Preparation of Cross-Linked Graphene Oxide on Polyethersulfone Membrane for Pharmaceuticals and Personal Care Products Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of the Membranes
2.2.1. Pretreatment of Support Layer
2.2.2. Preparation of Cross-Linked GO Self-Supporting Film
2.3. Membrane Characterization
2.3.1. Morphology and Microstructure
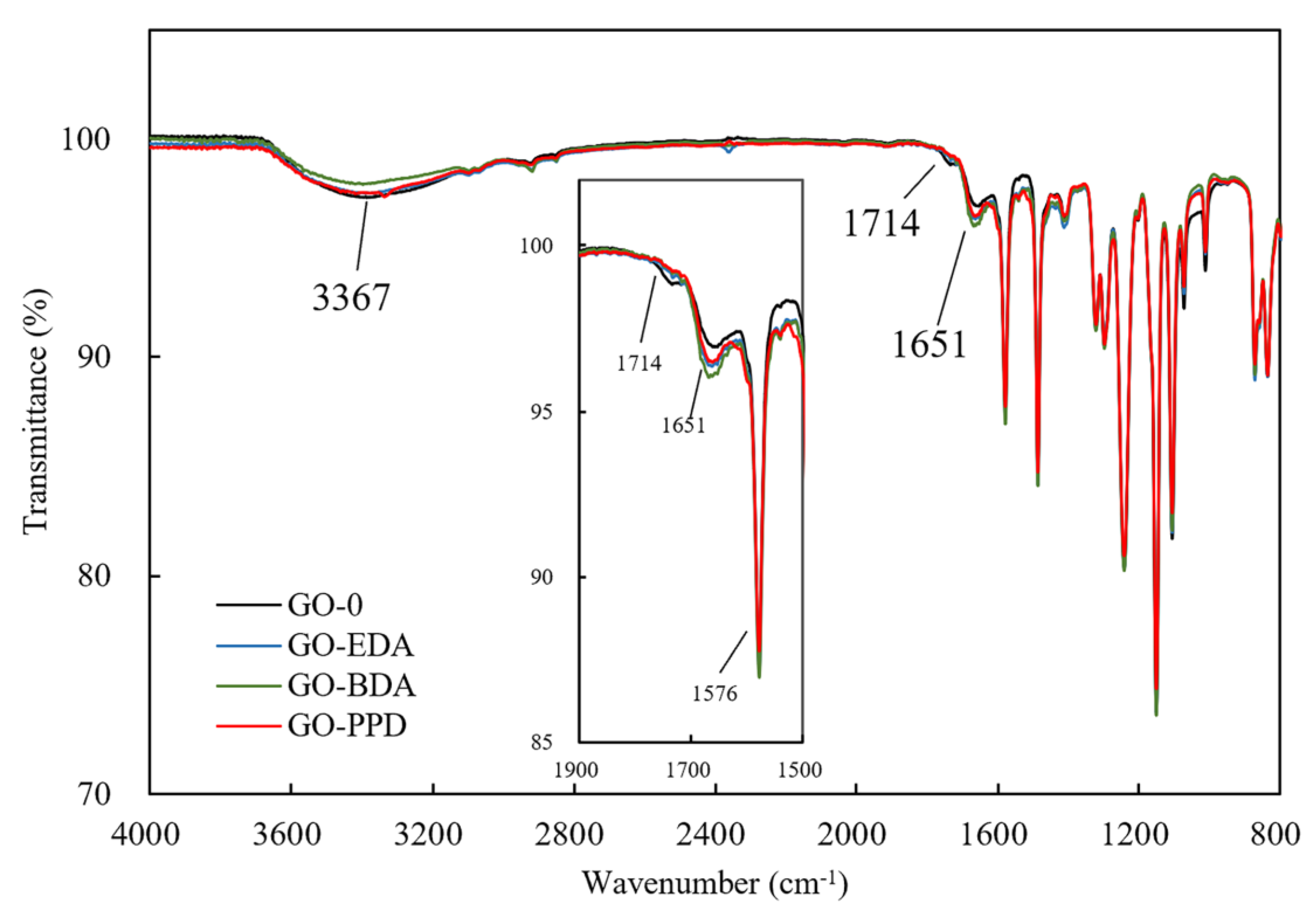
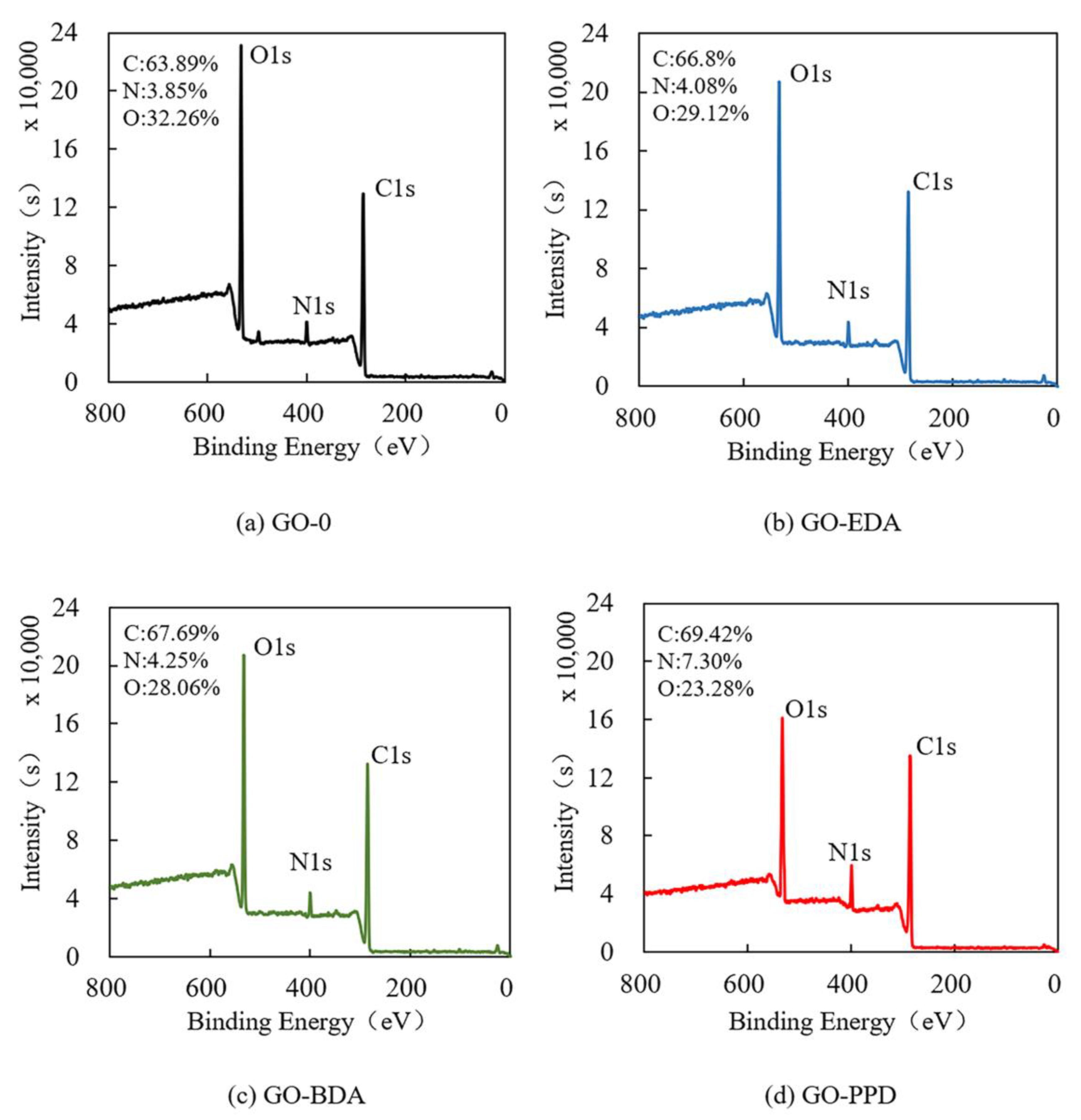
2.3.2. Chemical Properties
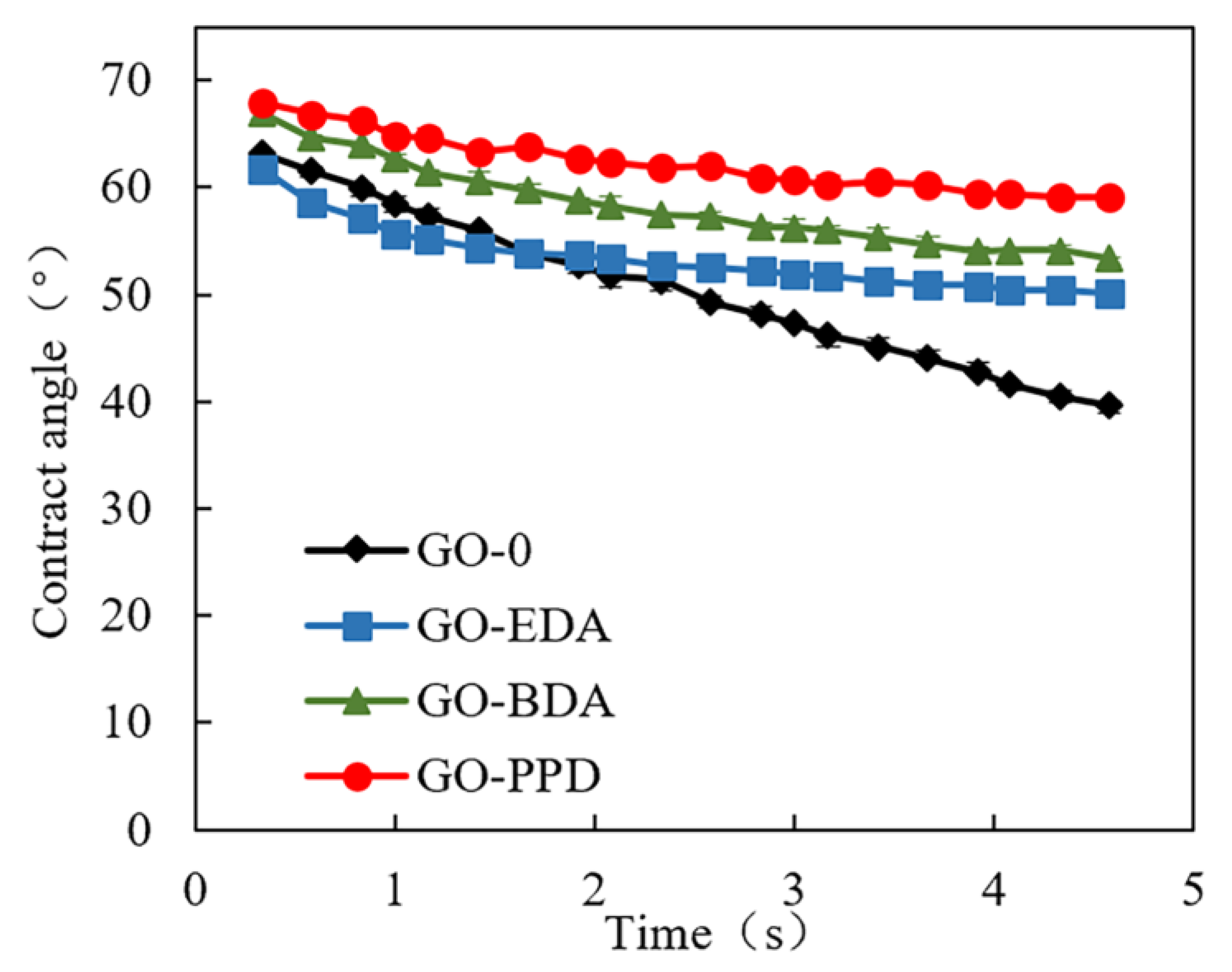
2.3.3. Surface Hydrophilicity
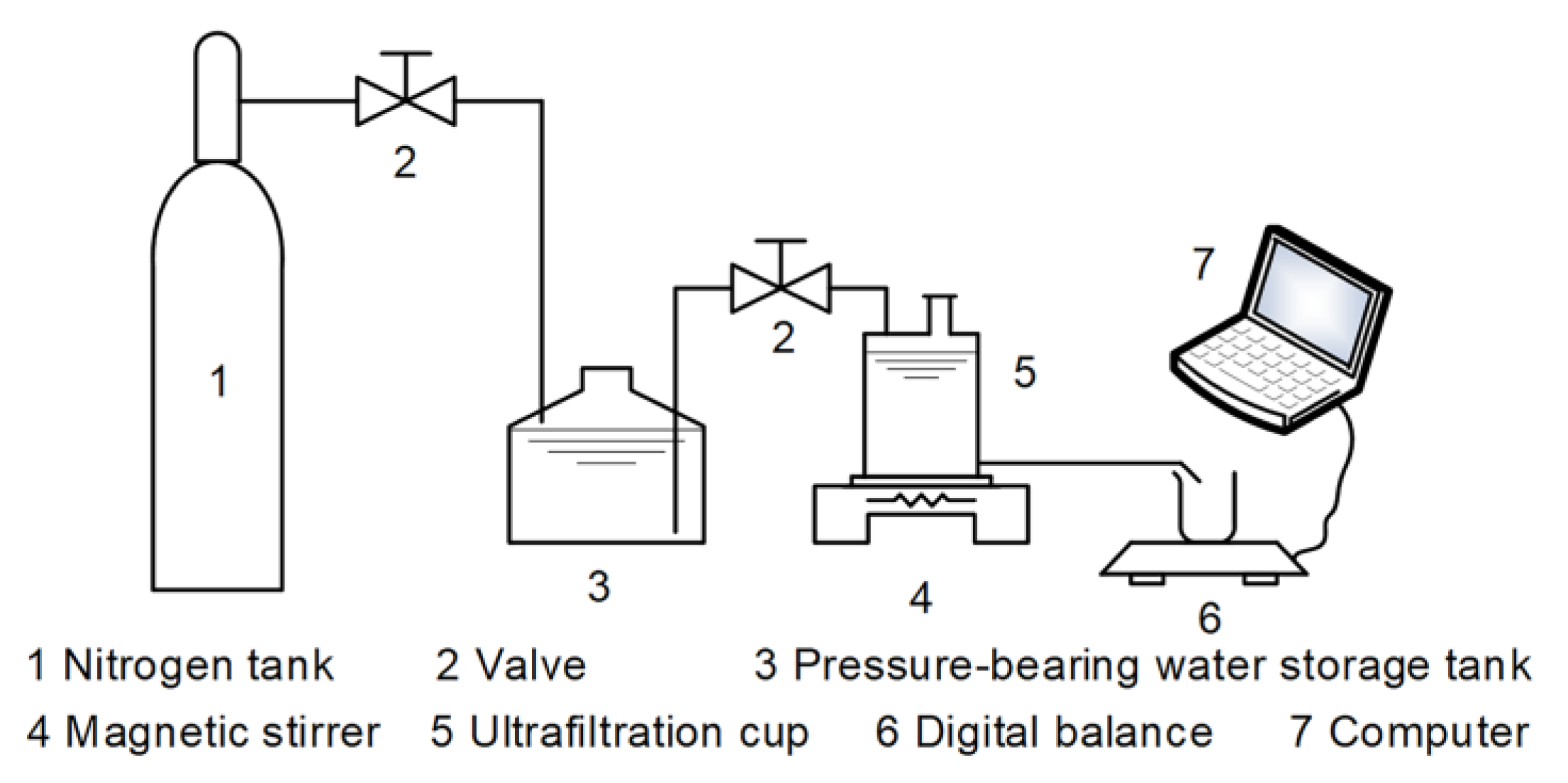
2.3.4. Filtration Experiments
2.3.5. Instruments and Methods Used for Water Analysis
3. Results and Discussion
3.1. Characterization of Cross-Linked GO Self-Supporting Membranes
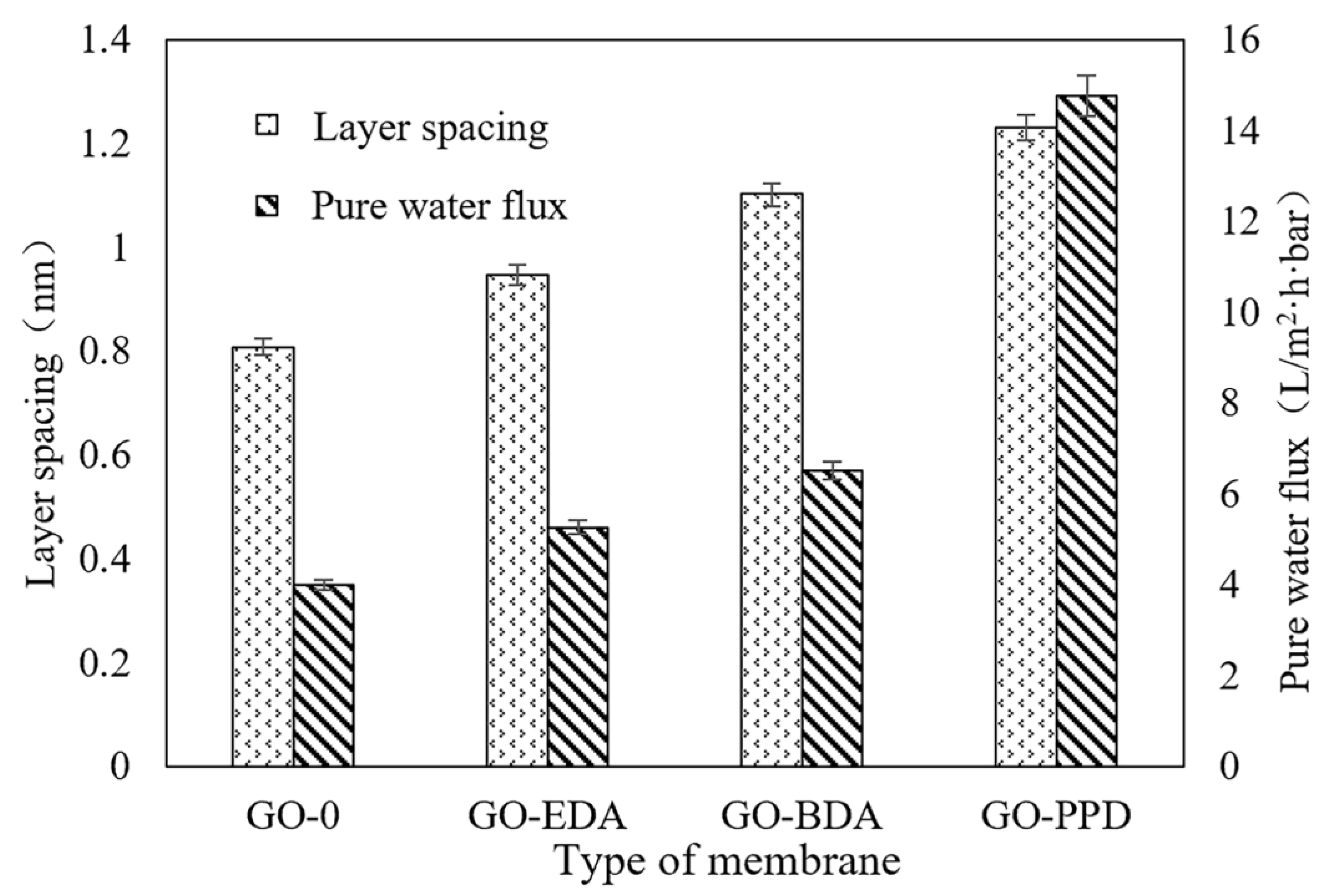
3.2. Flux of Cross-Linked GO Self-Supporting Membrane
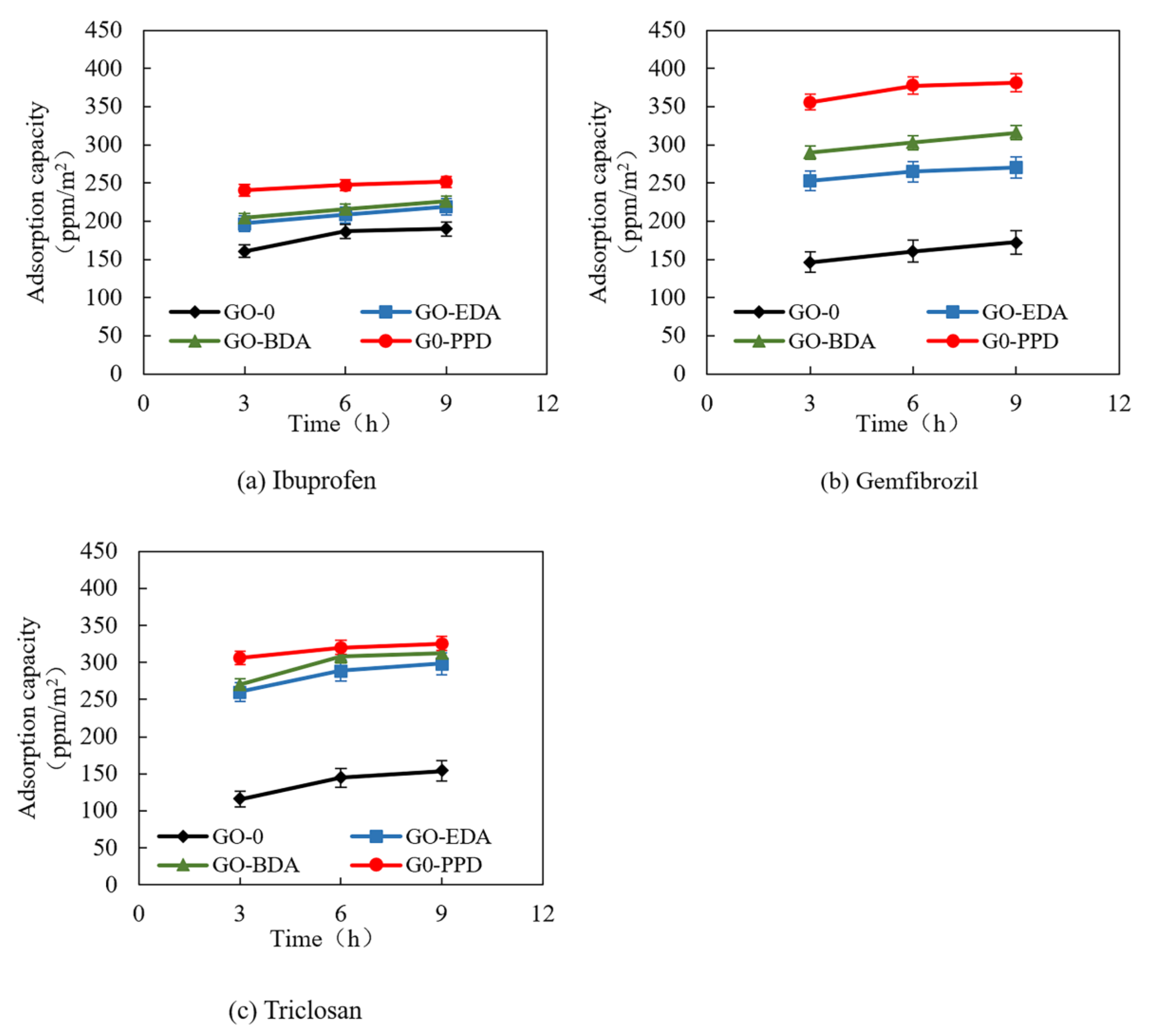
3.3. Adsorption of PPCPs by Cross-Linked GO Self-Supporting Membranes
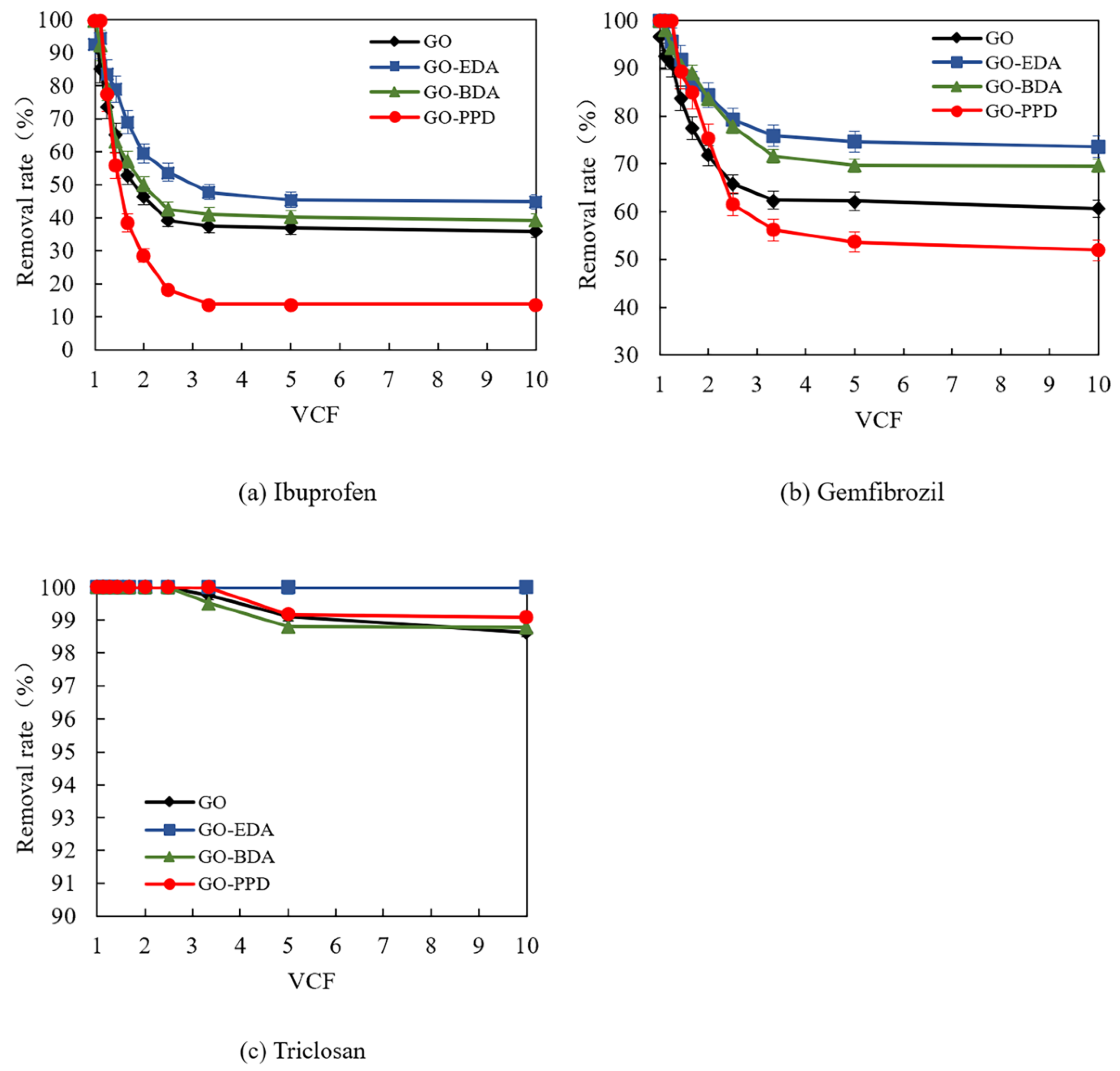
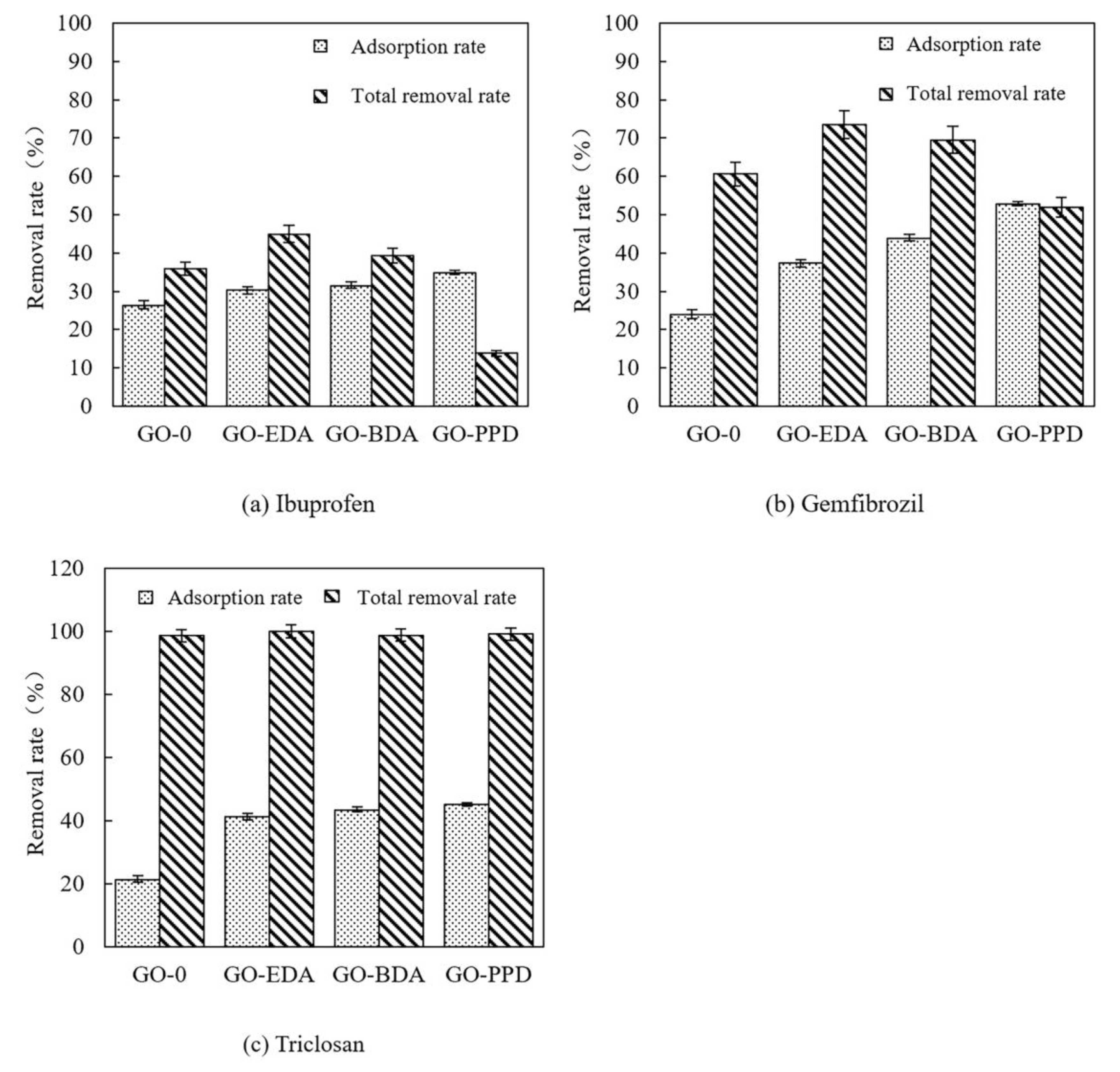
3.4. Removal of PPCPs by Cross-Linked Self-Supporting GO Membranes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Padhye, L.P.; Yao, H.; Kung’u, F.T.; Huang, C.-H. Year-long evaluation on the occurrence and fate of pharmaceuticals, personal care products, and endocrine disrupting chemicals in an urban drinking water treatment plant. Water Res. 2014, 51, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Loos, R.; Carvalho, R.; Antonio, D.C.; Cornero, S.; Locoro, G.; Tavazzi, S.; Paracchini, B.; Ghiani, M.; Lettieri, T.; Blaha, L.; et al. EU-wide monitoring survey on emerging polar organic contaminants in wastewater treatment plant effluents. Water Res. 2013, 47, 6475–6487. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Westerhoff, P.; Snyder, S.A.; Wert, E.C.; Yoon, J. Removal of endocrine disrupting compounds and pharmaceuticals by nanofiltration and ultrafiltration membranes. Desalination 2007, 202, 16–23. [Google Scholar] [CrossRef]
