Study of the Compatibilization Effect of Different Reactive Agents in PHB/Natural Fiber-Based Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Rice Husk Fibers Preparation
2.3. Composites Preparation
2.4. Methods
3. Results
3.1. Influence of Reactive Agents in PHB/Cellulose Composites
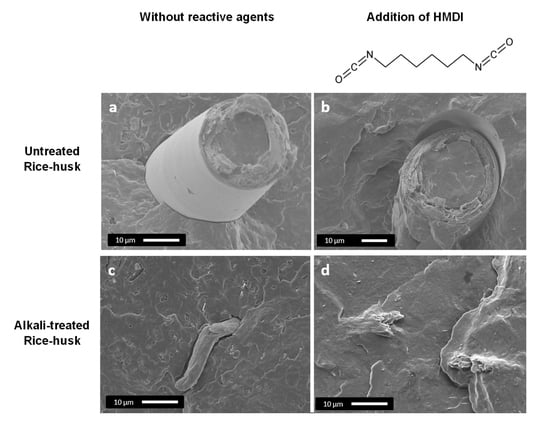
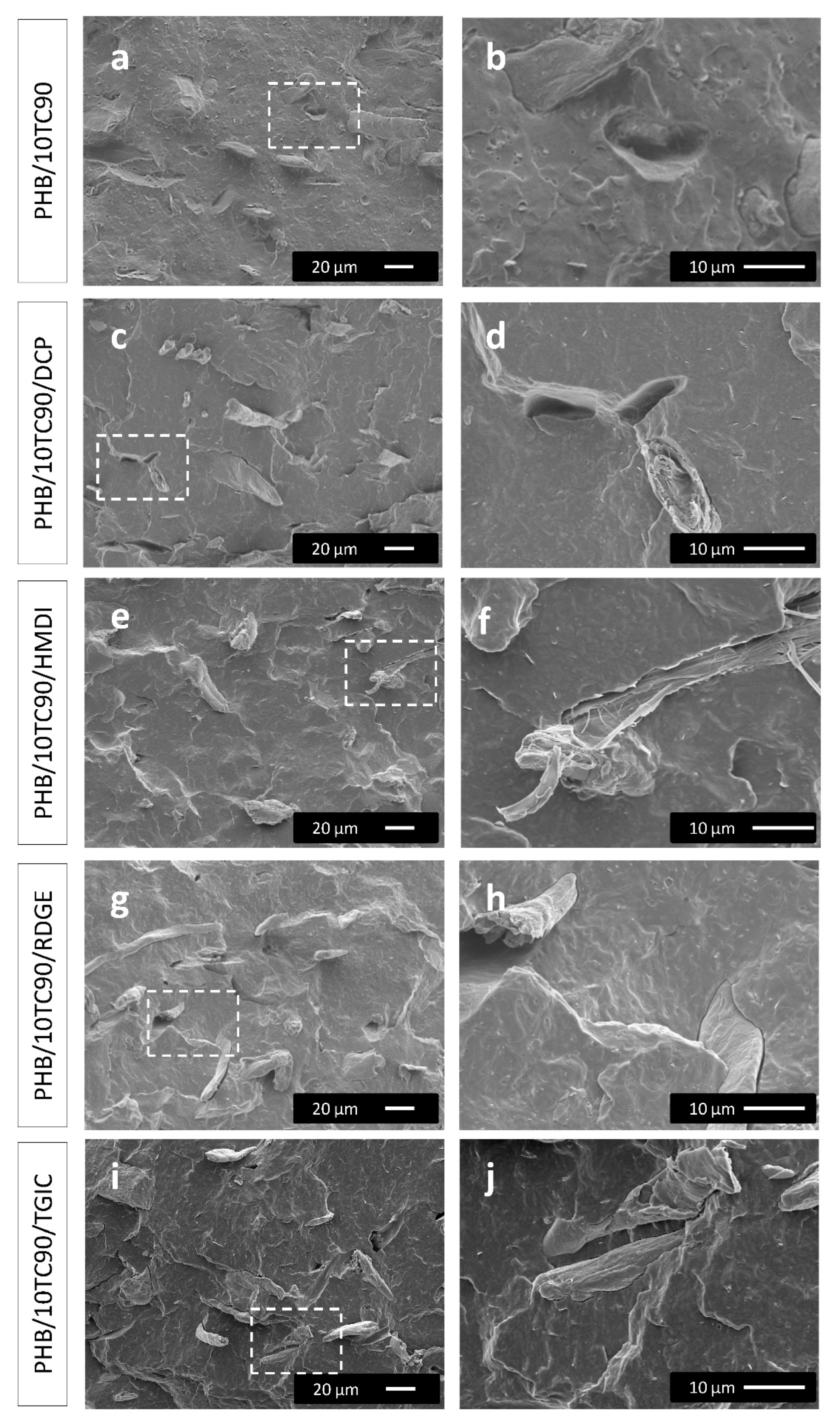
3.1.1. Morphological Analysis
3.1.2. Thermal Properties
3.1.3. Mechanical Properties
3.2. Influence of Fiber Purity
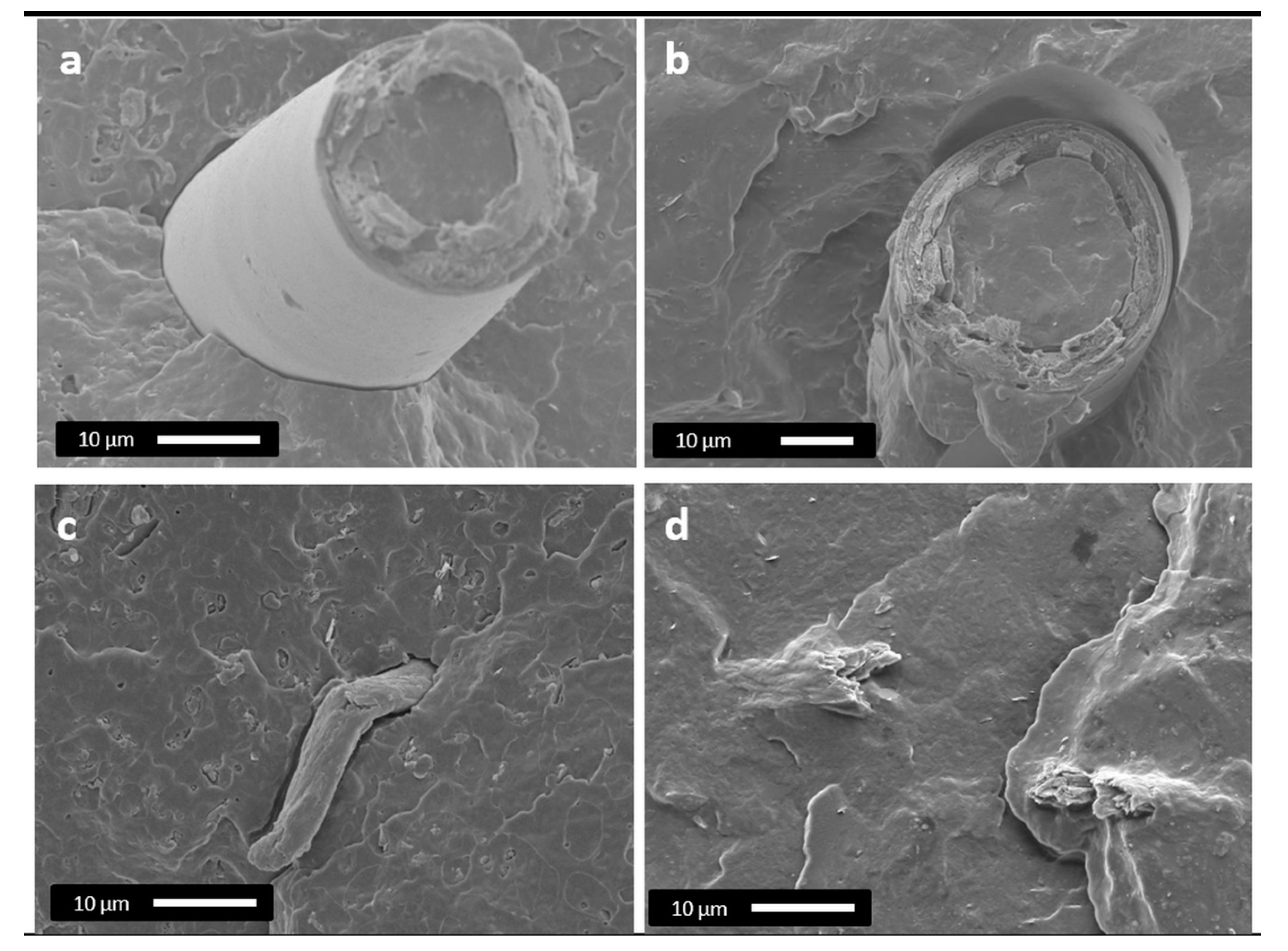
3.2.1. Morphological Analysis
3.2.2. Mechanical Properties
3.2.3. Thermoforming Ability
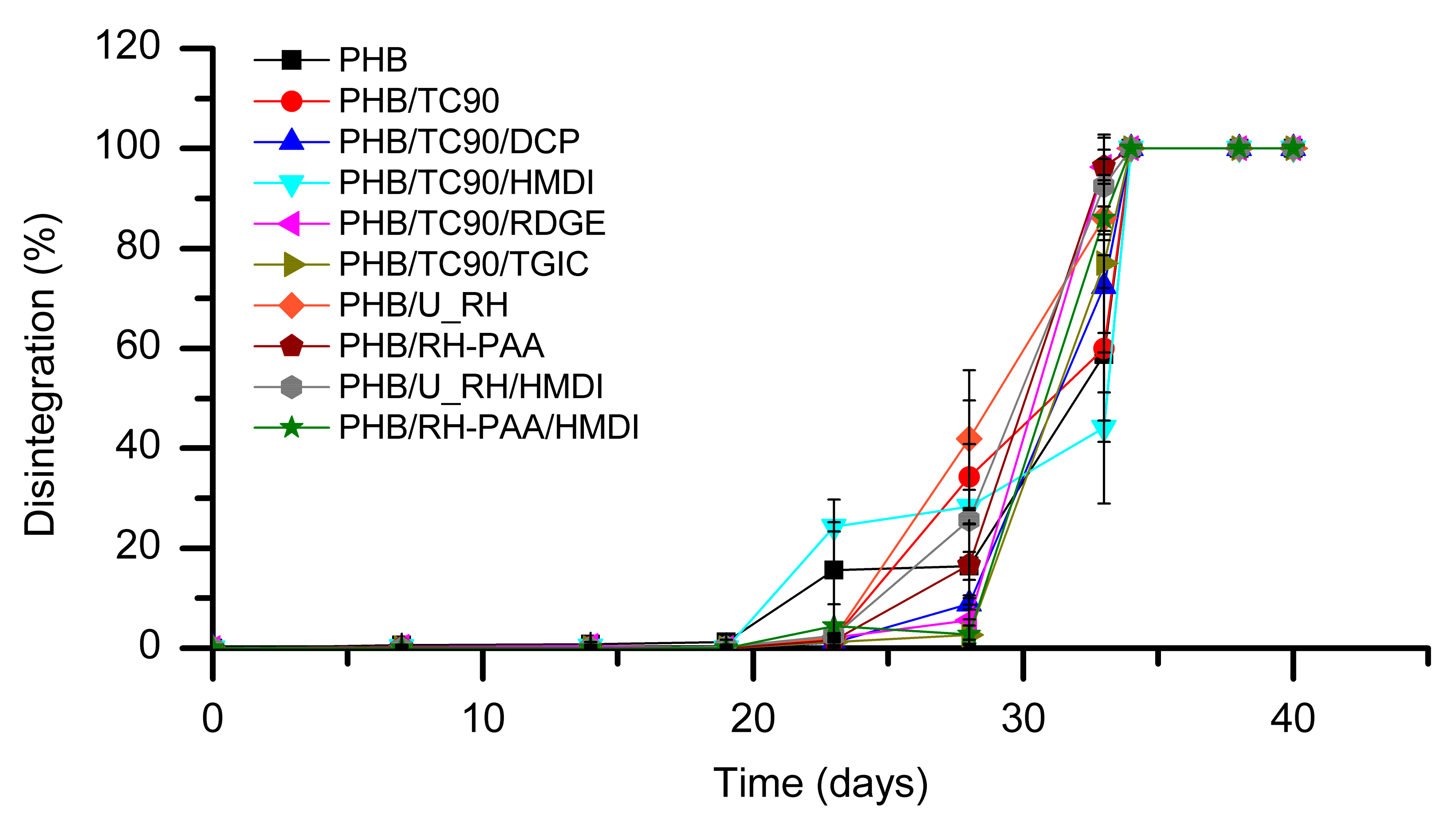
3.2.4. Biodisintegration in Composting Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muneer, F.; Rasul, I.; Azeem, F.; Siddique, M.H.; Zubair, M.; Nadeem, H. Microbial Polyhydroxyalkanoates (PHAs): Efficient Replacement of Synthetic Polymers. J. Polym. Environ. 2020, 28, 2301–2323. [Google Scholar] [CrossRef]
- Kumar, M.; Rathour, R.; Singh, R.; Sun, Y.; Pandey, A.; Gnansounou, E.; Andrew Lin, K.Y.; Tsang, D.C.W.; Thakur, I.S. Bacterial polyhydroxyalkanoates: Opportunities, challenges, and prospects. J. Clean. Prod. 2020, 263, 121500. [Google Scholar] [CrossRef]
- Rujnić-Sokele, M.; Pilipović, A. Challenges and opportunities of biodegradable plastics: A mini review. Waste Manag. Res. 2017, 35, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, P.B.S.; Malafaia, C.B. Perspectives on the production, structural characteristics and potential applications of bioplastics derived from polyhydroxyalkanoates. Int. J. Biol. Macromol. 2018, 107, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Peelman, N.; Ragaert, P.; Ragaert, K.; De Meulenaer, B.; Devlieghere, F.; Cardon, L. Heat resistance of new biobased polymeric materials, focusing on starch, cellulose, PLA, and PHA. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Keskin, G.; Kızıl, G.; Bechelany, M.; Pochat-Bohatier, C.; Öner, M. Potential of polyhydroxyalkanoate (PHA) polymers family as substitutes of petroleum based polymers for packaging applications and solutions brought by their composites to form barrier materials. Pure Appl. Chem. 2017, 89, 1841–1848. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, J.; Chen, G.Q. Polyhydroxyalkanoates, challenges and opportunities. Curr. Opin. Biotechnol. 2014, 30, 59–65. [Google Scholar] [CrossRef]
