pH-Responsive Gamma-Irradiated Poly(Acrylic Acid)-Cellulose-Nanocrystal-Reinforced Hydrogels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cellulose and Cellulose Nanocrystals (CNCs)
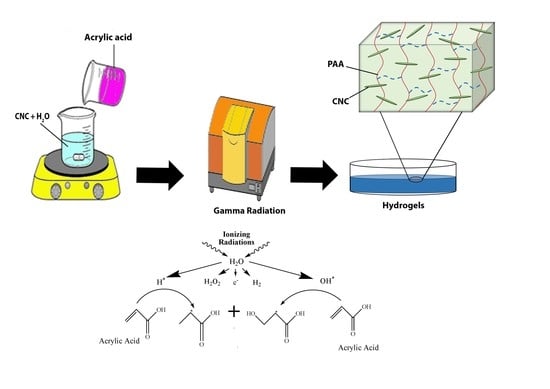
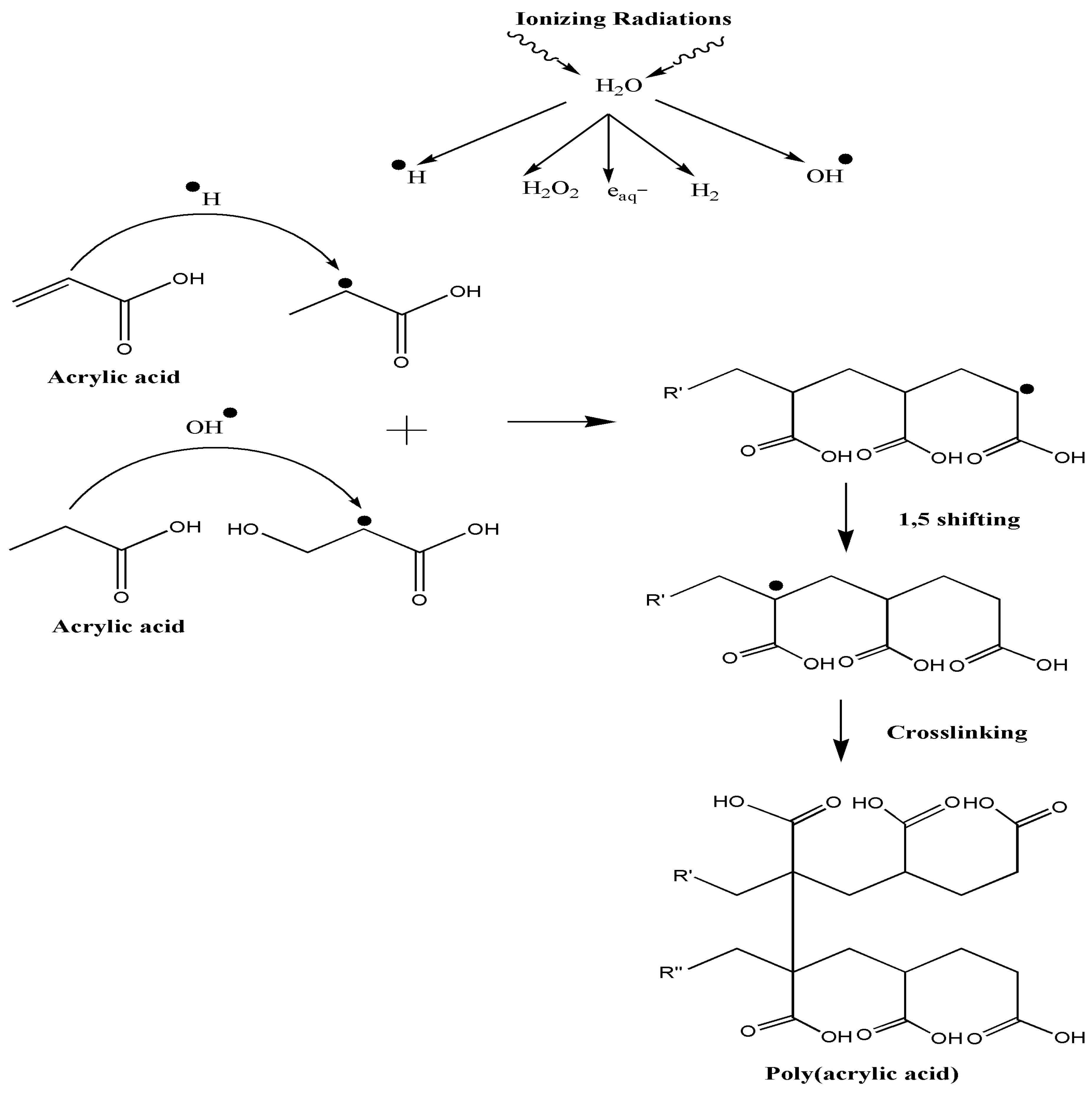
2.3. Preparation of Hydrogels
2.4. Morphological Observation
2.5. Fourier-Transform Infrared (FTIR) Spectroscopy
2.6. Gel Fraction Test
2.7. Swelling Test
2.8. Rheology Test
3. Results
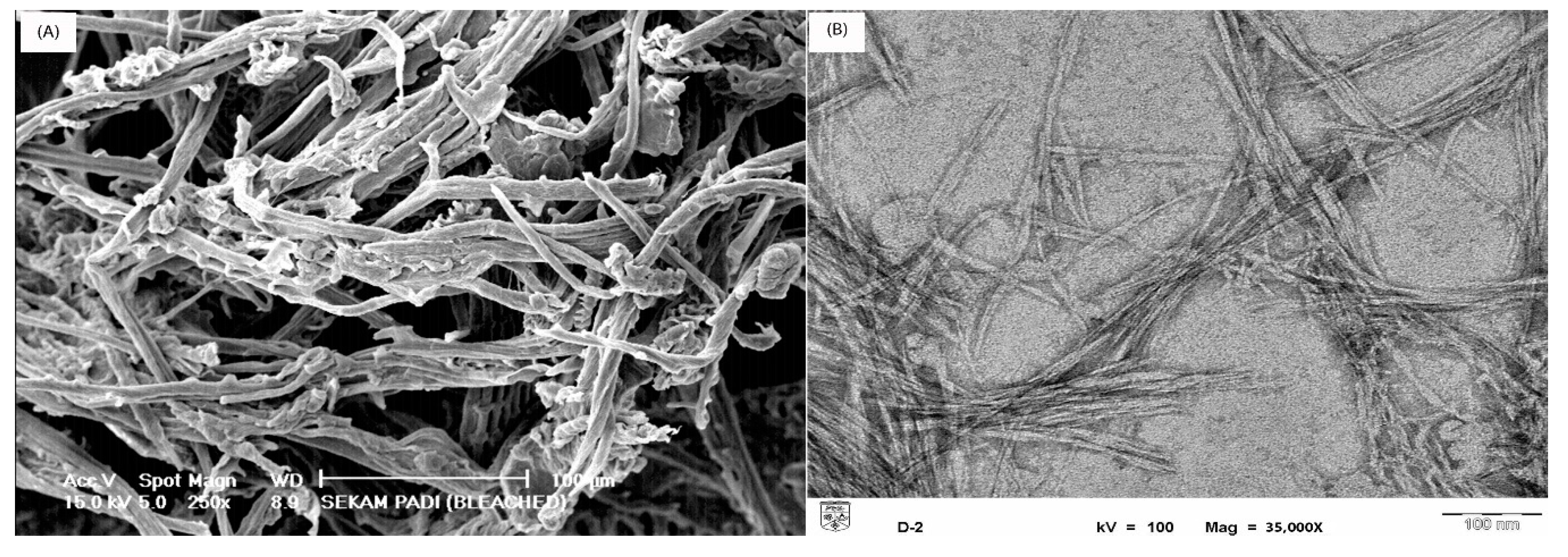
3.1. Morphological Observation of Cellulose and Cellulose Nanocrystals
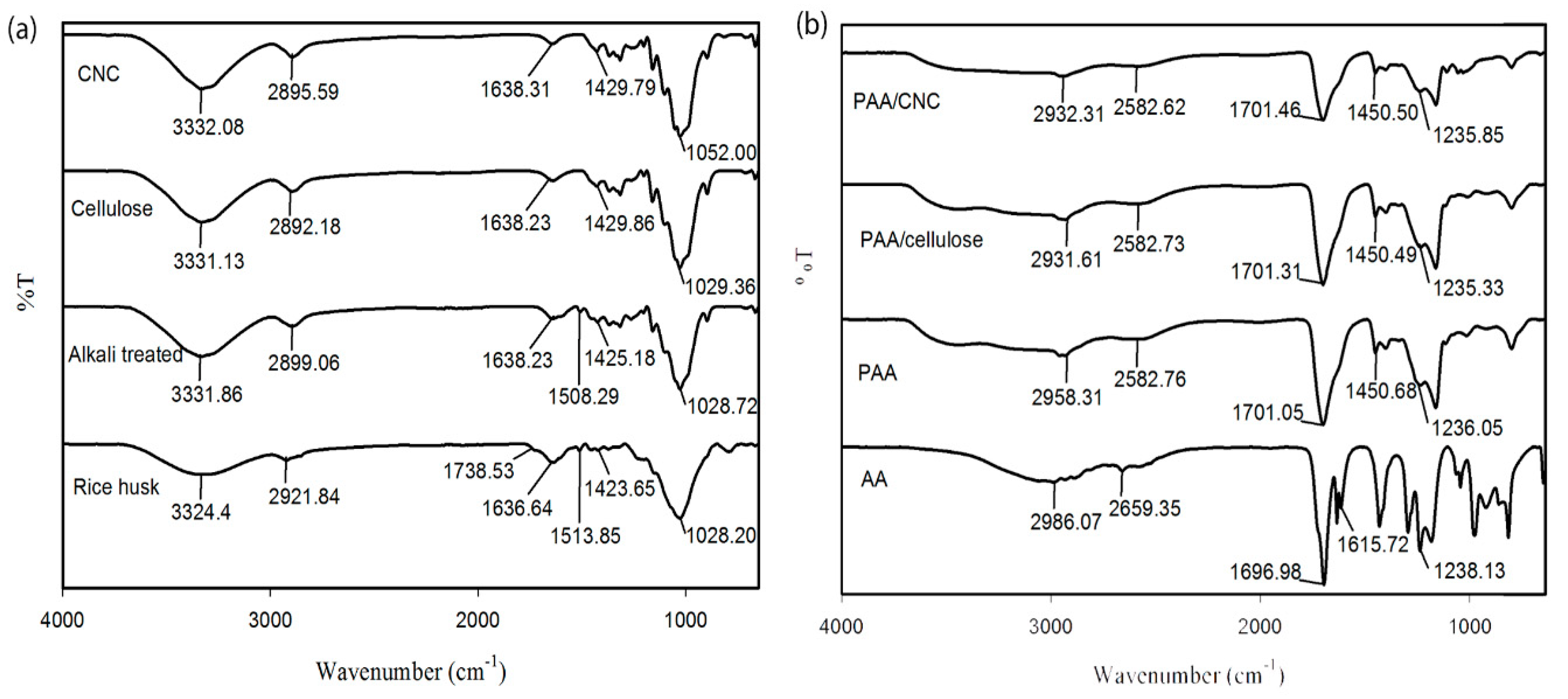
3.2. Fourier-Transform Infrared (FTIR) Spectroscopy
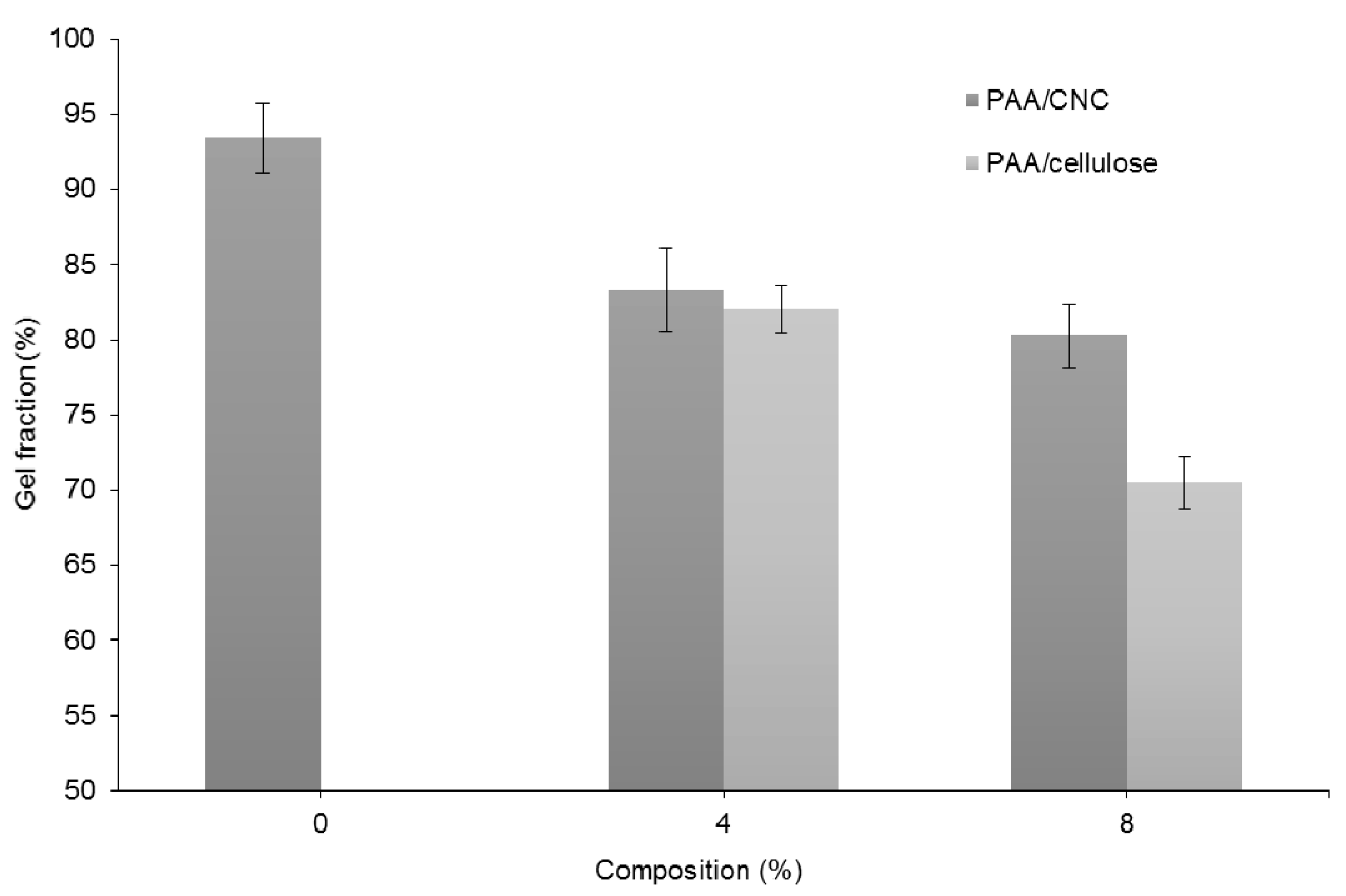
3.3. Gel Fraction Test
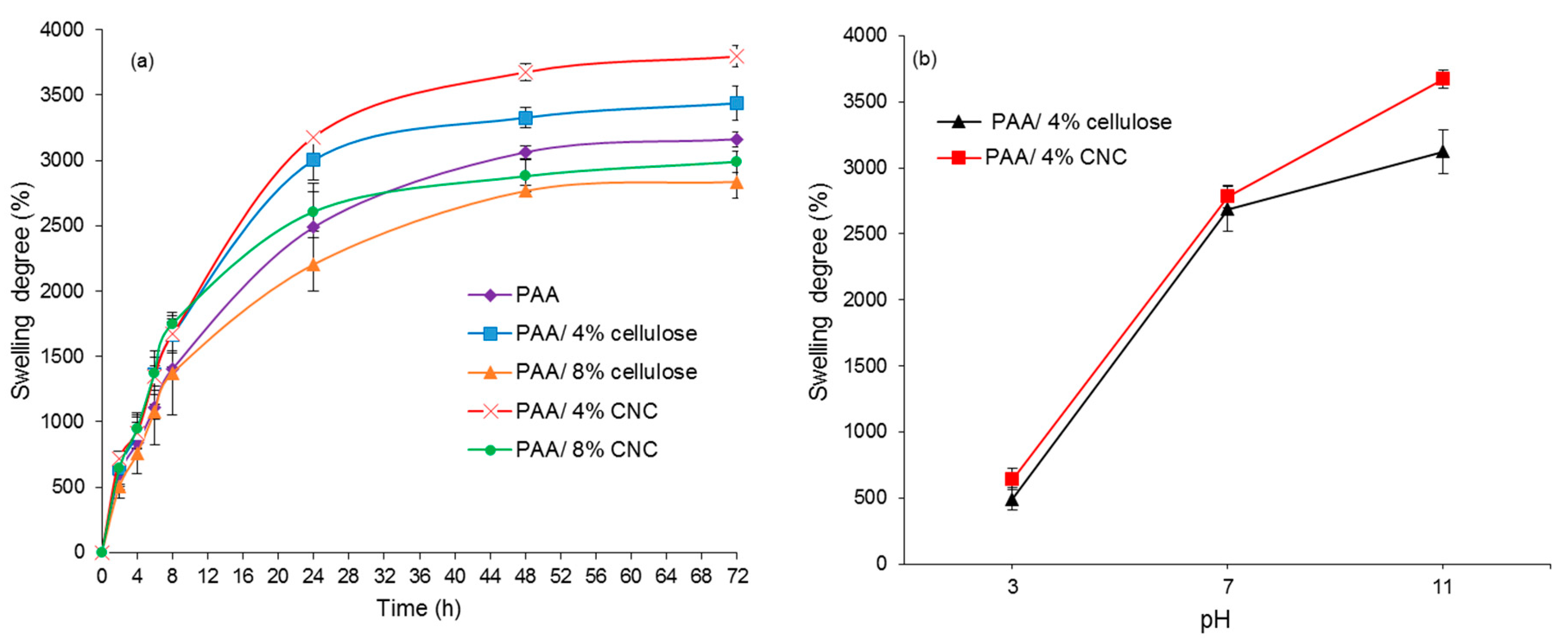
3.4. Swelling Test
3.4.1. Effect of Different Temperature
3.4.2. pH Response
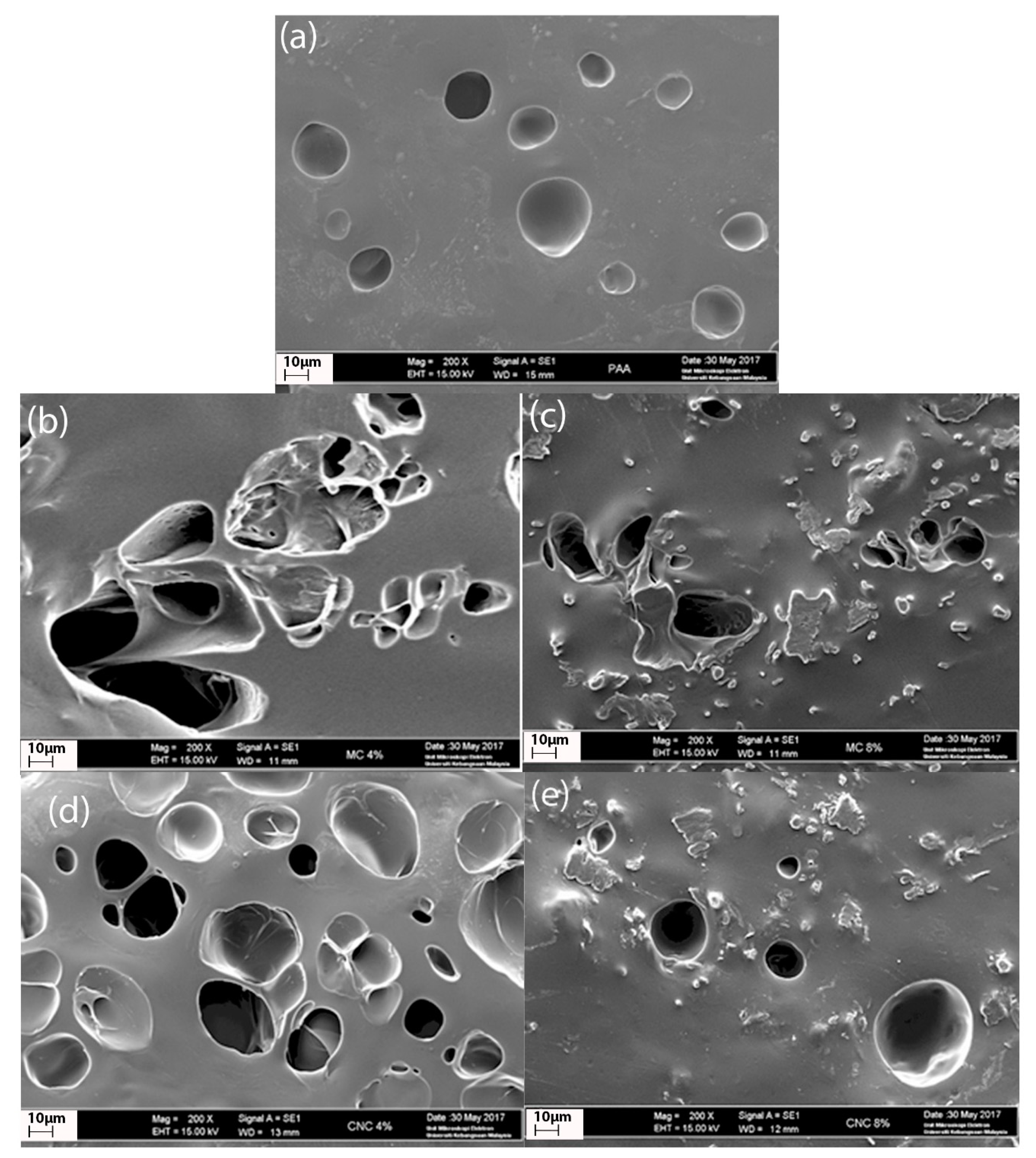
3.5. Scanning Electron Microscopy (SEM)
3.6. Rheology Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rueda, J.C.; Santillán, F.; Komber, H.; Voit, B. Synthesis and Characterization of Stiff, Self-Crosslinked Thermoresponsive DMAA Hydrogels. Polymers 2020, 12, 1401. [Google Scholar] [CrossRef] [PubMed]
