Development and Characterization of Membranes with PVA Containing Silver Particles: A Study of the Addition and Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Membrane Fabrication—Hybrid Method
2.2. Fourier Transform Infrared Spectroscopy
2.3. Scanning Electron Microscopy and Energy Dispersive X-ray Spectroscopy
2.4. Atomic Absorption Spectroscopy
2.5. X-ray Photoelectron Spectroscopy
2.6. Equilibrium Solution Content
2.7. Permeability and Retention
2.8. Anti-Bacterial Experiments
3. Results and Discussion
3.1. Membrane Characterization
3.2. Characterization—Atomic Absorption Content
3.3. Characterization—X-ray Photoelectron Spectroscopy
3.4. Equilibrium Solution Content
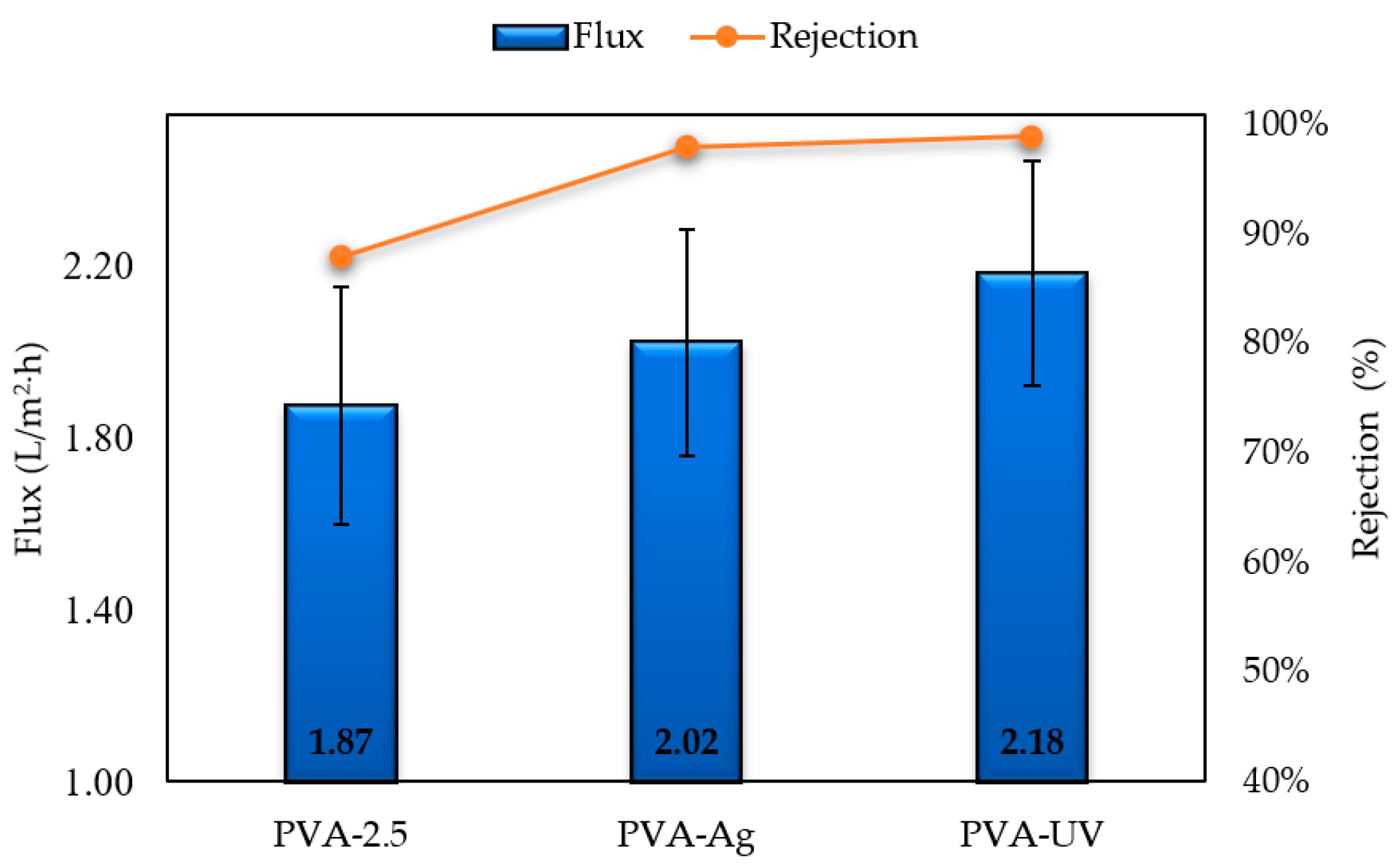
3.5. Membrane Performance
3.6. Anti-Bacterial Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, R.; Fan, W.; Liu, X.; Fan, W.; Meng, S.; Cai, W. Effect of magnesium ion on polysaccharide fouling. Chem. Eng. J. 2020, 379, 122351. [Google Scholar] [CrossRef]
- Zhao, C.; Lv, J.; Xu, X.; Zhang, G.-Q.; Yang, Y.; Yang, F. Highly antifouling and antibacterial performance of poly (vinylidene fluoride) ultrafiltration membranes blending with copper oxide and graphene oxide nanofillers for effective wastewater treatment. J. Colloid Interface Sci. 2017, 505, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Meng, X.; Fan, W.; Liang, D.; Wang, L.; Zhang, W.; Liu, Y. The role of transparent exopolymer particles (TEP) in membrane fouling: A critical review. Water Res. 2020, 181, 115930. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-Q. Molecular Insights into Extracellular Polymeric Substances in Activated Sludge. Environ. Sci. Technol. 2020, 54, 7742–7750. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, S.; Wang, Z.; Wu, J.; Wang, J.; Wang, S. In situ immobilization of silver nanoparticles for improving permeability, antifouling and anti-bacterial properties of ultrafiltration membrane. J. Membr. Sci. 2016, 499, 269–281. [Google Scholar] [CrossRef]
- Rus, A.; Leordean, V.-D.; Berce, P. Silver Nanoparticles (AgNP) impregnated filters in drinking water disinfection. MATEC Web Conf. 2017, 137, 7007. [Google Scholar] [CrossRef]
- Dong, X.; Shannon, H.D.; Amirsoleimani, A.; Brion, G.M.; Escobar, I.C. Thiol-Affinity Immobilization of Casein-Coated Silver Nanoparticles on Polymeric Membranes for Biofouling Control. Polymers 2019, 11, 2057. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, Y.; Wang, Z.; Zou, X.; Zhao, Y.; Sun, L. Fabrication of silver nanoparticles embedded into polyvinyl alcohol (Ag/PVA) composite nanofibrous films through electrospinning for antibacterial and surface-enhanced Raman scattering (SERS) activities. Mater. Sci. Eng. C 2016, 69, 462–469. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]
