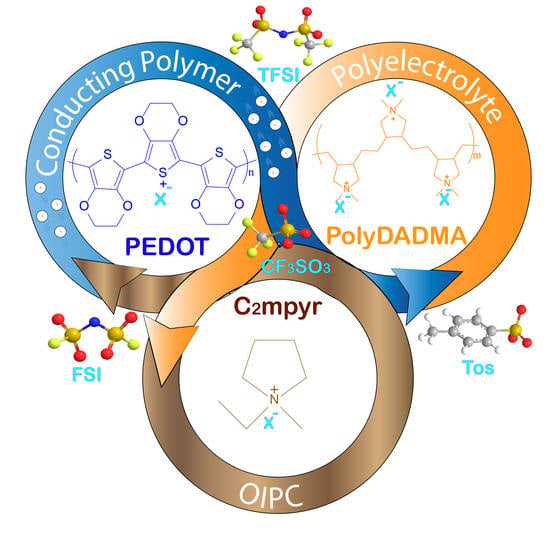
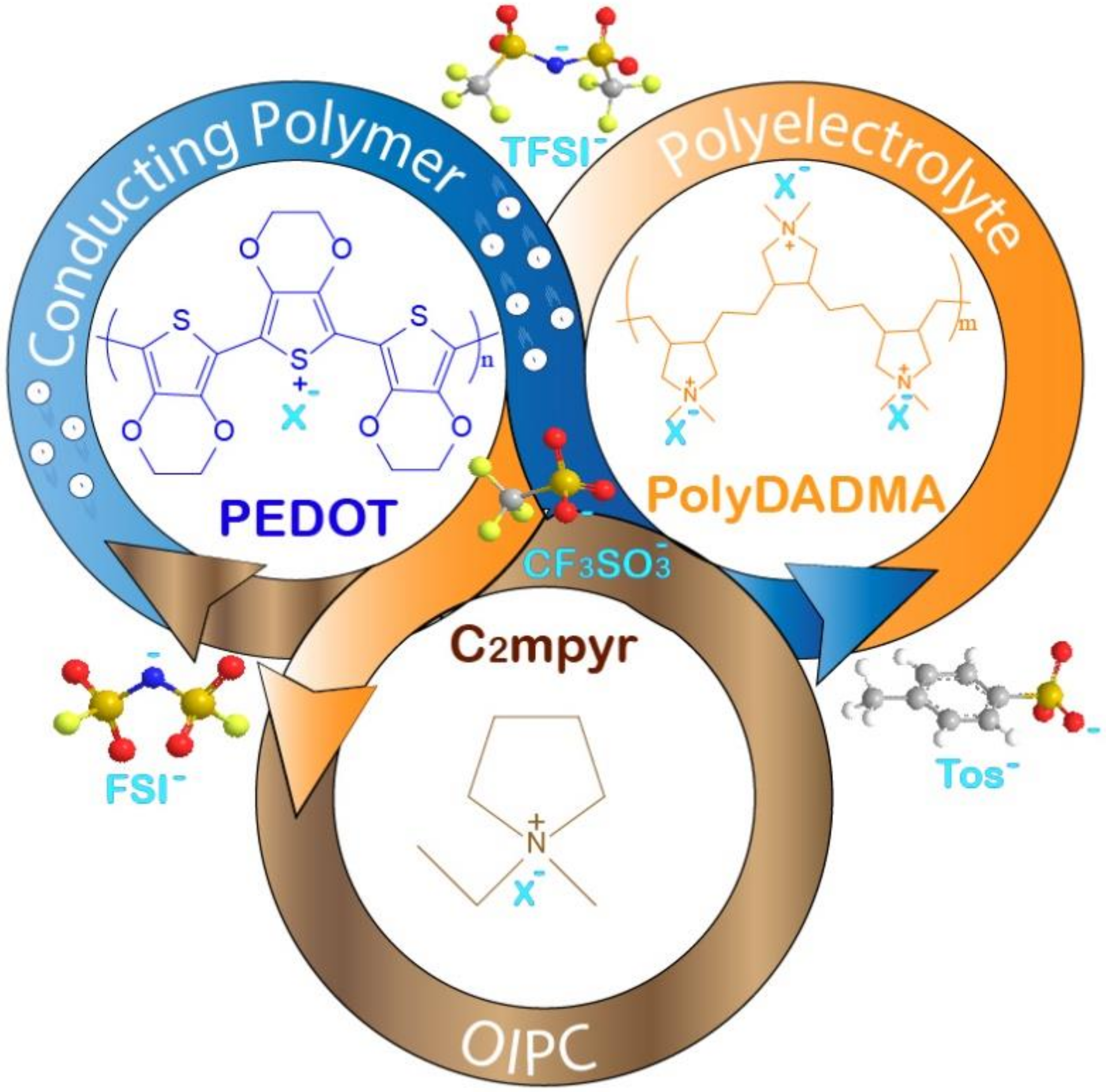
Mixed Ionic-Electronic Conductors Based on PEDOT:PolyDADMA and Organic Ionic Plastic Crystals
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
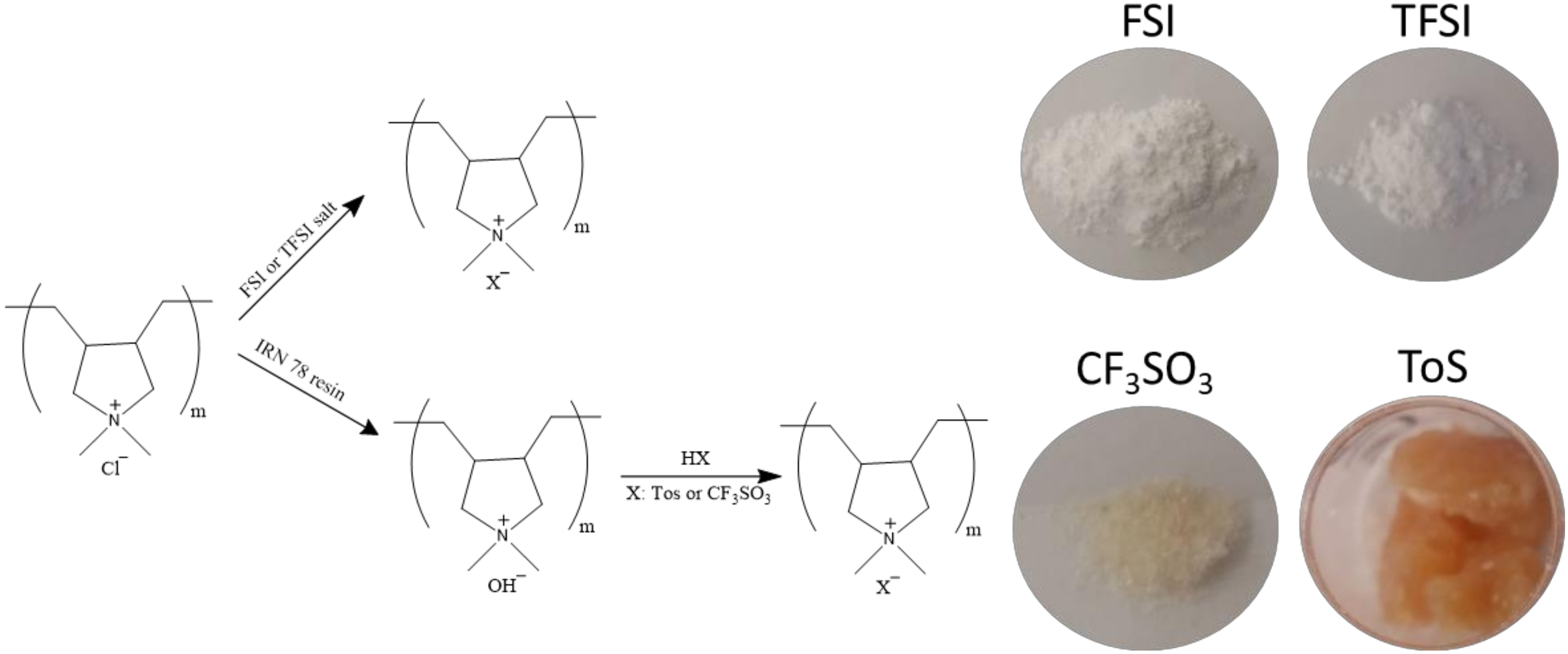
2.2. Synthesis of [C2mpyr][FSI]
2.3. Synthesis of PolyDADMA FSI and PolyDADMA TFSI
2.4. Synthesis of [C2mpyr][CF3SO3], [C2mpyr][Tos], PolyDADMACF3SO3 and PolyDADMA Tos
2.5. Synthesis of PEDOT:PolyDADMA FSI and PEDOT:PolyDADMA TFSI
2.6. Synthesis of PEDOT:PolyDADMA Cl, CF3SO3 and PEDOT:PolyDADMA Tos
3. Results and Discussion
3.1. Neat PolyDADMA X
3.1.1. Thermal Analysis
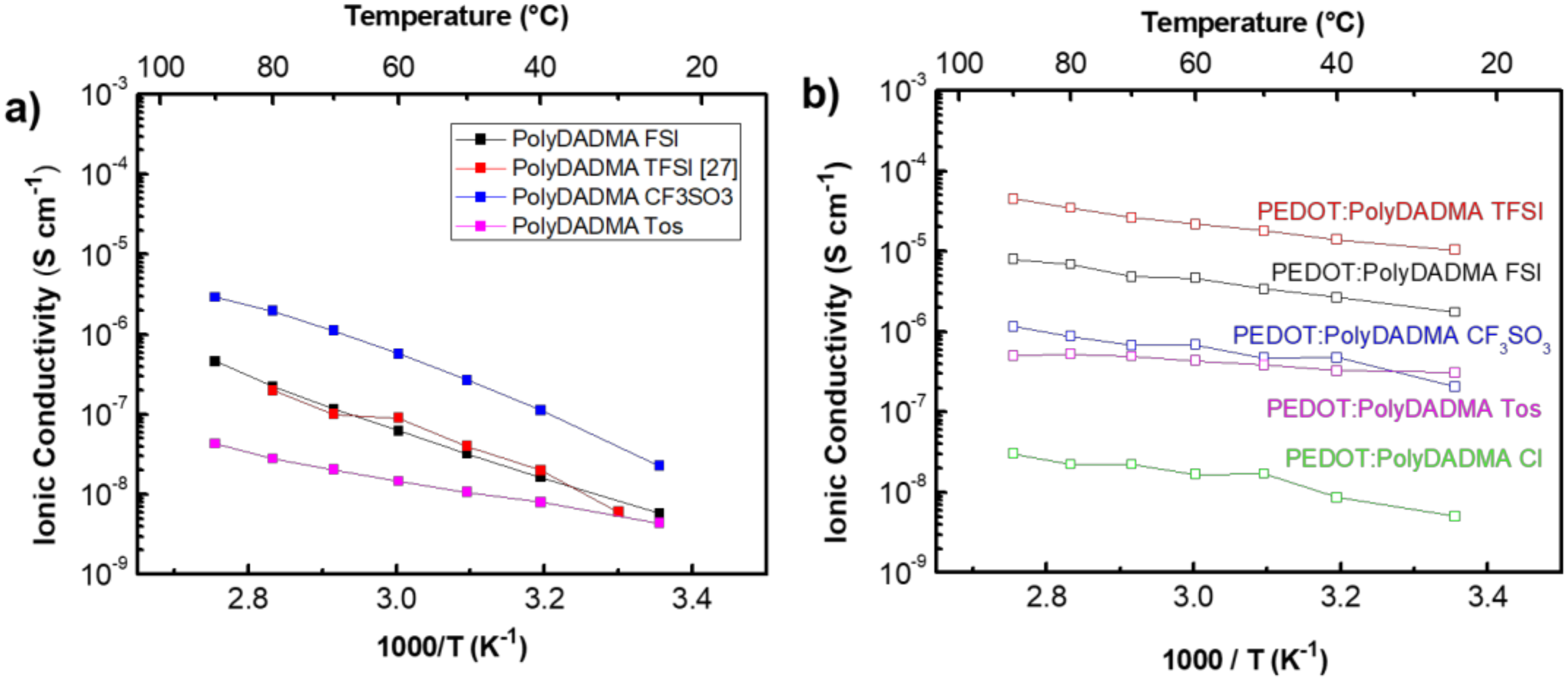
3.1.2. Ionic Conductivity
3.2. PEDOT:PolyDADMA X
3.2.1. Thermal Analysis
3.2.2. Ionic Conductivity
3.2.3. Electronic Conductivity
3.3. Neat OIPC
3.3.1. Thermal Analysis
3.3.2. Ionic Conductivity
3.4. PEDOT:PolyDADMA X + OIPC
3.4.1. Electronic Conductivity
3.4.2. Ionic Conductivity
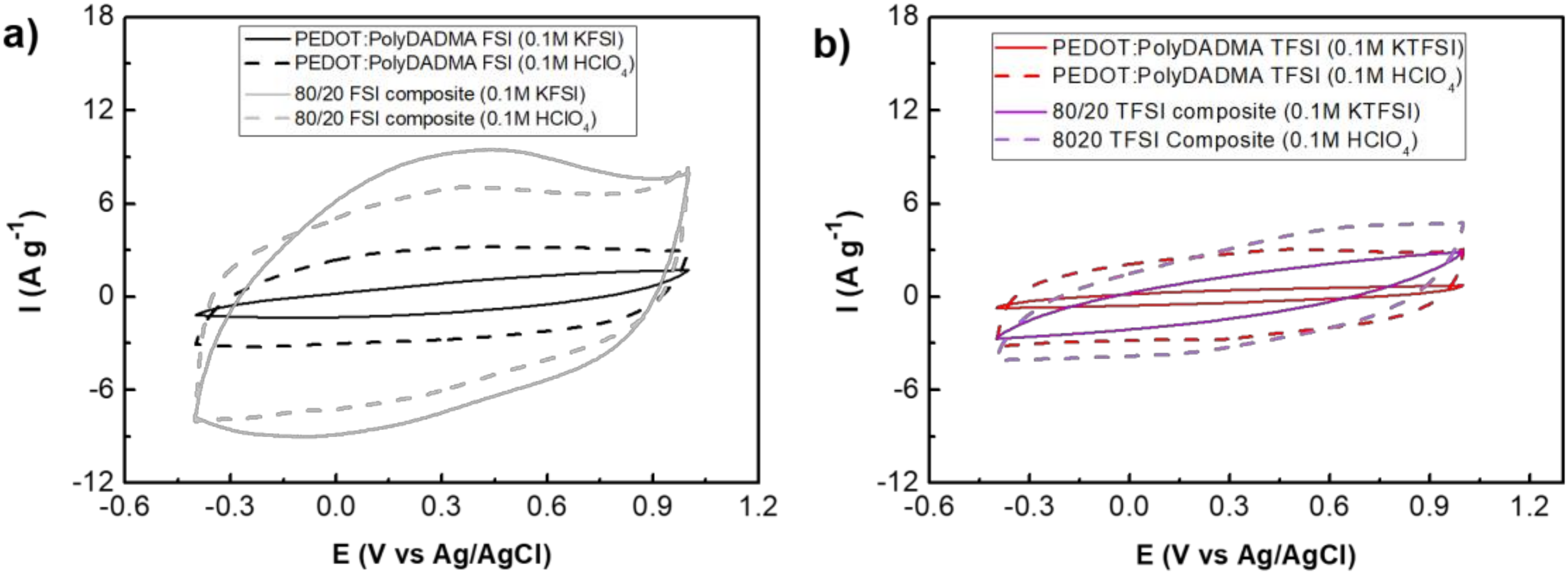
3.4.3. Cyclic Voltammetry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, C.; Sunarso, J.; Liu, S. Designing CO2-resistant oxygen-selective mixed ionic-electronic conducting membranes: Guidelines, recent advances, and forward directions. Chem. Soc. Rev. 2017, 22, 2941–3005. [Google Scholar] [CrossRef]
- Wang, S.; Yan, M.; Li, Y.; Vinado, C.; Yang, J. Separating electronic and ionic conductivity in mix-conducting layered lithium transition-metal oxides. J. Power Sources 2018, 393, 75–82. [Google Scholar] [CrossRef]
- Paulsen, B.D.; Tybrandt, K.; Stavrinidou, E.; Rivnay, J. Organic mixed ionic – electronic conductors. Nat. Mater. 2020, 19, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Moia, D.; Giovannitti, A.; Szumska, A.A.; Maria, I.P.; Rezasoltani, E.; Sachs, M.; Schnurr, M.; Barnes, P.R.F.; Nelson, J. Design and evaluation of conjugated polymers with polar side chains as electrode materials for electrochemical energy storage in aqueous electrolytes. Energ. Environ. Sci. 2019, 12, 1349–1357. [Google Scholar] [CrossRef]
- Nilsson, D.; Robinson, N.D.; Isaksson, J.; All, P.K.J.; Berggren, M.; Richter-dahlfors, A. Electronic control of Ca2+ signalling in neuronal cells using an organic electronic ion pump. Nat. Mater. 2007, 6, 673–679. [Google Scholar]