- Cleuvers, M. Aquatic ecotoxicity of pharmaceuticals including the assessment of combination effects. Toxicol. Lett. 2003, 142, 185–194. [Google Scholar] [CrossRef]
- Loos, R.; Gawlik, B.M.; Locoro, G.; Rimaviciute, E.; Contini, S.; Bidoglio, G. EU-wide survey of polar organic persistent pollutants in European river waters. Enviorn. Pollut. 2009, 157, 561–568. [Google Scholar] [CrossRef]
- Schwab, B.W.; Hayes, E.P.; Fiori, J.M.; Mastrocco, F.J.; Roden, N.M.; Cragin, D.; Meyerhoff, R.D.; D’Aco, V.J.; Anderson, P.D. Human pharmaceuticals in US surface waters: A human health risk assessment. Regul. Toxicol. Pharmacol. 2005, 42, 296–312. [Google Scholar] [CrossRef]
- Araujo, L.; Villa, N.; Camargo, N.; Bustos, M.; Garcia, T.; de Jesus Prieto, A. Persistence of gemfibrozil, naproxen and mefenamic acid in natural waters. Environ. Chem. Lett. 2011, 9, 13–18. [Google Scholar] [CrossRef]
- Prindiville, J.S.; Mennigen, J.A.; Zamora, J.M.; Moon, T.W.; Weber, J.-M. The fibrate drug gemfibrozil disrupts lipoprotein metabolism in rainbow trout. Toxicol. Appl. pharmacol. 2011, 251, 201–208. [Google Scholar] [CrossRef]
- Tato, T.; Salgueiro-Gonzalez, N.; Leon, V.M.; Gonzalez, S.; Beiras, R. Ecotoxicological evaluation of the risk posed by bisphenol A, triclosan, and 4-nonylphenol in coastal waters using early life stages of marine organisms (Isochrysis galbana, Mytilus galloprovincialis, Paracentrotus lividus, and Acartia clausi). Environ. Pollut. 2018, 232, 173–182. [Google Scholar] [CrossRef]
- Jachero, L.; Ahumada, I.; Fuentes, E.; Richter, P. Decreases in the bioconcentration of triclosan in wheat plants according to increasing amounts of biosolids added to soil. Geoderma 2016, 276, 19–25. [Google Scholar] [CrossRef]