- Ilić-Stojanović, S.; Urošević, M.; Nikolić, L.; Petrović, D.; Stanojević, J.; Najman, S.; Nikolić, V. Intelligent Poly (N-Isopropylmethacrylamide) Hydrogels: Synthesis, Structure Characterization, Stimuli-Responsive Swelling Properties, and Their Radiation Decomposition. Polymers 2020, 12, 1112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chu, L.-Y.; Li, Y.-K.; Lee, Y.M. Dual thermo-and pH-sensitive poly (N-isopropylacrylamide-co-acrylic acid) hydrogels with rapid response behaviors. Polymer 2007, 48, 1718–1728. [Google Scholar] [CrossRef]
- Chen, Y.; Pang, X.H.; Dong, C.M. Dual Stimuli-Responsive Supramolecular Polypeptide-Based Hydrogel and Reverse Micellar Hydrogel Mediated by Host–Guest Chemistry. Adv. Funct. Mater. 2010, 20, 579–586. [Google Scholar] [CrossRef]
- Lim, L.S.; Rosli, N.A.; Ahmad, I.; Mat Lazim, A.; Mohd Amin, M.C.I. Synthesis and swelling behavior of pH-sensitive semi-IPN superabsorbent hydrogels based on poly (acrylic acid) reinforced with cellulose nanocrystals. Nanomaterials 2017, 7, 399. [Google Scholar] [CrossRef]
- Tuan, H.N.A.; Nhu, V.T.T. Synthesis and Properties of pH-Thermo Dual Responsive Semi-IPN Hydrogels Based on N, N’-Diethylacrylamide and Itaconamic Acid. Polymers 2020, 12, 1139. [Google Scholar] [CrossRef]