- Keshavarz, T.; Roy, I. Polyhydroxyalkanoates: Bioplastics with a green agenda. Curr. Opin. Microbiol. 2010, 13, 321–326. [Google Scholar] [CrossRef]
- Możejko-Ciesielska, J.; Kiewisz, R. Bacterial polyhydroxyalkanoates: Still fabulous? Microbiol. Res. 2016, 192, 271–282. [Google Scholar] [CrossRef]
- Dilkes-Hoffman, L.S.; Lant, P.A.; Laycock, B.; Pratt, S. The rate of biodegradation of PHA bioplastics in the marine environment: A meta-study. Mar. Pollut. Bull. 2019, 142, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Arcos-Hernandez, M.V.; Laycock, B.; Pratt, S.; Donose, B.C.; Nikolić, M.A.; Luckman, P.; Werker, A.; Lant, P.A. Biodegradation in a soil environment of activated sludge derived polyhydroxyalkanoate (PHBV). Polym. Degrad. Stab. 2012, 97, 2301–2312. [Google Scholar] [CrossRef]
- Hermann, B.G.; Debeer, L.; De Wilde, B.; Blok, K.; Patel, M.K. To compost or not to compost: Carbon and energy footprints of biodegradable materials’ waste treatment. Polym. Degrad. Stab. 2011, 96, 1159–1171. [Google Scholar] [CrossRef]
- De Koning, G.J.M.; Scheeren, A.H.C.; Lemstra, P.J.; Peeters, M.; Reynaers, H. Crystallization phenomena in bacterial poly[(R)-3-hydroxybutyrate]: 3. Toughening via texture changes. Polymer 1994, 35, 4598–4605. [Google Scholar] [CrossRef]
- Alata, H.; Aoyama, T.; Inoue, Y. Effect of Aging on the Mechanical Properties of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Macromolecules 2007, 40, 4546–4551. [Google Scholar] [CrossRef]
- Corre, Y.-M.; Bruzaud, S.; Audic, J.-L.; Grohens, Y. Morphology and functional properties of commercial polyhydroxyalkanoates: A comprehensive and comparative study. Polym. Test. 2012, 31, 226–235. [Google Scholar] [CrossRef]
- Esposito, A.; Delpouve, N.; Causin, V.; Dhotel, A.; Delbreilh, L.; Dargent, E. From a Three-Phase Model to a Continuous Description of Molecular Mobility in Semicrystalline Poly(hydroxybutyrate-co-hydroxyvalerate). Macromolecules 2016, 49, 4850–4861. [Google Scholar] [CrossRef]
