. [Google Scholar] [CrossRef]
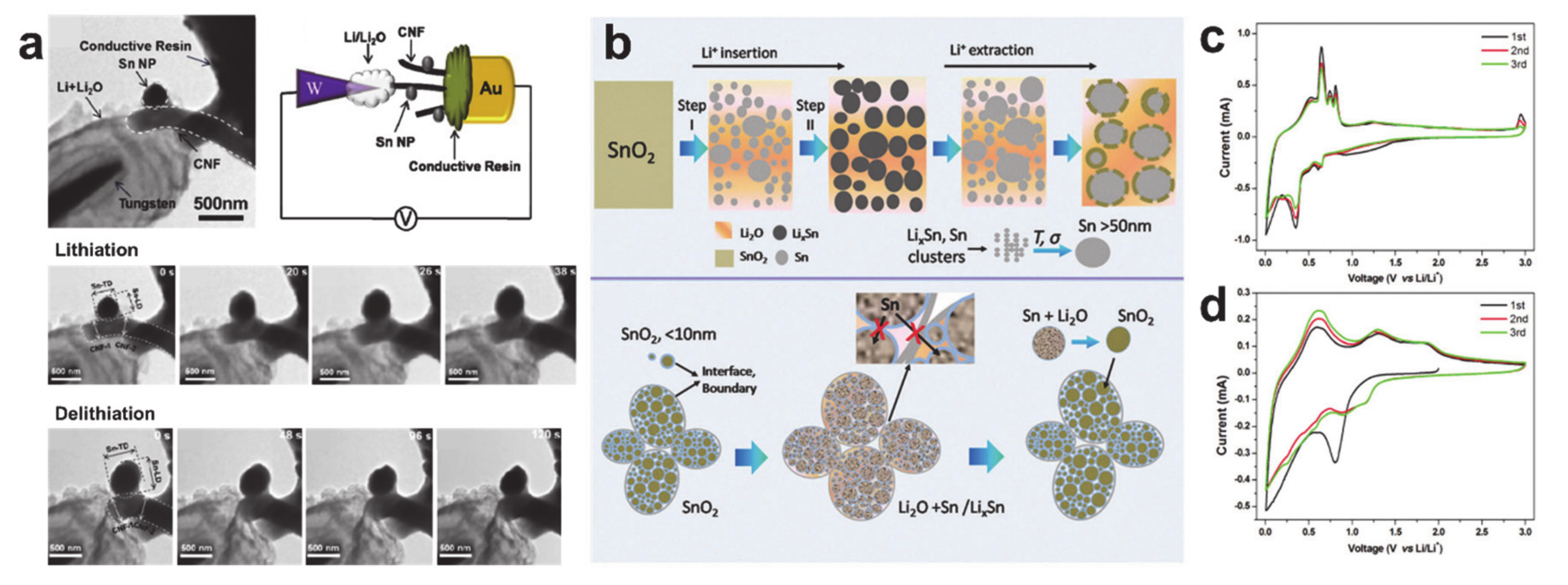
- Hu, R.; Chen, D.; Waller, G.; Ouyang, Y.; Chen, Y.; Zhao, B.; Rainwater, B.; Yang, C.; Zhu, M.; Liu, M. Dramatically enhanced reversibility of Li2O in SnO2-based electrodes: The effect of nanostructure on high initial reversible capacity. Energy Environ. Sci. 2016, 9, 595–603. [Google Scholar] [CrossRef]
- Wang, H.; Lu, X.; Li, L.; Li, B.; Cao, D.; Wu, Q.; Li, Z.; Yang, G.; Guo, B.; Niu, C. Synthesis of SnO2 versus Sn crystals within N-doped porous carbon nanofibers via electrospinning towards high-performance lithium ion batteries. Nanoscale 2016, 8, 7595–7603. [Google Scholar] [CrossRef]
- Dufficy, M.K.; Huang, S.-Y.; Khan, S.A.; Fedkiw, P.S. Effects of composition and structure on the performance of tin/graphene-containing carbon nanofibers for Li-ion anodes. RSC Adv. 2017, 7, 15428–15438. [Google Scholar] [CrossRef]
- Zou, L.; Gan, L.; Lv, R.; Wang, M.; Huang, Z.-h.; Kang, F.; Shen, W. A film of porous carbon nanofibers that contain Sn/SnOx nanoparticles in the pores and its electrochemical performance as an anode material for lithium ion batteries. Carbon 2011, 49, 89–95. [Google Scholar] [CrossRef]
- Lorie Lopez, J.L.; Grandinetti, P.J.; Co, A.C. Phase transformations and capacity fade mechanism in LixSn nanoparticle electrodes revealed by operando 7Li NMR. J. Mater. Chem. A 2019, 7, 10781–10794. [Google Scholar] [CrossRef]
- Kong, J.; Liu, Z.; Yang, Z.; Tan, H.R.; Xiong, S.; Wong, S.Y.; Li, X.; Lu, X. Carbon/SnO2/carbon core/shell/shell hybrid nanofibers: Tailored nanostructure for the anode of lithium ion batteries with high reversibility and rate capacity. Nanoscale 2012, 4, 525–530. [Google Scholar] [CrossRef]
- Aravindan, V.; Sundaramurthy, J.; Kumar, E.N.; Kumar, P.S.; Ling, W.C.; von Hagen, R.; Mathur, S.; Ramakrishna, S.; Madhavi, S. Does carbon coating really improves the electrochemical performance of electrospun SnO2 anodes? Electrochim. Acta 2014, 121, 109–115. [Google Scholar] [CrossRef]
- Zhou, X.; Dai, Z.; Liu, S.; Bao, J.; Guo, Y.-G. Ultra-Uniform SnOx/Carbon Nanohybrids toward Advanced Lithium-Ion Battery Anodes. Adv. Mater. 2014, 26, 3943–3949. [Google Scholar] [CrossRef]
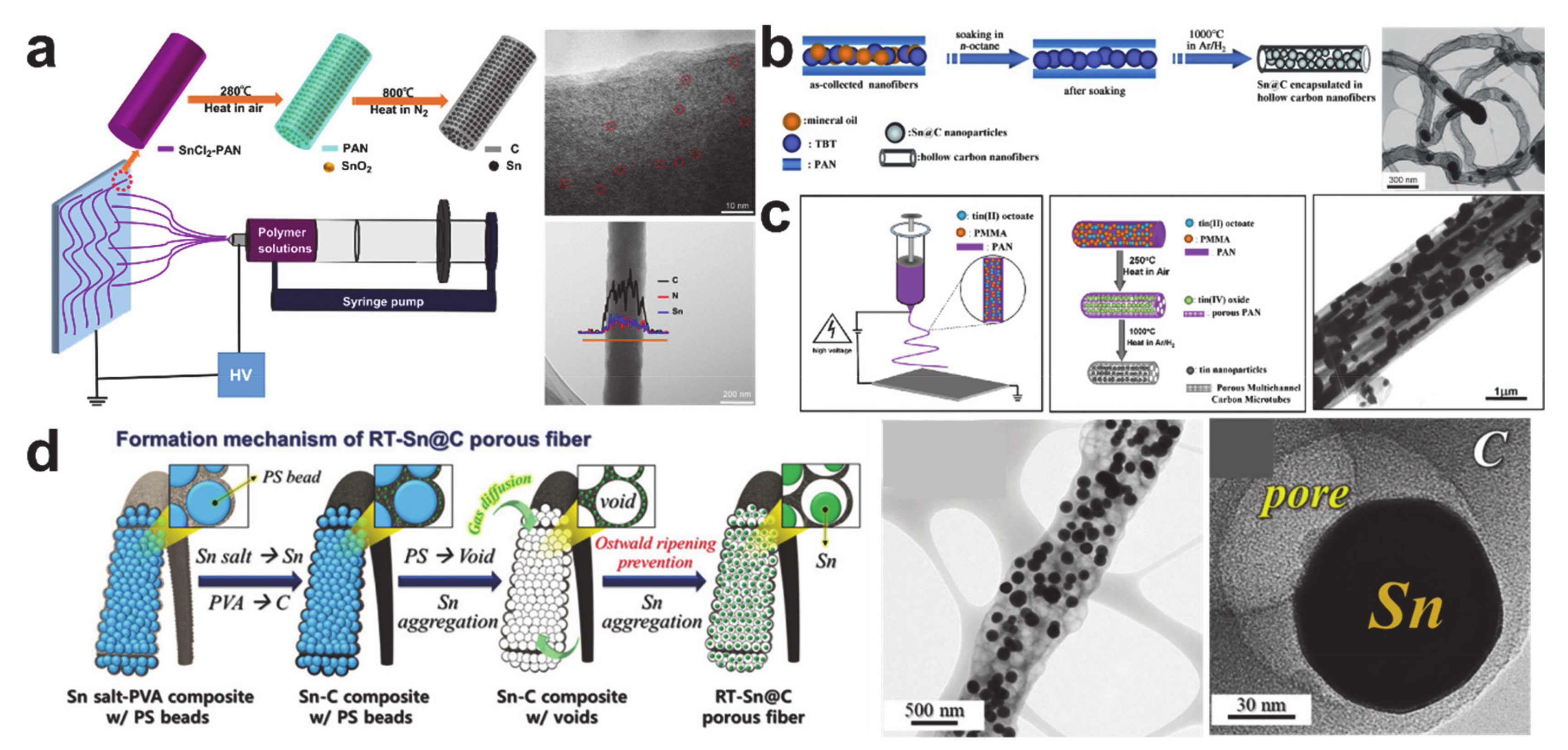
- Yu, Y.; Gu, L.; Wang, C.; Dhanabalan, A.; van Aken, P.A.; Maier, J. Encapsulation of Sn@carbon Nanoparticles in Bamboo-like Hollow Carbon Nanofibers as an Anode Material in Lithium-Based Batteries. Angew. Chem. Int. Ed. 2009, 48, 6485–6489. [Google Scholar] [CrossRef]