- Gonzalez-Pleiter, M.; Rioboo, C.; Reguera, M.; Abreu, I.; Leganes, F.; Cid, A.; Fernandez-Pinas, F. Calcium mediates the cellular response of Chlamydomonas reinhardtii to the emerging aquatic pollutant Triclosan. Aquat. Toxicol. 2017, 186, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Westerhoff, P.; Snyder, S.A.; Wert, E.C. Nanofiltration and ultrafiltration of endocrine disrupting compounds, pharmaceuticals and personal care products. J. Membr. Sci. 2006, 270, 88–100. [Google Scholar] [CrossRef]
- Wang, X.; Li, N.; Zhao, Y.; Xia, S. Preparation of graphene oxide incorporated polyamide thin-film composite membranes for PPCPs removal. Membr. Water Treat. 2018, 9, 211–220. [Google Scholar] [CrossRef]
- Yang, H.; Wang, X. Mechanism of removal of pharmaceuticals and personal care products by nanofiltration membranes. Desalin. Water Treat. 2015, 53, 2816–2824. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, L.; Ju, Z.; Feng, G. A review on the natural organic matter fouling and the removal of PPCPs by nanofiltration membrane. Membr. Sci. Technol. 2019, 39, 116–122. [Google Scholar]
- Yang, X.; He, Y.; Zeng, G.; Chen, X.; Shi, H.; Qing, D.; Li, F.; Chen, Q. Bio-inspired method for preparation of multiwall carbon nanotubes decorated superhydrophilic poly(vinylidene fluoride) membrane for oil/water emulsion separation. Chem. Eng. J. 2017, 321, 245–256. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Z.; Shan, M.; Zhou, B.; Li, Y.; Li, B.; Niu, J.; Qian, X. Synergetic effects of oxidized carbon nanotubes and graphene oxide on fouling control and anti-fouling mechanism of polyvinylidene fluoride ultrafiltration membranes. J. Membr. Sci. 2013, 448, 81–92. [Google Scholar] [CrossRef]