- Jang, J.; Ha, J.; Cho, J. Fabrication of Water-Dispersible Polyaniline-Poly(4-styrenesulfonate) Nanoparticles For Inkjet-Printed Chemical-Sensor Applications. Adv. Mater. 2007, 19, 1772–1775. [Google Scholar] [CrossRef]
- Zajdel, T.J.; Baruch, M.; Méhes, G.; Stavrinidou, E.; Berggren, M.; Maharb, M.M.; Simon, D.T.; Ajo-franklin, C.M. PEDOT:PSS-based Multilayer Bacterial-Composite Films for Bioelectronics. Sci. Rep. 2018, 8, 15293. [Google Scholar] [CrossRef]
- Zheng, Y.; Zeng, H.; Zhu, Q.; Xu, J. Recent advances in conducting poly(3,4-ethylenedioxythiophene):polystyrene sulfonate hybrids for thermoelectric applications. J. Mater. Chem. C 2018, 6, 8858–8873. [Google Scholar] [CrossRef]
- Yu, Z.; Xia, Y.; Du, D.; Ouyang, J. PEDOT:PSS Films with Metallic Conductivity through a Treatment with Common Organic Solutions of Organic Salts and Their Application as a Transparent Electrode of Polymer Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 11629–11638. [Google Scholar] [CrossRef]
- Wang, X.; Ko, K.; Yin, C.; Wang, F.; Zhu, Q.; Tang, T. Enhancement of thermoelectric performance of PEDOT:PSS films by post-treatment with a superacid. RSC Adv. 2018, 8, 18334–18340. [Google Scholar] [CrossRef]
- Armel, V.; Rivnay, J.; Malliaras, G.; Winther, J.B. Unexpected Interaction between PEDOT and Phosphonium Ionic Liquids. J. Am. Chem. Soc. 2013, 135, 11309–11313. [Google Scholar] [CrossRef] [PubMed]
- Malti, A.; Edberg, J.; Granberg, H.; Khan, Z.U.; Andreasen, J.W.; Liu, X.; Zhao, D.; Zhang, H.; Yao, Y.; Brill, J.W.; et al. An Organic Mixed Ion–Electron Conductor for Power Electronics. Adv. Sci. 2016, 3, 150035. [Google Scholar] [CrossRef] [PubMed]
- Salcedo-abraira, P.; Santiago-portillo, A.; Atienzar, P.; Bordet, P.; Salles, F.; Guillou, N.; Elkaim, E.; Garcia, H.; Navalon, S.; Horcajada, P. A highly conductive nanostructured PEDOT polymer confined into the mesoporous MIL-100(Fe). Dalton Trans. 2019, 48, 9807–9817. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Sun, L.; Lee, D.; Lee, S.; Wang, M.; Zhao, J.; Shao-horn, Y.; Dinc, M.; Palacios, T.; et al. High electrical conductivity and carrier mobility in oCVD PEDOT thin films by engineered crystallization and acid treatment. Sci. Adv. 2018, 4, 5780. [Google Scholar] [CrossRef]