- Nam, D.-H.; Kim, J.W.; Lee, J.-H.; Lee, S.-Y.; Shin, H.-A.S.; Lee, S.-H.; Joo, Y.-C. Tunable Sn structures in porosity-controlled carbon nanofibers for all-solid-state lithium-ion battery anodes. J. Mater. Chem. A 2015, 3, 11021–11030. [Google Scholar] [CrossRef]
- Yu, Y.; Gu, L.; Zhu, C.; van Aken, P.A.; Maier, J. Tin Nanoparticles Encapsulated in Porous Multichannel Carbon Microtubes: Preparation by Single-Nozzle Electrospinning and Application as Anode Material for High-Performance Li-Based Batteries. J. Am. Chem. Soc. 2009, 131, 15984–15985. [Google Scholar] [CrossRef]
- Lee, J.H.; Oh, S.H.; Jeong, S.Y.; Kang, Y.C.; Cho, J.S. Rattle-type porous Sn/C composite fibers with uniformly distributed nanovoids containing metallic Sn nanoparticles for high-performance anode materials in lithium-ion batteries. Nanoscale 2018, 10, 21483–21491. [Google Scholar] [CrossRef]
- Chen, Y.; Ge, D.; Zhang, J.; Chu, R.; Zheng, J.; Wu, C.; Zeng, Y.; Zhang, Y.; Guo, H. Ultrafine Mo-doped SnO2 nanostructure and derivative Mo-doped Sn/C nanofibers for high-performance lithium-ion batteries. Nanoscale 2018, 10, 17378–17387. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, X.; Yu, Y.; Yang, X. Eco-Friendly Fabricated Porous Carbon Nanofibers Decorated with Nanosized SnOx as High-Performance Lithium-Ion Battery Anodes. ACS Sustain. Chem. Eng. 2016, 4, 2951–2959. [Google Scholar] [CrossRef]
- Wang, W.; Liang, Y.; Kang, Y.; Liu, L.; Xu, Z.; Tian, X.; Mai, W.; Fu, H.; Lv, H.; Teng, K.; et al. Carbon-coated SnO2@carbon nanofibers produced by electrospinning-electrospraying method for anode materials of lithium-ion batteries. Mater. Chem. Phys. 2019, 223, 762–770. [Google Scholar] [CrossRef]
- Abe, J.; Takahashi, K.; Kawase, K.; Kobayashi, Y.; Shiratori, S. Self-Standing Carbon Nanofiber and SnO2 Nanorod Composite as a High-Capacity and High-Rate-Capability Anode for Lithium-Ion Batteries. ACS Appl. Nano Mater. 2018, 1, 2982–2989. [Google Scholar] [CrossRef]
- Pham-Cong, D.; Kim, J.Y.; Park, J.S.; Kim, J.H.; Kim, J.-P.; Jeong, E.-D.; Kim, J.; Jeong, S.-Y.; Cho, C.-R. Conductive framework supported high rate performance of SnO2 hollow nanofibers for lithium battery anodes. Electrochim. Acta 2015, 161, 1–9. [Google Scholar] [CrossRef]
- Pham-Cong, D.; Park, J.S.; Kim, J.H.; Kim, J.; Braun, P.V.; Choi, J.H.; Kim, S.J.; Jeong, S.Y.; Cho, C.R. Enhanced cycle stability of polypyrrole-derived nitrogen-doped carbon-coated tin oxide hollow nanofibers for lithium battery anodes. Carbon 2017, 111, 28–37. [Google Scholar] [CrossRef]
- Cho, J.S.; Kang, Y.C. Nanofibers Comprising Yolk–Shell Sn@void@SnO/SnO2 and Hollow SnO/SnO2 and SnO2 Nanospheres via the Kirkendall Diffusion Effect and Their Electrochemical Properties. Small 2015, 11, 4673–4681. [Google Scholar] [CrossRef]
