Synthesis and Electrorheological Response of Graphene Oxide/Polydiphenylamine Microsheet Composite Particles
Abstract
1. Introduction
2. Experimental
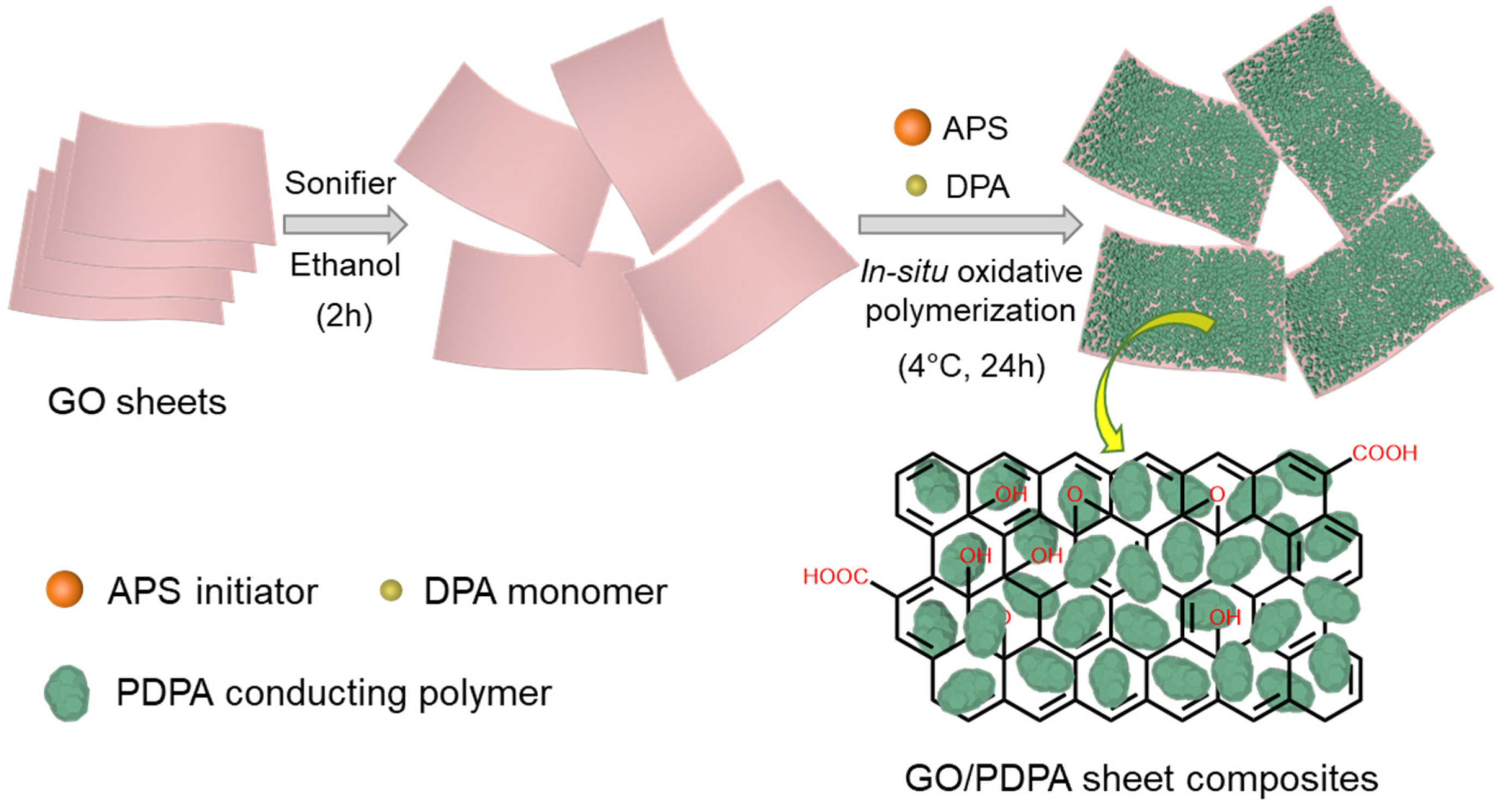
2.1. Materials and Synthesis
2.2. Characterization
3. Results and Discussion
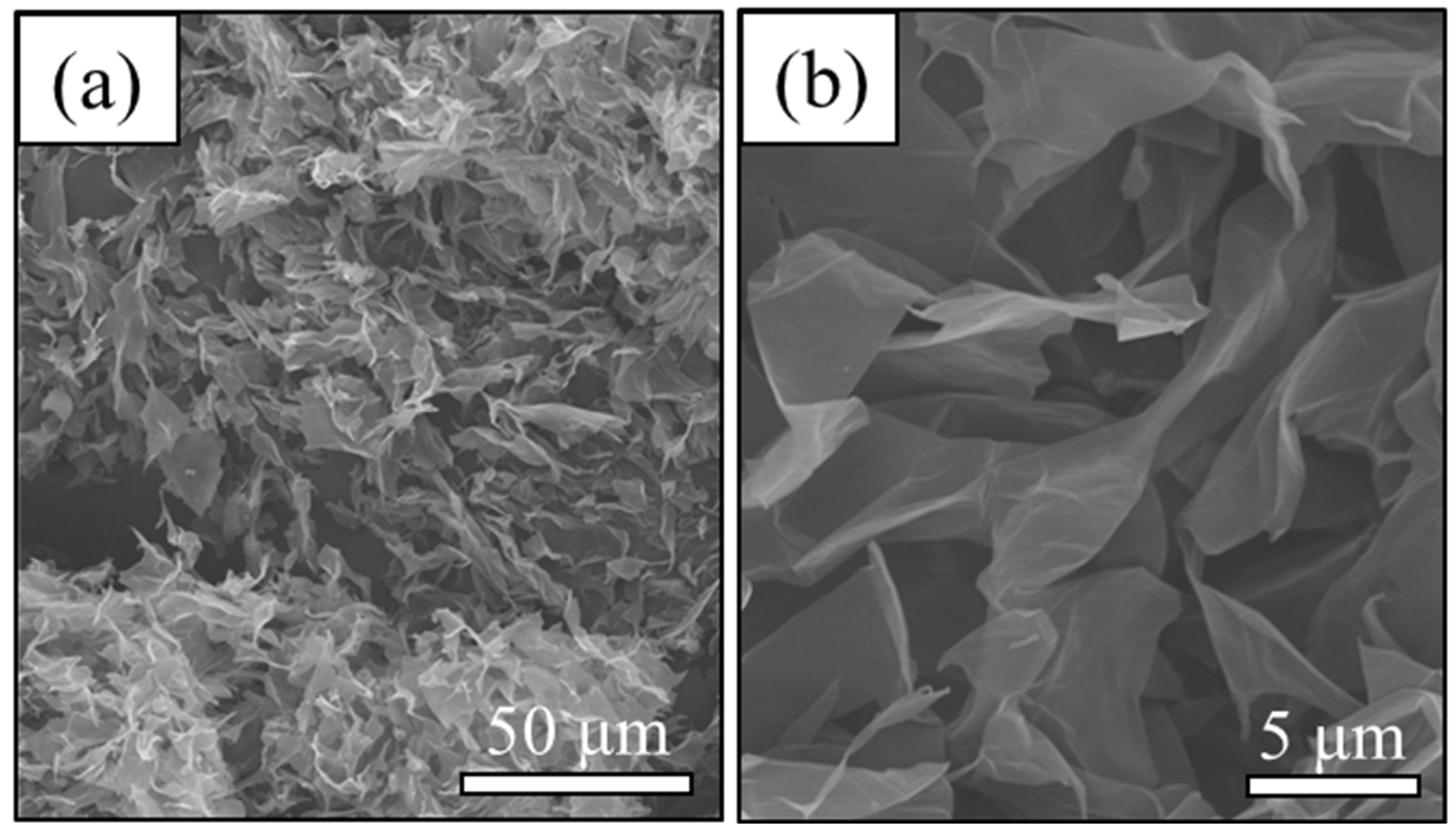
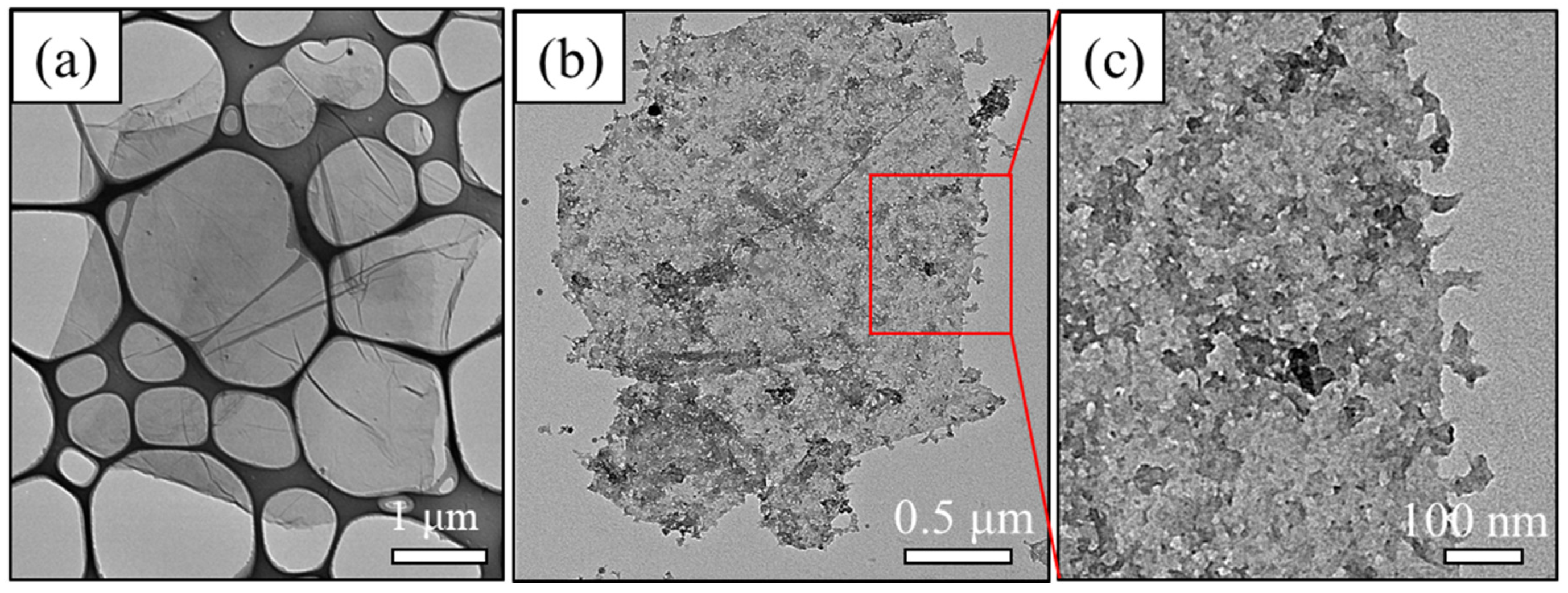
3.1. Material Property
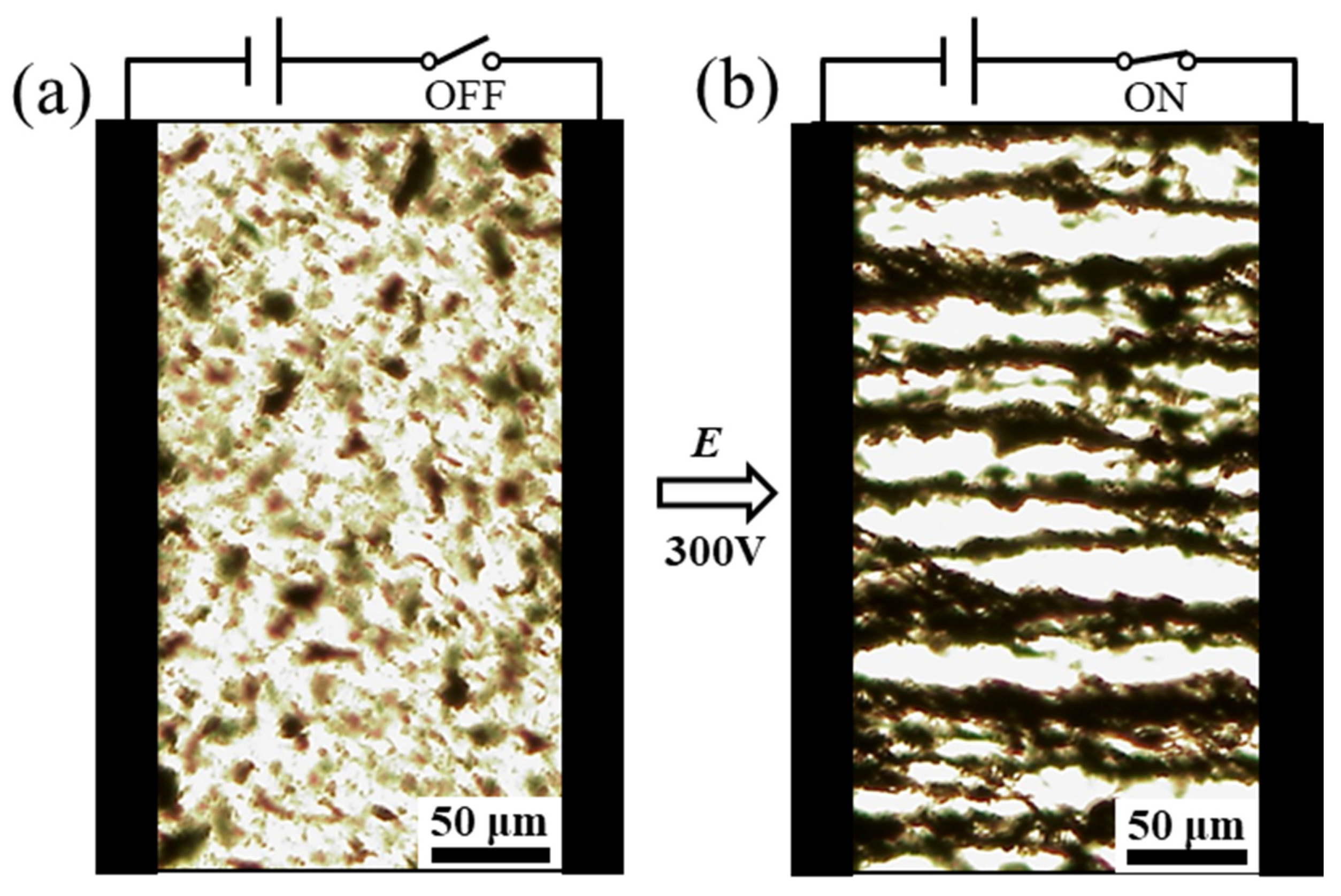
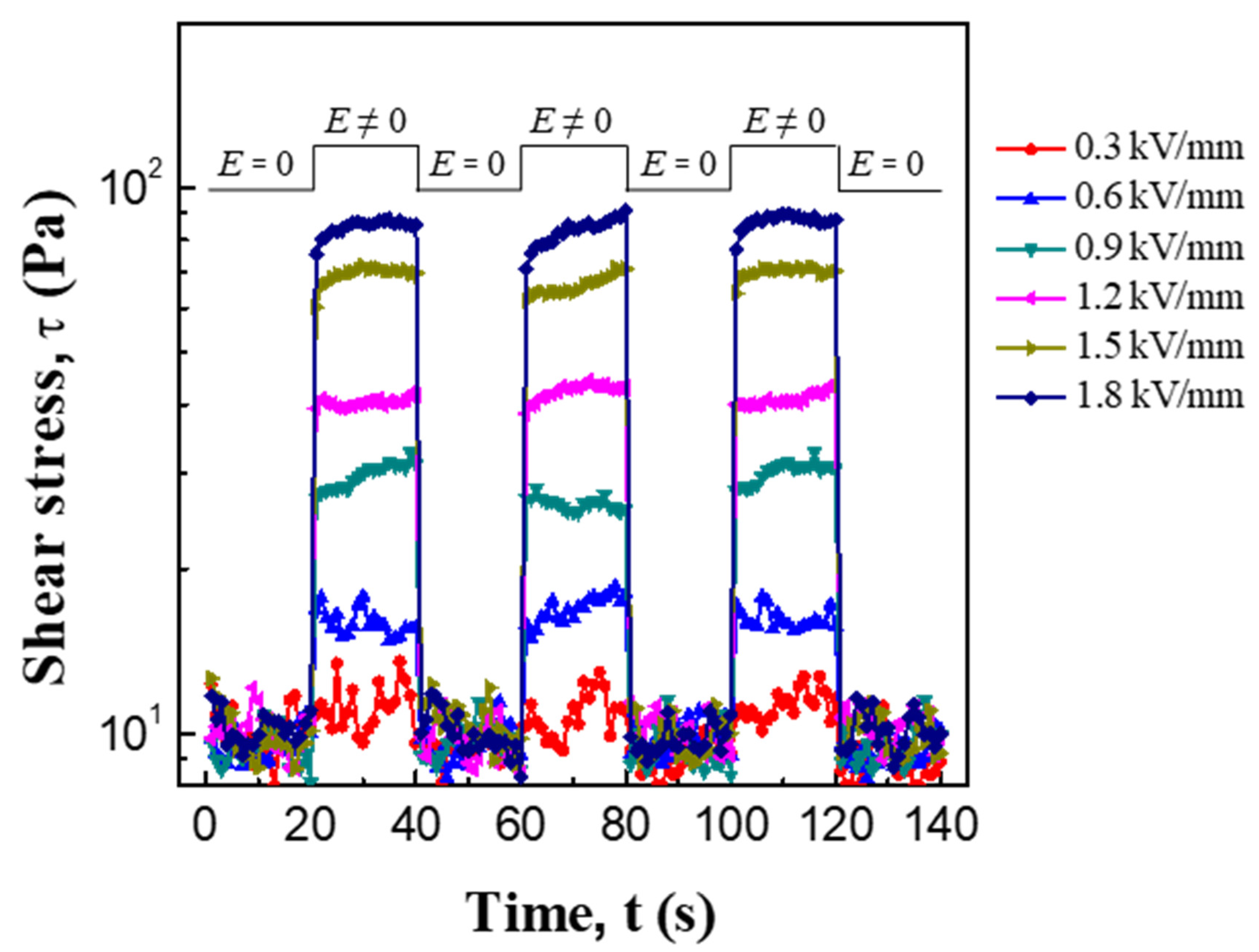
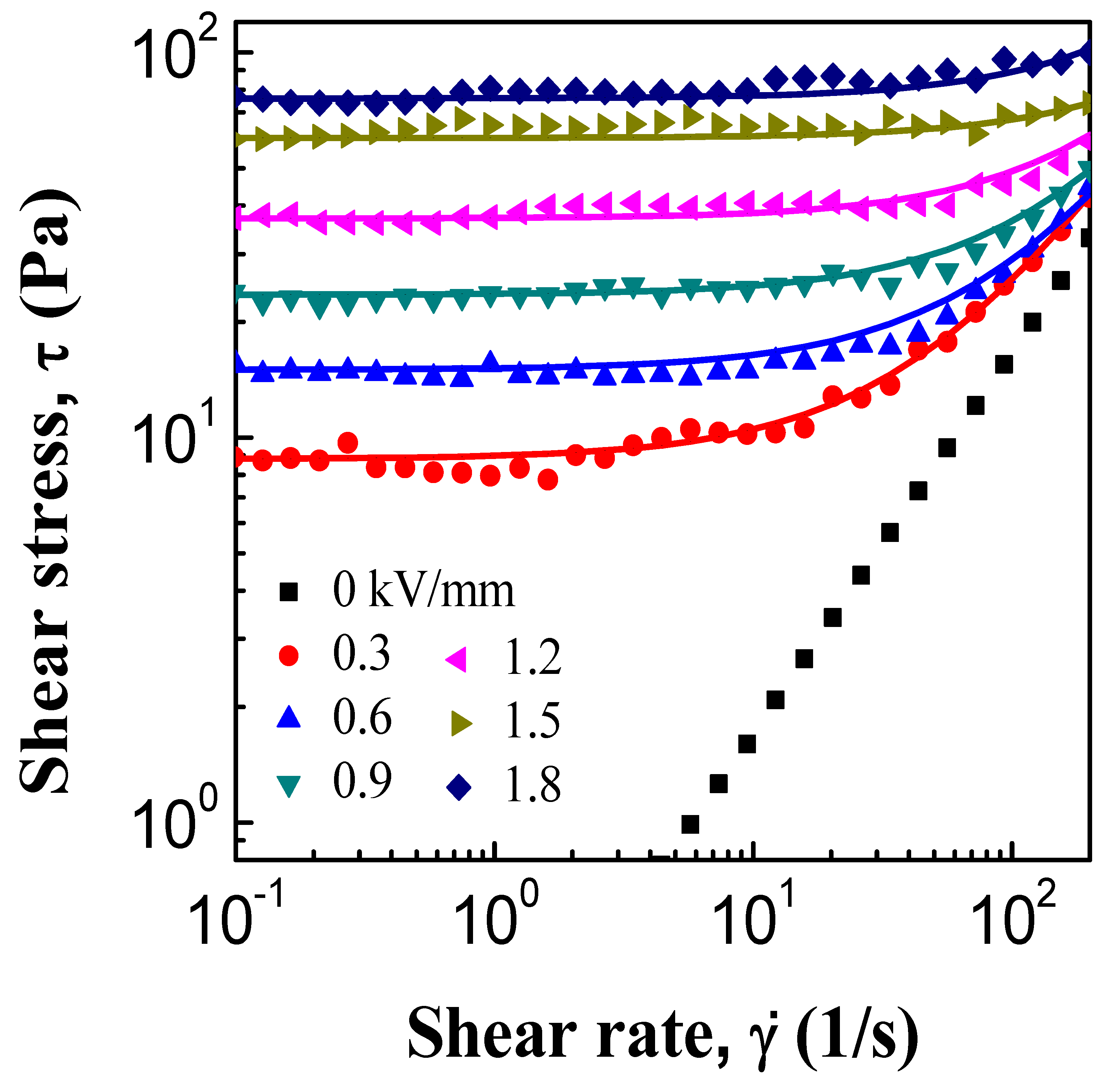
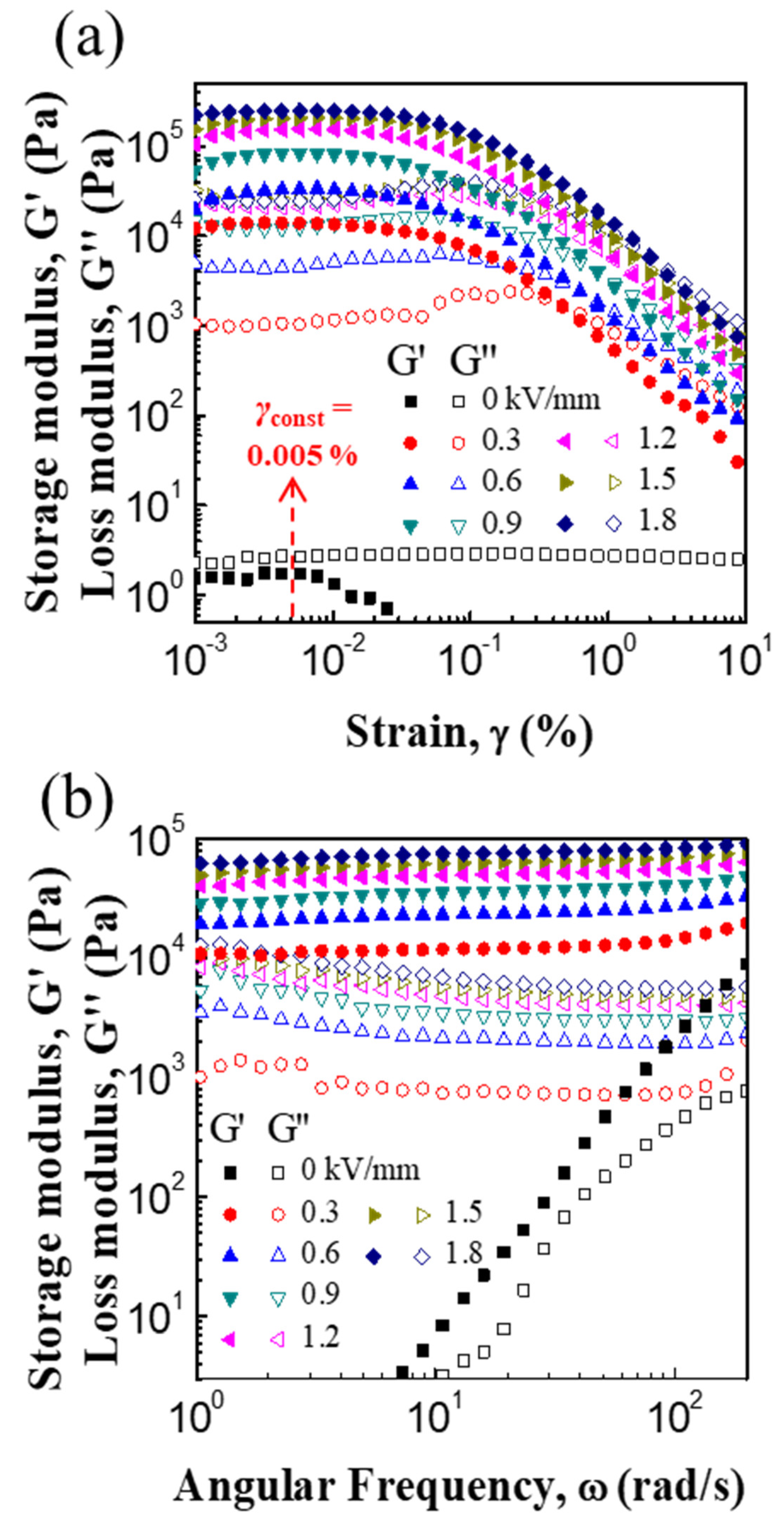
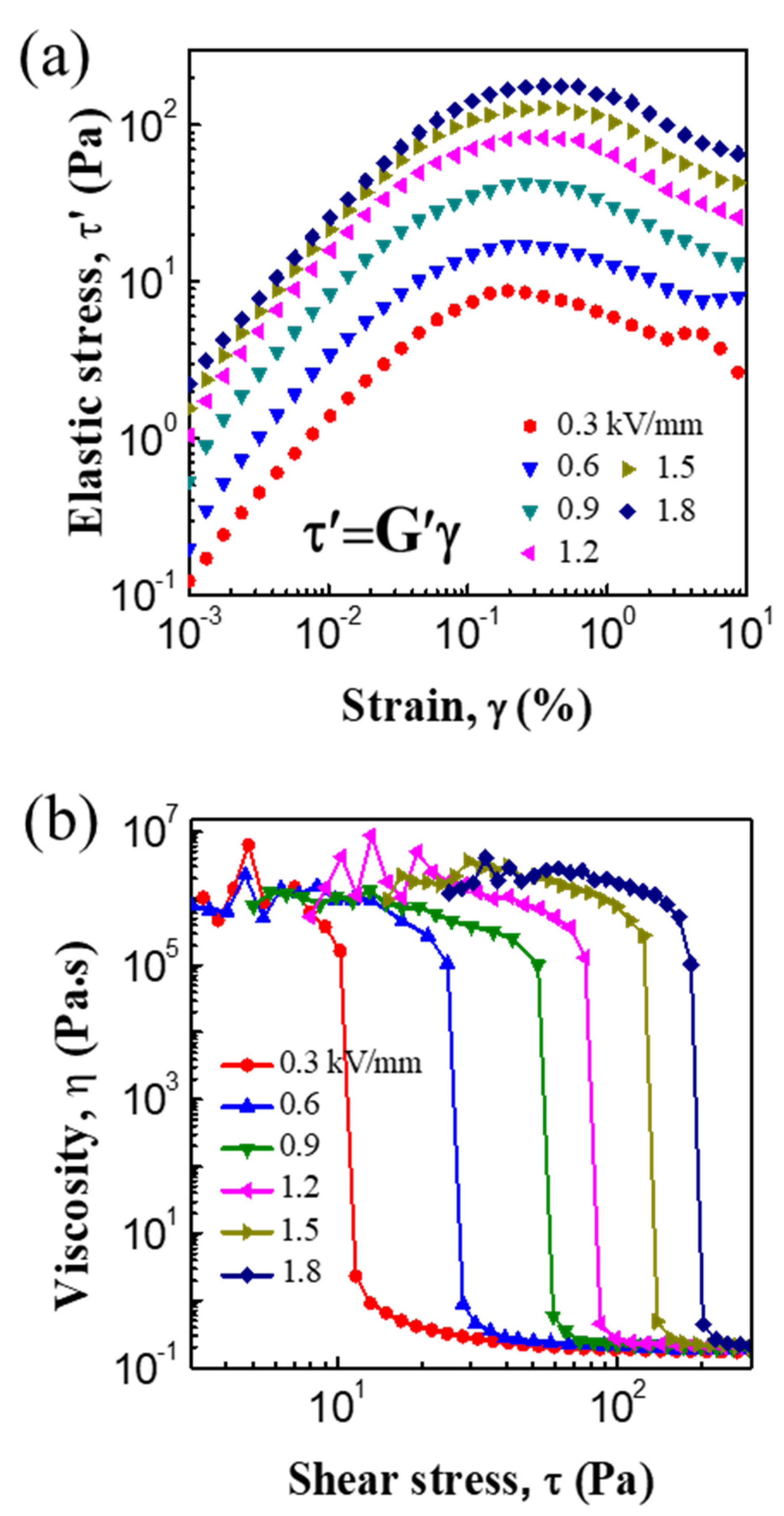
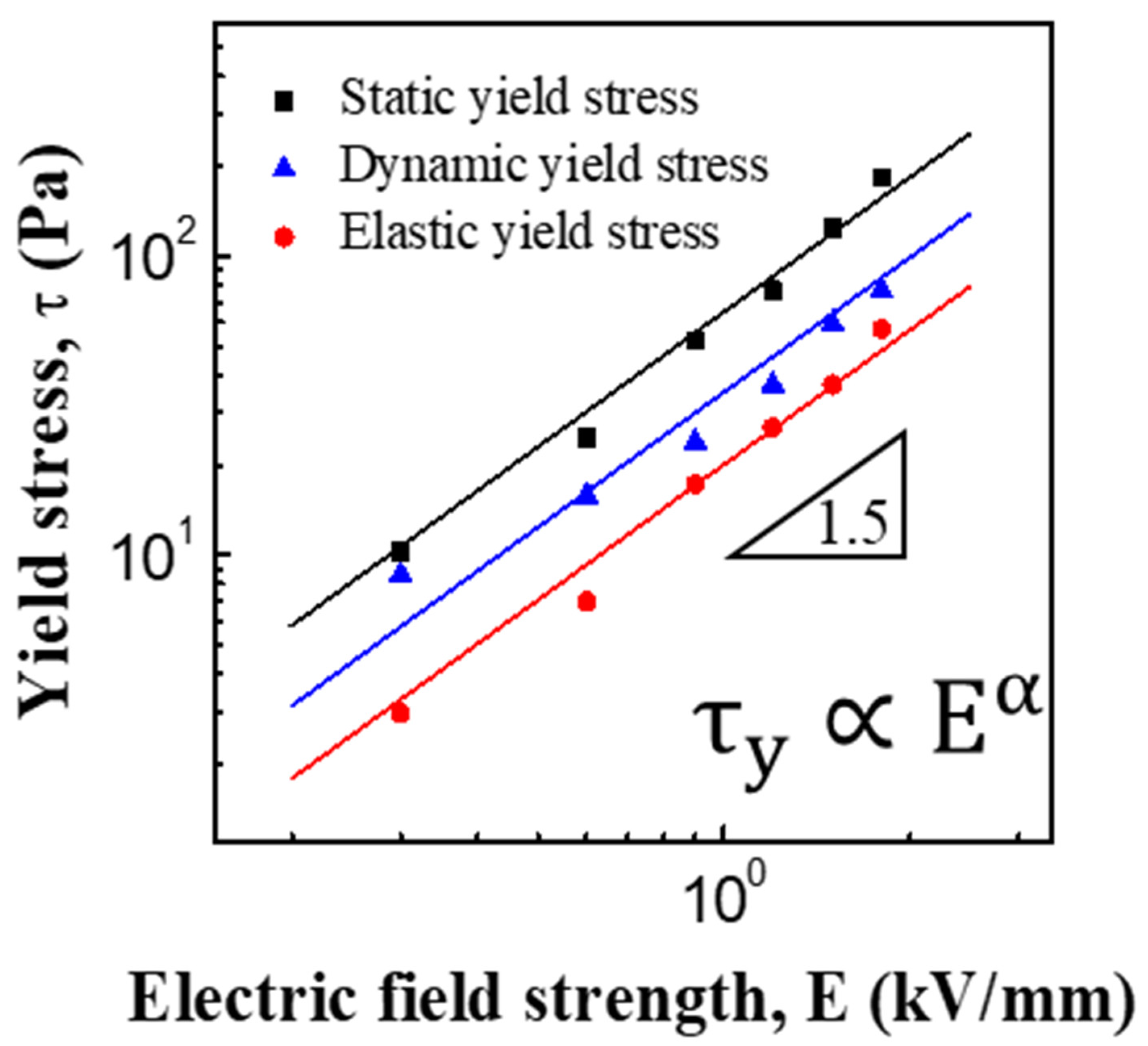
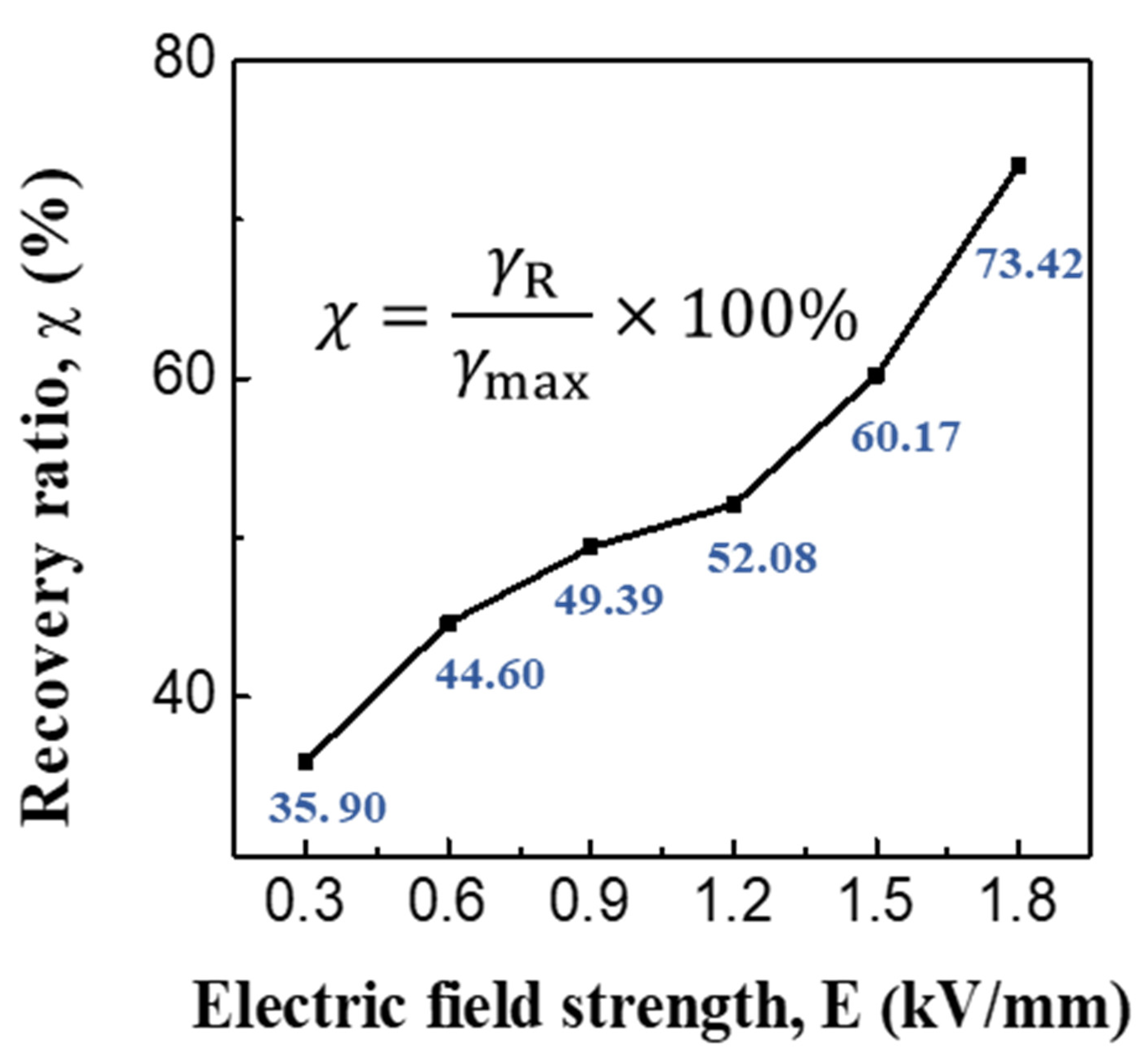
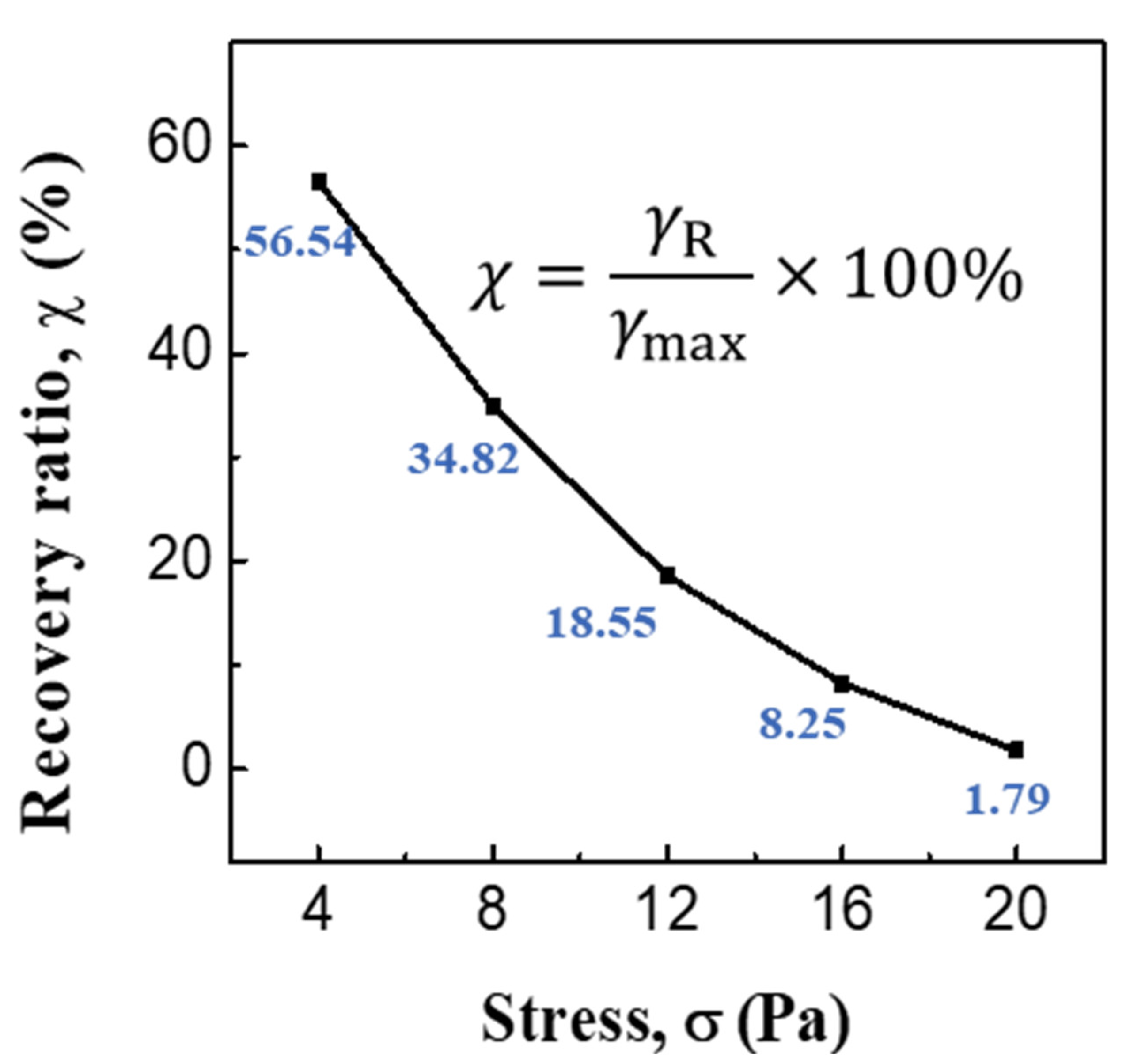
3.2. ER and Creep Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, B.; Tian, X.; He, K.; Ma, L.; Yu, S.; Hao, C.; Chen, K.; Lei, Q. Hollow PAQR nanostructure and its smart electrorheological activity. Polymer 2016, 83, 129–137. [Google Scholar] [CrossRef]
- Dai, S.; Ravi, P.; Tam, K.C. Thermo-and photo-responsive polymeric systems. Soft Matter 2009, 5, 2513–2533. [Google Scholar] [CrossRef]
- Cabuk, M.; Yavuz, M.; Unal, H.I. Electrokinetic, electrorheological and viscoelastic properties of Polythiophene-graft-Chitosan copolymer particles. Colloids Surf. A Physicochem. Eng. Asp. 2016, 510, 231–238. [Google Scholar] [CrossRef]
