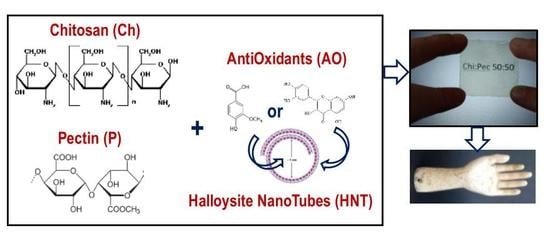
Bionanocomposite Films Containing Halloysite Nanotubes and Natural Antioxidants with Enhanced Performance and Durability as Promising Materials for Cultural Heritage Protection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- -
- Chitosan (Ch), low viscosity, deacetylation degree = 75–85% and average molecular weight = 120 kg·mol−1;
- -
- Pectin (P) from Citrus, Poly-D-galacturonic acid methyl ester with degree of methyl esterification 24%, Mw = 30–100 kg·mol−1;
- -
- Halloysite nanoclay (HNT), [Al2Si2O5(OH)4] × 2H2O, formula weight = 294.19 g·mol−1; pore volume = 1.26–1.34 mL/gm; pH = 4.5–7.0; diameter = 30–70 nm; length = 1–3 microns;
- -
- 4-Hydroxy-3-methoxybenzoic acid, named vanillic Acid (VA), Molecular Weight = 168.15 g·mol−1;
- -
- 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one hydrate, named quercetin (Q), molecular weight = 302.24 g·mol−1 (anhydrous basis).
2.2. Loading of Halloysite Nanotubes with Antioxidants
2.3. Preparation of Bio-Nanocomposite Films
2.4. Characterizations
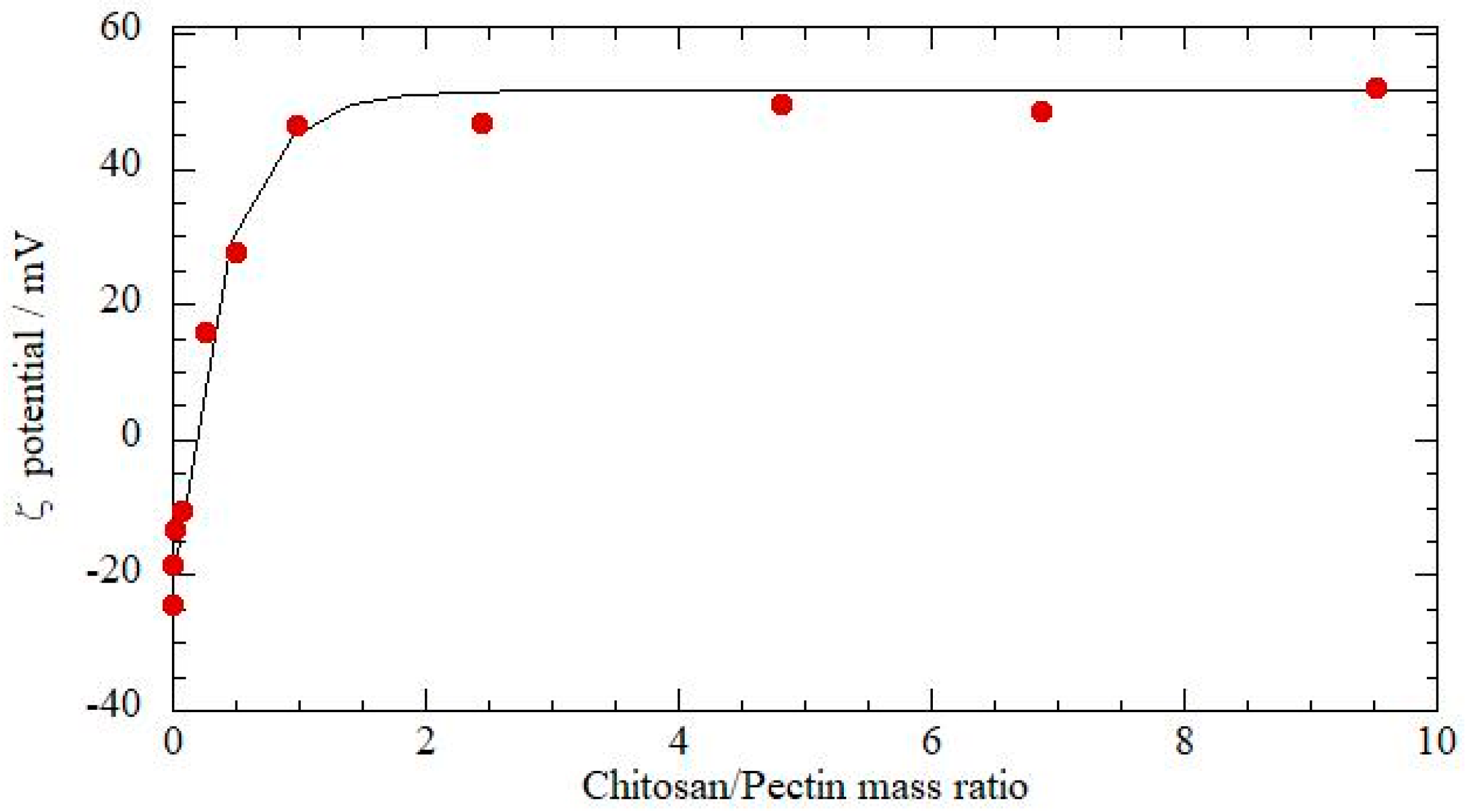
2.4.1. ζ-Potential and Isoelectric Point
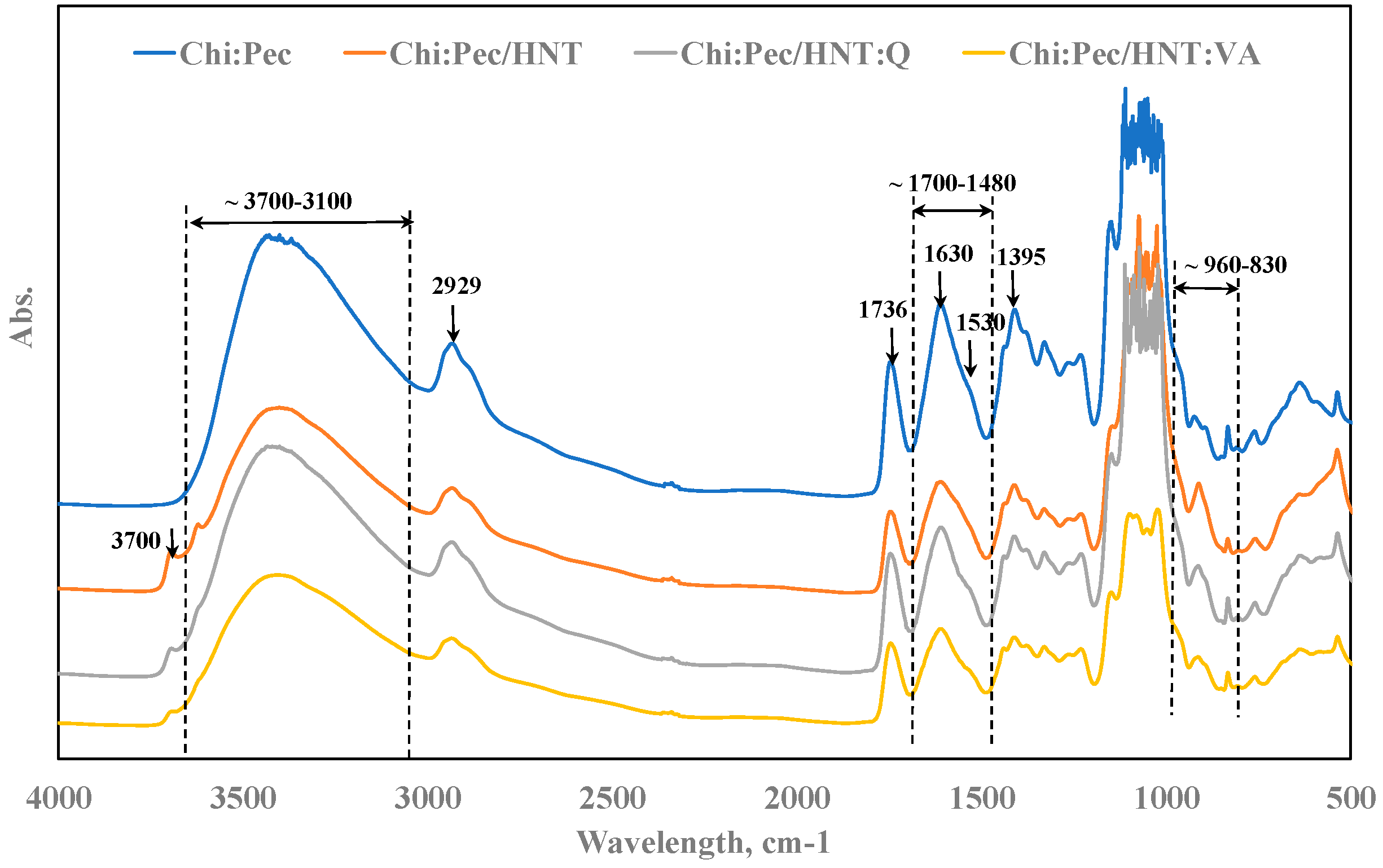
2.4.2. FT-IR Analysis
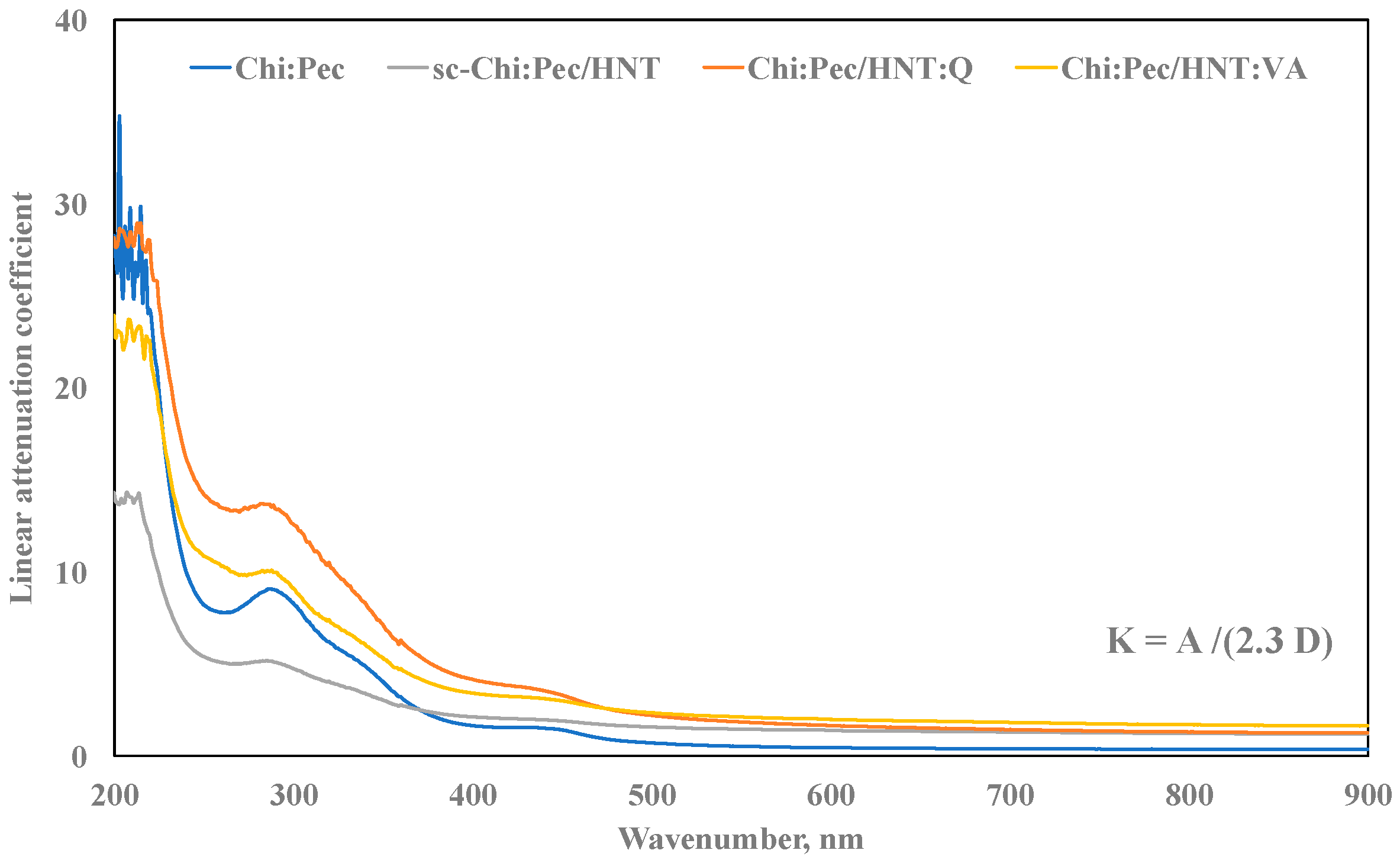
2.4.3. UV–visible Analysis
2.4.4. Contact Angle Measurements
2.4.5. Mechanical Characterization
2.4.6. Optical Observations
2.5. Photo-Oxidation Exposure
2.6. Solubility Theoretical Calculation
| Expressions for δ and δ-components | , B = 277, , , |
| Additive molar functions | Ft = ΣNiFt,i, Fp = ΣNiFp,i, V = ΣNiVi, ΔT (P) = ΣNi ΔT T,i (P), |
| Auxiliary equations | α(P) = 777 ΔT(P)/V, n = 0.5/ΔT(P), |
- Ft is the molar attraction function and Fp its polar contribution;
- V is the molar volume of the solvent molecule or the structural unit of the polymer;
- ΔT is the Lyndersen correction for non-ideal behavior, α is the molecular aggregation number;
- n is the number of repeating units per effective chain segment of the polymer;
- δtot is the solubility parameter;
- δp is the contribution of the polar forces;
- δh is the contribution of the hydrogen bonding;
- δd is the contribution of the dispersion forces.
3. Results and Discussion
3.1. Theoretical Solubility and z-Potential Estimation
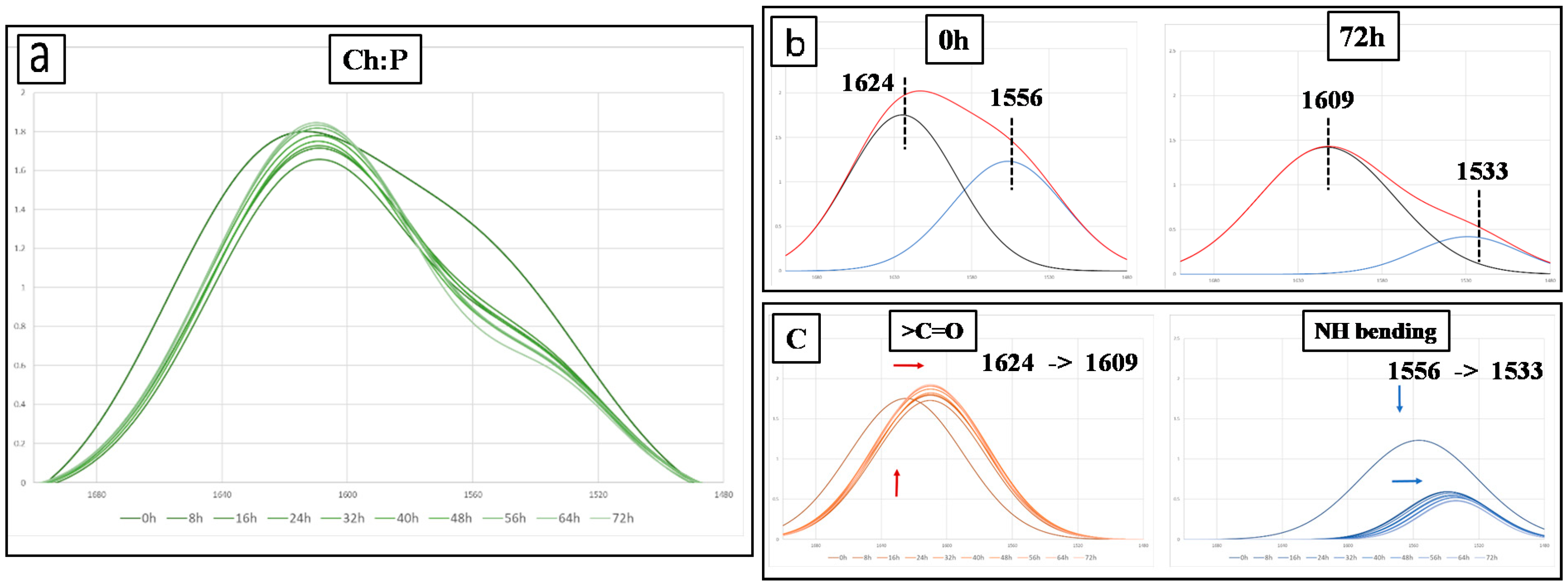
3.2. Spectroscopy and Contact Angle Analysis
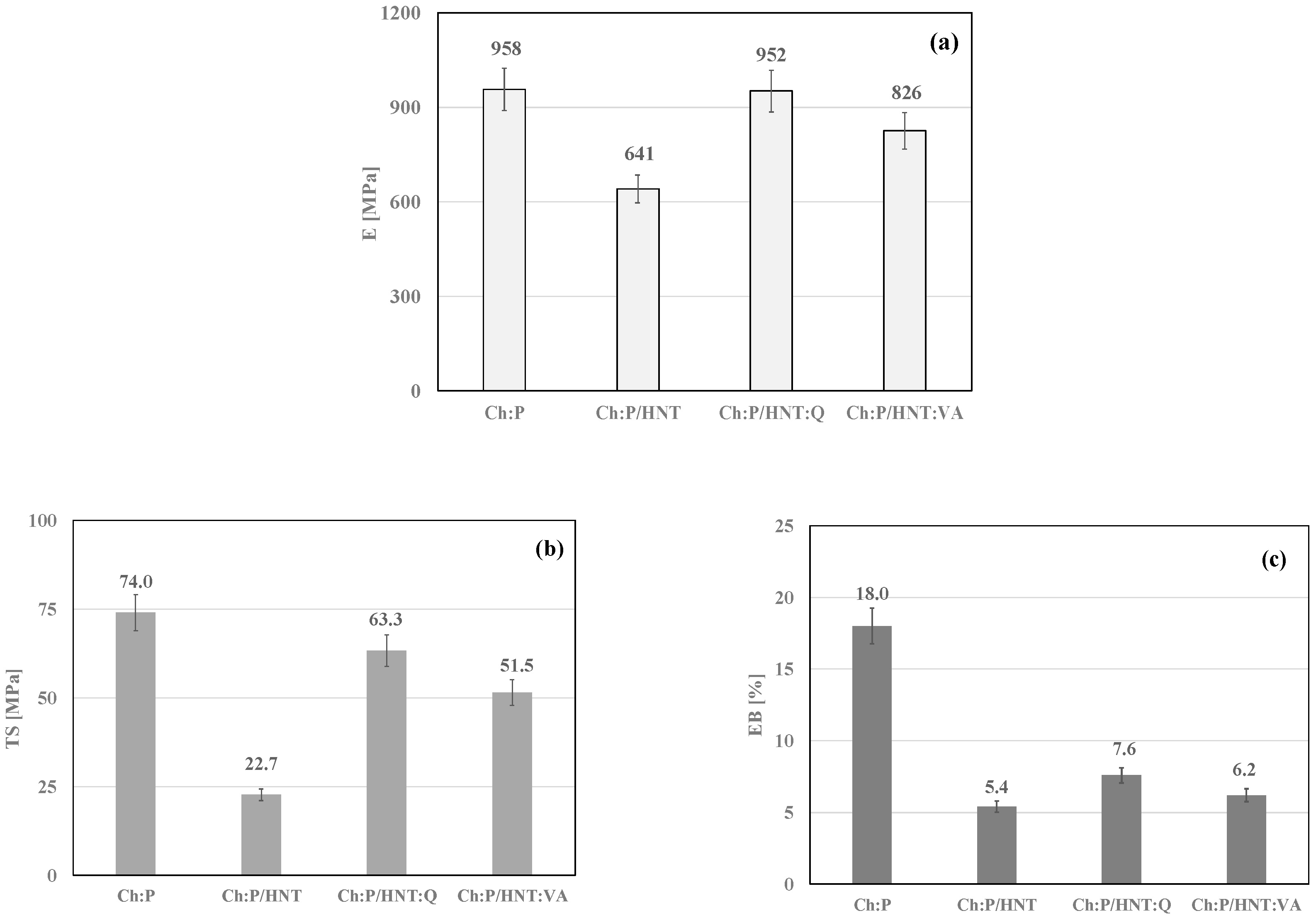
3.3. Mechanical Behavior and Optical Observations
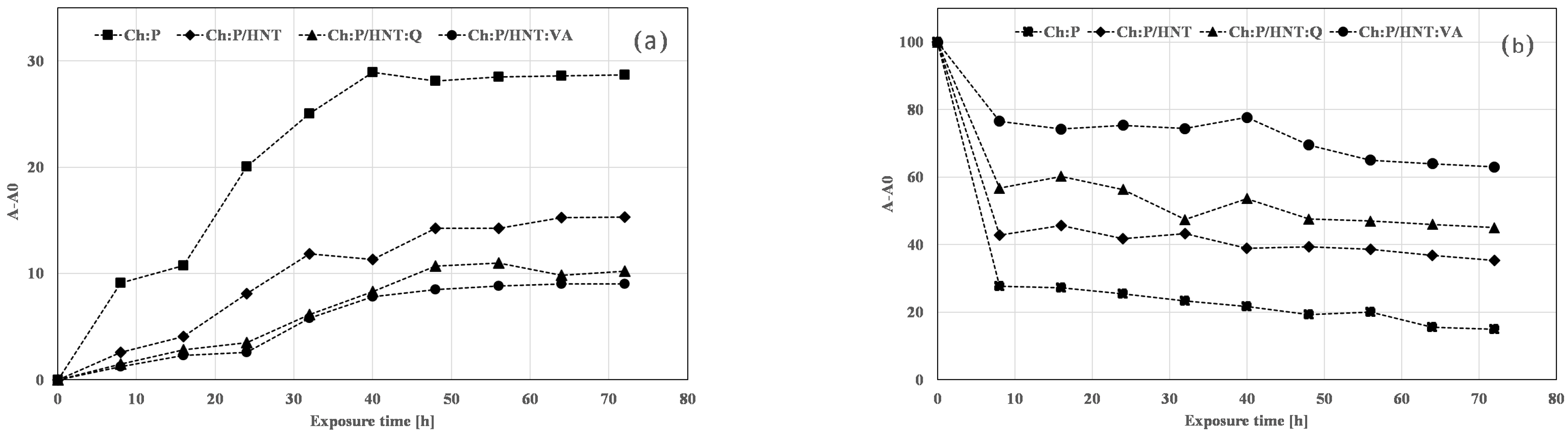