- Hong, Y.J.; Yoon, J.-W.; Lee, J.-H.; Kang, Y.C. A New Concept for Obtaining SnO2 Fiber-in-Tube Nanostructures with Superior Electrochemical Properties. Chem. Eur. J. 2015, 21, 371–376. [Google Scholar] [CrossRef]
- Liu, J.; Wen, Y.; van Aken, P.A.; Maier, J.; Yu, Y. In situ reduction and coating of SnS2 nanobelts for free-standing SnS@polypyrrole-nanobelt/carbon-nanotube paper electrodes with superior Li-ion storage. J. Mater. Chem. A 2015, 3, 5259–5265. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, L.; Zhuo, M.; Ni, W.; Wang, H.; Ma, J. Layered tin sulfide and selenide anode materials for Li- and Na-ion batteries. J. Mater. Chem. A 2018, 6, 12185–12214. [Google Scholar] [CrossRef]
- Xia, J.; Liu, L.; Jamil, S.; Xie, J.; Yan, H.; Yuan, Y.; Zhang, Y.; Nie, S.; Pan, J.; Wang, X.; et al. Free-standing SnS/C nanofiber anodes for ultralong cycle-life lithium-ion batteries and sodium-ion batteries. Energy Storage Mater. 2019, 17, 1–11. [Google Scholar] [CrossRef]
- Xia, J.; Yuan, Y.; Yan, H.; Liu, J.; Zhang, Y.; Liu, L.; Zhang, S.; Li, W.; Yang, X.; Shu, H.; et al. Electrospun SnSe/C nanofibers as binder-free anode for lithium–ion and sodium-ion batteries. J. Power Sources 2020, 449, 227559. [Google Scholar] [CrossRef]
- Zhang, F.; Xia, C.; Zhu, J.; Ahmed, B.; Liang, H.; Velusamy, D.B.; Schwingenschlögl, U.; Alshareef, H.N. SnSe2 2D Anodes for Advanced Sodium Ion Batteries. Adv. Energy Mater. 2016, 6, 1601188. [Google Scholar] [CrossRef]
- Ren, X.; Wang, J.; Zhu, D.; Li, Q.; Tian, W.; Wang, L.; Zhang, J.; Miao, L.; Chu, P.K.; Huo, K. Sn-C bonding riveted SnSe nanoplates vertically grown on nitrogen-doped carbon nanobelts for high-performance sodium-ion battery anodes. Nano Energy 2018, 54, 322–330. [Google Scholar] [CrossRef]
- Tan, H.; Feng, Y.; Rui, X.; Yu, Y.; Huang, S. Metal Chalcogenides: Paving the Way for High-Performance Sodium/Potassium-Ion Batteries. Small Methods 2020, 4, 1900563. [Google Scholar] [CrossRef]
- Xie, H.; Sayed, S.Y.; Kalisvaart, W.P.; Schaper, S.J.; Müller-Buschbaum, P.; Luber, E.J.; Olsen, B.C.; Haese, M.; Buriak, J.M. Adhesion and Surface Layers on Silicon Anodes Suppress Formation of c-Li3.75Si and Solid-Electrolyte Interphase. ACS Appl. Energy Mater. 2020, 3, 1609–1616. [Google Scholar] [CrossRef]
- Yang, H.-S.; Lee, B.-S.; You, B.-C.; Sohn, H.-J.; Yu, W.-R. Fabrication of carbon nanofibers with Si nanoparticle-stuffed cylindrical multi-channels via coaxial electrospinning and their anodic performance. RSC Adv. 2014, 4, 47389–47395. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, X. Electrospun carbon nanofibers containing silicon particles as an energy-storage medium. Carbon 2009, 47, 3219–3226. [Google Scholar] [CrossRef]