- Yin, J.; Xia, X.; Xiang, L.; Zhao, X. Coaxial cable-like polyaniline@ titania nanofibers: Facile synthesis and low power electrorheological fluid application. J. Mater. Chem. 2010, 20, 7096–7099. [Google Scholar] [CrossRef]
- Wang, C.; Ma, L.; Wen, Q.; Wang, B.; Han, R.; Hao, C.; Chen, K. Enhanced electrorheological characteristics of titanium oxide@H2Ti2O5 nanotube core/shell nanocomposite. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123641. [Google Scholar] [CrossRef]
- Bansevicius, R.; Virbalis, J. Two-dimensional Braille readers based on electrorheological fluid valves controlled by electric field. Mechatronics 2007, 17, 570–577. [Google Scholar] [CrossRef]
- Tao, R.; Tang, H.; Tawhid-Al-Islam, K.; Du, E.; Kim, J. Electrorheology leads to healthier and tastier chocolate. Proc. Nat. Acad. Sci. USA 2016, 113, 7399–7402. [Google Scholar] [CrossRef]
- Wei, K.; Bai, Q.; Meng, G.; Ye, L. Vibration characteristics of electrorheological elastomer sandwich beams. Smart Mater. Struct. 2011, 20, 055012. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J.; Zheng, C.; Liu, Y.; Zhao, X.; Yin, J. Enhanced interfacial polarization and electro-responsive characteristic of di-ionic poly(ionic liquid)s. Polymer 2019, 182, 121847. [Google Scholar] [CrossRef]