- Sharma, G.; Gupta, V.K.; Agarwal, S.; Bhogal, S.; Naushad, M.; Kumar, A.; Stadler, F.J. Fabrication and characterization of trimetallic nano-photocatalyst for remediation of ampicillin antibiotic. J. Mol. Liq. 2018, 260, 342–350. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Sharma, G.; Al-Muhtaseb, A.a.H.; Naushad, M.; Ghfar, A.A.; Stadler, F.J. Quaternary magnetic BiOCl/g-C3N4/Cu2O/Fe3O4 nano-junction for visible light and solar powered degradation of sulfamethoxazole from aqueous environment. Chem. Eng. J. 2018, 334, 462–478. [Google Scholar] [CrossRef]
- Abdel-Karim, A.; Leaper, S.; Alberto, M.; Vijayaraghavan, A.; Fan, X.; Holmes, S.M.; Souaya, E.R.; Badawy, M.I.; Gorgojo, P. High flux and fouling resistant flat sheet polyethersulfone membranes incorporated with graphene oxide for ultrafiltration applications. Chem. Eng. J. 2018, 334, 789–799. [Google Scholar] [CrossRef]
- Liu, L.; Xie, X.; Zambare, R.S.; Selvaraj, A.P.J.; Sowrirajalu, B.N.I.L.; Song, X.; Tang, C.Y.; Gao, C. Functionalized Graphene Oxide Modified Polyethersulfone Membranes for Low-Pressure Anionic Dye/Salt Fractionation. Polymers 2018, 10, 795. [Google Scholar] [CrossRef] [PubMed]
- Giwa, A.; Hasan, S.W. Novel polyethersulfone-functionalized graphene oxide (PES-fGO) mixed matrix membranes for wastewater treatment. Sep. Purif. Technol. 2020, 241, 116735. [Google Scholar] [CrossRef]
- Zhang, P.; Gong, J.-L.; Zeng, G.-M.; Deng, C.-H.; Yang, H.-C.; Liu, H.-Y.; Huan, S.-Y. Cross-linking to prepare composite graphene oxide-framework membranes with high-flux for dyes and heavy metal ions removal. Chem. Eng. J. 2017, 322, 657–666. [Google Scholar] [CrossRef]