- Ji, L.; Jung, K.-H.; Medford, A.J.; Zhang, X. Electrospun polyacrylonitrile fibers with dispersed Si nanoparticles and their electrochemical behaviors after carbonization. J. Mater. Chem. 2009, 19, 4992–4997. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, X. Evaluation of Si/carbon composite nanofiber-based insertion anodes for new-generation rechargeable lithium-ion batteries. Energy Environ. Sci. 2010, 3, 124–129. [Google Scholar] [CrossRef]
- Wang, L.; Ding, C.X.; Zhang, L.C.; Xu, H.W.; Zhang, D.W.; Cheng, T.; Chen, C.H. A novel carbon–silicon composite nanofiber prepared via electrospinning as anode material for high energy-density lithium ion batteries. J. Power Sources 2010, 195, 5052–5056. [Google Scholar] [CrossRef]
- Choi, H.S.; Lee, J.G.; Lee, H.Y.; Kim, S.W.; Park, C.R. Effects of surrounding confinements of Si nanoparticles on Si-based anode performance for lithium ion batteries. Electrochim. Acta 2010, 56, 790–796. [Google Scholar] [CrossRef]
- Xu, Z.-L.; Zhang, B.; Kim, J.-K. Electrospun carbon nanofiber anodes containing monodispersed Si nanoparticles and graphene oxide with exceptional high rate capacities. Nano Energy 2014, 6, 27–35. [Google Scholar] [CrossRef]
- Lee, D.J.; Lee, H.; Ryou, M.-H.; Han, G.-B.; Lee, J.-N.; Song, J.; Choi, J.; Cho, K.Y.; Lee, Y.M.; Park, J.-K. Electrospun Three-Dimensional Mesoporous Silicon Nanofibers as an Anode Material for High-Performance Lithium Secondary Batteries. ACS Appl. Mater. Interfaces 2013, 5, 12005–12010. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-K.; Kim, J.; Jung, Y.S.; Kang, K. Scalable Fabrication of Silicon Nanotubes and their Application to Energy Storage. Adv. Mater. 2012, 24, 5452–5456. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Choi, S.; Bok, T.; Park, S. Nanotubular structured Si-based multicomponent anodes for high-performance lithium-ion batteries with controllable pore size via coaxial electro-spinning. Nanoscale 2015, 7, 6126–6135. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-K.; Kim, J.; Lee, H.; Choi, J.; Choi, M.-J.; Sim, D.M.; Jung, Y.S.; Kang, K. Porous silicon nanowires for lithium rechargeable batteries. Nanotechnology 2013, 24, 424008. [Google Scholar] [CrossRef][Green Version]
- Favors, Z.; Bay, H.H.; Mutlu, Z.; Ahmed, K.; Ionescu, R.; Ye, R.; Ozkan, M.; Ozkan, C.S. Towards Scalable Binderless Electrodes: Carbon Coated Silicon Nanofiber Paper via Mg Reduction of Electrospun SiO2 Nanofibers. Sci. Rep. 2015, 5, 8246. [Google Scholar] [CrossRef]