- Choi, K.; Gao, C.Y.; Nam, J.D.; Choi, H.J. Cellulose-based smart fluids under applied electric fields. Materials 2017, 10, 1060. [Google Scholar] [CrossRef]
- He, K.; Wen, Q.; Wang, C.; Wang, B.; Yu, S.; Hao, C.; Chen, K. The preparation and electrorheological behavior of bowl-like titanium oxide nanoparticles. Soft Matter 2017, 13, 7677–7688. [Google Scholar] [CrossRef] [PubMed]
- Ilčíková, M.; Mrlík, M.; Babayan, V.; Kasák, P. Graphene oxide modified by betaine moieties for improvement of electrorheological performance. RSC Adv. 2015, 5, 57820–57827. [Google Scholar] [CrossRef]
- Zhang, W.L.; Liu, Y.D.; Choi, H.J.; Kim, S.G. Electrorheology of graphene oxide. ACS Appl. Mater. Inter. 2012, 4, 2267–2272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Liu, Y.D.; Choi, H.J. Graphene oxide coated core–shell structured polystyrene microspheres and their electrorheological characteristics under applied electric field. J. Mater. Chem. 2011, 21, 6916–6921. [Google Scholar] [CrossRef]
- Lee, S.; Noh, J.; Hong, S.; Kim, Y.K.; Jang, J. Dual stimuli-responsive smart fluid of graphene oxide-coated iron oxide/silica core/shell nanoparticles. Chem. Mater. 2016, 28, 2624–2633. [Google Scholar] [CrossRef]
- Zhang, W.L.; Park, B.J.; Choi, H.J. Colloidal graphene oxide/polyaniline nanocomposite and its electrorheology. Chem. Commun. 2010, 46, 5596–5598. [Google Scholar] [CrossRef]
