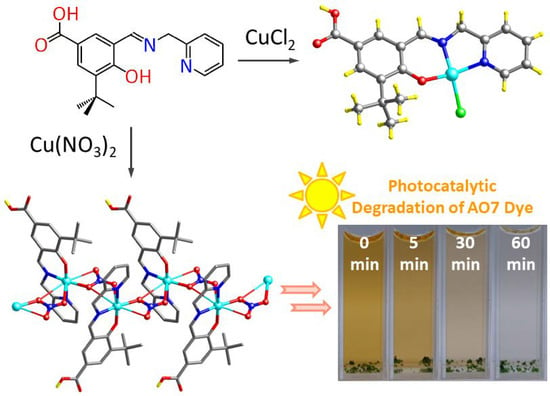
Anion-Dominated Copper Salicyaldimine Complexes—Structures, Coordination Mode of Nitrate and Decolorization Properties toward Acid Orange 7 Dye
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Synthesis of 3-Tert-butyl-4-hydroxy-5-(((pyridin-2-ylmethyl)imino)methyl)benzoic Acid (H2Lsalpyca)
2.3. Synthesis of [Cu(HLsalpyca)Cl] (1)
2.4. Synthesis of [Cu(HLsalpyca)(NO3)]n (2)
2.5. X-Ray Data Collection and Structure Refinement
3. Results and Discussion
3.1. Syntheses and Characterization
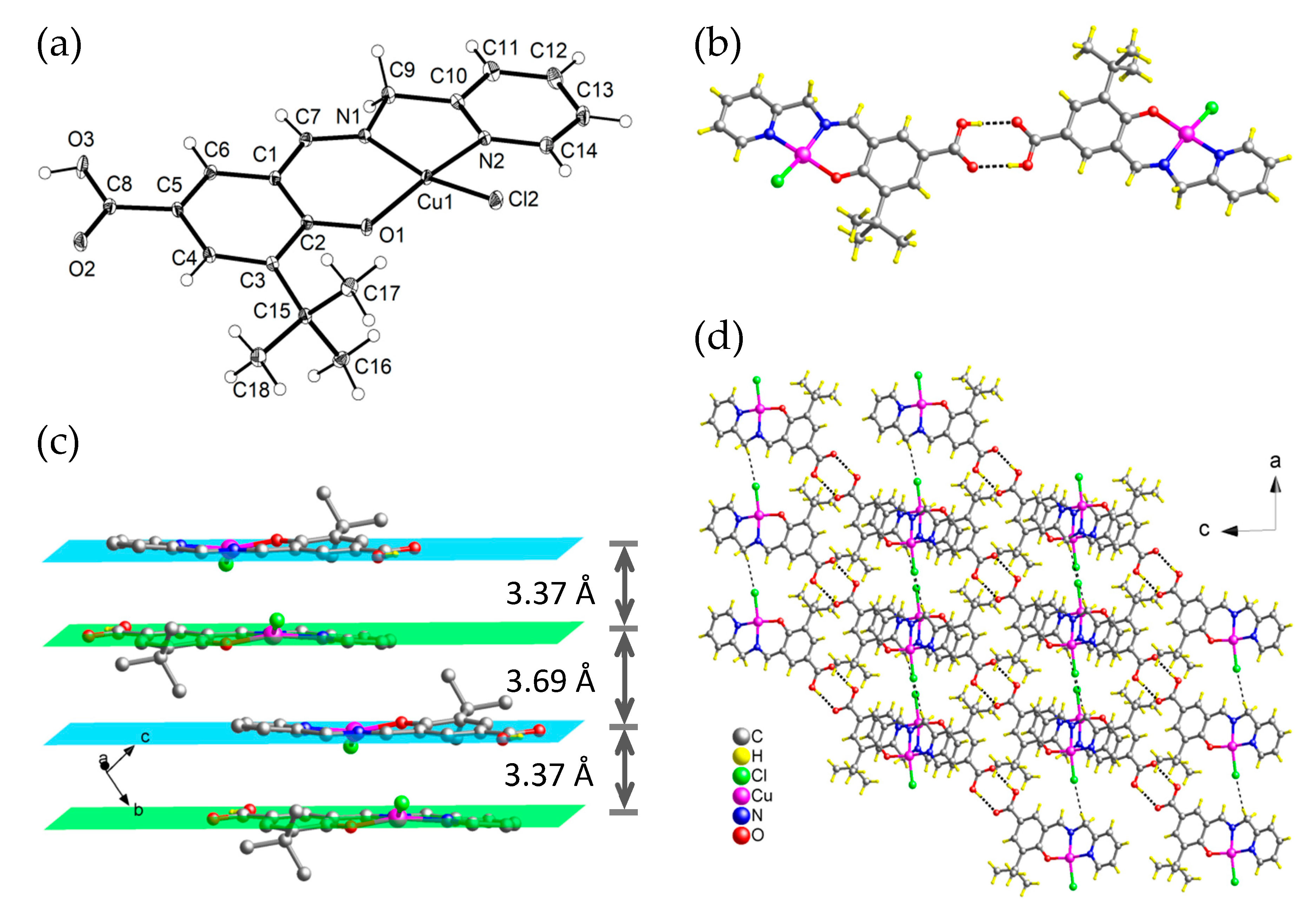
3.2. Crystal Structural Description of [Cu(HLsalpyca)Cl] (1)