3.4. Photo-Oxidation Resistance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Baglioni, P.; Chelazzi, D.; Giorgi, R.; Poggi, G. Colloid and materials science for the conservation of cultural heritage: Cleaning, consolidation, and deacidification. Langmuir 2013, 29, 5110–5122. [Google Scholar] [CrossRef] [PubMed]
- Chelazzi, D.; Poggi, G.; Jaidar, Y.; Toccafondi, N.; Giorgi, R.; Baglioni, P. Hydroxide nanoparticles for cultural heritage: Consolidation and protection of wall paintings and carbonate materials. J. Colloid Interface Sci. 2013, 392, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Fernandez, A.; Gomez-Villalba, L.S.; Rabanal, M.E.; Forta, R. New nanomaterials for applications in conservation and restoration of stony materials: A review. Mater. De Constr. 2017, 67, 107. [Google Scholar] [CrossRef]
- David, M.E.; Ion, R.M.; Grigorescu, R.M.; Iancu, L.; Andrei, E.R. Nanomaterials Used in Conservation and Restoration of Cultural Heritage: An Up-to-Date Overview. Materials 2020, 13, 2064. [Google Scholar] [CrossRef]
- Gross, R.A.; Kalra, B. Biodegradable polymers for the environment. Science 2002, 297, 803–807. [Google Scholar] [CrossRef]
- Basrioli, C. (Ed.) Handbook of Biodegradable Polymers; Rapra Technol. Ltd.: Shropshire, UK, 2005. [Google Scholar]
- Ebnesajjad, S. (Ed.) Handbook of Biopolymers and Biodegradable Plastics; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Luzi, F.; Torre, L.; Kenny, J.M.; Puglia, D. Bio- and Fossil-Based Polymeric Blends and Nanocomposites for Packaging: Structure–Property Relationship. Materials 2019, 12, 471. [Google Scholar] [CrossRef]
- Darroudi, M.; Bagherpour, M.; Hossein, H.A.; Ebrahimi, M. Biopolymer-assisted green synthesis and characterization of calcium hydroxide nanoparticles. Ceram. Int. 2016, 42, 3816–3819. [Google Scholar] [CrossRef]
- Andreotti, S.; Franzoni, E.; Degli Esposti, M.; Fabbri, P. Poly(hydroxyalkanoate)s-Based Hydrophobic Coatings for the Protection of Stone in Cultural Heritage. Materials 2018, 11, 165. [Google Scholar] [CrossRef]
- Valentini, F.; Carbone, M.; Palleschi, G. Carbon nanostructured materials for applications in nano-medicine, cultural heritage, and electrochemical biosensors. Anal. Bioanal. Chem. 2013, 405, 451–465. [Google Scholar] [CrossRef]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. A review of the recent developments in biocomposites based on natural fibres and their application perspectives. Compos. Part A Appl. Sci. Manuf. 2015, 77, 1–25. [Google Scholar] [CrossRef]
- Sun, M.; Wang, T.; Pang, J.; Chen, X.; Liu, Y. Hydroxybutyl Chitosan Centered Biocomposites for Potential Curative Applications: A Critical Review. Biomacromolecules 2020, 21, 1351–1367. [Google Scholar] [CrossRef] [PubMed]
