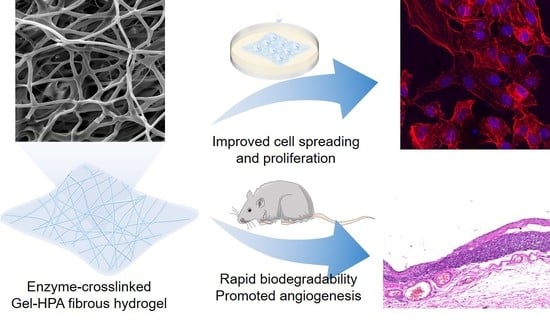
Enzyme-Crosslinked Electrospun Fibrous Gelatin Hydrogel for Potential Soft Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
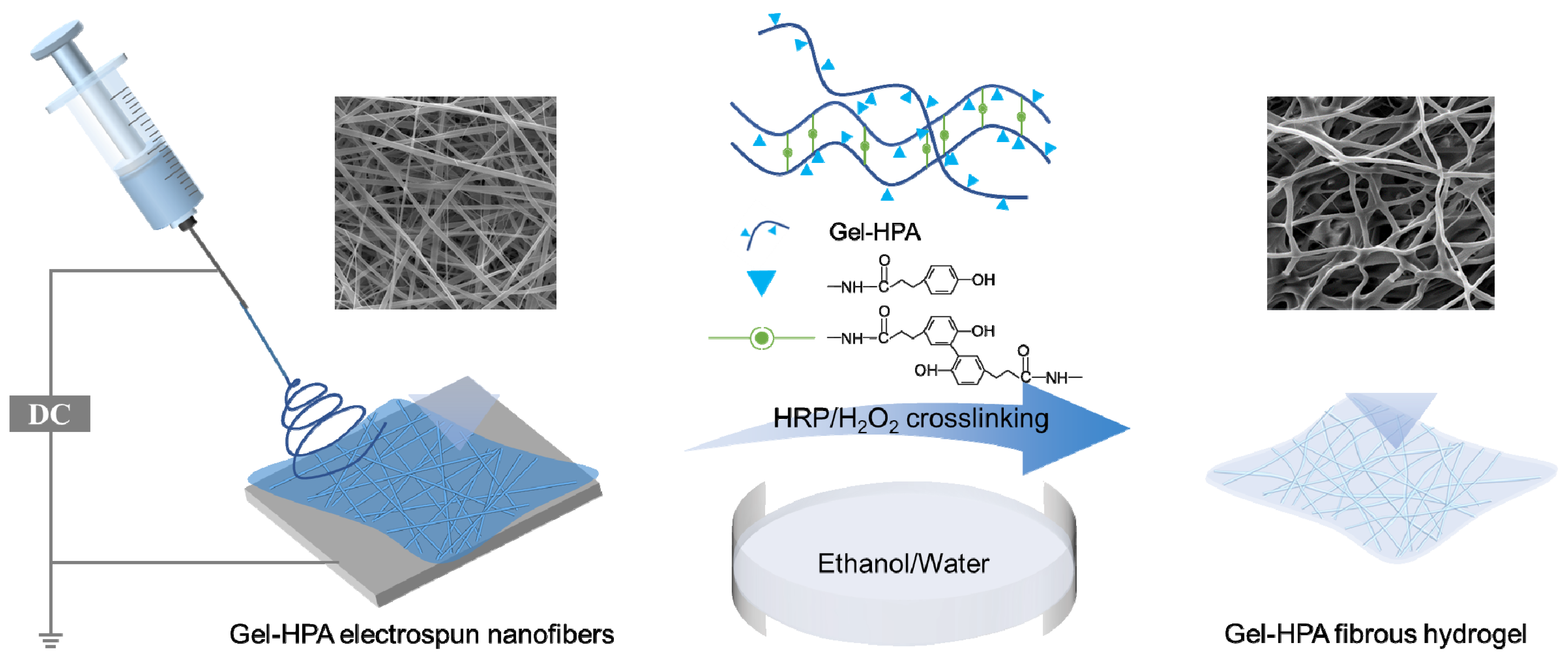
2.1. Synthesis of Gelatin–Hydroxyphenylpropionic Acid
2.2. 1H Nuclear Magnetic Resonance (NMR)
2.3. Electrospinning of Gel–HPA and Gelatin Electrospun Fibers
2.4. Crosslinking of Gel–HPA Fibrous Hydrogels and Gelatin Fibers
2.5. Scanning Electron Microscopy (SEM)
2.6. Water Uptake Measurements
2.7. Mechanical Testing
2.8. Measurement of Enzymatic Degradation
2.9. In Vitro Cell Viability, Spreading and Proliferation
2.10. In Vivo Implantation
2.11. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of Gel–HPA
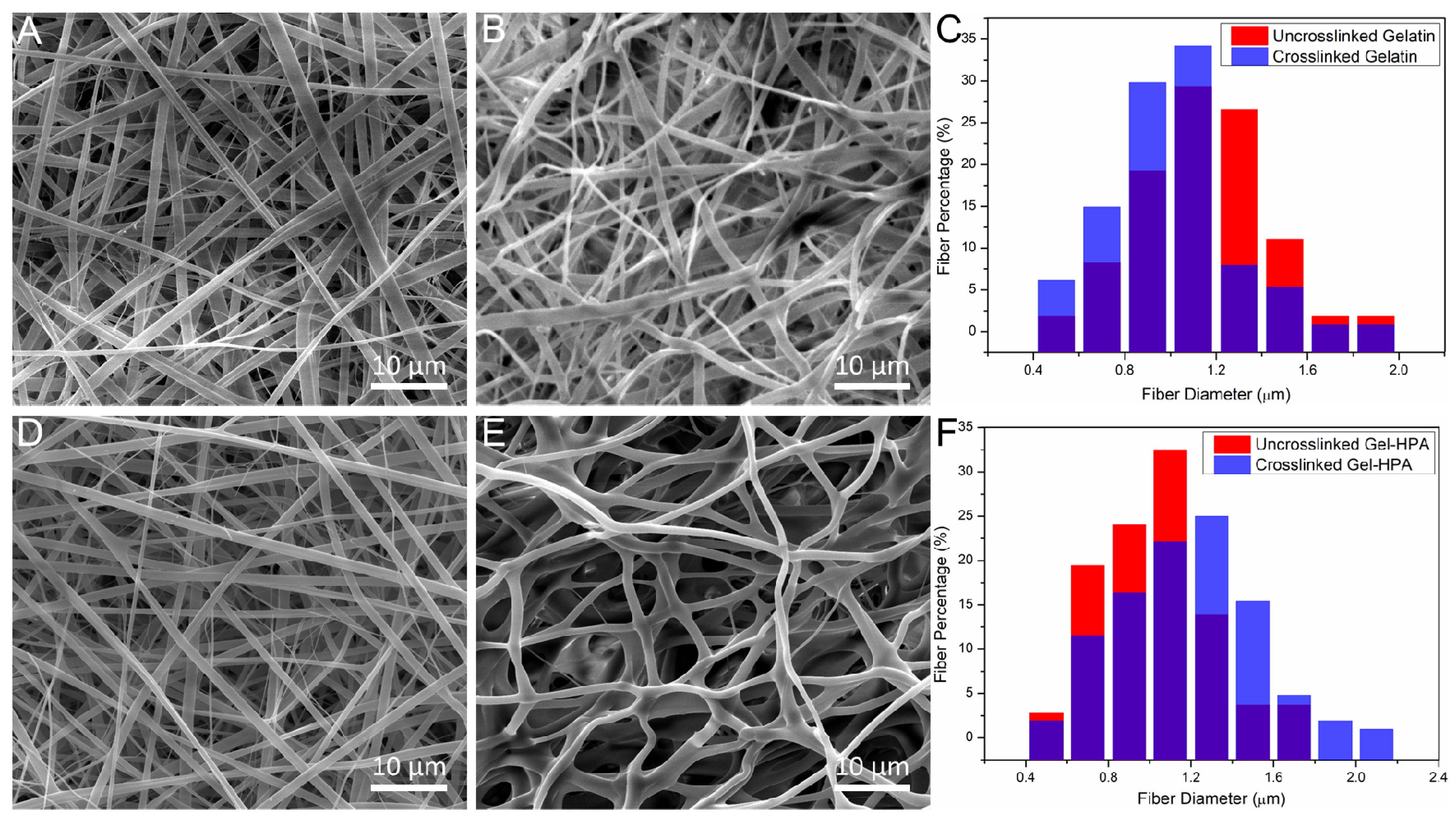
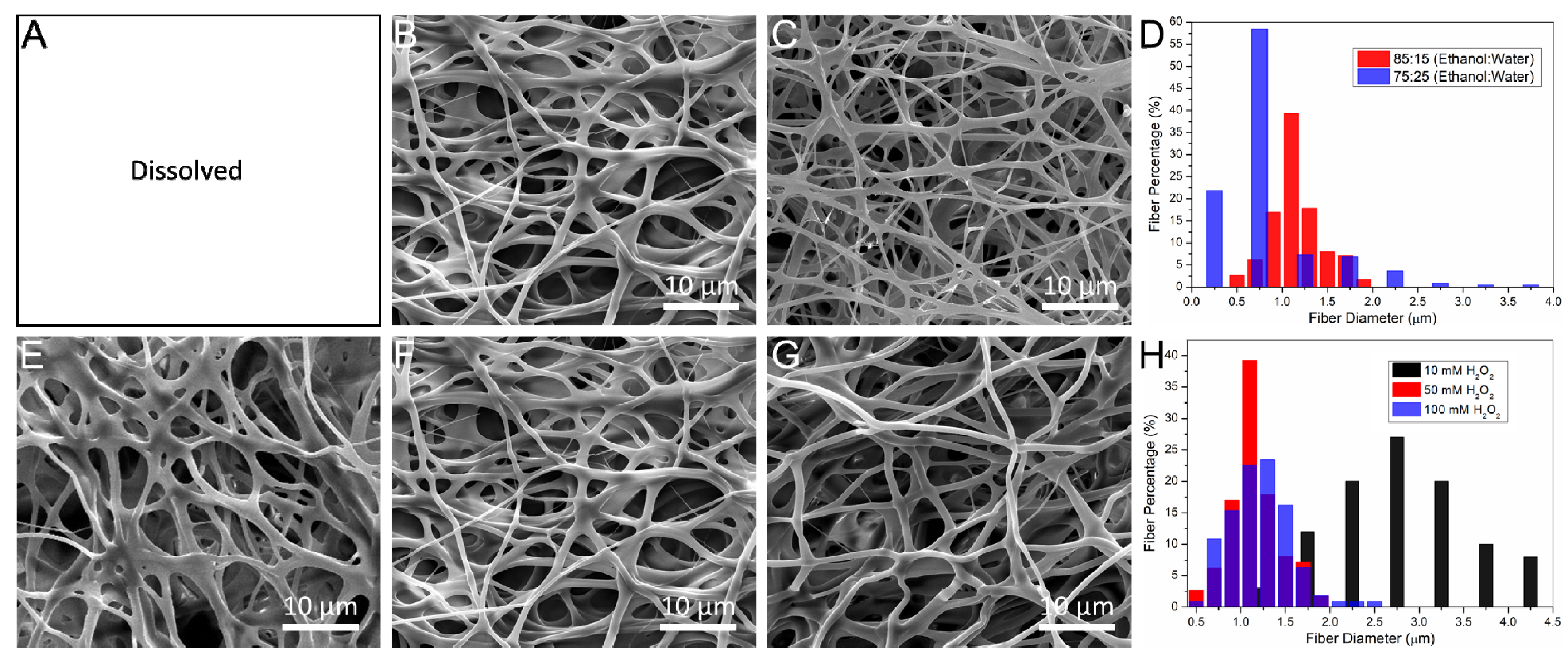
3.2. The Influence of Crosslinking Parameters on Morphology of Electrospun Scaffolds
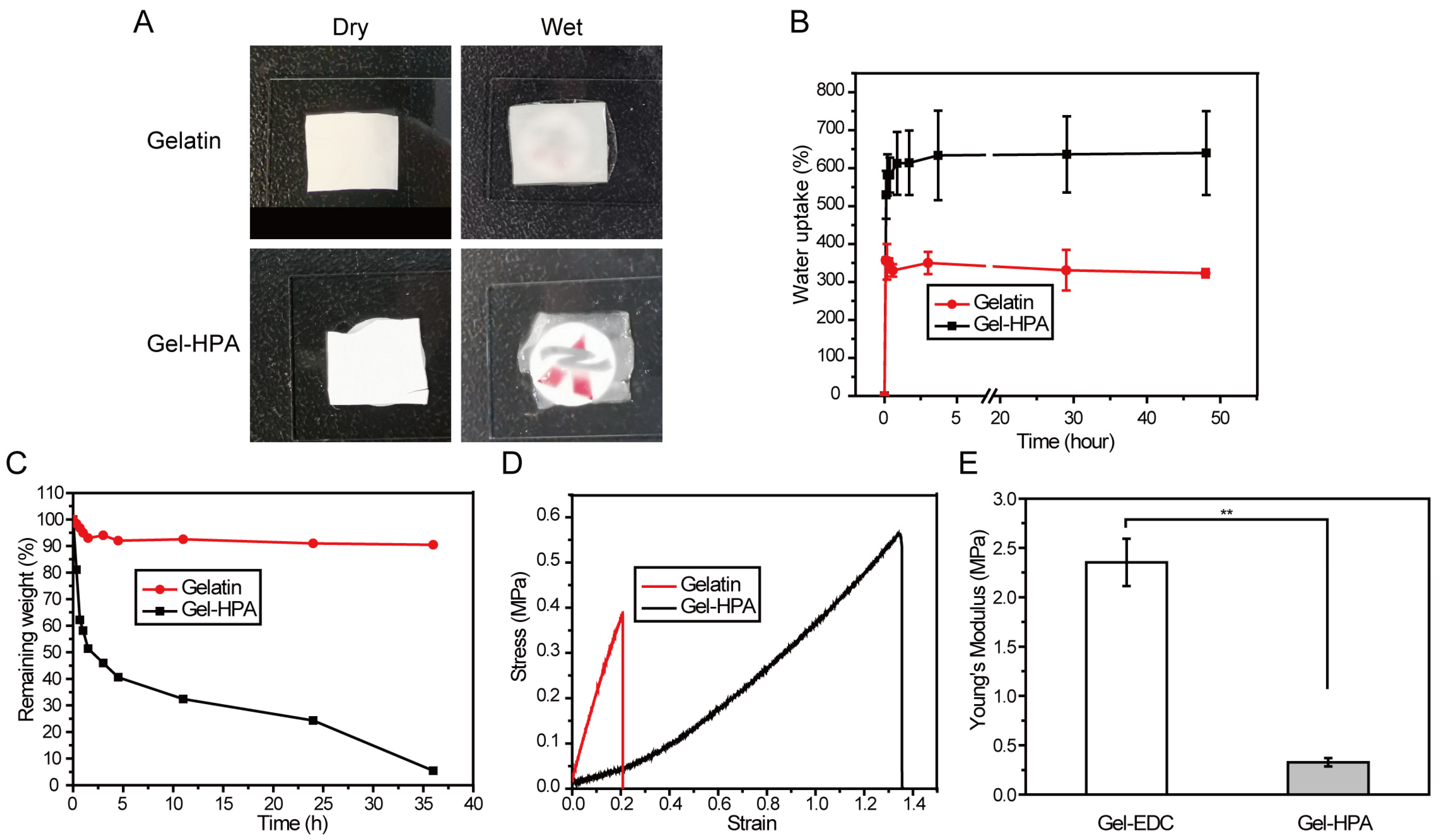
3.3. The Physical Properties of Electrospun Scaffolds
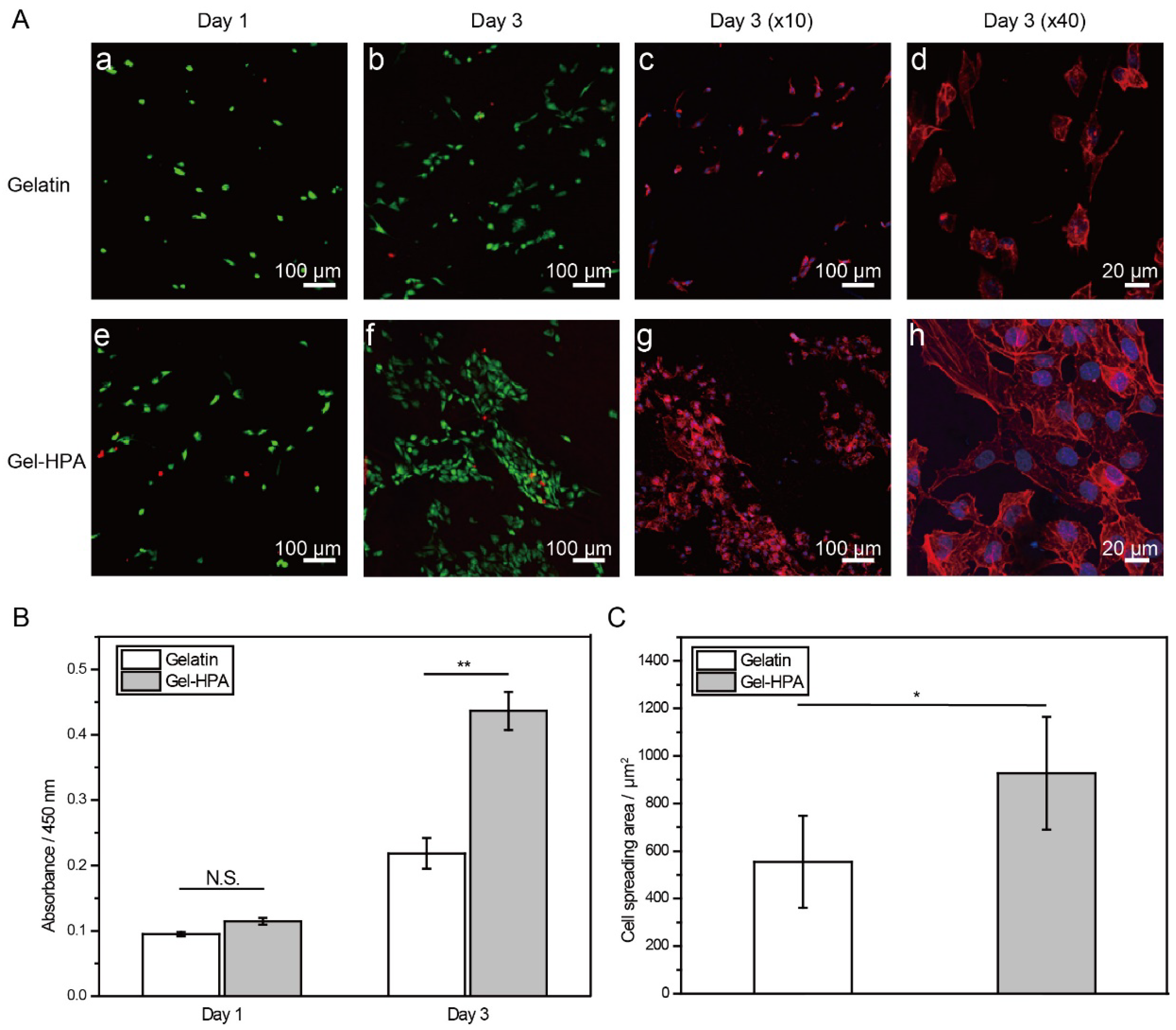
3.4. Cell Viability, Spreading and Proliferation on Electrospun Scaffolds
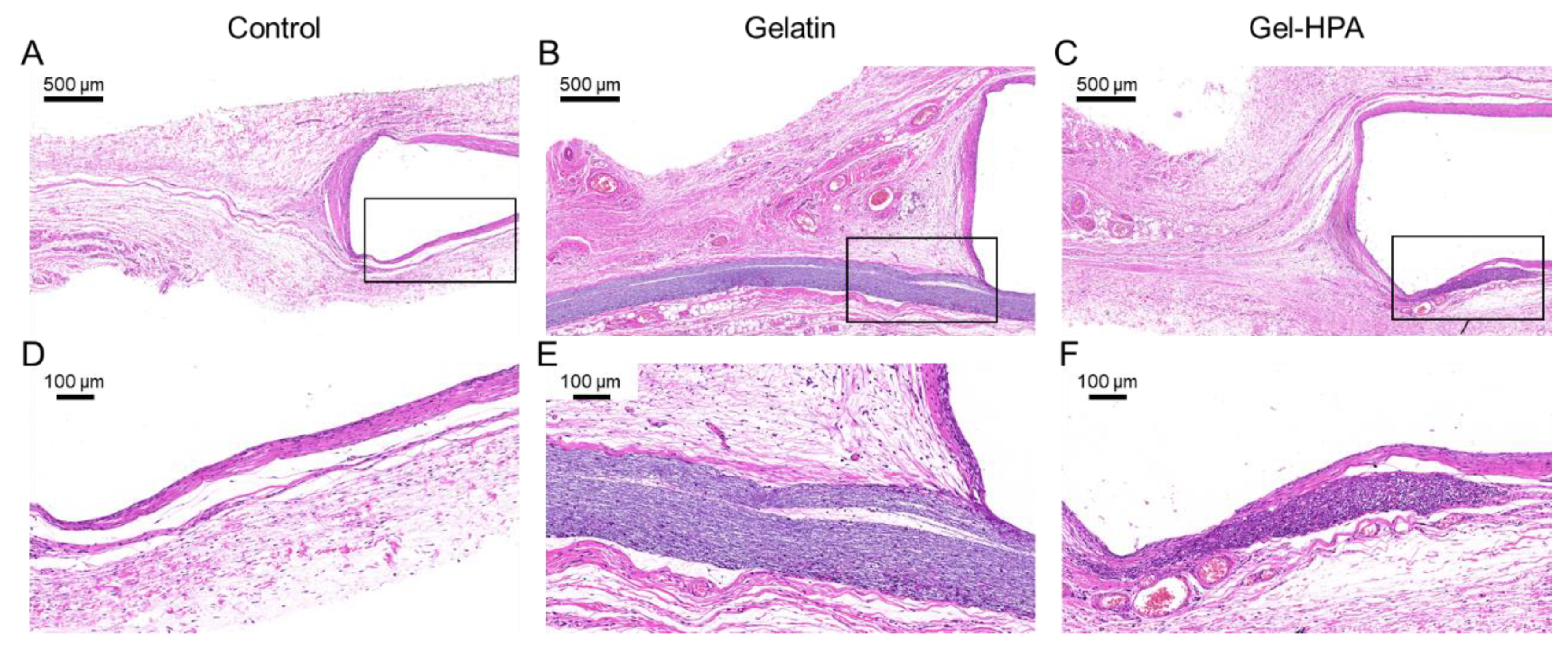
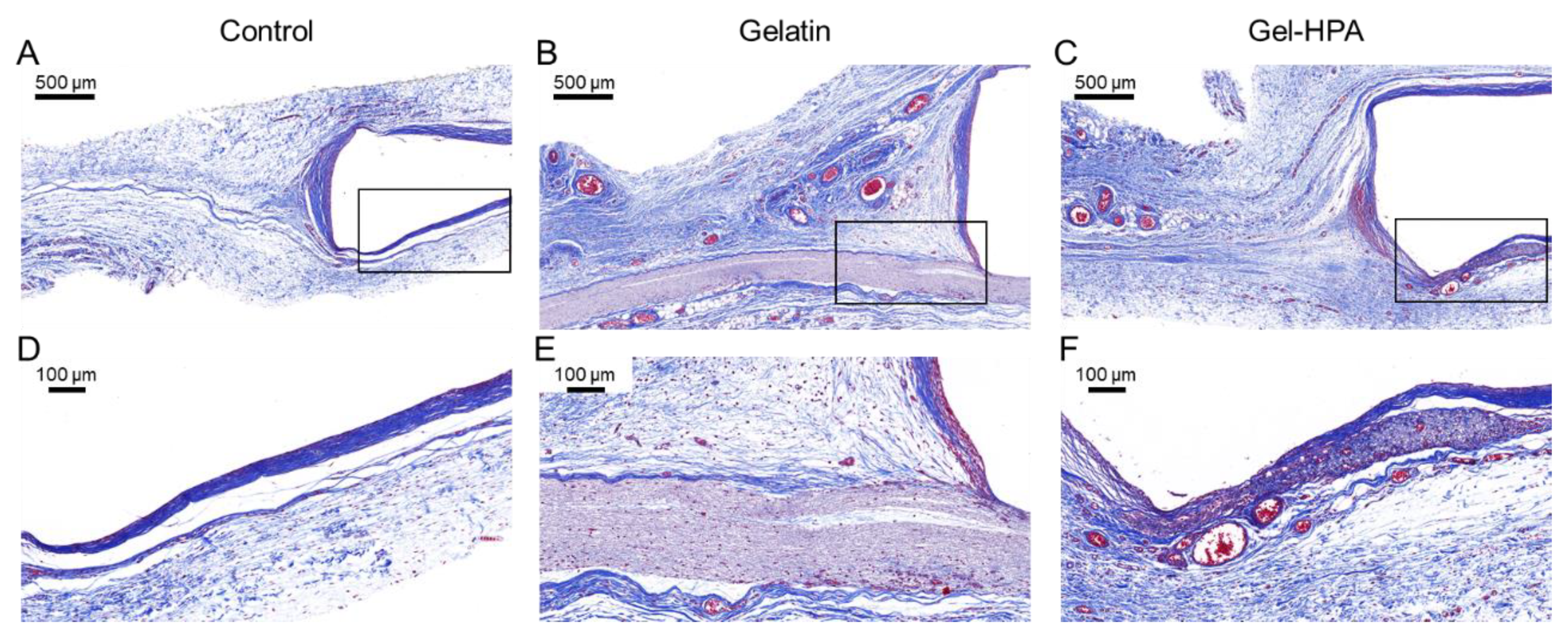
3.5. Histological Staining
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tytgat, L.; Van Damme, L.; Van Hoorick, J.; Declercq, H.; Thienpont, H.; Ottevaere, H.; Blondeel, P.; Dubruel, P.; Van Vlierberghe, S. Additive manufacturing of photo-crosslinked gelatin scaffolds for adipose tissue engineering. Acta Biomater. 2019, 94, 340–350. [Google Scholar] [CrossRef]
- Li, X.; Cho, B.; Martin, R.; Seu, M.; Zhang, C.; Zhou, Z.; Choi, J.S.; Jiang, X.; Chen, L.; Walia, G. Nanofiber-hydrogel composite–mediated angiogenesis for soft tissue reconstruction. Sci. Transl. Med. 2019, 11, eaau6210. [Google Scholar] [CrossRef]