- Wang, X.; Wu, C.; Zhu, T.; Li, P.; Xia, S. The hierarchical flower-like MoS2 nanosheets incorporated into PES mixed matrix membranes for enhanced separation performance. Chemosphere 2020, 256, 127099. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, M.; Wang, J.; Liu, G.; Liu, H.; Jiang, Y. Bioinspired Modification of Layer-Stacked Molybdenum Disulfide (MoS2) Membranes for Enhanced Nanofiltration Performance. ACS Omega 2019, 4, 4012–4022. [Google Scholar] [CrossRef]
- Jiang, Q.; Tian, H.; Zhang, K. Enhanced performance of poly(m-phenylene isophthalamide) (PMIA) composite hollow fiber ultrafiltration membranes by O-MoS2 nanosheets modification. Desalin. Water Treat. 2019, 166, 245–258. [Google Scholar] [CrossRef]
- Wu, C.; Wang, X.; Zhu, T.; Li, P.; Xia, S. Covalent organic frameworks embedded membrane via acetic-acid-catalyzed interfacial polymerization for dyes separation: Enhanced permeability and selectivity. Chemosphere 2020, 261, 127580. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Ma, Y.; Wu, X.; Meng, L.; Guo, Y.; Yu, G.; Liu, Y. Graphene-coated silica as a highly efficient sorbent for residual organophosphorus pesticides in water. J. Mater. Chem. A 2013, 1, 1875–1884. [Google Scholar] [CrossRef]
- Balasubramani, K.; Sivarajasekar, N.; Naushad, M. Effective adsorption of antidiabetic pharmaceutical (metformin) from aqueous medium using graphene oxide nanoparticles: Equilibrium and statistical modelling. J. Mol. Liq. 2020, 301, 112426. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, F.; Sun, Z.; Hasan, T.; Ferrari, A.C. Graphene photonics and optoelectronics. Nat. Photonics 2010, 4, 611–622. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Lerf, A.; He, H.Y.; Forster, M.; Klinowski, J. Structure of graphite oxide revisited. JPCB 1998, 102, 4477–4482. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Fang, M.; Zhang, Z.; Li, J.; Zhang, H.; Lu, H.; Yang, Y. Constructing hierarchically structured interphases for strong and tough epoxy nanocomposites by amine-rich graphene surfaces. J. Mater. Chem. 2010, 20, 9635–9643. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, Y.; Jiang, X.; Yu, L.; Wang, C. Highly Dual Antifouling and Antibacterial Ultrafiltration Membranes Modified with Silane Coupling Agent and Capsaicin-Mimic Moieties. Polymers 2020, 12, 412. [Google Scholar] [CrossRef]
- Eren, E.; Sarihan, A.; Eren, B.; Gumus, H.; Kocak, F.O. Preparation, characterization and performance enhancement of polysulfone ultrafiltration membrane using PBI as hydrophilic modifier. J. Membr. Sci. 2015, 475, 1–8. [Google Scholar] [CrossRef]
- Mozia, S.; Grylewicz, A.; Zgrzebnicki, M.; Darowna, D.; Czyzewski, A. Investigations on the Properties and Performance of Mixed-Matrix Polyethersulfone Membranes Modified with Halloysite Nanotubes. Polymers 2019, 11, 671. [Google Scholar] [CrossRef]
- Fang, X.; Li, J.; Li, X.; Pan, S.; Sun, X.; Shen, J.; Han, W.; Wang, L.; Van der Bruggen, B. Iron-tannin-framework complex modified PES ultrafiltration membranes with enhanced filtration performance and fouling resistance. J. Colloid Interface Sci. 2017, 505, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Hegab, H.M.; Zou, L. Graphene oxide-assisted membranes: Fabrication and potential applications in desalination and water purification. J. Membr. Sci. 2015, 484, 95–106. [Google Scholar] [CrossRef]
- Hung, W.-S.; Tsou, C.-H.; De Guzman, M.; An, Q.-F.; Liu, Y.-L.; Zhang, Y.-M.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Cross-Linking with Diamine Monomers To Prepare Composite Graphene Oxide-Framework Membranes with Varying d-Spacing. Chem. Mater. 2014, 26, 2983–2990. [Google Scholar] [CrossRef]