- González-Ausejo, J.; Sanchez-Safont, E.; Lagaron, J.M.; Olsson, R.T.; Gamez-Perez, J.; Cabedo, L. Assessing the thermoformability of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/poly(acid lactic) blends compatibilized with diisocyanates. Polym. Test. 2017, 62, 235–245. [Google Scholar] [CrossRef]
- Pereira, P.H.F.; de Freitas, M.R.; Cioffi, M.O.H.; de Caravalho, K.C.C.B.; Milanese, A.C.; Voorwald, H.J.C.; Mulinari, D.R. Vegetal fibers in polymeric composites: A review. Polímeros 2015, 25, 9–22. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.; Hinrichsen, G. Biofibres, biodegradable polymers and biocomposites: An overview. Macromol. Mater. Eng. 2000, 276–277, 1–24. [Google Scholar] [CrossRef]
- Lagarón, J.M.; Lopez-Rubio, A.; Fabra, M.J. Bio-based packaging. J. Appl. Polym. Sci. 2015, 133. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Mohanty, A.K.; Drzal, L.T.; Pourboghrat, F.; Misra, M. Renewable resource-based green composites from recycled cellulose fiber and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) bioplastic. Biomacromolecules 2006, 7, 2044–2051. [Google Scholar] [CrossRef] [PubMed]
- Gunning, M.A.; Geever, L.M.; Killion, J.A.; Lyons, J.G.; Higginbotham, C.L. Mechanical and biodegradation performance of short natural fibre polyhydroxybutyrate composites. Polym. Test. 2013, 32, 1603–1611. [Google Scholar] [CrossRef]
- Torres-Tello, E.V.; Robledo-Ortíz, J.R.; González-García, Y.; Pérez-Fonseca, A.A.; Jasso-Gastinel, C.F.; Mendizábal, E. Effect of agave fiber content in the thermal and mechanical properties of green composites based on polyhydroxybutyrate or poly(hydroxybutyrate-co-hydroxyvalerate). Ind. Crops Prod. 2017, 99, 117–125. [Google Scholar] [CrossRef]
- Batista, K.C.; Silva, D.A.K.; Coelho, L.A.F.; Pezzin, S.H.; Pezzin, A.P.T. Soil Biodegradation of PHBV/Peach Palm Particles Biocomposites. J. Polym. Environ. 2010, 18, 346–354. [Google Scholar] [CrossRef]
- Barkoula, N.M.; Garkhail, S.K.; Peijs, T. Biodegradable composites based on flax/polyhydroxybutyrate and its copolymer with hydroxyvalerate. Ind. Crops Prod. 2010, 31, 34–42. [Google Scholar] [CrossRef]
- Michel, A.T.; Billington, S.L. Characterization of poly-hydroxybutyrate films and hemp fiber reinforced composites exposed to accelerated weathering. Polym. Degrad. Stab. 2012, 97, 870–878. [Google Scholar] [CrossRef]