- Patel, K.M.; Hill, L.M.; Gatti, M.E.; Nahabedian, M.Y. Management of massive mastectomy skin flap necrosis following autologous breast reconstruction. Ann. Plast. Surg. 2012, 69, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, B.; Ziolkowski, N.I.; Thoma, A.; Campbell, K.; O’Reilly, D.; Goeree, R. Safety of tissue expander/implant versus autologous abdominal tissue breast reconstruction in postmastectomy breast cancer patients: A systematic review and meta-analysis. Plast. Reconstr. Surg. 2014, 133, 234–249. [Google Scholar] [CrossRef] [PubMed]
- Largo, R.D.; Tchang, L.A.; Mele, V.; Scherberich, A.; Harder, Y.; Wettstein, R.; Schaefer, D.J. Efficacy, safety and complications of autologous fat grafting to healthy breast tissue: A systematic review. Plast. Reconstr. Surg. 2014, 67, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, Z.; Xu, Y.; Wang, X.; Xiao, Y.; Zhang, S.; Wang, J. Remodeling of inherent antimicrobial nanofiber dressings with melamine-modified fibroin into neoskin. J. Mater. Chem. B 2019, 7, 3412–3423. [Google Scholar] [CrossRef]
- Pérez-Madrigal, M.M.; Shaw, J.E.; Arno, M.C.; Hoyland, J.A.; Richardson, S.M.; Dove, A.P. Robust alginate/hyaluronic acid thiol–yne click-hydrogel scaffolds with superior mechanical performance and stability for load-bearing soft tissue engineering. Biomater. Sci. 2020, 8, 405–412. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, W.; Du, Y.; Xiao, Y.; Wang, X.; Zhang, S.; Wang, J.; Mao, C. Green Gas-Mediated Cross-Linking Generates Biomolecular Hydrogels with Enhanced Strength and Excellent Hemostasis for Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 13622–13633. [Google Scholar] [CrossRef]
- Lee, K.; Chen, Y.; Li, X.; Wang, Y.; Kawazoe, N.; Yang, Y.; Chen, G. Solution viscosity regulates chondrocyte proliferation and phenotype during 3D culture. J. Mater. Chem. B 2019, 7, 7713–7722. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Li, X.; Sun, Q.; Li, Q.; Kawazoe, N.; Chen, G. Functional hydrogels with tunable structures and properties for tissue engineering applications. Front. Chem. 2018, 6, 499. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Chen, Y.; Li, X.; Kawazoe, N.; Yang, Y.; Chen, G. Influence of viscosity on chondrogenic differentiation of mesenchymal stem cells during 3D culture in viscous gelatin solution-embedded hydrogels. J. Mater. Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Sun, X.; Zheng, R.; Cheng, L.; Zhao, X.; Jin, R.; Zhang, L.; Zhang, Y.; Zhang, Y.; Cui, W. Two-dimensional electrospun nanofibrous membranes for promoting random skin flap survival. RSC Adv. 2016, 6, 9360–9369. [Google Scholar] [CrossRef]
- Gloria, A.; Russo, T.; D’Amora, U.; Santin, M.; De Santis, R.; Ambrosio, L. Customised multiphasic nucleus/annulus scaffold for intervertebral disc repair/regeneration. Connect. Tissue Res. 2020, 61, 152–162. [Google Scholar] [CrossRef]
- Russo, T.; D’Amora, U.; Gloria, A.; Tunesi, M.; Sandri, M.; Rodilossi, S.; Albani, D.; Forloni, G.; Giordano, C.; Cigada, A. Systematic analysis of injectable materials and 3D rapid prototyped magnetic scaffolds: From CNS applications to soft and hard tissue repair/regeneration. Procedia Eng. 2013, 59, 233–239. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Kawazoe, N.; Chen, G. Influence of microporous gelatin hydrogels on chondrocyte functions. J. Mater. Chem. B 2017, 5, 5753–5762. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Yang, F. Recent progress in developing injectable matrices for enhancing cell delivery and tissue regeneration. Adv. Healthc. Mater. 2018, 7, 1701065. [Google Scholar] [CrossRef]
- Abalymov, A.; Parakhonskiy, B.; Skirtach, A.G. Polymer-and Hybrid-Based Biomaterials for Interstitial, Connective, Vascular, Nerve, Visceral and Musculoskeletal Tissue Engineering. Polymers 2020, 12, 620. [Google Scholar] [CrossRef]
- Wu, S.; Duan, B.; Qin, X.; Butcher, J.T. Living nano-micro fibrous woven fabric/hydrogel composite scaffolds for heart valve engineering. Acta Biomater. 2017, 51, 89–100. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, Y.; Liu, C. The horizon of materiobiology: A perspective on material-guided cell behaviors and tissue engineering. Chem. Rev. 2017, 117, 4376–4421. [Google Scholar] [CrossRef]