- Inal, S.; Rivnay, J.; Leleux, P.; Ferro, M.; Ramuz, M.; Brendel, J.C.; Schmidt, M.M.; Thelakkat, M.; Malliaras, G.G. A High Transconductance Accumulation Mode Electrochemical Transistor. Adv. Mater. 2014, 26, 7450–7455. [Google Scholar] [CrossRef]
- Kim, T.Y.; Park, C.M.; Kim, J.E.; Suh, K.S. Electronic, chemical and structural change induced by organic solvents in tosylate-doped poly(3,4-ethylenedioxythiophene) (PEDOT-OTs). Synth. Met. 2005, 149, 169–174. [Google Scholar] [CrossRef]
- Kim, H.; Jeong, K.; Yu, C.; Nam, H.; Soh, H.; Lee, J. The effects of the surface morphology of poly (3,4-ethylenedioxythiophene) electrodes on the growth of pentacene, and the electrical performance of the bottom contact pentacene transistor. Solid State Electron. 2012, 67, 70–73. [Google Scholar] [CrossRef]
- Olmedo-mart, J.L.; Meabe, L.; Basterretxea, A.; Mecerreyes, D.; Müller, A.J. Effect of Chemical Structure and Salt Concentration on the Crystallization and Ionic Conductivity of Aliphatic Polyethers. Polymers 2019, 11, 452. [Google Scholar] [CrossRef]
- Demarteau, J.; Fdz de Añastro, A.; Shaplov, A.S. Poly(diallyldimethylammonium) based poly(ionic liquid) di- and triblock copolymers by PISA as matrices for ionogel membranes. Polym. Chem. 2020, 11, 1481–1488. [Google Scholar] [CrossRef]
- Kee, S.; Kim, N.; Kim, B.S.; Park, S.; Jang, Y.H.; Lee, S.H.; Kim, J.; Kim, J.; Kwon, S.; Lee, K. Controlling Molecular Ordering in Aqueous Conducting Polymers Using Ionic Liquids. Adv. Mater. 2016, 28, 8625–8631. [Google Scholar] [CrossRef]
- Mazaheripour, A.; Majumdar, S.; Hanemann-rawlings, D.; Thomas, E.M.; Mcguiness, C.; Alencon, L.; Chabinyc, M.L.; Segalman, R.A. Tailoring the Seebeck Coefficient of PEDOT:PSS by Controlling Ion Stoichiometry in Ionic Liquid Additives. Chem. Mater. 2018, 30, 4816–4822. [Google Scholar] [CrossRef]