- Andrade, P.F.; De Faria, A.F.; Oliveira, S.R.; Arruda, M.A.Z.; Gonçalves, M.D.C. Improved antibacterial activity of nanofiltration polysulfone membranes modified with silver nanoparticles. Water Res. 2015, 81, 333–342. [Google Scholar] [CrossRef]
- Chede, S.; Anaya, N.M.; Oyanedel-Craver, V.; Gorgannejad, S.; Harris, T.A.; Al-Mallahi, J.; Abu-Dalo, M.A.; Abu Qdais, H.; Escobar, I.C.; Al-Mallahi, J. Desalination using low biofouling nanocomposite membranes: From batch-scale to continuous-scale membrane fabrication. Desalination 2019, 451, 81–91. [Google Scholar] [CrossRef]
- Liu, C.; De Faria, A.F.; Ma, J.; Elimelech, M. Mitigation of Biofilm Development on Thin-Film Composite Membranes Functionalized with Zwitterionic Polymers and Silver Nanoparticles. Environ. Sci. Technol. 2016, 51, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.S.; Shao, G.N.; Imran, S.M.; Park, S.S.; Abbas, N.; Tahir, M.S.; Hussain, M.; Bae, W.; Kim, H.T. Aminated polyethersulfone-silver nanoparticles (AgNPs-APES) composite membranes with controlled silver ion release for antibacterial and water treatment applications. Mater. Sci. Eng. C 2016, 62, 732–745. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Singh, N.; Charan, S.; Subbarao, V.; Gokhale, R.; Mulik, U. Synthesis and characterization of Ag/PVA nanocomposite by chemical reduction method. Mater. Chem. Phys. 2005, 93, 117–121. [Google Scholar] [CrossRef]
- Ali, I.O. Synthesis and characterization of Ag0/PVA nanoparticles via photo- and chemical reduction methods for antibacterial study. Colloids and Surfaces A: Physicochem. Eng. Asp. 2013, 436, 922–929. [Google Scholar] [CrossRef]
- Lee, H.J.; Yeo, S.Y.; Jeong, S.H. Antibacterial effect of nanosized silver colloidalsolution on textile fabrics. J. Mater. Sci. 2003, 38, 2199–2204. [Google Scholar] [CrossRef]
- Lee, H.J.; Jeong, S.H. Bacteriostasis of Nanosized Colloidal Silver on Polyester Nonwovens. Text. Res. J. 2004, 74, 442–447. [Google Scholar] [CrossRef]
- Dumée, L.F.; Maina, J.W.; Merenda, A.; Reis, R.; He, L.; Kong, L. Hybrid thin film nano-composite membrane reactors for simultaneous separation and degradation of pesticides. J. Membr. Sci. 2017, 528, 217–224. [Google Scholar] [CrossRef]