- Abdul Khalil, H.P.S.; Chaturbhuj, K.S.; Adnan, A.S.; Fazita, M.N.; Syakir, M.I.; Davoudpour, Y.; Rafatullah, M.; Abdullah, K.C.; Haafiza, M.K.M.; Dungani, R. A review on chitosan-cellulose blends and nanocellulose reinforced chitosan biocomposites: Properties and their applications. Carbohydr. Polym. 2016, 150, 216–226. [Google Scholar] [CrossRef]
- Julkapli, M.N.; Akil, H.M.D.; Ahmad, Z. Preparation, Properties and Applications of Chitosan-Based Biocomposites/Blend Materials: A Review. Compos. Interfaces 2011, 18, 6. [Google Scholar] [CrossRef]
- Martău, G.A.; Mihai, M.; Vodnar, D.C. The Use of Chitosan, Alginate, and Pectin in the Biomedical and Food Sector—Biocompatibility, Bioadhesiveness and Biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef]
- Branca, C.; D’Angelo, G.; Crupi, C.; Khouzami, K.; Rifici, S.; Ruello, G.; Wanderlingh, U. Role of the OH and NH vibrational groups in polysaccharide-nanocomposite interactions: A FTIR-ATR study on chitosan andchitosan/clayfilms. Polymer 2016, 99, 614–622. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengíbar, M.; Harris, R.; Paños, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, A. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar] [CrossRef]
- Epure, V.; Griffon, M.; Pollet, E.; Avérous, L. Structure and properties of glycerol-plasticized chitosan obtained by mechanical kneading. Carbohydr. Polym. 2011, 83, 947–952. [Google Scholar] [CrossRef]
- Sun, S.; Wang, Y.; Li, L.; Huang, Z.; Zhou, H. Thermoplastic biomass transparent films directly fabricated by chitosan nanospheres. Polymer 2020, 192, 122335. [Google Scholar] [CrossRef]
- Yapo, B.M. Pectin Substances: From Simple Pectic Polysaccharides to Complex Pectins-A New Hypothetical Model. Carbohydr. Polym. 2011, 86, 373–385. [Google Scholar] [CrossRef]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and Pectin-Based Composite Materials: Beyond Food Texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef]
- Fraeye, I.; Doungla, E.; Duvetter, T.; Moldenaers, P.; Loey, A.V.; Hendrickx, M. Influence of Intrinsic and Extrinsic Factors on Rheology of Pectin–calcium Gels. Food Hydrocoll. 2009, 23, 2069–2077. [Google Scholar] [CrossRef]