- Teo, M.Y.; Kim, N.; Kee, S.; Kim, B.S.; Kim, G.; Hong, S.; Jung, S.; Lee, K. Highly Stretchable and Highly Conductive PEDOT: PSS / Ionic Liquid Composite Transparent Electrodes for Solution-Processed Stretchable Electronics. ACS Appl. Mater. Interfaces 2017, 9, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, X.; Zhu, H.; Armand, M.; Forsyth, M.; Greene, G.W.; Pringle, J.M.; Howlett, P.C. N-ethyl-N-methylpyrrolidinium bis(fluorosulfonyl)imide-electrospun polyvinylidene fluoride composite electrolytes: Characterization and lithium cell studies. Phys. Chem. Chem. Phys. 2017, 19, 2225–2234. [Google Scholar] [CrossRef] [PubMed]
- Ponrouch, A.; Monti, D.; Boschin, A.; Steen, B.; Johansson, P.; Palacin, M.R. Non-Aqueous Electrolytes for Sodium-Ion Batteries. J. Mater. Chem. A 2015, 3, 22–42. [Google Scholar] [CrossRef]
- Nti, F.; Porcarelli, L.; Greene, G.W.; Zhu, H.; Makhlooghiazad, F.; Mecerreyes, D.; Howlett, P.C.; Forsyth, M.; Wang, X. The influence of interfacial interactions on the conductivity and phase behaviour of organic ionic plastic crystal/polymer nanoparticle composite electrolytes. J. Mater. Chem. A 2020, 8, 5350–5362. [Google Scholar] [CrossRef]
- Yoshizawa-fujita, M.; Kishi, E.; Suematsu, M.; Takekawa, T.; Rikukawa, M. A Plastic Electrolyte Material in a Highly Desirable Temperature Range: N -Ethyl- N -methylpyrrolidinium Bis(fluorosulfonyl)amide. Chem. Lett. 2014, 43, 1909–1911. [Google Scholar] [CrossRef]
- Fdz de Añastro, A.; Casado, N.; Wang, X.; Rehmen, J.; Evans, D.; Mecerreyes, D.; Forsyth, M.; Pozo-gonzalo, C. Poly(ionic liquid) iongels for all-solid rechargeable zinc/PEDOT batteries. Electrochim. Acta 2018, 278, 271–278. [Google Scholar] [CrossRef]
- Gouveia, A.S.L.; Tome, L.C.; Marrucho, I.M. Density, Viscosity, and Refractive Index of Ionic Liquid Mixtures Containing Cyano and Amino Acid-Based Anions. J. Chem. Eng. Data 2015, 61, 83–93. [Google Scholar] [CrossRef]
- Pozo-gonzalo, C.; Marcilla, R.; Salsamendi, M.; Mecerreyes, D.; Pomposo, J.A.; Rodri, J. PEDOT:Poly(1-vinyl-3-ethylimidazolium) dispersions as alternative materials for optoelectronic devices. J. Polym. Sci. 2008, 46, 3150–3154. [Google Scholar] [CrossRef]
- Yano, H.; Kudo, K.; Marumo, K.; Okuzaki, H. Fully soluble self-doped an electrical conductivity greater than 1000 S cm−1. Sci. Adv. 2019, 5, 1–10. [Google Scholar] [CrossRef]