- De Lima, G.G.; Lyons, S.; Devine, D.M.; Nugent, M.J. Electrospinning of hydrogels for biomedical applications. In Hydrogels; Springer International Publishing: Cham, Switzerland, 2018; pp. 219–258. [Google Scholar]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xu, Y.; Li, Y.; Jia, L.; Mo, X.; Jiang, G.; Zhou, G. 3D printing electrospinning fiber-reinforced decellularized extracellular matrix for cartilage regeneration. Chem. Eng. J. 2020, 382, 122986. [Google Scholar] [CrossRef]
- Xu, F.; Sheardown, H.; Hoare, T. Reactive electrospinning of degradable poly (oligoethylene glycol methacrylate)-based nanofibrous hydrogel networks. Chem. Commun. 2016, 52, 1451–1454. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Ma, J.; Lv, D.; Liu, Q.; Jiao, W.; Wang, P. A core–shell fiber-constructed pH-responsive nanofibrous hydrogel membrane for efficient oil/water separation. J. Mater. Chem. A 2017, 5, 19398–19405. [Google Scholar] [CrossRef]
- Sheehy, E.; Cunniffe, G.; O’Brien, F. Collagen-based biomaterials for tissue regeneration and repair. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Elsevier: Aalborg, Denmark, 2018; pp. 127–150. [Google Scholar]
- Ming, J.; Li, M.; Han, Y.; Chen, Y.; Li, H.; Zuo, B.; Pan, F. Novel two-step method to form silk fibroin fibrous hydrogel. Mater. Sci Eng. C 2016, 59, 185–192. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, X.; Yildirimer, L.; Lang, Q.; Lin, Z.Y.W.; Zheng, R.; Zhang, Y.; Cui, W.; Annabi, N.; Khademhosseini, A. Cell infiltrative hydrogel fibrous scaffolds for accelerated wound healing. Acta Biomater. 2017, 49, 66–77. [Google Scholar] [CrossRef]
- Furuike, T.; Chaochai, T.; Okubo, T.; Mori, T.; Tamura, H. Fabrication of nonwoven fabrics consisting of gelatin nanofibers cross-linked by glutaraldehyde or N-acetyl-d-glucosamine by aqueous method. Int. J. Biol. Macromol. 2016, 93, 1530–1538. [Google Scholar] [CrossRef]