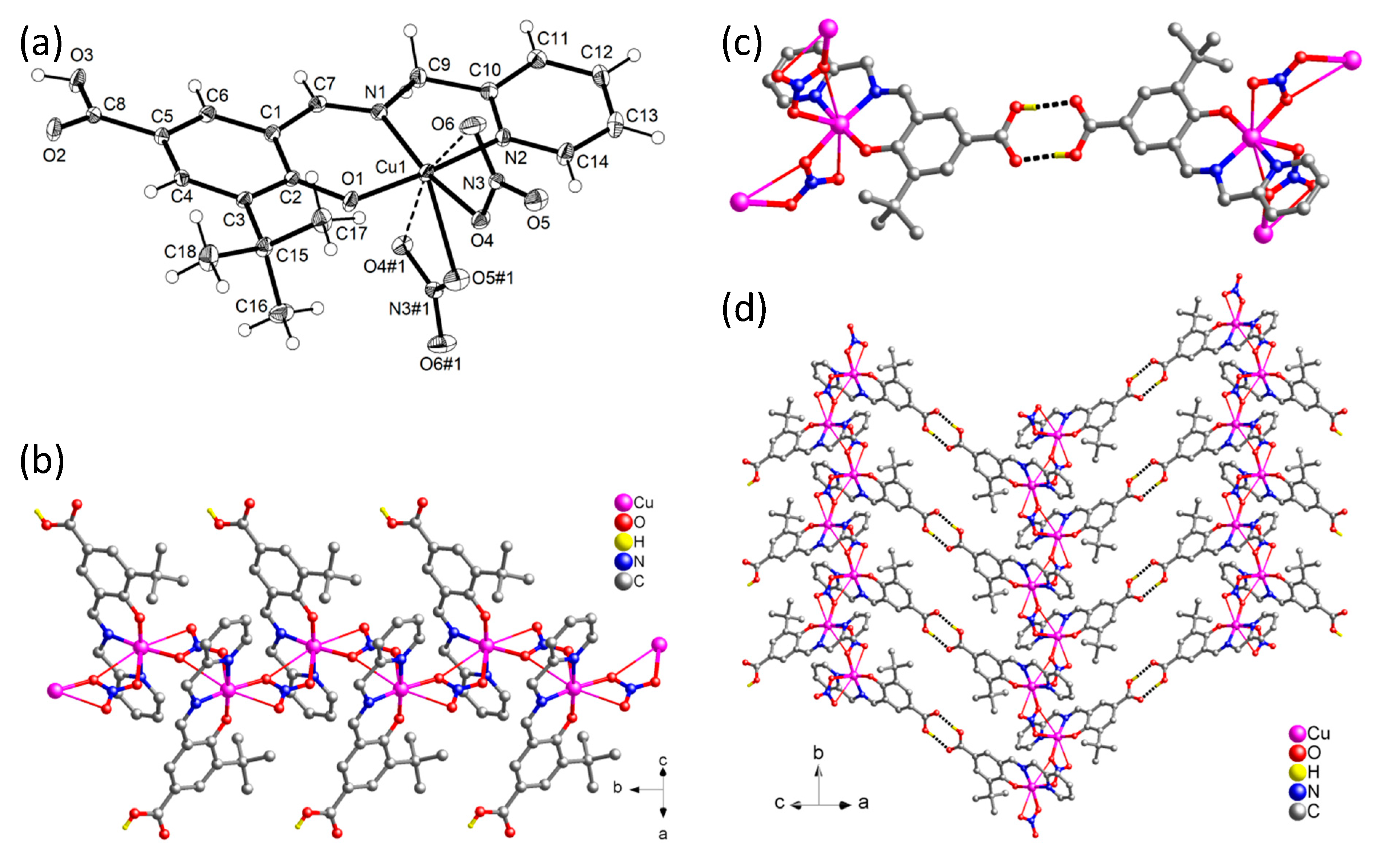
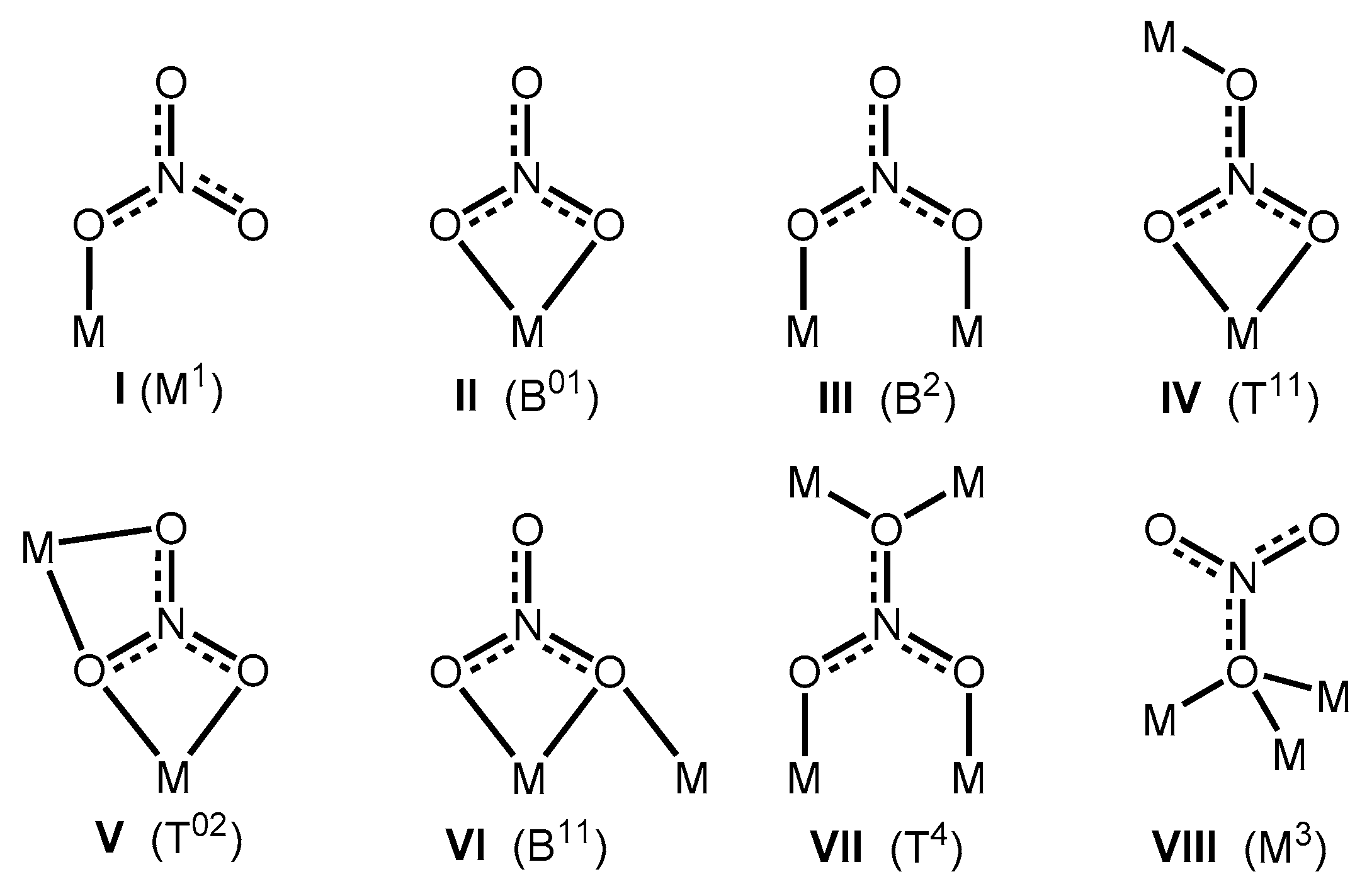
3.3. Crystal Structural Description of [Cu(HLsalpyca)(NO3)]n (2)
3.4. Thermogravimetric (TG) Analysis
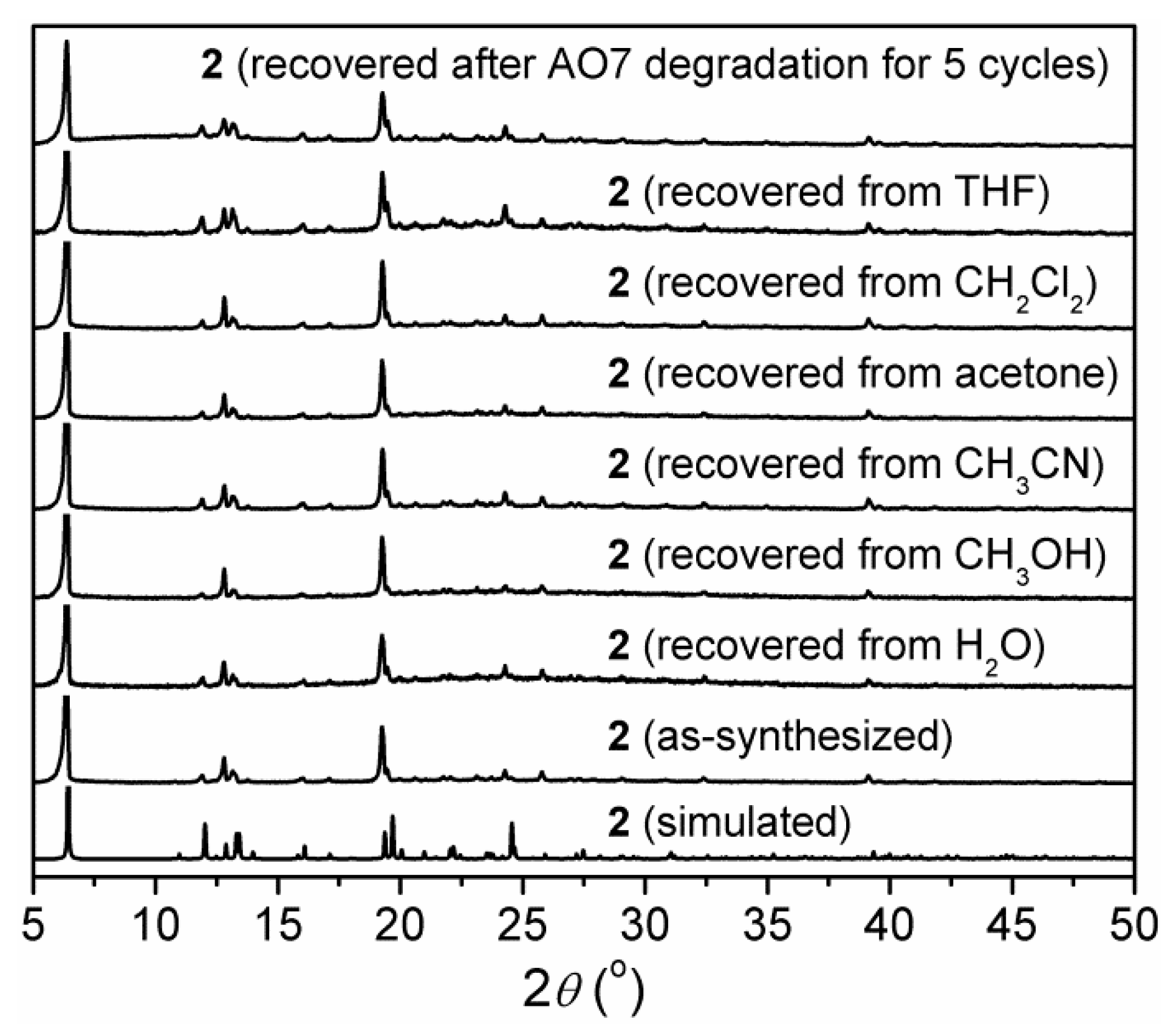
3.5. Stability of 2
3.6. Photophysical Properties
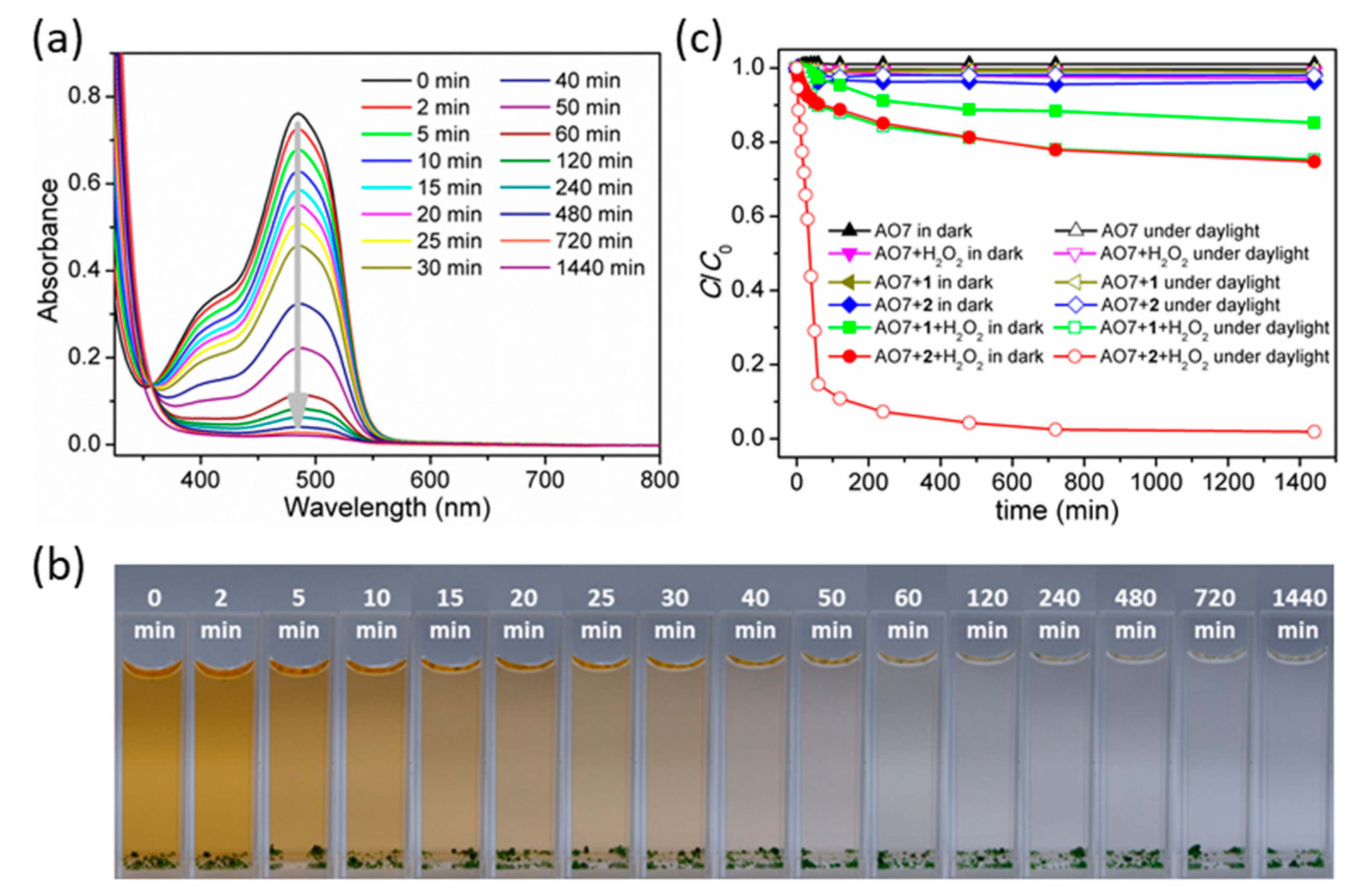
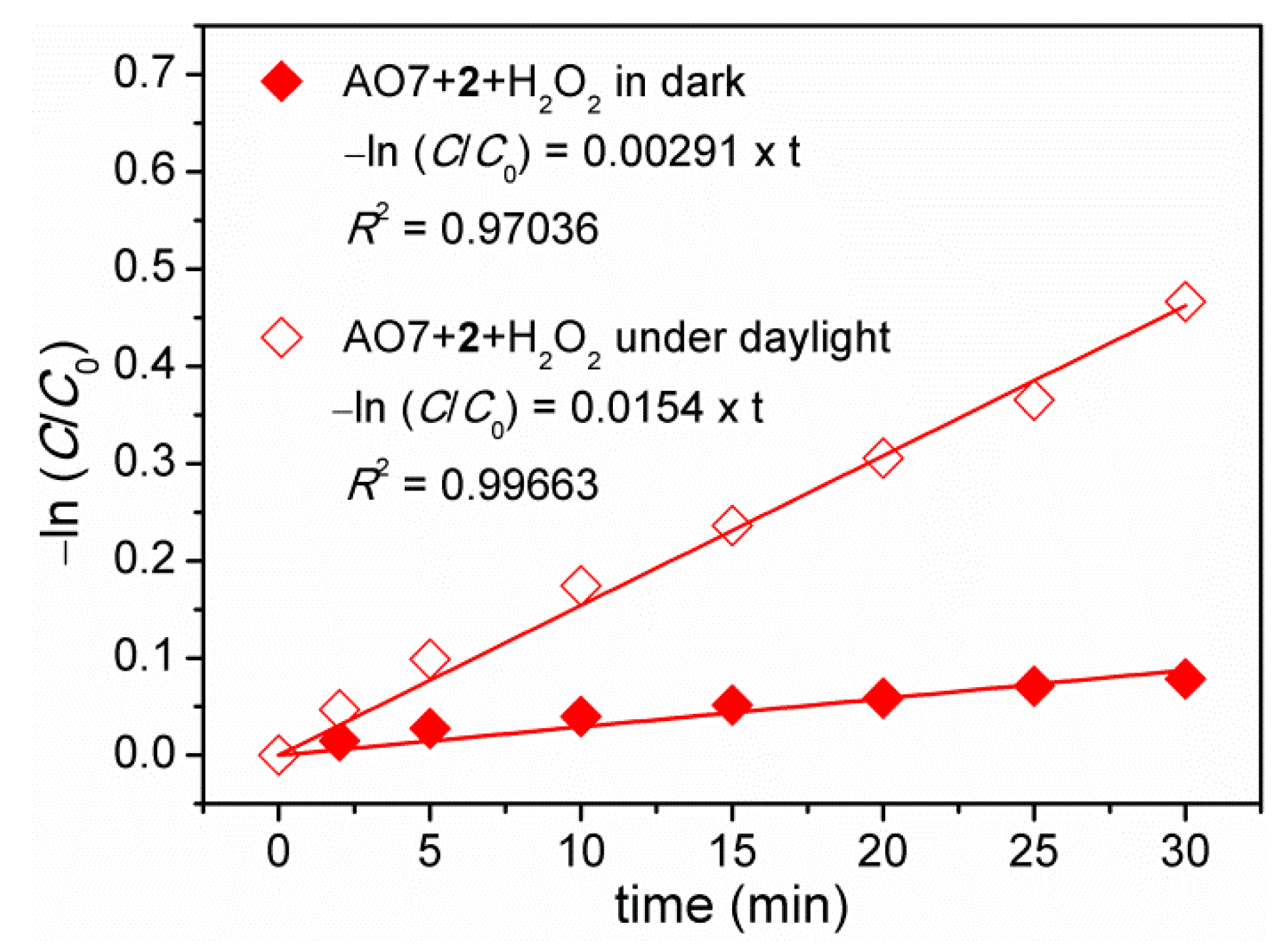
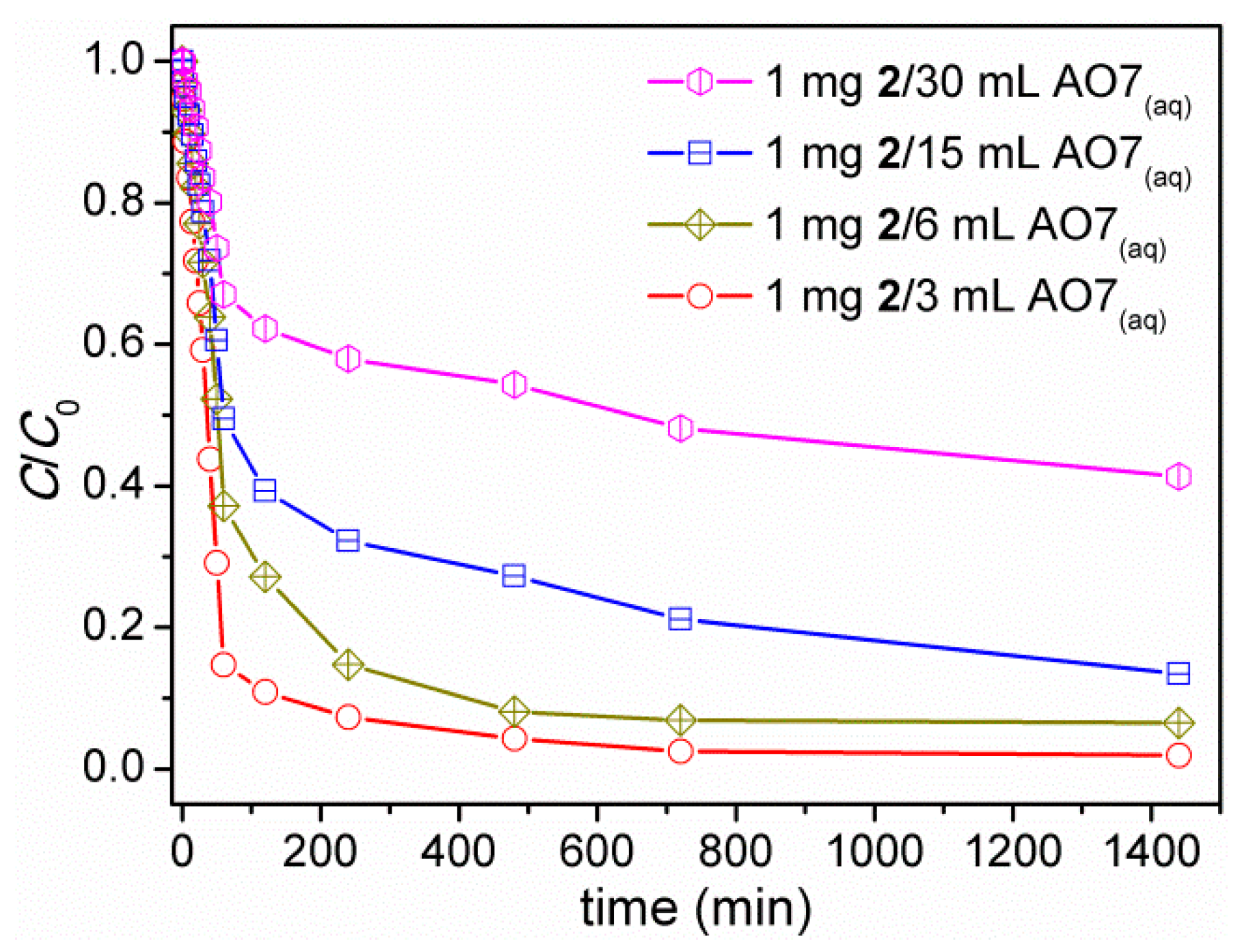
3.7. Decolorization of Acid Orange 7 (AO7) Dye
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yamada, S. Advancement in stereochemical aspects of Schiff base metal complexes. Chem. Rev. 1999, 190–192, 537–555. [Google Scholar] [CrossRef]