- Yunis, R.; Girard, G.M.A.; Wang, X.; Zhu, H.; Bhattacharyya, A.J.; Howlett, P.; Macfarlane, D.R.; Forsyth, M. The anion effect in ternary electrolyte systems using poly (diallyldimethylammonium) and phosphonium-based ionic liquid with high lithium salt concentration. Solid State Ionics 2018, 327, 83–92. [Google Scholar] [CrossRef]
- Pereiro, A.B.; Martinho, S.; Alves, F.; Nunes, S.; Matias, A.; Duarte, C.M.M.; Rebelo, L.P.N.; Marrucho, I.M. Fluorinated Ionic Liquids: Properties and Applications. ACS Sustain. Chem. Eng. 2013, 1, 427–439. [Google Scholar] [CrossRef]
- Eftekharnia, M.; Hasanpoor, M.; Forsyth, M.; Kerr, R.; Howlett, P.C. Toward Practical Li Metal Batteries: Importance of Separator Compatibility Using Ionic Liquid Electrolytes. ACS Appl. Energ. Mater. 2019, 2, 6655–6663. [Google Scholar] [CrossRef]
- Agrawal, S.L.; Rai, N. DMA and Conductivity Studies in PVA:NH4SCN:DMSO:MWNT Nanocomposite Polymer Dried Gel Electrolytes. J. Nanomater. 2015, 2015. [Google Scholar] [CrossRef]
- Ue, M.; Murakami, A.; Nakamura, S. A Convenient Method to Estimate Ion Size for Electrolyte Materials Design. J. Electrochem. Soc. 2002, 149, 1385–1388. [Google Scholar] [CrossRef]
- Han, H.; Zhou, S.; Zhang, D.; Feng, S.; Li, L.; Liu, K. Lithium bis(fluorosulfonyl) imide (LiFSI) as conducting salt for nonaqueous liquid electrolytes for lithium-ion batteries: Physicochemical and electrochemical properties. J. Power Sources 2011, 196, 3623–3632. [Google Scholar] [CrossRef]
- Beichel, W.; Eiden, P.; Krossing, I. Establishing Consistent van der Waals Volumes of Polyatomic Ions from Crystal Structures. ChemPhysCHem 2013, 14, 3221–3226. [Google Scholar] [CrossRef]
- Mamun, A.; Tewfik, M.; Rahman, S.M.M.; Al-harthi, S.H.; Munam, A. Miscibility and Thermal Stability of Ethyl Vinyl Acetate and Ethylene-Octane Copolymer Blends. Polym. Blends 2017, 59, 397–404. [Google Scholar] [CrossRef]
- Shimogama, N.; Uda, M.; Oyama, K.; Hanochi, H.; Hirai, T. Hydrophobic poly(3,4-ethylenedioxythiophene) particles synthesized by aqueous oxidative coupling polymerization and their use as near-infrared-responsive liquid marble stabilizer. Polym. J. 2019, 51, 761–770. [Google Scholar] [CrossRef]
- Gonzalez, F.; Tiemblo, P.; Hoyos, M. In-Situ Approaches for the Preparation of Polythiophene-Derivative Cellulose Composites with High Flexibility and Conductivity. Appl. Sci. 2019, 9, 3371. [Google Scholar] [CrossRef]