- Rastogi, V.K.; Samyn, P. Novel processing of polyhydroxybutyrate with micro-to nanofibrillated cellulose and effect of fiber morphology on crystallization behaviour of composites. Express Polym. Lett. 2020, 14, 115–133. [Google Scholar] [CrossRef]
- Wei, L.; McDonald, A. A Review on Grafting of Biofibers for Biocomposites. Materials 2016, 9, 303. [Google Scholar] [CrossRef]
- Anderson, S.; Zhang, J.; Wolcott, M.P. Effect of Interfacial Modifiers on Mechanical and Physical Properties of the PHB Composite with High Wood Flour Content. J. Polym. Environ. 2013, 21, 631–639. [Google Scholar] [CrossRef]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Reactive compatibilization and performance evaluation of miscanthus biofiber reinforced poly(hydroxybutyrate-co-hydroxyvalerate) biocomposites. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, F.; Qian, J.; Huang, J.; Wolcott, M.; Liu, L.; Zhang, J. Reinforcing and Toughening Effects of Bamboo Pulp Fiber on Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Fiber Composites. Ind. Eng. Chem. Res. 2010, 49, 572–577. [Google Scholar] [CrossRef]
- Nagarajan, V.; Misra, M.; Mohanty, A.K. New engineered biocomposites from poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV)/poly(butylene adipate-co-terephthalate) (PBAT) blends and switchgrass: Fabrication and performance evaluation. Ind. Crops Prod. 2013, 42, 461–468. [Google Scholar] [CrossRef]
- Hao, M.; Wu, H. Effect of in situ reactive interfacial compatibilization on structure and properties of polylactide/sisal fiber biocomposites. Polym. Compos. 2017, 39, E174–E187. [Google Scholar] [CrossRef]
- Hao, M.; Wu, H.; Qiu, F.; Wang, X. Interface Bond Improvement of Sisal Fibre Reinforced Polylactide Composites with Added Epoxy Oligomer. Materials 2018, 11, 398. [Google Scholar] [CrossRef]
- Wei, L.; Stark, N.M.; McDonald, A.G. Interfacial improvements in biocomposites based on poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) bioplastics reinforced and grafted with α-cellulose fibers. Green Chem. 2015, 17, 4800–4814. [Google Scholar] [CrossRef]