- Li, R.; Barbari, T. Performance of poly (vinyl alcohol) thin-gel composite ultrafiltration membranes. J. Membr. Sci. 1995, 105, 71–78. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Yusuf, N.; Ooi, B.S. Preparation and modification of poly (vinyl) alcohol membrane: Effect of crosslinking time towards its morphology. Desalination 2012, 287, 35–40. [Google Scholar] [CrossRef]
- Omrani, A.A.; Taghavinia, N. Photo-induced growth of silver nanoparticles using UV sensitivity of cellulose fibers. Appl. Surf. Sci. 2012, 258, 2373–2377. [Google Scholar] [CrossRef]
- Zhu, X.; Bai, R.; Wee, K.-H.; Liu, C.; Tang, S.-L. Membrane surfaces immobilized with ionic or reduced silver and their anti-biofouling performances. J. Membr. Sci. 2010, 363, 278–286. [Google Scholar] [CrossRef]
- Kotel’Nikova, N.E.; Wegener, G.; Paakkari, T.; Serimaa, R.; Demidov, V.N.; Serebriakov, A.S.; Shchukarev, A.; Gribanov, A.V. Silver Intercalation into Cellulose Matrix. An X-ray Scattering, Solid-State 13C NMR, IR, X-ray Photoelectron, and Raman Study. Russ. J. Gen. Chem. 2003, 73, 418–426. [Google Scholar] [CrossRef]
- Kotel’Nikova, N.E.; Demidov, V.N.; Wegener, G.; Windeisen, E. Mechanisms of Diffusion-Reduction Interaction of Microcrystalline Cellulose and Silver Ions. Russ. J. Gen. Chem. 2003, 73, 427–433. [Google Scholar] [CrossRef]
- Gu, L.; Xie, M.-Y.; Jin, Y.; He, M.; Xing, X.-Y.; Yu, Y.; Wu, Q.-Y. Construction of Antifouling Membrane Surfaces through Layer-by-Layer Self-Assembly of Lignosulfonate and Polyethyleneimine. Polymers 2019, 11, 1782. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.R.; Tak, T.-M.; Kwon, Y.-N. Preparation and applications of poly vinyl alcohol (PVA) modified cellulose acetate (CA) membranes for forward osmosis (FO) processes. Desalin. Water Treat. 2013, 53, 1–7. [Google Scholar] [CrossRef]
- Thompson, A.; Nguyen, D.; Nave, F. Characterization of PVA-IDA Hydrogel Crosslinked with 1.25%, 2.5% and 5% Glutaraldehyde. GSTF Int. J. Educ. 2013, 1, 1. [Google Scholar] [CrossRef]
- Dai, W.; Barbari, T. Hydrogel membranes with mesh size asymmetry based on the gradient cross linking of poly (vinyl alcohol). J. Membr. Sci. 1998, 156, 67–79. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Sorour, M.; Talaat, H.A.; Ali, S. Functional analysis of cellulose acetate flat membranes prepared via casting technique. Desalin. Water Treat. 2010, 21, 115–121. [Google Scholar] [CrossRef]
- Nave, F.M.; Luo, Y.Z.; Coleman, M.R. Impact of Mobile Phase Parameters on Transport Properties of Metal Affinity Hydrogel Membranes. Sep. Sci. Technol. 2008, 43, 4075–4098. [Google Scholar] [CrossRef]
- McCabe, W.; Smith, J.; Harriott, P. Unit Operations of Chemical Engineering; McGraw-Hill: New York, NY, USA, 2005. [Google Scholar]