- Wu, H.; Chan, G.; Choi, J.W.; Ryu, I.; Yao, Y.; McDowell, M.T.; Lee, S.W.; Jackson, A.; Yang, Y.; Hu, L.; et al. Stable cycling of double-walled silicon nanotube battery anodes through solid–electrolyte interphase control. Nat. Nanotechnol. 2012, 7, 310–315. [Google Scholar] [CrossRef]
- Yao, F.; Li, B.; So, K.; Chang, J.; Ly, T.H.; Vu, A.Q.; Mun, H.; Cojocaru, C.S.; Yue, H.; Xie, S.; et al. A strategy to overcome the limits of carbon-based materials as lithium-ion battery anodes. Carbon 2014, 79, 563–571. [Google Scholar] [CrossRef]
- Zhou, X.; Wan, L.-J.; Guo, Y.-G. Electrospun Silicon Nanoparticle/Porous Carbon Hybrid Nanofibers for Lithium-Ion Batteries. Small 2013, 9, 2684–2688. [Google Scholar] [CrossRef]
- Ma, X.; Hou, G.; Ai, Q.; Zhang, L.; Si, P.; Feng, J.; Ci, L. A heart-coronary arteries structure of carbon nanofibers/graphene/silicon composite anode for high performance lithium ion batteries. Sci. Rep. 2017, 7, 9642. [Google Scholar] [CrossRef]
- An, G.-H.; Kim, H.; Ahn, H.-J. Improved Ionic Diffusion through the Mesoporous Carbon Skin on Silicon Nanoparticles Embedded in Carbon for Ultrafast Lithium Storage. ACS Appl. Mater. Interfaces 2018, 10, 6235–6244. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Yun, J.; Lee, J.Y.; Park, G.; Kim, S.-S.; Lee, K.J. Mass-Production of Electrospun Carbon Nanofiber Containing SiOx for Lithium-Ion Batteries with Enhanced Capacity. Macromol. Mater. Eng. 2019, 304, 1800564. [Google Scholar] [CrossRef]
- Sivonxay, E.; Aykol, M.; Persson, K.A. The lithiation process and Li diffusion in amorphous SiO2 and Si from first-principles. Electrochim. Acta 2020, 331, 135344. [Google Scholar] [CrossRef]
- Lee, B.-S.; Yu, W.-R. Electrospun carbon nanofibers as a functional composite platform: A review of highly tunable microstructures and morphologies for versatile applications. Funct. Compos. Struct. 2020, 2, 012001. [Google Scholar] [CrossRef]
- Gu, M.; Li, Y.; Li, X.; Hu, S.; Zhang, X.; Xu, W.; Thevuthasan, S.; Baer, D.R.; Zhang, J.-G.; Liu, J.; et al. In Situ TEM Study of Lithiation Behavior of Silicon Nanoparticles Attached to and Embedded in a Carbon Matrix. ACS Nano 2012, 6, 8439–8447. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, Y.; Shao, J.; Shen, Z.; Chen, R.; Zhang, X.; He, X.; Song, Y.; Xing, X. Pyrolytic carbon-coated silicon/carbon nanofiber composite anodes for high-performance lithium-ion batteries. J. Power Sources 2015, 298, 130–137. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, Y.; Shen, Z.; Chen, R.; He, X.; Zhang, X.; Zhang, Y.; Wu, K. Sandwich structure of graphene-protected silicon/carbon nanofibers for lithium-ion battery anodes. Electrochim. Acta 2016, 210, 53–60. [Google Scholar] [CrossRef]