- Sánchez-Safont, E.L.; Aldureid, A.; Lagarón, J.M.; Gamez-Perez, J.; Cabedo, L. Effect of the Purification Treatment on the Valorization of Natural Cellulosic Residues as Fillers in PHB-Based Composites for Short Shelf Life Applications. Waste Biomass Valorization 2020, 1, 3. [Google Scholar] [CrossRef]
- Zhao, X.; van der Heide, E.; Zhang, T.; Liu, D. Delignification of sugarcane bagasse with alkali and peracetic acid and characterization of the pulp. BioResources 2010, 5, 1565–1580. [Google Scholar]
- Mariano, M.; Cercená, R.; Soldi, V. Thermal characterization of cellulose nanocrystals isolated from sisal fibers using acid hydrolysis. Ind. Crops Prod. 2016, 94, 454–462. [Google Scholar] [CrossRef]
- Carli, L.N.; Crespo, J.S.; Mauler, R.S. PHBV nanocomposites based on organomodified montmorillonite and halloysite: The effect of clay type on the morphology and thermal and mechanical properties. Compos. Part A Appl. Sci. Manuf. 2011, 42, 1601–1608. [Google Scholar] [CrossRef]
- UNE-EN ISO UNE-EN ISO 20200. Determinación del Grado de Desintegración de Materiales Plásticos Bajo Condiciones de Compostaje Simuladas en un Laboratorio; Amigos Museo del Prado: Madrid, Spain, 2006. [Google Scholar]
- Zhang, K.; Misra, M.; Mohanty, A.K. Toughened sustainable green composites from poly(3-hydroxybutyrate-co-3-hydroxyvalerate) based ternary blends and miscanthus biofiber. ACS Sustain. Chem. Eng. 2014, 2, 2345–2354. [Google Scholar] [CrossRef]
- Crétois, R.; Chenal, J.M.; Sheibat-Othman, N.; Monnier, A.; Martin, C.; Astruz, O.; Kurusu, R.; Demarquette, N.R. Physical explanations about the improvement of PolyHydroxyButyrate ductility: Hidden effect of plasticizer on physical ageing. Polymers 2016, 102, 176–182. [Google Scholar] [CrossRef]
- De Koning, G.J.M.; Lemstra, P.J. Crystallization phenomena in bacterial poly[(R)-3-hydroxybutyrate]: 2. Embrittlement and rejuvenation. Polymer 1993, 34, 4089–4094. [Google Scholar] [CrossRef]
- Fei, B.; Chen, C.; Chen, S.; Peng, S.; Zhuang, Y.; An, Y.; Dong, L. Crosslinking of poly[(3-hydroxybutyrate)-co-(3-hydroxyvalerate)] using dicumyl peroxide as initiator. Polym. Int. 2004, 53, 937–943. [Google Scholar] [CrossRef]
- Crist, B.; Schultz, J.M. Polymer spherulites: A critical review. Prog. Polym. Sci. 2016, 56, 1–63. [Google Scholar] [CrossRef]