- Turhan, H.; Bicak, N. Selective Dinitramide Removal from Aqueous Solution by Crosslinked PolyDADMAC Gels. Propellants Explos. Pyrotech. 2020, 45, 1–8. [Google Scholar] [CrossRef]
- Zozoulenko, I.; Singh, A.; Singh, S.K.; Gueskine, V.; Crispin, X.; Berggren, M. Polarons, Bipolarons, And Absorption Spectroscopy of PEDOT. ACS Appl. Polym. Mater. 2018, 1, 83–94. [Google Scholar] [CrossRef]
- Shuzhong, H.; Masakazu, M.; Kazuhiro, K.; Lingyun, L.; Qingshuo, W. Reversible Protonic Doping in Poly(3,4-Ethylenedioxythiophene). Polymers 2018, 10, 1065. [Google Scholar]
- Marcilla, R.; Salsamendi, M.; Pozo-gonzalo, C.; Carrasco, P.M.; Pomposo, J.A.; Mecerreyes, D. Influence of Ionic Liquids on the Electrical Conductivity and Morphology of PEDOT: PSS Films. Chem. Mater. 2007, 19, 2147–2149. [Google Scholar]
- Yamada, H.; Miyachi, Y.; Takeoka, Y.; Rikukawa, M.; Yoshizawa-fujita, M. Pyrrolidinium-based organic ionic plastic crystals: Relationship between side chain length and properties. Electrochim. Acta 2019, 303, 293–298. [Google Scholar] [CrossRef]
- Ignat, N.V.; Barthen, P.; Kucheryna, A.; Willner, H.; Sartori, P. A Convenient Synthesis of Triflate Anion Ionic Liquids and Their Properties. Molecules 2012, 17, 5319–5338. [Google Scholar] [CrossRef] [PubMed]
- Dhahri, A.; El Ghali, A.; Baouab, M.H.V. Synthesis of a new 2,4,5-triphenyl-1H-imidazolium-paratoluenesulfonic acid salt: Thermal and electrochemical stability. J. Tunis. Chem. Soc. 2016, 19, 139–143. [Google Scholar]
- Timmermans, J. Plastic crystalis: A historical review. J. Phys. Chem. Solids 1961, 18, 1–8. [Google Scholar] [CrossRef]
- Basile, A.; Hilder, M.; Makhlooghiazad, F.; Pozo-gonzalo, C.; Macfarlane, D.R.; Howlett, P.C.; Forsyth, M. Ionic Liquids and Organic Ionic Plastic Crystals: Advanced Electrolytes for Safer High Performance Sodium Energy Storage Technologies. Adv. Energ. Mater. 2018, 8, 1703491. [Google Scholar] [CrossRef]
- Shekibi, Y.; Pas, S.J.; Rocher, N.M.; Clare, B.R.; Hill, A.J.; Macfarlane, D.R.; Forsyth, M. Surprising effect of nanoparticle inclusion on ion conductivity in a lithium doped organic ionic plastic crystal. J. Mater.Chem. 2009, 19, 1635–1642. [Google Scholar] [CrossRef]