- Younis, H.G.R.; Zhao, G. Physicochemical properties of the edible films from the blends of high methoxyl apple pectin and chitosan. Int. J. Biol. Macromol. 2019, 131, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lei, Y.; Lu, Y.; Zhu, R.; Xiao, D.; Jiao, C.; Xia, R.; Zhang, Z.; Shen, G.; Liu, Y.; et al. Effect of citric acid induced crosslinking on the structure and properties of potato starch/chitosan composite films. Food Hydrocoll. 2019, 97, 105208. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, G.; Lei, M.; Guo, Y.; Song, Y.; Lu, T.; Wang, Y. Multi-scale investigation on the phase miscibility of polylactic acid/o-carboxymethyl chitosan blends. Polymer 2019, 176, 159–167. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; Infurna, G.; Baiamonte, M.; D’Anna, F. Natural compounds as sustainable additives for biopolymers. Polymers 2020, 12, 732. [Google Scholar] [CrossRef]
- Corrasi, G. Dispersion of halloysite loaded with natural antimicrobials into pectins: Characterization and controlled release analysis. Carbohydr. Polym. 2015, 127, 47–53. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Effects of halloysite content on the thermo-mechanical performances of composite bioplastics. Appl. Clay Sci. 2020, 185, 105416. [Google Scholar] [CrossRef]
- Singh, A.A.; Sharma, S.; Srivastava, M.; Majumdar, A. Modulating the Properties of Polylactic Acid for Packaging Applications Using Biobased Plasticizers and Naturally Obtained Fillers. Int. J. Biol. Macromol. 2020, 153, 1165–1175. [Google Scholar] [CrossRef]
- Liu, M.; Wu, C.; Jiao, Y.; Xiong, S.; Zhou, C. Chitosan–halloysite nanotubes nanocomposite scaffolds for tissue engineering. J. Mater. Chem. B 2013, 1, 2078–2089. [Google Scholar] [CrossRef]
- Naumenko, E.; Fakhrullin, R. Halloysite Nanoclay/Biopolymers Composite Materials in Tissue Engineering. Biotechnol. J. 2019, 14, 1900055. [Google Scholar] [CrossRef]
- Suner, S.S.; Demirci, S.; Yetiskin, B.; Fakhrullin, R.; Naumenko, E.; Okay, O.; Ayyala, R.S.; Sahiner, N. Cryogel Composites Based on Hyaluronic Acid and Halloysite Nanotubes as Scaffold for Tissue Engineering. Int. J. Biol. Macromol. 2019, 130, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Huang, K.; Yang, F.; Wang, R.; Han, L.; Yu, H.; Ye, Z.; Wu, F. Chitosan Nanocomposite Films Based on Halloysite Nanotubes Modification for Potential Biomedical Applications. J. Mater. Chem. B 2020, 151, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Bugatti, V.; Sorrentino, A.; Gorrasi, G. Encapsulation of Lysozyme into halloysite nanotubes and dispersion in PLA: Structural and physical properties and controlled release analysis. Eur. Polym. J. 2017, 93, 495–506. [Google Scholar] [CrossRef]