- Cao, Y.; Choi, H.J.; Zhang, W.L.; Wang, B.; Hao, C.; Liu, J. Eco-friendly mass production of poly (p-phenylenediamine)/graphene oxide nanoplatelet composites and their electrorheological characteristics. Compos. Sci. Technol. 2016, 122, 36–41. [Google Scholar]
- Min, T.H.; Lee, C.J.; Choi, H.J. Pickering emulsion polymerized core-shell structured poly (methyl methacrylate)/graphene oxide particles and their electrorheological characteristics. Polym. Test. 2018, 66, 195–202. [Google Scholar] [CrossRef]
- Kim, M.H.; Bae, D.H.; Choi, H.J.; Seo, Y. Synthesis of semiconducting poly(diphenylamine) particles and analysis of their electrorheological properties. Polymer 2017, 119, 40–49. [Google Scholar] [CrossRef]
- Showkat, A.M.; Lee, K.P.; Gopalan, A.I.; Kim, S.H. Synthesis and chiro-optical properties of water processable conducting poly (diphenylamine) nanocomposites. Macromol. Res. 2007, 15, 575–580. [Google Scholar] [CrossRef]
- Kim, M.H.; Choi, H.J. Core–shell structured semiconducting poly (diphenylamine)-coated polystyrene microspheres and their electrorheology. Polymer 2017, 131, 120–131. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Smaranda, I.; Benito, A.M.; Maser, W.K.; Baltog, I.; Baibarac, M. Electrochemical Grafting of Reduced Graphene Oxide with Polydiphenylamine Doped with Heteropolyanions and Its Optical Properties. J. Phys. Chem. C 2014, 118, 25704–25717. [Google Scholar] [CrossRef]