- Fu, K.; Lu, Y.; Dirican, M.; Chen, C.; Yanilmaz, M.; Shi, Q.; Bradford, P.D.; Zhang, X. Chamber-confined silicon–carbon nanofiber composites for prolonged cycling life of Li-ion batteries. Nanoscale 2014, 6, 7489–7495. [Google Scholar] [CrossRef]
- Hieu, N.T.; Suk, J.; Kim, D.W.; Chung, O.H.; Park, J.S.; Kang, Y. Silicon nanoparticle and carbon nanotube loaded carbon nanofibers for use in lithium-ion battery anodes. Synth. Met. 2014, 198, 36–40. [Google Scholar] [CrossRef]
- Wang, M.-S.; Song, W.-L.; Wang, J.; Fan, L.-Z. Highly uniform silicon nanoparticle/porous carbon nanofiber hybrids towards free-standing high-performance anodes for lithium-ion batteries. Carbon 2015, 82, 337–345. [Google Scholar] [CrossRef]
- Dirican, M.; Yildiz, O.; Lu, Y.; Fang, X.; Jiang, H.; Kizil, H.; Zhang, X. Flexible binder-free silicon/silica/carbon nanofiber composites as anode for lithium–ion batteries. Electrochim. Acta 2015, 169, 52–60. [Google Scholar] [CrossRef]
- Park, S.-W.; Shim, H.-W.; Kim, J.-C.; Kim, D.-W. Uniform Si nanoparticle-embedded nitrogen-doped carbon nanofiber electrodes for lithium ion batteries. J. Alloy. Compd. 2017, 728, 490–496. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, G.; Liu, N.; Carney, T.J.; Yang, Y.; Cui, Y. Engineering Empty Space between Si Nanoparticles for Lithium-Ion Battery Anodes. Nano Lett. 2012, 12, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-S.; Son, S.-B.; Park, K.-M.; Seo, J.-H.; Lee, S.-H.; Choi, I.-S.; Oh, K.-H.; Yu, W.-R. Fabrication of Si core/C shell nanofibers and their electrochemical performances as a lithium-ion battery anode. J. Power Sources 2012, 206, 267–273. [Google Scholar] [CrossRef]
- Lee, B.-S.; Son, S.-B.; Seo, J.-H.; Park, K.-M.; Lee, G.; Lee, S.-H.; Oh, K.H.; Ahn, J.-P.; Yu, W.-R. Facile conductive bridges formed between silicon nanoparticles inside hollow carbon nanofibers. Nanoscale 2013, 5, 4790–4796. [Google Scholar] [CrossRef]
- Hieu, N.T.; Suk, J.; Kim, D.W.; Park, J.S.; Kang, Y. Electrospun nanofibers with a core–shell structure of silicon nanoparticles and carbon nanotubes in carbon for use as lithium-ion battery anodes. J. Mater. Chem. A 2014, 2, 15094–15101. [Google Scholar] [CrossRef]
- Wu, J.; Qin, X.; Miao, C.; He, Y.-B.; Liang, G.; Zhou, D.; Liu, M.; Han, C.; Li, B.; Kang, F. A honeycomb-cobweb inspired hierarchical core–shell structure design for electrospun silicon/carbon fibers as lithium-ion battery anodes. Carbon 2016, 98, 582–591. [Google Scholar] [CrossRef]