- Chou, S.-F.; Luo, L.J.; Lai, J.Y.; Ma, D.H.K. Role of solvent-mediated carbodiimide cross-linking in fabrication of electrospun gelatin nanofibrous membranes as ophthalmic biomaterials. Mater. Sci. Eng. C 2017, 71, 1145–1155. [Google Scholar] [CrossRef]
- Sun, X.; Lang, Q.; Zhang, H.; Cheng, L.; Zhang, Y.; Pan, G.; Zhao, X.; Yang, H.; Zhang, Y.; Santos, H.A. Electrospun photocrosslinkable hydrogel fibrous scaffolds for rapid in vivo vascularized skin flap regeneration. Adv. Funct. Mater. 2017, 27, 1604617. [Google Scholar] [CrossRef]
- Reyna-Urrutia, V.A.; Mata-Haro, V.; Cauich-Rodriguez, J.V.; Herrera-Kao, W.A.; Cervantes-Uc, J.M. Effect of two crosslinking methods on the physicochemical and biological properties of the collagen-chitosan scaffolds. Eur. Polym. J. 2019, 117, 424–433. [Google Scholar] [CrossRef]
- Gu, L.; Shan, T.; Ma, Y.-x.; Tay, F.R.; Niu, L. Novel biomedical applications of crosslinked collagen. Trends Biotechnol. 2019, 37, 464–491. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Lee, H.; Kim, H.S.; Kim, M.G.; Lee, L.P.; Lee, J.Y. Transdermal thiol–acrylate polyethylene glycol hydrogel synthesis using near infrared light. Nanoscale 2016, 8, 14213–14221. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ren, Y.; Wang, W.; Hao, H.; Tang, M.; Zhang, Z.; Yang, J.; Zheng, Y.; Shi, X. Transglutaminase-catalyzed encapsulation of individual mammalian cells with biocompatible and cytoprotective gelatin nanoshells. ACS Biomater. Sci. Eng. 2020, 6, 2336–2345. [Google Scholar] [CrossRef]
- Yao, M.; Zhang, J.; Gao, F.; Chen, Y.; Ma, S.; Zhang, K.; Liu, H.; Guan, F. New BMSC-laden gelatin hydrogel formed in situ by dual-enzymatic cross-linking accelerates dermal wound healing. ACS Omega 2019, 4, 8334–8340. [Google Scholar] [CrossRef] [PubMed]