- Forsyth, M.; Chimdi, T.; Seeber, A.; Gunzelmann, D.; Howlett, P. Structure and dynamics in an organic ionic plastic crystal, N-ethyl-N-methyl pyrrolidinium bis(trifluoromethanesulfonyl) amide, mixed with a sodium salt. J. Mater. Chem. A 2014, 2, 3993–4003. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, H.; Greene, G.W.; Zhou, Y.; Yoshizawa-fujita, M.; Miyachi, Y.; Armand, M.; Forsyth, M.; Pringle, J.M.; Howlett, P.C. Organic Ionic Plastic Crystal-Based Composite Electrolyte with Surface Enhanced Ion Transport and Its Use in All-Solid-State Lithium Batteries. Adv. Mater. Technol. 2017, 2, 1700046. [Google Scholar] [CrossRef]
- Henderson, W.A.; Seo, D.M.; Zhou, Q.; Boyle, P.D.; Shin, J.; De Long, H.C.; Trulove, P.C.; Passerini, S. An Alternative Ionic Conductivity Mechanism for Plastic Crystalline Salt–Lithium Salt Electrolyte Mixtures. Adv. Energ. Mater. 2012, 2, 1343–1350. [Google Scholar] [CrossRef]
- Macfarlane, D.R.; Huang, J.; Forsyth, M. Lithium-doped plastic crystal electrolytes exhibiting fast ion conduction for secondary batteries. Nature 1999, 402, 792–794. [Google Scholar] [CrossRef]
- Jin, L.; Howlett, P.; Efthimiadis, J.; Kar, M.; Forsyth, M. Lithium doped N,N-dimethyl pyrrolidinium tetrafluoroborate organic ionic plastic crystal electrolytes for solid state lithium batteries. J. Mater. Chem. 2011, 21, 10171–10178. [Google Scholar] [CrossRef]
- Krampa, F.D.; Aniweh, Y.; Awandare, G.A.; Kanyong, P. A Disposable Amperometric Sensor Based on High-Performance PEDOT:PSS/Ionic Liquid Nanocomposite Thin Film-Modified Screen-Printed Electrode for the Analysis of Catechol in Natural Water Samples. Sensors 2017, 17, 1716. [Google Scholar] [CrossRef]
- Malengier, B.; Deferme, W.; De Mey, G.; Van Langenhove, L. Charge-Discharge Characteristics of Textile Energy Storage Devices Having Different PEDOT: PSS Ratios and Conductive Yarns Configuration. Polymers 2019, 11, 345. [Google Scholar]


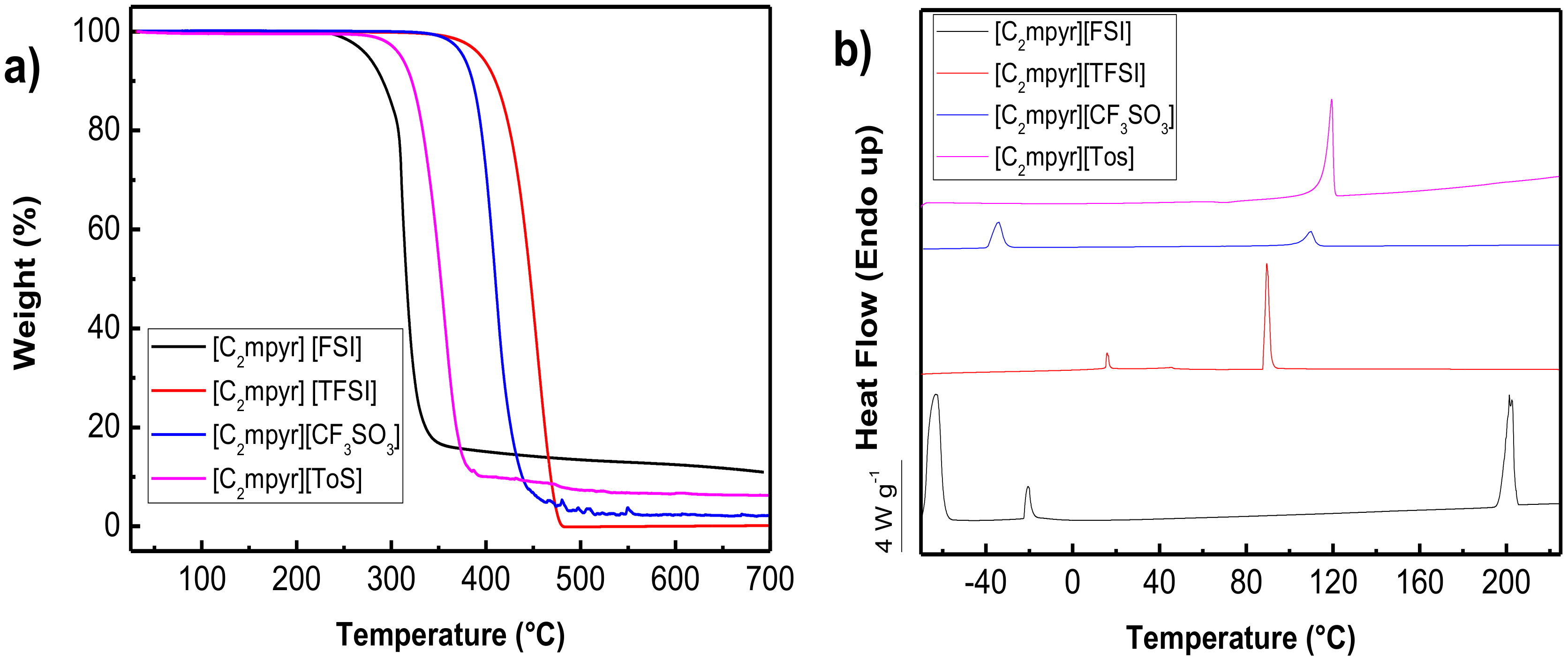
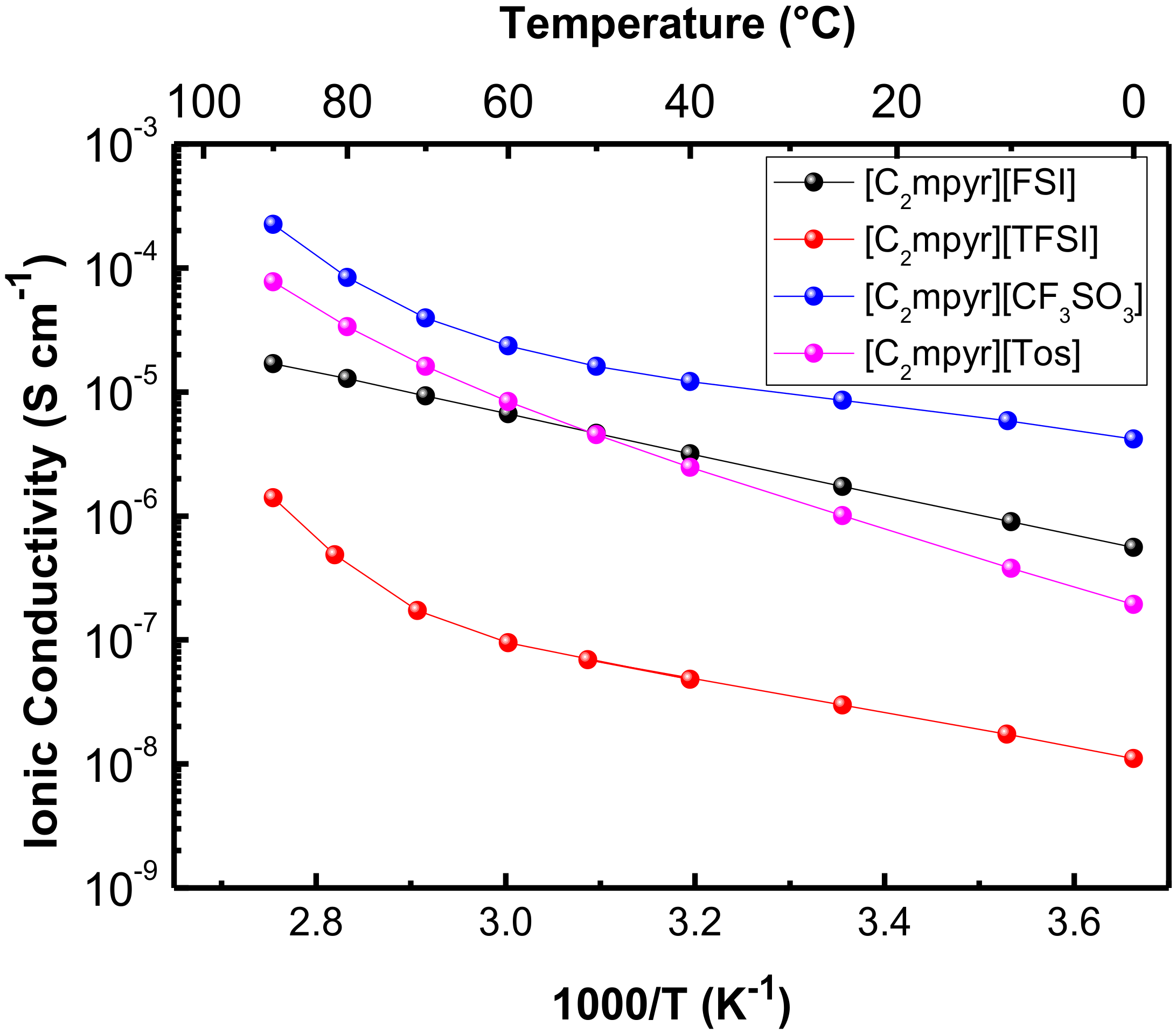
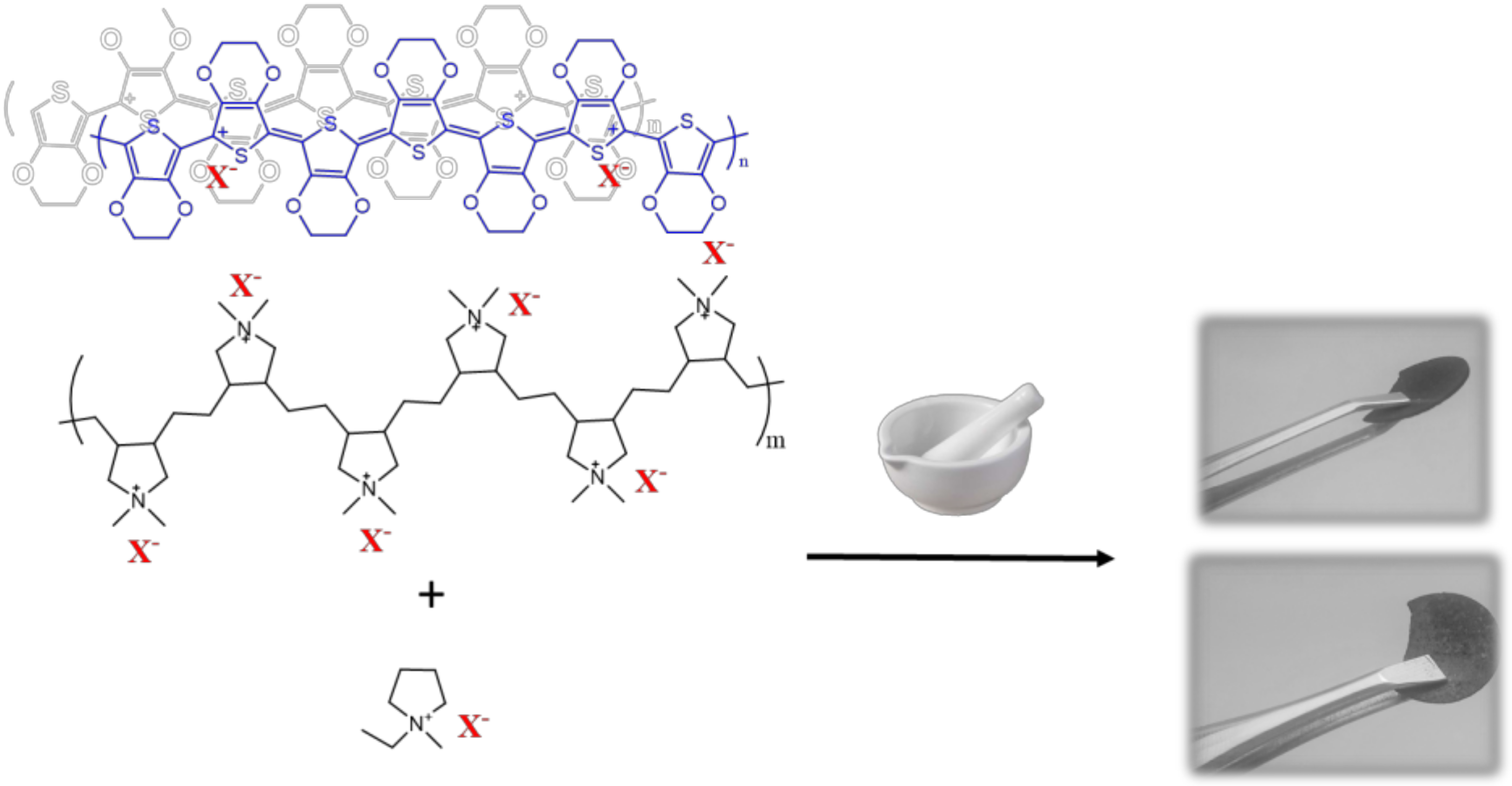
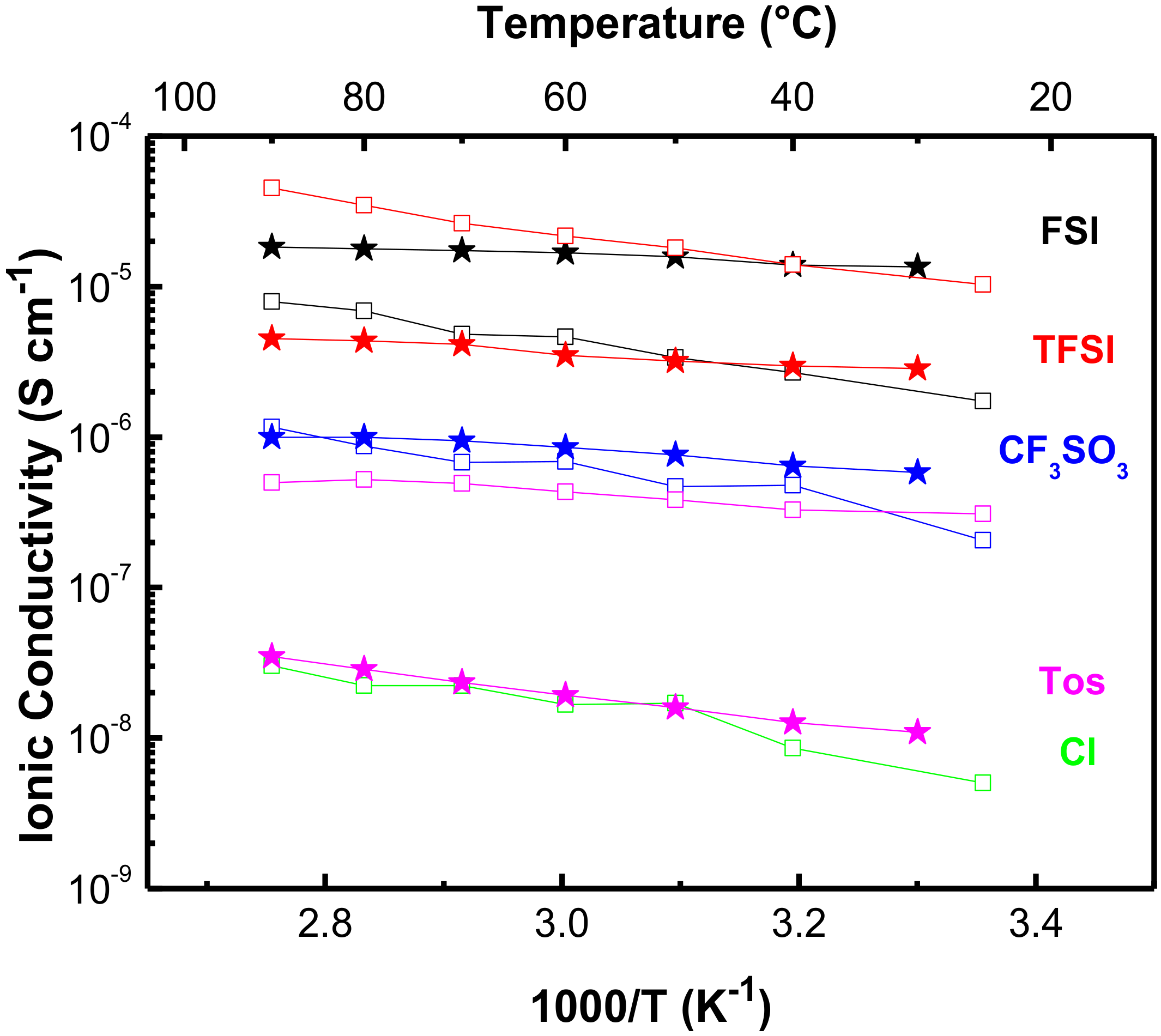
| Compound | Literature Values | Experimental Values | |
|---|---|---|---|