- Lee, B.-S.; Yang, H.-S.; Jung, H.; Jeon, S.-Y.; Jung, C.; Kim, S.-W.; Bae, J.; Choong, C.-L.; Im, J.; Chung, U.I.; et al. Novel multi-layered 1-D nanostructure exhibiting the theoretical capacity of silicon for a super-enhanced lithium-ion battery. Nanoscale 2014, 6, 5989–5998. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, X.; Wu, J.; He, Y.-B.; Du, H.; Li, B.; Kang, F. Electrospun core–shell silicon/carbon fibers with an internal honeycomb-like conductive carbon framework as an anode for lithium ion batteries. J. Mater. Chem. A 2015, 3, 7112–7120. [Google Scholar] [CrossRef]
- Xu, Y.; Yuan, T.; Bian, Z.; Sun, H.; Pang, Y.; Peng, C.; Yang, J.; Zheng, S. Electrospun flexible Si/C@CNF nonwoven anode for high capacity and durable lithium-ion battery. Compos. Commun. 2019, 11, 1–5. [Google Scholar] [CrossRef]
- Lee, B.-S.; Yang, H.-S.; Lee, K.H.; Han, S.; Yu, W.-R. Rational design of a Si–Sn–C ternary anode having exceptional rate performance. Energy Storage Mater. 2019, 17, 62–69. [Google Scholar] [CrossRef]
- Li, W.; Yang, Z.; Jiang, Y.; Yu, Z.; Gu, L.; Yu, Y. Crystalline red phosphorus incorporated with porous carbon nanofibers as flexible electrode for high performance lithium-ion batteries. Carbon 2014, 78, 455–462. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, D.; Liang, Z.; Zhao, J.; Yan, K.; Cui, Y. Lithium-coated polymeric matrix as a minimum volume-change and dendrite-free lithium metal anode. Nat. Commun. 2016, 7, 10992. [Google Scholar] [CrossRef]
- Zinigrad, E.; Larush-Asraf, L.; Gnanaraj, J.S.; Gottlieb, H.E.; Sprecher, M.; Aurbach, D. Calorimetric studies of the thermal stability of electrolyte solutions based on alkyl carbonates and the effect of the contact with lithium. J. Power Sources 2005, 146, 176–179. [Google Scholar] [CrossRef]
- Lang, J.; Jin, Y.; Luo, X.; Liu, Z.; Song, J.; Long, Y.; Qi, L.; Fang, M.; Li, Z.; Wu, H. Surface graphited carbon scaffold enables simple and scalable fabrication of 3D composite lithium metal anode. J. Mater. Chem. A 2017, 5, 19168–19174. [Google Scholar] [CrossRef]
- Liang, Z.; Lin, D.; Zhao, J.; Lu, Z.; Liu, Y.; Liu, C.; Lu, Y.; Wang, H.; Yan, K.; Tao, X.; et al. Composite lithium metal anode by melt infusion of lithium into a 3D conducting scaffold with lithiophilic coating. Proc. Natl. Acad. Sci. USA 2016, 113, 2862. [Google Scholar] [CrossRef]
- Qian, J.; Adams, B.D.; Zheng, J.; Xu, W.; Henderson, W.A.; Wang, J.; Bowden, M.E.; Xu, S.; Hu, J.; Zhang, J.-G. Anode-Free Rechargeable Lithium Metal Batteries. Adv. Funct. Mater. 2016, 26, 7094–7102. [Google Scholar] [CrossRef]