- Dromel, P.C.; Singh, D.; Alexander-Katz, A.; Kurisawa, M.; Spector, M.; Young, M. Injectable gelatin hydroxyphenyl propionic acid hydrogel protects human retinal progenitor cells (hRPCs) from shear stress applied during small-bore needle injection. Appl. Mater. Today 2020, 19, 100602. [Google Scholar] [CrossRef]
- Hu, M.; Kurisawa, M.; Deng, R.; Teo, C.M.; Schumacher, A.; Thong, Y.-X.; Wang, L.; Schumacher, K.M.; Ying, J.Y. Cell immobilization in gelatin–hydroxyphenylpropionic acid hydrogel fibers. Biomaterials 2009, 30, 3523–3531. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Du, C.; Chung, J.E.; Kurisawa, M. Enzymatically cross-linked gelatin-phenol hydrogels with a broader stiffness range for osteogenic differentiation of human mesenchymal stem cells. Acta Biomater. 2012, 8, 1826–1837. [Google Scholar] [CrossRef]
- Wang, L.S.; Du, C.; Toh, W.S.; Wan, A.C.; Gao, S.J.; Kurisawa, M. Modulation of chondrocyte functions and stiffness-dependent cartilage repair using an injectable enzymatically crosslinked hydrogel with tunable mechanical properties. Biomaterials 2014, 35, 2207–2217. [Google Scholar] [CrossRef]
- Liu, H.Y.; Korc, M.; Lin, C.C. Biomimetic and enzyme-responsive dynamic hydrogels for studying cell-matrix interactions in pancreatic ductal adenocarcinoma. Biomaterials 2018, 160, 24–36. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Gimeno-Alcaniz, J.V.; Ocio, M.J.; Lagaron, J.M. Comparative performance of electrospun collagen nanofibers cross-linked by means of different methods. ACS Appl. Mater. Interfaces 2009, 1, 218–223. [Google Scholar] [CrossRef]
- Chen, C.; Tang, J.; Gu, Y.; Liu, L.; Liu, X.; Deng, L.; Martins, C.; Sarmento, B.; Cui, W.; Chen, L. Bioinspired hydrogel electrospun fibers for spinal cord regeneration. Adv. Funct. Mater. 2019, 29, 1806899. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Li, J.; Wang, X.; Zhang, J.; Kawazoe, N.; Chen, G. 3D culture of chondrocytes in gelatin hydrogels with different stiffness. Polymers 2016, 8, 269. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.; Kawazoe, N.; Chen, G. Fabrication of highly crosslinked gelatin hydrogel and its influence on chondrocyte proliferation and phenotype. Polymers 2017, 9, 309. [Google Scholar] [CrossRef]