| σionic/S cm−1 | σionic/S cm−1 | Ea/KJ mol−1 | |
| PolyDADMA FSI | 2·× 10−7 [31] | 1·× 10−7 | 25.9 ± 0.4 |
| PolyDADMA TFSI | 1·× 10−7 [27] | - | 25.8 ± 2.5 [27] |
| PolyDADMA CF3SO3 | - | 1·× 10−6 | 29.2 ± 1.3 |
| PolyDADMA Tos | - | 2·× 10−8 | 13.6 ± 0.4 |
| X | Td PEDOT:X/°C | Td PolyDADMA X/°C | T1, d PEDOT:PolyDADMA X/°C | T2, d PEDOT:PolyDADMA X/°C |
|---|---|---|---|---|
| Cl | 300 [39] | 300 and 450 [41] | 281, 295 | 378 |
| FSI | - | 300 [31] | - | 340 |
| TFSI | - | 450 [31] | - | 423 |
| CF3SO3 | 360 [40] | 427 | 301 | 345 |
| Tos | 343 [40] | 382 | 296 | 343 |
| Coating | Pellet | |||
|---|---|---|---|---|
| Thickness/μm | σelectronic/S cm−1 | Thickness/μm | σelectronic/S cm−1 | |
| FSI | 70 | 0.60 | 400 | 0.04 |
| TFSI | 75 | 0.25 | 500 | 0.02 |
| Cl | - | - | 250 | 0.10 |
| CF3SO3 | - | - | 350 | 0.30 |
| Tos | - | - | 250 | 0.10 |
| X | PEDOT:PolyDADMA X | 80/20 wt% PEDOT:PolyDADMA X/C2mpyr X | ||
|---|---|---|---|---|
| σionic/S cm−1 | Ea/KJ mol−1 | σionic/S cm−1 | Ea/KJ mol−1 | |
| FSI | 5·10−6 | 8.4 | 2·10−5 | 2.3 ± 0.2 |
| TFSI | 3·10−5 | 8.6 | 4·10−6 | 3.4 ± 0.2 |
| Cl | 2·10−8 | 8.9 | - | - |
| CF3SO3 | 7·10−7 | 7.5 | 1·10−6 | 3.6 ± 0.3 |
| Tos | 5·10−7 | 4.0 | 2·10−8 | 8.7 ± 0.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Olmo, R.; Casado, N.; Olmedo-Martínez, J.L.; Wang, X.; Forsyth, M. Mixed Ionic-Electronic Conductors Based on PEDOT:PolyDADMA and Organic Ionic Plastic Crystals. Polymers 2020, 12, 1981. https://doi.org/10.3390/polym12091981
Del Olmo R, Casado N, Olmedo-Martínez JL, Wang X, Forsyth M. Mixed Ionic-Electronic Conductors Based on PEDOT:PolyDADMA and Organic Ionic Plastic Crystals. Polymers. 2020; 12(9):1981. https://doi.org/10.3390/polym12091981
Chicago/Turabian StyleDel Olmo, Rafael, Nerea Casado, Jorge L. Olmedo-Martínez, Xiaoen Wang, and Maria Forsyth. 2020. "Mixed Ionic-Electronic Conductors Based on PEDOT:PolyDADMA and Organic Ionic Plastic Crystals" Polymers 12, no. 9: 1981. https://doi.org/10.3390/polym12091981
APA StyleDel Olmo, R., Casado, N., Olmedo-Martínez, J. L., Wang, X., & Forsyth, M. (2020). Mixed Ionic-Electronic Conductors Based on PEDOT:PolyDADMA and Organic Ionic Plastic Crystals. Polymers, 12(9), 1981. https://doi.org/10.3390/polym12091981