- Majumder, A.; Rosair, G.M.; Mallick, A.; Chattopadhyay, N.; Mitra, S. Synthesis, structures and fluorescence of nickel, zinc and cadmium complexes with the N,N,O-tridentate Schiff base N-2-pyridylmethylidene-2-hydroxy-phenylamine. Polyhedron 2006, 25, 1753–1762. [Google Scholar] [CrossRef]
- Basak, S.; Sen, S.; Marschner, C.; Baumgartner, J.; Batten, S.R.; Turner, D.R.; Mitra, S. Synthesis, crystal structures and fluorescence properties of two new di- and polynuclear Cd(II) complexes with N2O donor set of a tridentate Schiff base ligand. Polyhedron 2008, 27, 1193–1200. [Google Scholar] [CrossRef]
- Li, S.-N.; Zhai, Q.-G.; Hu, M.-C.; Jiang, Y.-C. Synthesis, crystal structures and characterization of three novel complexes with N-[2-(2-hydroxybenzylideneamino)ethyl]-4-methyl-benzene-sulfonamide as ligand. Inorg. Chim. Acta 2009, 362, 2217–2221. [Google Scholar] [CrossRef]
- Gupta, K.C.; Sutar, A.K. Catalytic activities of Schiff base transition metal complexes. Coord. Chem. Rev. 2008, 252, 1420–1450. [Google Scholar] [CrossRef]
- Zhou, X.-X.; Fang, H.-C.; Ge, Y.-Y.; Zhou, Z.-Y.; Gu, Z.-G.; Gong, X.; Zhao, G.; Zhan, Q.-G.; Zeng, R.-H.; Cai, Y.-P. Assembly of a Series of Trinuclear Zinc(II) Compounds with N2O2 Donor Tetradentate Symmetrical Schiff Base Ligand. Cryst. Growth Des. 2010, 10, 4014–4022. [Google Scholar] [CrossRef]