- He, H.; Xiao, Z.; Zhou, Y.; Xuan, X.; Li, Y.; Zheng, J.; Xiao, J.; Wu, J.; Chen, A. Zwitterionic poly(sulfobetaine methacrylate) hydrogels with optimal mechanical properties for improving wound healing in vivo. J. Mater. Chem. B 2019, 7, 1697–1707. [Google Scholar] [CrossRef]
- Chwalek, K.; Levental, K.R.; Tsurkan, M.V.; Zieris, A.; Freudenberg, U.; Werner, C. Two-tier hydrogel degradation to boost endothelial cell morphogenesis. Biomaterials 2011, 32, 9649–9657. [Google Scholar] [CrossRef] [PubMed]
- Hanjaya-Putra, D.; Wong, K.T.; Hirotsu, K.; Khetan, S.; Burdick, J.A.; Gerecht, S. Spatial control of cell-mediated degradation to regulate vasculogenesis and angiogenesis in hyaluronan hydrogels. Biomaterials 2012, 33, 6123–6131. [Google Scholar] [CrossRef]
- Li, L.; Bae, K.H.; Ng, S.; Yamashita, A.; Kurisawa, M. Peroxidase-immobilized porous silica particles for in situ formation of peroxidase-free hydrogels with attenuated immune responses. Acta Biomater. 2018, 81, 103–114. [Google Scholar] [CrossRef]
- Loebel, C.; Szczesny, S.E.; Cosgrove, B.D.; Alini, M.; Zenobi-Wong, M.; Mauck, R.L.; Eglin, D. Cross-Linking Chemistry of Tyramine-Modified Hyaluronan Hydrogels Alters Mesenchymal Stem Cell Early Attachment and Behavior. Biomacromolecules 2017, 18, 855–864. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Yao, D.; Jiang, J.; Guo, X.; Gao, Y.; Li, Q.; Shen, C. Effects of aligned and random fibers with different diameter on cell behaviors. Colloids Surf. B 2018, 171, 461–467. [Google Scholar] [CrossRef]
- Shi, F.; Wang, Y.C.; Hu, Z.B.; Xu, H.Y.; Sun, J.; Gao, Y.; Li, X.T.; Yang, C.B.; Xie, C.; Li, C.F. Simulated microgravity promotes angiogenesis through RhoA-dependent rearrangement of the actin cytoskeleton. Cell. Physiol. Biochem. 2017, 41, 227–238. [Google Scholar] [CrossRef]