- Liu, M.; Fakhrullin, R.; Novikov, A.; Panchal, A.; Lvov, Y. Tubule Nanoclay-Organic Heterostructures for Biomedical Applications. Macromol. Biosci. 2019, 19, 1800419. [Google Scholar] [CrossRef] [PubMed]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Layered composite based on halloysite and natural polymers: A carrier for the pH controlled release of drugs. New J. Chem. 2019, 43, 10887–10893. [Google Scholar] [CrossRef]
- Liu, F.; Bai, L.; Zhang, H.; Song, H.; Hu, L.; Wu, Y.; Ba, X. Smart H2O2-Responsive Drug Delivery System Made by Halloysite Nanotubes and Carbohydrate Polymers. ACS Appl. Mater. Interfaces 2017, 9, 31626–31633. [Google Scholar] [CrossRef] [PubMed]
- Gorrasi, G.; Bugatti, V.; Ussia, M.; Mendichi, R.; Zampino, D.; Puglisi, C.; Carroccio, S.C. Halloysite nanotubes and thymol as photo protectors of biobased polyamide 11. Polym. Degrad. Stab. 2018, 152, 43–51. [Google Scholar] [CrossRef]
- Cavallaro, G.; Milioto, S.; Konnova, S.; Fakhrullina, G.; Akhatova, F.; Lazzara, G. Halloysite/Keratin Nanocomposite for Human Hair Photoprotection Coating. ACS Appl. Mater. Interfaces 2020, 12, 24348–24362. [Google Scholar] [CrossRef]
- Cavallaro, G.; Milioto, S.; Nigamatzyanova, L.; Akhatova, F.; Fakhrullin, R.; Lazzara, G. Pickering Emulsion Gels Based on Halloysite Nanotubes and Ionic Biopolymers: Properties and Cleaning Action on Marble Surface. ACS Appl. Nano Mater. 2019, 2, 3169–3176. [Google Scholar] [CrossRef]
- Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite Nanotubes: Interfacial Properties and Applications in Cultural Heritage. Langmuir 2020, 36, 3677–3689. [Google Scholar] [CrossRef]
- Pasbakhsh, P.; Churchman, G.J.; Keeling, J.L. Characterisation of properties of various halloysites relevant to their use as nanotubes and microfibre fillers. Appl. Clay Sci. 2013, 74, 47–57. [Google Scholar] [CrossRef]
- Cavallaro, G.; Chiappisi, L.; Pasbakhsh, P.; Gradzielski, M.; Lazzara, G. A structural comparison of halloysite nanotubes of different origin by Small-Angle Neutron Scattering (SANS) and Electric Birefringence. Appl. Clay Sci. 2018, 160, 71–80. [Google Scholar] [CrossRef]
- Liu, Y.; Guan, H.; Zhang, J.; Zhao, Y.; Yang, J.-H.; Zhang, B. Polydopamine-coated halloysite nanotubes supported AgPd nanoalloy: An efficient catalyst for hydrolysis of ammonia borane. Int. J. Hydrog. Energy 2018, 43, 2754–2762. [Google Scholar] [CrossRef]
- Sadjadi, S.; Heravi, M.M.; Kazemi, S.S. Ionic liquid decorated chitosan hybridized with clay: A novel support for immobilizing Pd nanoparticles. Carbohydr. Polym. 2018, 200, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhou, X.; Yang, J.; Gao, X.; Yin, L.; Zhao, Y.; Zhang, B. Encapsulation of Ammonia Borane in Pd/Halloysite Nanotubes for Efficient Thermal Dehydrogenation. ACS Sustain. Chem. Eng. 2020, 8, 2122–2129. [Google Scholar] [CrossRef]