- Huang, S.; Tang, L.; Najafabadi, H.S.; Chen, S.; Ren, Z. A highly flexible semi-tubular carbon film for stable lithium metal anodes in high-performance batteries. Nano Energy 2017, 38, 504–509. [Google Scholar] [CrossRef]
- Xiang, J.; Yuan, L.; Shen, Y.; Cheng, Z.; Yuan, K.; Guo, Z.; Zhang, Y.; Chen, X.; Huang, Y. Improved Rechargeability of Lithium Metal Anode via Controlling Lithium-Ion Flux. Adv. Energy Mater. 2018, 8, 1802352. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, S.; Li, G.; Zhang, C.; Liu, X.; Luo, J. Incorporating Ionic Paths into 3D Conducting Scaffolds for High Volumetric and Areal Capacity, High Rate Lithium-Metal Anodes. Adv. Mater. 2018, 30, 1801328. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yao, Y.; He, S.; Xie, H.; Hitz, E.; Hu, L. Ultrafine Silver Nanoparticles for Seeded Lithium Deposition toward Stable Lithium Metal Anode. Adv. Mater. 2017, 29, 1702714. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-D.; Lim, H.-K.; Xing, X.; Lee, B.-S.; Liu, H.; Coaty, C.; Kim, H.; Liu, P. Solid Electrolyte Layers by Solution Deposition. Adv. Mater. Interfaces 2018, 5, 1701328. [Google Scholar] [CrossRef]
- Maroni, F.; Bruni, P.; Suzuki, N.; Aihara, Y.; Croce, F. Electrospun tin-carbon nanocomposite as anode material for all solid state lithium-ion batteries. J. Solid State Electrochem. 2019, 23, 1697–1703. [Google Scholar] [CrossRef]
- Kim, K.-B.; Dunlap, N.A.; Han, S.S.; Jeong, J.J.; Kim, S.C.; Oh, K.H.; Lee, S.-H. Nanostructured Si/C Fibers as a Highly Reversible Anode Material for All-Solid-State Lithium-Ion Batteries. J. Electrochem. Soc. 2018, 165, A1903–A1908. [Google Scholar] [CrossRef]
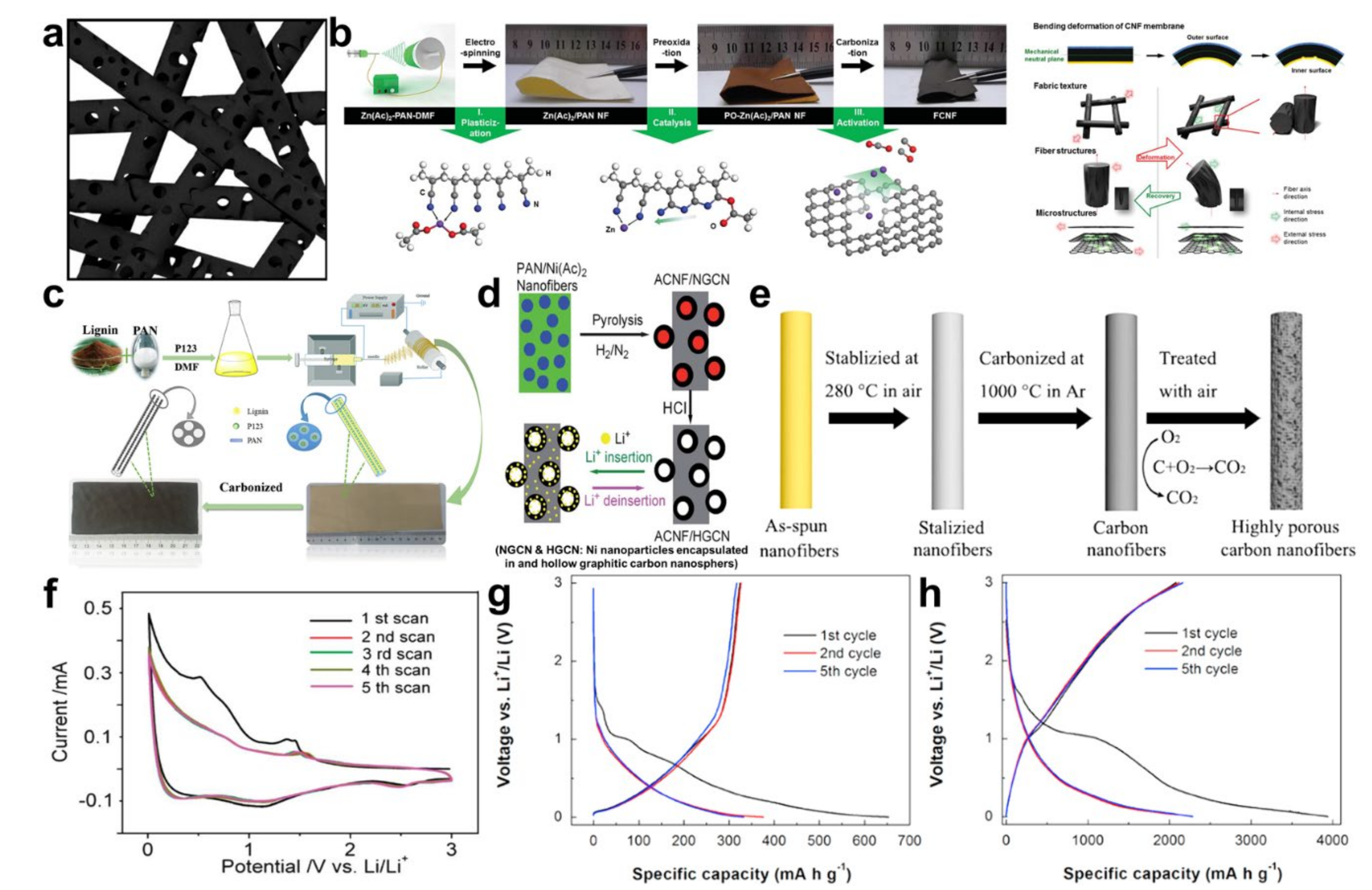
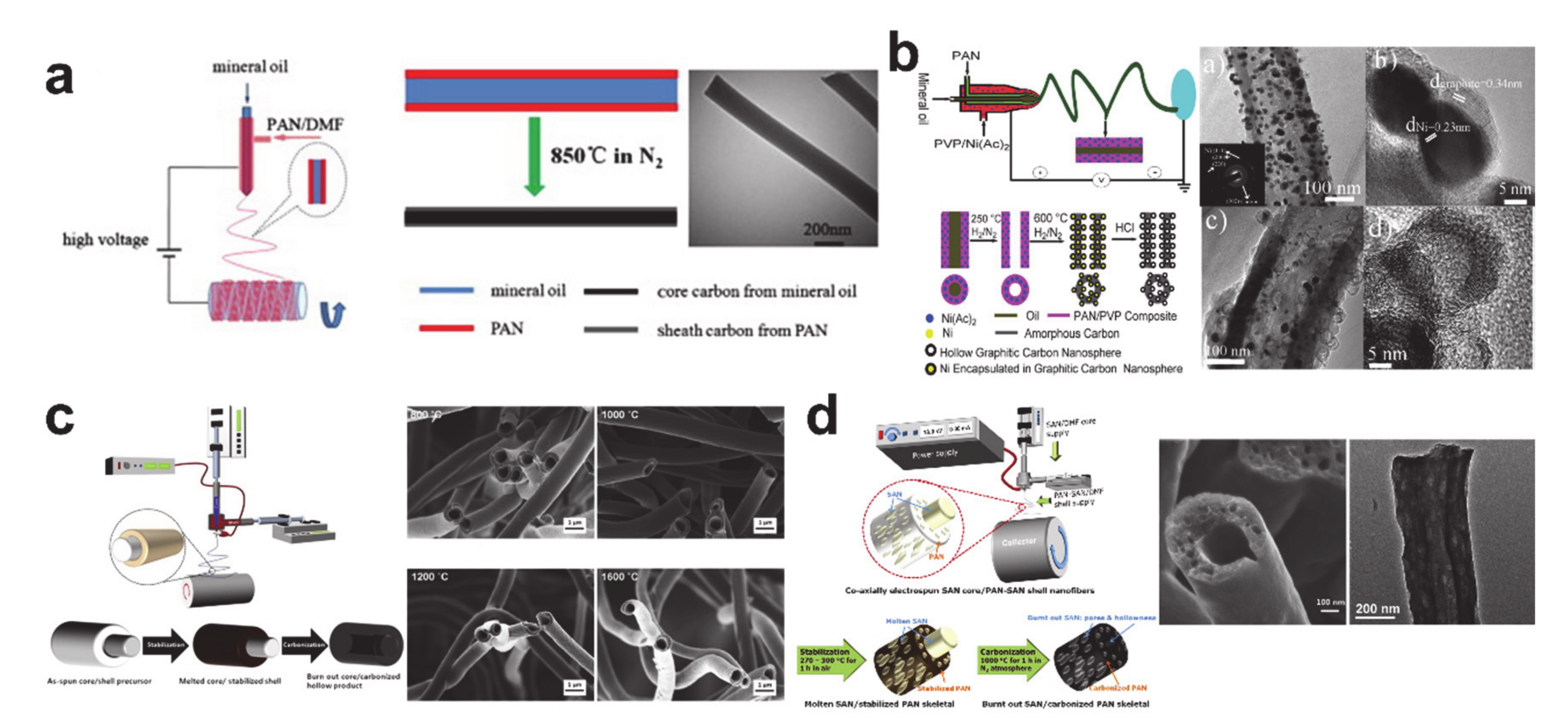
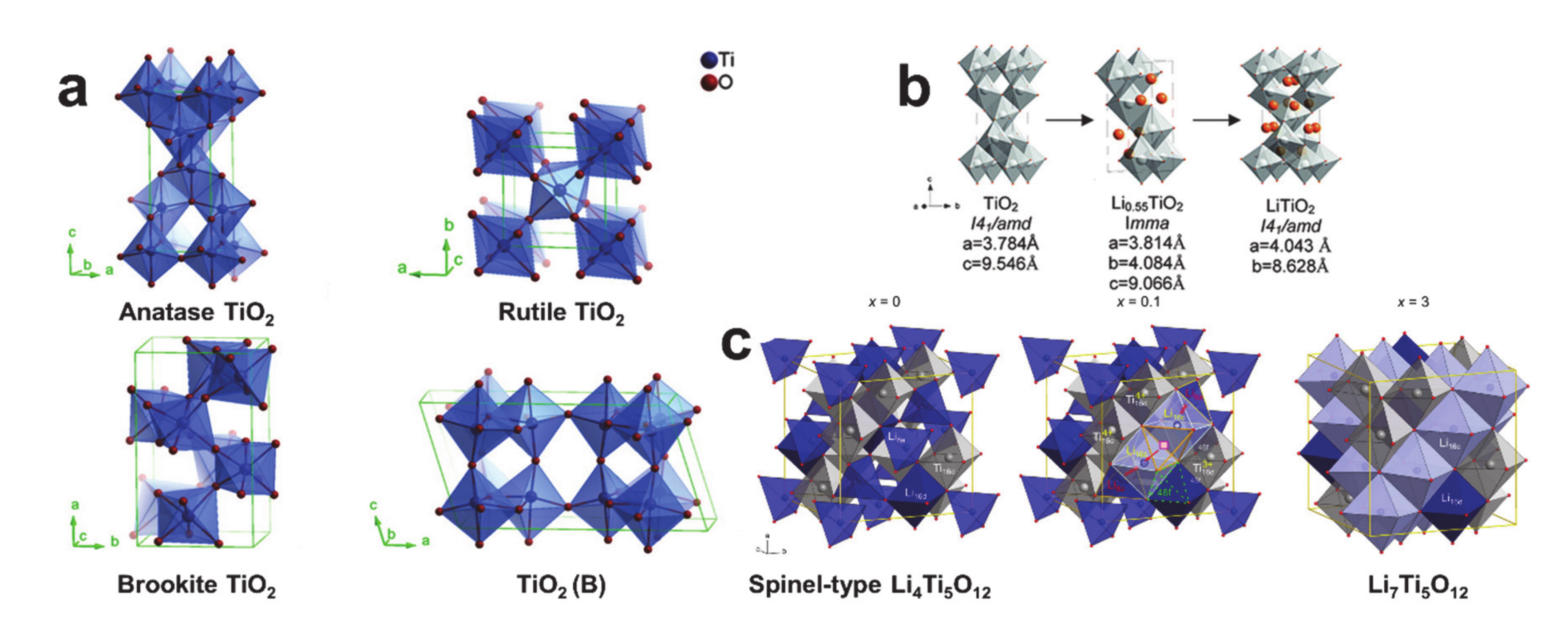
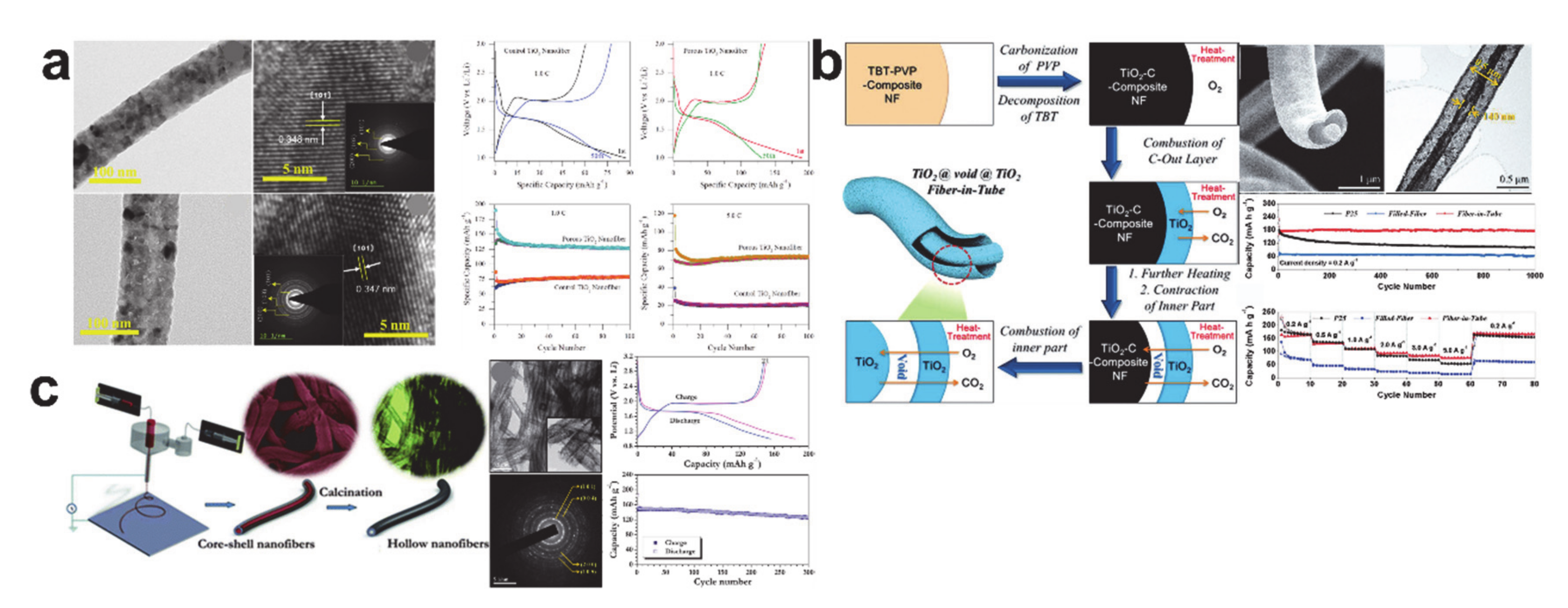
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.-S. A Review of Recent Advancements in Electrospun Anode Materials to Improve Rechargeable Lithium Battery Performance. Polymers 2020, 12, 2035. https://doi.org/10.3390/polym12092035
Lee B-S. A Review of Recent Advancements in Electrospun Anode Materials to Improve Rechargeable Lithium Battery Performance. Polymers. 2020; 12(9):2035. https://doi.org/10.3390/polym12092035
Chicago/Turabian StyleLee, Byoung-Sun. 2020. "A Review of Recent Advancements in Electrospun Anode Materials to Improve Rechargeable Lithium Battery Performance" Polymers 12, no. 9: 2035. https://doi.org/10.3390/polym12092035
APA StyleLee, B.-S. (2020). A Review of Recent Advancements in Electrospun Anode Materials to Improve Rechargeable Lithium Battery Performance. Polymers, 12(9), 2035. https://doi.org/10.3390/polym12092035