- Wu, J.; He, C.; He, H.; Cheng, C.; Zhu, J.; Xiao, Z.; Zhang, H.; Li, X.; Zheng, J.; Xiao, J. Importance of zwitterionic incorporation into polymethacrylate-based hydrogels for simultaneously improving optical transparency, oxygen permeability, and antifouling properties. J. Mater. Chem B 2017, 5, 4595–4606. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.; Hubbell, J.A. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials 2010, 31, 7836–7845. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, H.; He, J.; Cheng, R.; Cao, Y.; Che, K.; Cheng, L.; Zhang, L.; Pan, G.; Ni, P. Adjustable hardness of hydrogel for promoting vascularization and maintaining stemness of stem cells in skin flap regeneration. Appl. Mater. Today 2018, 13, 54–63. [Google Scholar] [CrossRef]
- Wade, R.J.; Bassin, E.J.; Rodell, C.B.; Burdick, J.A. Protease-degradable electrospun fibrous hydrogels. Nat. Commun. 2015, 6, 1–10. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, K.; Han, S.; Yang, J.; Sun, Q.; Wang, X.; Li, X.; Li, Q. Enzyme-Crosslinked Electrospun Fibrous Gelatin Hydrogel for Potential Soft Tissue Engineering. Polymers 2020, 12, 1977. https://doi.org/10.3390/polym12091977
Nie K, Han S, Yang J, Sun Q, Wang X, Li X, Li Q. Enzyme-Crosslinked Electrospun Fibrous Gelatin Hydrogel for Potential Soft Tissue Engineering. Polymers. 2020; 12(9):1977. https://doi.org/10.3390/polym12091977
Chicago/Turabian StyleNie, Kexin, Shanshan Han, Jianmin Yang, Qingqing Sun, Xiaofeng Wang, Xiaomeng Li, and Qian Li. 2020. "Enzyme-Crosslinked Electrospun Fibrous Gelatin Hydrogel for Potential Soft Tissue Engineering" Polymers 12, no. 9: 1977. https://doi.org/10.3390/polym12091977
APA StyleNie, K., Han, S., Yang, J., Sun, Q., Wang, X., Li, X., & Li, Q. (2020). Enzyme-Crosslinked Electrospun Fibrous Gelatin Hydrogel for Potential Soft Tissue Engineering. Polymers, 12(9), 1977. https://doi.org/10.3390/polym12091977