- Muthusankar, E.; Ponnusamyc, V.K.; Ragupathy, D. Electrochemically sandwiched poly(diphenylamine)/phosphotungstic acid/graphene nanohybrid as highly sensitive and selective urea biosensor. Synth. Met. 2019, 254, 134–140. [Google Scholar] [CrossRef]
- Yang, H.; Jiang, J.; Zhou, W.; Lai, L.; Xi, L.; Lam, Y.M.; Shen, Z.; Khezri, B.; Yu, T. Influences of graphene oxide support on the electrochemical performances of graphene oxide-MnO2 nanocomposites. Nanoscale Res. Lett. 2011, 6, 531. [Google Scholar] [CrossRef]
- Krishna, R.; Titus, E.; Okhay, O.; Gil, J.C.; Ventura, J.; Ramana, E.V.; Gracio, J.J.A. Rapid electrochemical synthesis of hydrogenated graphene oxide using Ni nanoparticles. Int. J. Electrochem. Sci. 2014, 9, 4054–4069. [Google Scholar]
- Han, Y.; Lu, Y. Characterization and electrical properties of conductive polymer/colloidal graphite oxide nanocomposites. Compos. Sci. Technol. 2009, 69, 1231–1237. [Google Scholar] [CrossRef]
- Gopalan, A.I.; Lee, K.P.; Manesh, K.M.; Santhosh, P. Poly(vinylidene fluoride)–polydiphenylamine composite electrospun membrane as high-performance polymer electrolyte for lithium batteries. J. Membrane Sci. 2008, 318, 422–428. [Google Scholar] [CrossRef]