- Nau, M.; Trosien, S.; Seelinger, D.; Boehm, A.K.; Biesalski, M. Spatially resolved crosslinking of hydroxypropyl cellulose esters for the generation of functional surface-attached organogels. Front. Chem. 2019, 7, 367. [Google Scholar] [CrossRef]
- Arunbabu, D.; Shahsavan, H.; Zhang, W.; Zhao, B. Poly (AAc-co-MBA) hydrogel films: Adhesive and mechanical properties in aqueous medium. J. Phys. Chem. B 2013, 117, 441–449. [Google Scholar] [CrossRef]
- Peppas, N.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Baker, J.P.; Blanch, H.W.; Prausnitz, J.M. Equilibrium swelling properties of weakly lonizable 2-hydroxyethyl methacrylate (HEMA)-based hydrogels. J. Appl. Polym. Sci. 1994, 52, 783–788. [Google Scholar] [CrossRef]
- Zhao, Y.; Kang, J.; Tan, T. Salt-, pH-and temperature-responsive semi-interpenetrating polymer network hydrogel based on poly (aspartic acid) and poly (acrylic acid). Polymer 2006, 47, 7702–7710. [Google Scholar] [CrossRef]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Barbucci, R.; Leone, G.; Vecchiullo, A. Novel carboxymethylcellulose-based microporous hydrogels suitable for drug delivery. J. Biomater. Sci. Polym. Ed. 2004, 15, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.-T.; Liu, J.-H. Fabrication and characterization of poly (vinyl alcohol)/chitosan hydrogel thin films via UV irradiation. Eur. Polym. J. 2013, 49, 4201–4211. [Google Scholar] [CrossRef]