- Sua, Z.; Zhanga, H.; Gao, Y.; Huo, L.; Wu, Y.; Ba, X. Coumarin-anchored halloysite nanotubes for highly selective detection and removal of Zn(II). Chem. Eng. J. 2020, 393, 124695. [Google Scholar] [CrossRef]
- Von Klitzing, R.; Stehl, D.; Pogrzeba, T.; Schomäcker, R.; Minullina, R.; Panchal, A.; Konnova, S.; Fakhrullin, R.; Koetz, J.; Möhwald, H.; et al. Halloysites Stabilized Emulsions for Hydroformylation of Long Chain Olefins. Adv. Mater. Interfaces 2017, 4, 1600435. [Google Scholar] [CrossRef]
- Chao, C.; Guan, H.; Zhang, J.; Liu, Y.; Zhao, Y.; Zhang, B. Immobilization of Laccase Onto Porous Polyvinyl Alcohol/Halloysite Hybrid Beads for Dye Removal. Water Sci. Technol. 2018, 77, 809–818. [Google Scholar] [CrossRef]
- Zhao, X.; Luo, Y.; Tan, P.; Liu, M.; Zhou, C. Hydrophobically modified chitin/halloysite nanotubes composite sponges for high efficiency oil-water separation. Int. J. Biol. Macromol. 2019, 132, 406–415. [Google Scholar] [CrossRef]
- Zhao, X.; Wan, Q.; Fu, X.; Meng, X.; Ou, X.; Zhong, R.; Zhou, Q.; Liu, M. Toxicity Evaluation of One-Dimensional Nanoparticles Using Caenorhabditis elegans: A Comparative Study of Halloysite Nanotubes and Chitin Nanocrystals. ACS Sustain. Chem. Eng. 2019, 7, 18965–18975. [Google Scholar] [CrossRef]
- Wang, X.; Gong, J.; Rong, R.; Gui, Z.; Hu, T.; Xu, X. Halloysite Nanotubes-Induced Al Accumulation and Fibrotic Response in Lung of Mice after 30-Day Repeated Oral Administration. J. Agric. Food Chem. 2018, 66, 2925–2933. [Google Scholar] [CrossRef] [PubMed]
- Tarasova, E.; Naumenko, E.; Rozhina, E.; Akhatova, F.; Fakhrullin, R. Cytocompatibility and uptake of polycations-modified halloysite clay nanotubes. Appl. Clay Sci. 2019, 169, 21–30. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; Arrigo, R.; Gambarotti, C.; Carroccio, S.C.; Filippone, G.; Cicogna, F.; Guenzi, M. α-Tocopherol-induced radical scavenging activity in carbon nanotubes for thermo-oxidation resistant ultra-high molecular weight polyethylene-based nanocomposites. Carbon 2014, 74, 14–21. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; Arrigo, R.; Morici, E.; Gambarotti, C.; Carroccio, S.C.; Cicogna, F.; Filippone, G. Multi-functional hindered amine light stabilizers-functionalized carbon nanotubes for advanced ultra-high molecular weight Polyethylene-based nanocomposites. Compos. Part B Eng. 2015, 82, 196–204. [Google Scholar] [CrossRef]