- Jang, W.H.; Kim, J.W.; Choi, H.J.; Jhon, M.S. Synthesis and electrorheology of camphorsulfonic acid doped polyaniline suspensions. Colloid Polym. Sci. 2001, 279, 823–827. [Google Scholar] [CrossRef]
- Erol, O.; Unal, H.I. Core/shell-structured, covalently bonded TiO2/poly(3,4-ethylenedioxythiophene) dispersions and their electrorheological response: The effect of anisotropy. RSC Adv. 2015, 5, 103159–103171. [Google Scholar] [CrossRef]
- Yin, J.; Xia, X.; Xiang, L.; Zhao, X. Temperature effect of electrorheological fluids based on polyaniline derived carbonaceous nanotubes. Smart Mater. Struct. 2011, 20, 015002. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, S.G.; Choi, H.J.; Suh, M.S.; Shin, M.J.; Jhon, M.S. Synthesis and electrorheological characterization of polyaniline and Na+-montmorillonite clay nanocomposite. Int. J. Mod. Phys. B 2001, 15, 657–664. [Google Scholar] [CrossRef]
- Kutalkova, E.; Mrlik, M.; Ilcikova, M.; Osicka, J.; Sedlacik, M.; Mosnacek, J. Enhanced and Tunable Electrorheological Capability using Surface Initiated Atom Transfer Radical Polymerization Modification with Simultaneous Reduction of the Graphene Oxide by Silyl-Based Polymer Grafting. Nanomaterials 2019, 9, 308. [Google Scholar] [CrossRef] [PubMed]
- Mrlik, M.; Ilcikova, M.; Plachy, T.; Moucka, R.; Pavlinek, V.; Mosnacek, J. Tunable electrorheological performance of silicone oil suspensions based on controllably reduced graphene oxide by surface initiated atom transfer radical polymerization of poly(glycidyl methacrylate). J. Ind. Eng. Chem. 2018, 57, 104–112. [Google Scholar] [CrossRef]