- Balta, S.; Sotto, A.; Luis, P.; Benea, L.; Van der Bruggen, B.; Kim, J. A new outlook on membrane enhancement with nanoparticles: The alternative of ZnO. J. Membr. Sci. 2012, 389, 155–161. [Google Scholar] [CrossRef]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef]
- Idris, A.; Zain, N.M.; Noordin, M.Y. Synthesis, characterization and performance of asymmetric polyethersulfone (PES) ultrafiltration membranes with polyethylene glycol of different molecular weights as additives. Desalination 2007, 207, 324–339. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Compton, O.C.; Dommett, G.H.B.; Ruoff, R.S.; Nguyen, S.T. Systematic Post-assembly Modification of Graphene Oxide Paper with Primary Alkylamines. Chem. Mater. 2010, 22, 4153–4157. [Google Scholar] [CrossRef]
- Naushad, M.; Sharma, G.; Kumar, A.; Sharma, S.; Ghfar, A.A.; Bhatnagar, A.; Stadler, F.J.; Khan, M.R. Efficient removal of toxic phosphate anions from aqueous environment using pectin based quaternary amino anion exchanger. Int. J. Biol Macromol. 2018, 106, 1–10. [Google Scholar] [CrossRef]
- Doederer, K.; Farre, M.J.; Pidou, M.; Weinberg, H.S.; Gernjak, W. Rejection of disinfection by-products by RO and NF membranes: Influence of solute properties and operational parameters. J. Membr. Sci. 2014, 467, 195–205. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Schafer, A.I. Adsorption and transport of trace contaminant estrone in NF/RO membranes. Environ. Eng. Sci. 2002, 19, 441–451. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Schafer, A.I.; Waite, T.D. Adsorptive interactions between membranes and trace contaminants. Desalination 2002, 147, 269–274. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Schafer, A.I.; Waite, T.D. Adsorption of estrone on nanofiltration and reverse osmosis membranes in water and wastewater treatment. Water Sci. Technol. 2002, 46, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Duranceau, S.J.; Taylor, J.S.; Mulford, L.A. SOC Removal in a Membrane Softening Process. J. Am. Water Work. Assoc. 1992, 84, 68–78. [Google Scholar] [CrossRef]