- Arrigo, R.; Dintcheva, N.T.; Guenzi, M.; Gambarotti, C.; Filippone, G.; Coiai, S.; Carroccio, S.C. Thermo-oxidative resistant nanocomposites containing novel hybrid-nanoparticles based on natural polyphenol and carbon nanotubes. Polym. Degrad. Stab. 2015, 115, 129–137. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; Al-Malaika, S. Photo-stabilization of biopolymers-based nanocomposites with UV-modified layered silicates. Polym. Degrad. Stab. 2020, 179, 109252. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Pasbakhsh, P.; Milioto, S.; Lazzara, G. Why does vacuum drive to the loading of halloysite nanotubes? The key role of water confinement. J. Colloid Interface Sci. 2019, 547, 361–369. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S. Dispersions of Nanoclays of Different Shapes into Aqueous and Solid Biopolymeric Matrices. Extended Physicochemical Study. Langmuir 2011, 27, 1158–1167. [Google Scholar] [CrossRef]
- Van Krevelen, D.W.; Nijenhuis, K. Cohesive properties and solubility. In Properties of Polymers; Van Krevelen, D.W., Nijenhuis, K., Eds.; Elsevier Ltd.: Oxford, UK, 2009. [Google Scholar]
- Bertolino, V.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F. Biopolymer-Targeted Adsorption onto Halloysite Nanotubes in Aqueous Media. Langmuir 2017, 33, 3317–3323. [Google Scholar] [CrossRef]
- Cavallaro, G.; Ines Donato, D.; Lazzara, G.; Milioto, S. Films of Halloysite Nanotubes Sandwiched between Two Layers of Biopolymer: From the Morphology to the Dielectric, Thermal, Transparency, and Wettability Properties. J. Phys. Chem. C 2011, 115, 20491–20498. [Google Scholar] [CrossRef]
- Arcudi, F.; Cavallaro, G.; Lazzara, G.; Massaro, M.; Milioto, S.; Noto, R.; Riela, S. Selective Functionalization of Halloysite Cavity by Click Reaction: Structured Filler for Enhancing Mechanical Properties of Bionanocomposite Films. J. Phys. Chem. C 2014, 118, 15095–15101. [Google Scholar] [CrossRef]
- Nuzzo, A.; Coiai, S.; Carroccio, S.C.; Dintcheva, N.T.; Gambarotti, C.; Filippone, G. Heat-resistant fully bio-based nanocomposite blends based on Poly(lactic acid). Macromol. Mater. Eng. 2014, 299, 31–40. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; D’Anna, F. Anti-/Pro-Oxidant Behavior of Naturally Occurring Molecules in Polymers and Biopolymers: A Brief Review. ACS Sustain. Chem. Eng. 2019, 7, 12656–12670. [Google Scholar] [CrossRef]
- Szymańska, E.K.; Winnicka, K. Stability of Chitosan-A Challenge for Pharmaceutical and Biomedical Applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef] [PubMed]