- Wen, Q.; Ma, L.; Wang, C.; Wang, B.; Han, R.; Hao, C.; Chen, K. Preparation of core–shell structured metal–organic framework@PANI nanocomposite and its electrorheological properties. RSC Adv. 2019, 9, 14520–14530. [Google Scholar] [CrossRef]
- Gao, C.Y.; Kim, M.W.; Bae, D.H.; Dong, Y.Z.; Piao, S.H.; Choi, H.J. Fe3O4 nanoparticle-embedded polystyrene composite particles fabricated via a Shirasu porous glass membrane technique and their magnetorheology. Polymer 2017, 125, 21–29. [Google Scholar] [CrossRef]
- Gumus, O.Y.; Unal, H.I. Effect of surfactant on the dielectric and electrorheological properties of zinc borate/silicone oil dispersions. Int. J. Miner. Metal. Mater. 2019, 26, 1523–1530. [Google Scholar] [CrossRef]
- Niu, C.; Dong, X.; Lan, Y.; Niu, L.; Xiong, X.; Zhao, B.; Qi, M. Creep and recovery behaviors of electrorheological elastomers and time-electric field superposition principle. Smart Mater. Struct. 2020, 29, 025009. [Google Scholar] [CrossRef]
- Chotpattananont, D.; Sirivat, A.; Jamieson, A.M. Creep and recovery behaviors of a polythiophene-based electrorheological fluid. Polymer 2006, 47, 3568–3575. [Google Scholar] [CrossRef]