- Hu, X.; Gao, Z.; Tan, H.; Wang, H.; Mao, X.; Pang, J. An injectable hyaluronic acid-based composite hydrogel by DA click chemistry with pH sensitive nanoparticle for biomedical application. Front. Chem. 2019, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lu, J.; Xiao, C. Thermal and mechanical properties of cationic guar gum/poly (acrylic acid) hydrogel membranes. Polym. Degrad. Stab. 2007, 92, 1072–1081. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Yuan, Z.; Han, H.; Li, T.; Li, L.; Guo, X. Chitosan cross-linked poly (acrylic acid) hydrogels: Drug release control and mechanism. Colloids Surf. B Biointerfaces 2017, 152, 252–259. [Google Scholar] [CrossRef]
- Al-qudah, Y.H.; Mahmoud, G.A.; Khalek, M.A. Radiation crosslinked poly (vinyl alcohol)/acrylic acid copolymer for removal of heavy metal ions from aqueous solutions. J. Radiat. Res. Appl. Sci. 2014, 7, 135–145. [Google Scholar] [CrossRef]
- Wan Ishak, W.H.; Ahmad, I.; Ramli, S.; Mohd Amin, M.C.I. Gamma irradiation-assisted synthesis of cellulose nanocrystal-reinforced gelatin hydrogels. Nanomaterials 2018, 8, 749. [Google Scholar] [CrossRef]
- Morehouse, K.M.; Komolprasert, V. Irradiation of food and packaging: An overview. ACS Publ. 2004. [Google Scholar] [CrossRef]