- Berthet, M.-A.; Angellier-Coussy, H.; Machado, D.; Hilliou, L.; Staebler, A.; Vicente, A.A.; Gontard, N. Exploring the potentialities of using lignocellulosic fibres derived from three food by-products as constituents of biocomposites for food packaging. Ind. Crop. Prod. 2015, 69, 110–122. [Google Scholar] [CrossRef]
- Srubar, W.V.; Wright, Z.C.; Tsui, A.; Michel, A.T.; Billington, S.L.; Frank, C.W. Characterizing the effects of ambient aging on the mechanical and physical properties of two commercially available bacterial thermoplastics. In Polymer Degradation and Stability, Proceedings of the 3rd International Conference on Biodegradable and Biobased Polymers (BIOPOL-2011), Strasbourg, Austria, 29–31 August 2011; Elsevier: Amsterdam, The Netherlands, 2012; Volume 97, pp. 1922–1929. [Google Scholar]
- Saba, N.; Jawaid, M.; Alothman, O.Y.; Paridah, M.T. A review on dynamic mechanical properties of natural fibre reinforced polymer composites. Constr. Build. Mater. 2016, 106, 149–159. [Google Scholar] [CrossRef]
- Yu, L. Biodegradable Polymer Blends and Composites from Renewable Resources; John Wiley and Sons: Hoboken, NJ, USA, 2009; ISBN 9780470146835. [Google Scholar]
- Giménez, E.; Lagarón, J.M.; Cabedo, L.; Gavara, R.; Saura, J.J. Study of the thermoformability of ethylene-vinyl alcohol copolymer based barrier blends of interest in food packaging applications. J. Appl. Polym. Sci. 2004, 91, 3851–3855. [Google Scholar] [CrossRef]
- Giménez, E.; Lagarón, J.M.; Maspoch, M.L.; Cabedo, L.; Saura, J.J. Uniaxial tensile behavior and thermoforming characteristics of high barrier EVOH-based blends of interest in food packaging. Polym. Eng. Sci. 2004, 44, 598–608. [Google Scholar] [CrossRef]
- Sánchez-Safont, E.L.; González-Ausejo, J.; Gámez-Pérez, J.; Lagarón, J.M.; Cabedo, L. Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate)/Purified Cellulose Fiber Composites by Melt Blending: Characterization and Degradation in Composting Conditions. J. Renew. Mater. 2016, 4, 123–132. [Google Scholar] [CrossRef]
- Sánchez-Safont, E.L.; Aldureid, A.; Lagarón, J.M.; Gámez-Pérez, J.; Cabedo, L. Biocomposites of different lignocellulosic wastes for sustainable food packaging applications. Compos. Part B Eng. 2018, 145, 215–225. [Google Scholar] [CrossRef]