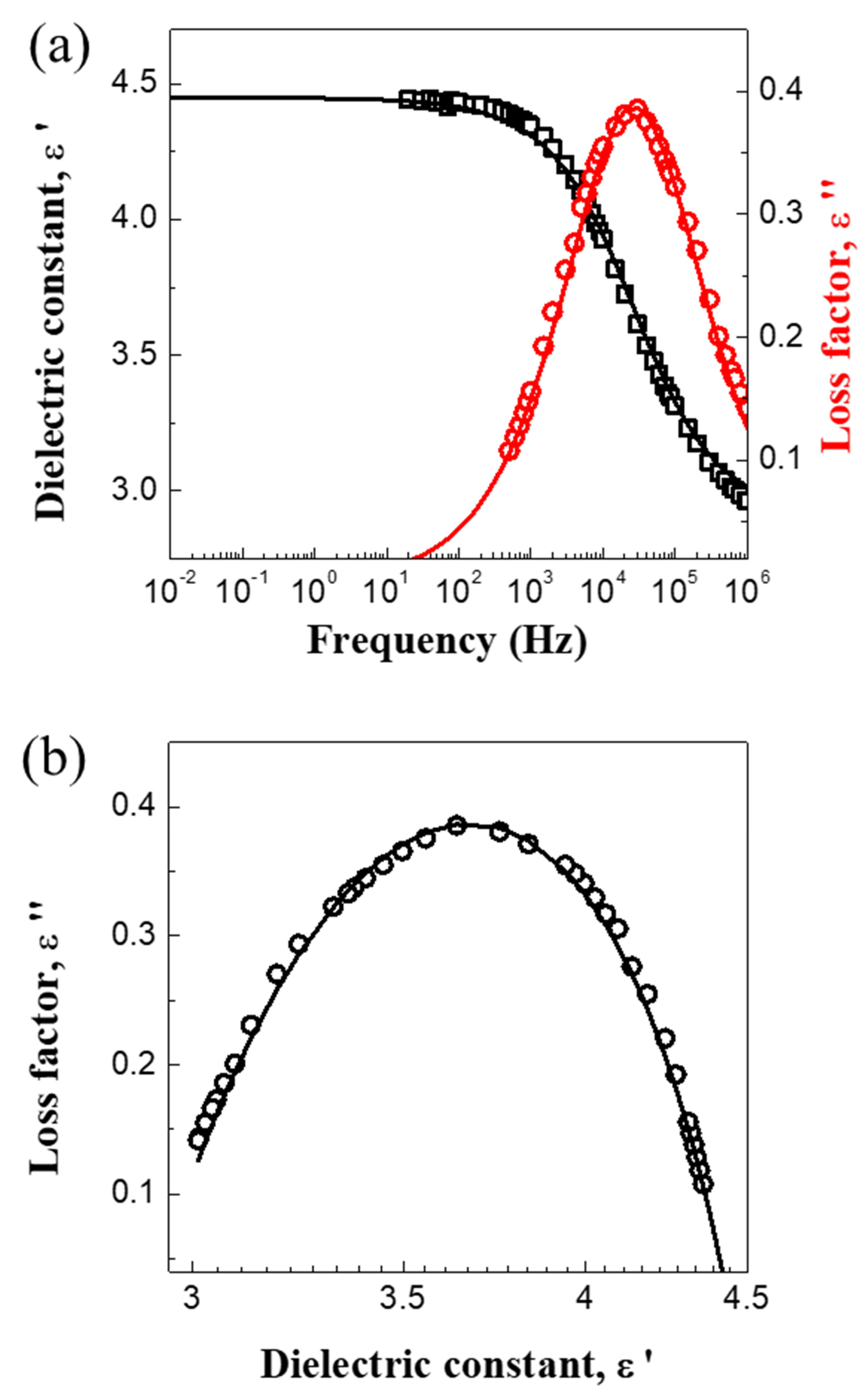


| Parameters | ε0 | ε∞ | Δ ε = ε0 − ε∞ | λ (s) | α |
|---|---|---|---|---|---|
| value | 4.45 | 2.91 | 1.54 | 6 10−6 | 0.41 |
| Model | Parameters | Electric Field Strength (kV/mm) | |||||
| 0.3 | 0.6 | 0.9 | 1.2 | 1.5 | 1.8 | ||
| Bingham | 8.8 | 15.1 | 23.5 | 37.1 | 59.9 | 76.1 | |
| η | 0.17 | 0.14 | 0.13 | 0.12 | 0.07 | 0.13 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, C.Y.; Kim, M.H.; Jin, H.-J.; Choi, H.J. Synthesis and Electrorheological Response of Graphene Oxide/Polydiphenylamine Microsheet Composite Particles. Polymers 2020, 12, 1984. https://doi.org/10.3390/polym12091984
Gao CY, Kim MH, Jin H-J, Choi HJ. Synthesis and Electrorheological Response of Graphene Oxide/Polydiphenylamine Microsheet Composite Particles. Polymers. 2020; 12(9):1984. https://doi.org/10.3390/polym12091984
Chicago/Turabian StyleGao, Chun Yan, Min Hwan Kim, Hyoung-Joon Jin, and Hyoung Jin Choi. 2020. "Synthesis and Electrorheological Response of Graphene Oxide/Polydiphenylamine Microsheet Composite Particles" Polymers 12, no. 9: 1984. https://doi.org/10.3390/polym12091984
APA StyleGao, C. Y., Kim, M. H., Jin, H.-J., & Choi, H. J. (2020). Synthesis and Electrorheological Response of Graphene Oxide/Polydiphenylamine Microsheet Composite Particles. Polymers, 12(9), 1984. https://doi.org/10.3390/polym12091984