- Lu, Z.; Yuan, M.; Pan, F.; Gao, S.; Zhang, D.; Zhu, D. Syntheses, Crystal Structures, and Magnetic Characterization of Five New Dimeric Manganese(III) Tetradentate Schiff Base Complexes Exhibiting Single-Molecule-Magnet Behavior. Inorg. Chem. 2006, 45, 3538–3548. [Google Scholar] [CrossRef]
- Whiteoak, C.J.; Salassa, G.; Kleij, A.W. Recent advances with π-conjugated salen systems. Chem. Soc. Rev. 2012, 41, 622–631. [Google Scholar] [CrossRef]
- Escudero-Adán, E.C.; Benet-Buchholz, J.; Kleij, A.W. Expedient Method for the Transmetalation of Zn(II)-Centered Salphen Complexes. Inorg. Chem. 2007, 46, 7265–7267. [Google Scholar] [CrossRef]
- Houjou, H.; Iwasaki, A.; Ogihara, T.; Kanesato, M.; Akabori, S.; Hiratani, K. The architecture of dinuclear Ni and Cu complexes: Twisted and parallel forms controlled by the self-assembly of Schiff bas ligands. New J. Chem. 2003, 27, 886–889. [Google Scholar] [CrossRef]
- Yoshida, N.; Oshio, H.; Ito, T. Slef-assembling tetranuclear copper(II) complex of a bis-bidentate Schiff base: Double-helical structure induced by aromatic π∙∙∙π and CH∙∙∙π interactions. Chem. Commun. 1998, 63–64. [Google Scholar] [CrossRef]
- Kitaura, R.; Onoyama, G.; Sakamoto, H.; Matsuda, R.; Noro, S.