- Chong, E.L.; Ahmad, I.; Dahlan, H.M.; Abdullah, I. Reinforcement of Natural rubber/High Density Polyethylene blends with Electron Beam Irradiated Liquid Natural Rubber-coated Rice Husk. Radiat. Phys. Chem. 2010, 79, 906–911. [Google Scholar] [CrossRef]
- Shanmugarajah, B.; Kiew, P.L.; Chew, I.M.L.; Choong, T.S.Y.; Tan, K.W. Isolation of nanocrystalline cellulose (NCC) from palm oil empty fruit bunch (EFB): Preliminary result on FTIR and DLS analysis. Chem. Eng. Trans. 2015, 45, 1705–1710. [Google Scholar] [CrossRef]
- Ooi, S.Y.; Ahmad, I.; Amin, M.C.I.M. Cellulose nanocrystals extracted from rice husks as a reinforcing material in gelatin hydrogels for use in controlled drug delivery systems. Ind. Crop. Prod. 2016, 93, 227–234. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Wu, C.; Guo, B.; Luo, Z. Fabrication of mechanically tough and self-recoverable nanocomposite hydrogels from polyacrylamide grafted cellulose nanocrystal and poly (acrylic acid). Carbohydr. Polym. 2018, 198, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Abeer, M.M.; Amin, M.C.I.M.; Lazim, A.M.; Pandey, M.; Martin, C. Synthesis of a novel acrylated abietic acid-g-bacterial cellulose hydrogel by gamma irradiation. Carbohydr. Polym. 