| Target Compound | CAS Number | Molecular Weight | Acid Dissociation Constant (pKa) | N-Octanol/Water Partition Coefficient (logKOW) | Chemical Structural Formula |
|---|---|---|---|---|---|
| Ibuprofen | 15687-27-1 | 206.3 | 4.91 | 3.97 |  |
| Gemfibrozil | 25812-30-0 | 250.3 | 4.7 | 4.77 |  |
| Triclosan | 3380-34-5 | 289.5 | 7.9 | 4.76 | 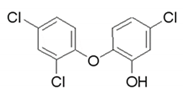 |
| Membrane Type | GO-0 | GO-EDA | GO-BDA | GO-PPD |
|---|---|---|---|---|
| Layer spacing | 0.81 nm | 0.95 nm | 1.10 nm | 1.23 nm |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, Y.; Tan, F.J.; Zeng, R.; Wang, M.; Li, P.; Xia, S. Preparation of Cross-Linked Graphene Oxide on Polyethersulfone Membrane for Pharmaceuticals and Personal Care Products Removal. Polymers 2020, 12, 1921. https://doi.org/10.3390/polym12091921
Lou Y, Tan FJ, Zeng R, Wang M, Li P, Xia S. Preparation of Cross-Linked Graphene Oxide on Polyethersulfone Membrane for Pharmaceuticals and Personal Care Products Removal. Polymers. 2020; 12(9):1921. https://doi.org/10.3390/polym12091921
Chicago/Turabian StyleLou, Yanyan, Fibor J. Tan, Rong Zeng, Mengen Wang, Pan Li, and Shengji Xia. 2020. "Preparation of Cross-Linked Graphene Oxide on Polyethersulfone Membrane for Pharmaceuticals and Personal Care Products Removal" Polymers 12, no. 9: 1921. https://doi.org/10.3390/polym12091921
APA StyleLou, Y., Tan, F. J., Zeng, R., Wang, M., Li, P., & Xia, S. (2020). Preparation of Cross-Linked Graphene Oxide on Polyethersulfone Membrane for Pharmaceuticals and Personal Care Products Removal. Polymers, 12(9), 1921. https://doi.org/10.3390/polym12091921