- Einhorn-Stoll, U.; Kastner, H.; Urbisch, A.; Kroh, L.W.; Drusch, S. Thermal degradation of citrus pectin in low-moisture environment—Influence of acidic and alkaline pre-treatment. Food Hydrocoll. 2019, 86, 104–115. [Google Scholar] [CrossRef]
| Sample | δtot, (J/cm3)1/2 | δp, (J/cm3)1/2 | δh, (J/cm3)1/2 | δd, (J/cm3)1/2 |
|---|---|---|---|---|
| Chitosan (Ch) | 29.54 | 17.99 | 18.71 | 14.10 |
| Pectin (p) | 28.26 | 17.26 | 17.57 | 13.85 |
| Chitosan | Pectin | ||
|---|---|---|---|
| ν cm−1 | Attribution | ν cm−1 | Attribution |
| 2927 | symmetric C–H stretching | 2929 | symmetric-CH2 stretching |
| 1736 | >C=O stretching | 1739 | –O–C=O |
| 1626 | C=O of ion COO stretching of secondary amide group (amide I) | 1634 | –O(−)–C=O |
| 1530 | N–H bending (residue of amide II) | 1015 | –C–O–C– |
| 1395 | C=N stretching (amide III band) | 955 | rhamnogalacturonan (uronic acid) |
| 1353 | N–H in plan deformation | 923 | d-glucopyranosyl |
| 955 | piranose ring | 890, 852 | α-, β-glucosidic linkage |
| 890 | C–N fingerprint band | 832 | α-d-mannopyranose |
| Sample | Left [°] | Right [°] | Average [°] |
|---|---|---|---|
| Ch:P | 73.7 | 74.0 | 73.9 |
| Ch:P/HNT | 65.9 | 66.0 | 66.0 |
| Ch:P/HNT:Q | 68.7 | 67.3 | 68.0 |
| Ch:P/HNT:VA | 68.6 | 68.3 | 68.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Infurna, G.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Dintcheva, N.T. Bionanocomposite Films Containing Halloysite Nanotubes and Natural Antioxidants with Enhanced Performance and Durability as Promising Materials for Cultural Heritage Protection. Polymers 2020, 12, 1973. https://doi.org/10.3390/polym12091973
Infurna G, Cavallaro G, Lazzara G, Milioto S, Dintcheva NT. Bionanocomposite Films Containing Halloysite Nanotubes and Natural Antioxidants with Enhanced Performance and Durability as Promising Materials for Cultural Heritage Protection. Polymers. 2020; 12(9):1973. https://doi.org/10.3390/polym12091973
Chicago/Turabian StyleInfurna, Giulia, Giuseppe Cavallaro, Giuseppe Lazzara, Stefana Milioto, and Nadka Tzankova Dintcheva. 2020. "Bionanocomposite Films Containing Halloysite Nanotubes and Natural Antioxidants with Enhanced Performance and Durability as Promising Materials for Cultural Heritage Protection" Polymers 12, no. 9: 1973. https://doi.org/10.3390/polym12091973
APA StyleInfurna, G., Cavallaro, G., Lazzara, G., Milioto, S., & Dintcheva, N. T. (2020). Bionanocomposite Films Containing Halloysite Nanotubes and Natural Antioxidants with Enhanced Performance and Durability as Promising Materials for Cultural Heritage Protection. Polymers, 12(9), 1973. https://doi.org/10.3390/polym12091973