2014, 110, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.; Kumar, M.; Varshney, L. Radiation synthesis of superabsorbent poly (acrylic acid)–carrageenan hydrogels. Radiat. Phys. Chem. 2004, 69, 481–486. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: A new ageless bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Sheltami, R.M.; Abdullah, I.; Ahmad, I.; Dufresne, A.; Kargarzadeh, H. Extraction of cellulose nanocrystals from mengkuang leaves (Pandanus tectorius). Carbohydr. Polym. 2012, 88, 772–779. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues—Wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef]
- Mohd Amin, M.C.I.; Ahmad, N.; Halib, N.; Ahmad, I. Synthesis and characterization of thermo- and pH-responsive bacterial cellulose/acrylic acid hydrogels for drug delivery. Carbohydr. Polym. 2012, 88, 465–473. [Google Scholar] [CrossRef]
- Nho, Y.-C.; Park, J.-S.; Lim, Y.-M. Preparation of Poly(acrylic acid) Hydrogel by Radiation Crosslinking and Its Application for Mucoadhesives. Polymers 2014, 6, 890. [Google Scholar] [CrossRef]
- Tokarev, I.; Minko, S. Stimuli-responsive hydrogel thin films. Soft Matter 2009, 5, 511–524. [Google Scholar] [CrossRef]
- Razmjou, A.; Simon, G.P.; Wang, H. Effect of particle size on the performance of forward osmosis desalination by stimuli-responsive polymer hydrogels as a draw agent. Chem. Eng. J. 2013, 215–216, 913–920. [Google Scholar] [CrossRef]