- Weng, Y.-X.; Wang, Y.-Z.Y.; Wang, X.-L.; Wang, Y.-Z.Y. Biodegradation behavior of PHBV films in a pilot-scale composting condition. Polym. Test. 2010, 29, 579–587. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; Rayón, E.; Jiménez, A. Disintegrability under composting conditions of plasticized PLA–PHB blends. Polym. Degrad. Stab. 2014, 108, 307–318. [Google Scholar] [CrossRef]
- Tran, T.P.T.; Bénézet, J.-C.; Bergeret, A. Rice and Einkorn wheat husks reinforced poly(lactic acid) (PLA) biocomposites: Effects of alkaline and silane surface treatments of husks. Ind. Crops Prod. 2014, 58, 111–124. [Google Scholar] [CrossRef]







| Sample | Component (phr) | |||||||
|---|---|---|---|---|---|---|---|---|
| PHB | TC90 | U_RH | T_RH | DCP | HMDI | RDGE | TGIC | |
| PHB | 100 | - | - | - | - | - | ||
| PHB/DCP | 100 | - | 1 | - | - | - | ||
| PHB/HMDI | 100 | - | 1 | - | - | |||
| PHB/RDGE | 100 | - | - | - | 1 | - | ||
| PHB/TGIC | 100 | - | - | - | - | 1 | ||
| PHB/TC90 | 100 | 10 | - | - | - | - | - | |
| PHB/TC90/DCP | 100 | 10 | - | 1 | - | - | - | |
| PHB/TC90/HMDI | 100 | 10 | - | - | 1 | - | - | |
| PHB/TC90/RDGE | 100 | 10 | - | - | - | 1 | - | |
| PHB/TC90/TGIC | 100 | 10 | - | - | - | - | 1 | |
| PHB/U_RH | 100 | 10 | - | - | - | - | - | |
| PHB/U_RH/HMDI | 100 | 10 | - | - | - | - | - | |
| PHB/T_RH | 100 | 10 | - | - | - | - | ||
| PHB/T_RH/HMDI | 100 | 10 | - | 1 | - | - | ||
| 1st Heating Scan | Cooling Scan | 2nd Heating Scan | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 days | 100 days | Tc (°C) | ΔHc (J/g) | Tm (°C) | ΔHm (J/g) | Xc (%) | |||||
| Tm (°C) | ΔHm (J/g) | Xc (%) | Tm (°C) | ΔHm (J/g) | Xc (%) | ||||||
| PHB | 175 | 72 | 49 | 170 | 86 | 59 | 117 | 91 | 170 | 94 | 64 |
| PHB/DCP | 167 | 71 | 49 | 167 | 73 | 50 | 115 | 81 | 160 | 84 | 58 |
| PHB/HMDI | 173 | 69 | 48 | 173 | 79 | 55 | 106 | 86 | 167 | 90 | 62 |
| PHB/RDGE | 172 | 75 | 52 | 173 | 81 | 56 | 115 | 88 | 168 | 91 | 63 |
| PHB/TGIC | 173 | 77 | 53 | 172 | 80 | 56 | 116 | 89 | 168 | 92 | 64 |
| PHB/TC90 | 173 | 74 | 56 | 173 | 73 | 55 | 117 | 82 | 169 | 84 | 63 |
| PHB/TC90/DCP | 166 | 67 | 51 | 165 | 66 | 50 | 118 | 75 | 161 | 78 | 59 |
| PHB/TC90/HMDI | 173 | 71 | 49 | 173 | 72 | 55 | 111 | 78 | 169 | 81 | 62 |