- Kim, E.-S.; Hwang, G.; El-Din, M.G.; Liu, Y. Development of nanosilver and multi-walled carbon nanotubes thin-film nanocomposite membrane for enhanced water treatment. J. Membr. Sci. 2012, 394, 37–48. [Google Scholar] [CrossRef]
- Molleman, B.; Hiemstra, T. Surface Structure of Silver Nanoparticles as a Model for Understanding the Oxidative Dissolution of Silver Ions. Langmuir 2015, 31, 13361–13372. [Google Scholar] [CrossRef] [PubMed]
- Peretyazhko, T.S.; Zhang, Q.; Colvin, V.L. Size-Controlled Dissolution of Silver Nanoparticles at Neutral and Acidic pH Conditions: Kinetics and Size Changes. Environ. Sci. Technol. 2014, 48, 11954–11961. [Google Scholar] [CrossRef] [PubMed]
- Prince, J.; Bhuvana, S.; Boodhoo, K.; Anbharasi, V.; Singh, G. Synthesis and characterization of PEG-Ag immobilized PES hollow fiber ultrafiltration membranes with long lasting antifouling properties. J. Membr. Sci. 2014, 454, 538–548. [Google Scholar] [CrossRef]
- Kaushik, V.K. XPS core level spectra and Auger parameters for some silver compounds. J. Electron Spectrosc. Relat. Phenom. 1991, 56, 273–277. [Google Scholar] [CrossRef]
- Hoflund, G.B.; Hazos, Z.F.; Salaita, G.N. Surface characterization study of Ag, AgO, and Ag2O using x-ray photoelectron spectroscopy and electron energy-loss spectroscopy. Phys. Rev. B 2000, 62, 11126–11133. [Google Scholar] [CrossRef]
- Bao, X.; Schedel-Niedrig, T.; Muhler, M.; Schlögl, R. Interaction of oxygen with silver at high temperature and atmospheric pressure: A spectroscopic and structural analysis of a strongly bound surface species. Phys. Rev. B 1996, 54, 2249–2262. [Google Scholar] [CrossRef]
- Calderon, S.; Cavaleiro, A.; Carvalho, S. Chemical and structural characterization of ZrCNAg coatings: XPS, XRD and Raman spectroscopy. Appl. Surf. Sci. 2015, 346, 240–247. [Google Scholar] [CrossRef]
- Fonseca, A.M.; Neves, I. Study of silver species stabilized in different microporous zeolites. Microporous Mesoporous Mater. 2013, 181, 83–87. [Google Scholar] [CrossRef]
- Moulder, J.F.; William, F.S.; Sobol, P.E.; Bomber, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Waltham, MA, USA; Physical Electronic Division: Chanhassen, MN, USA, 1992. [Google Scholar]
- Son, W.; Youk, J.; Park, W. Antimicrobial cellulose acetate nanofibers containins silver nanoparticles. Carbohydr. Polym. 2006, 65, 430–434. [Google Scholar] [CrossRef]
- Said, K.A.M.; Jamain, R.; Sutan, N.M.; Alipah, N. Enhanced permeation performance with incorporation of silver nitrate onto polymeric membrane. J. Mech. Eng. Sci. 2018, 12, 3811–3824. [Google Scholar] [CrossRef]