- Alvarado, A.G.; Cortés, J.; Pérez-Carrillo, L.A.; Rabelero, M.; Arellano, J.; Sánchez-Díaz, J.C.; Puig, J.E.; Arellano, M. Temperature and pH-Responsive Polyacrylamide/Poly(Acrylic Acid) Interpenetrating Polymer Network Nanoparticles. J. Macromol. Sci. Part B 2016, 55, 1086–1098. [Google Scholar] [CrossRef]
- Serpe, M.J.; Jones, C.D.; Lyon, L.A. Layer-by-Layer Deposition of Thermoresponsive Microgel Thin Films. Langmuir 2003, 19, 8759–8764. [Google Scholar] [CrossRef]
- Park, S.-E.; Nho, Y.-C.; Lim, Y.-M.; Kim, H.-I. Preparation of pH-sensitive poly(vinyl alcohol-g-methacrylic acid) and poly(vinyl alcohol-g-acrylic acid) hydrogels by gamma ray irradiation and their insulin release behavior. J. Appl. Polym. Sci. 2004, 91, 636–643. [Google Scholar] [CrossRef]
- Abd Alla, S.G.; Sen, M.; El-Naggar, A.W.M. Swelling and mechanical properties of superabsorbent hydrogels based on Tara gum/acrylic acid synthesized by gamma radiation. Carbohydr. Polym. 2012, 89, 478–485. [Google Scholar] [CrossRef]
- Aouada, F.A.; de Moura, M.R.; Orts, W.J.; Mattoso, L.H.C. Preparation and Characterization of Novel Micro- and Nanocomposite Hydrogels Containing Cellulosic Fibrils. J. Agric. Food Chem. 2011, 59, 9433–9442. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, Z.; Fan, Q.; Chen, L.; Zhu, M. Facile in-situ fabrication of novel organic nanoparticle hydrogels with excellent mechanical properties. J. Mater. Chem. 2009, 19, 7340–7346. [Google Scholar] [CrossRef]




| Composition | Cellulose/CNC (g) | Water (mL) | Acrylic Acid (AA) (g) |
|---|---|---|---|
| 0% | 0.00 | 30.00 | 5.00 |
| 4% | 0.20 | 30.00 | 4.80 |
| 8% | 0.40 | 30.00 | 4.60 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan Ishak, W.H.; Yong Jia, O.; Ahmad, I. pH-Responsive Gamma-Irradiated Poly(Acrylic Acid)-Cellulose-Nanocrystal-Reinforced Hydrogels. Polymers 2020, 12, 1932. https://doi.org/10.3390/polym12091932
Wan Ishak WH, Yong Jia O, Ahmad I. pH-Responsive Gamma-Irradiated Poly(Acrylic Acid)-Cellulose-Nanocrystal-Reinforced Hydrogels. Polymers. 2020; 12(9):1932. https://doi.org/10.3390/polym12091932
Chicago/Turabian StyleWan Ishak, Wan Hafizi, Oo Yong Jia, and Ishak Ahmad. 2020. "pH-Responsive Gamma-Irradiated Poly(Acrylic Acid)-Cellulose-Nanocrystal-Reinforced Hydrogels" Polymers 12, no. 9: 1932. https://doi.org/10.3390/polym12091932
APA StyleWan Ishak, W. H., Yong Jia, O., & Ahmad, I. (2020). pH-Responsive Gamma-Irradiated Poly(Acrylic Acid)-Cellulose-Nanocrystal-Reinforced Hydrogels. Polymers, 12(9), 1932. https://doi.org/10.3390/polym12091932