| PHB/TC90/RDGE | 172 | 76 | 52 | 173 | 73 | 56 | 115 | 79 | 169 | 85 | 65 |
| PHB/TC90/TGIC | 167 | 78 | 54 | 172 | 73 | 55 | 118 | 79 | 166 | 85 | 64 |
| 0 Days | 15 Days | 0 Days | 15 Days | 0 Days | 15 Days | 0 Days | 15 Days | |
|---|---|---|---|---|---|---|---|---|
| Elastic Modulus (GPa) | Tensile Strength (MPa) | Elongation at Break (%) | Static Toughness (mJ/m3) | |||||
| PHB | 1.41 | 3.04 | 25.89 | 23.03 | 4.96 | 0.98 | 0.84 | 0.13 |
| P309/DCP | 1.54 | 2.75 | 24.88 | 29.77 | 3.87 | 1.55 | 0.62 | 0.27 |
| P309/HMDI | 1.82 | 3.15 | 23.36 | 24.36 | 2.50 | 0.86 | 0.38 | 0.10 |
| P309/RDGE | 1.54 | 2.90 | 23.42 | 29.32 | 3.62 | 1.55 | 0.57 | 0.27 |
| P309/TGIC | 1.48 | 3.03 | 24.76 | 30.95 | 4.28 | 1.43 | 0.72 | 0.25 |
| P309/TC90 | 1.71 | 3.44 | 21.27 | 27.14 | 3.49 | 1.21 | 0.53 | 0.19 |
| P309/TC90/DCP | 1.92 | 3.19 | 23.64 | 26.83 | 2.23 | 1.15 | 0.34 | 0.18 |
| P309/TC90/HMDI | 2.31 | 3.49 | 26.75 | 32.11 | 2.04 | 1.16 | 0.35 | 0.20 |
| P309/TC90/RDGE | 2.10 | 3.40 | 21.71 | 25.06 | 2.42 | 1.07 | 0.37 | 0.16 |
| P309/TC90/TGIC | 2.02 | 3.59 | 23.89 | 28.06 | 3.22 | 1.22 | 0.56 | 0.21 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Safont, E.L.; Aldureid, A.; Lagarón, J.M.; Cabedo, L.; Gámez-Pérez, J. Study of the Compatibilization Effect of Different Reactive Agents in PHB/Natural Fiber-Based Composites. Polymers 2020, 12, 1967. https://doi.org/10.3390/polym12091967
Sánchez-Safont EL, Aldureid A, Lagarón JM, Cabedo L, Gámez-Pérez J. Study of the Compatibilization Effect of Different Reactive Agents in PHB/Natural Fiber-Based Composites. Polymers. 2020; 12(9):1967. https://doi.org/10.3390/polym12091967
Chicago/Turabian StyleSánchez-Safont, Estefanía Lidón, Abdulaziz Aldureid, José María Lagarón, Luis Cabedo, and José Gámez-Pérez. 2020. "Study of the Compatibilization Effect of Different Reactive Agents in PHB/Natural Fiber-Based Composites" Polymers 12, no. 9: 1967. https://doi.org/10.3390/polym12091967
APA StyleSánchez-Safont, E. L., Aldureid, A., Lagarón, J. M., Cabedo, L., & Gámez-Pérez, J. (2020). Study of the Compatibilization Effect of Different Reactive Agents in PHB/Natural Fiber-Based Composites. Polymers, 12(9), 1967. https://doi.org/10.3390/polym12091967