- Sawada, I.; Fachrul, R.; Ito, T.; Ohmukai, Y.; Maruyama, T.; Matsuyama, H. Development of a hydrophilic polymer membrane containing silver nanoparticles with both organic antifouling and antibacterial properties. J. Membr. Sci. 2012, 387, 1–6. [Google Scholar] [CrossRef]
- Koseoglu-Imer, D.Y.; Kose, B.; Altinbas, M.; Koyuncu, I. The production of polysulfone (PS) membrane with silver nanoparticles (AgNP): Physical properties, filtration performances, and biofouling resistances of membranes. J. Membr. Sci. 2013, 428, 620–628. [Google Scholar] [CrossRef]
- Sivakumar, M.; Moham, D.; Rangarajan, R. Preparation and Performance of Cellulose Acetate-Polyurethane Blend Membranes and their Applications; Part 1. Polym. Int. 1998, 47, 311–316. [Google Scholar]
- Mehta, A.; Zydney, A.L. Permeability and selectivity analysis for ultrafiltration membranes. J. Membr. Sci. 2005, 249, 245–249. [Google Scholar] [CrossRef]
- Hausman, R.; Escobar, I.C. A comparison of silver- and copper-charged polypropylene feed spacers for biofouling control. J. Appl. Polym. Sci. 2012, 128. [Google Scholar] [CrossRef]








| Ag3d5/2 (eV) | Ag3d3/2 (eV) | KE (eV) | AP (eV) | |
|---|---|---|---|---|
| PVA-Ag | 368.60 | 374.62 | 355.71 | 724.10 |
| PVA-UV | 368.23 | 374.25 | 354.19 | 722.38 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thompson, A.K.; Hackett, C.; Grady, T.L.; Enyinnia, S.; Moore, Q.C., III; Nave, F.M. Development and Characterization of Membranes with PVA Containing Silver Particles: A Study of the Addition and Stability. Polymers 2020, 12, 1937. https://doi.org/10.3390/polym12091937
Thompson AK, Hackett C, Grady TL, Enyinnia S, Moore QC III, Nave FM. Development and Characterization of Membranes with PVA Containing Silver Particles: A Study of the Addition and Stability. Polymers. 2020; 12(9):1937. https://doi.org/10.3390/polym12091937
Chicago/Turabian StyleThompson, Audie K., Cannon Hackett, Tony L. Grady, Silver Enyinnia, Quincy C. Moore, III, and Felecia M. Nave. 2020. "Development and Characterization of Membranes with PVA Containing Silver Particles: A Study of the Addition and Stability" Polymers 12, no. 9: 1937. https://doi.org/10.3390/polym12091937
APA StyleThompson, A. K., Hackett, C., Grady, T. L., Enyinnia, S., Moore, Q. C., III, & Nave, F. M. (2020). Development and Characterization of Membranes with PVA Containing Silver Particles: A Study of the Addition and Stability. Polymers, 12(9), 1937. https://doi.org/10.3390/polym12091937