-I.; Kitagawa, S. Immobilization of a Metallo Schiff Base into a Microporous Coordination Polymer. Angew. Chem. Int. Ed. 2004, 43, 2684–2687. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Xuan, W.; Cui, Y. Luminescent microporous metal–metallosalen frameworks with the primitive cubic net. Dalton Trans. 2012, 41, 3928–3932. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Chang, C.-Y.; Tsai, C.-J.; Lee, J.-J. Reversible Single-Crystal to Single-Crystal Transformations of a Zn(II)–Salicyaldimine Coordination Polymer Accompanying Changes in Coordination Sphere and Network Dimensionality upon Dehydration and Rehydration. Inorg. Chem. 2015, 54, 10918–10924. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-H.; Ma, B.; Nguyen, S.T.; Hupp, J.T.; Albrecht-Schmitt, T.E. A metal–organic framework material that functions as an enantioselective catalyst for olefin epoxidation. Chem. Commun. 2006, 2563–2565. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, E.N. Asymmetric Catalysis of Epoxide Ring-Opening Reactions. Acc. Chem. Res. 2000, 33, 421–431. [Google Scholar] [CrossRef]
- Bagai, R.; Abboud, K.A.; Christou, G. Ligand-induced distortion of a tetranuclear manganese butterfly complex. Dalton Trans. 2006, 3306–3312. [Google Scholar] [CrossRef]
- Pandey, R.; Kumar, P.; Singh, A.K.; Shahid, M.; Li, P.-Z.; Singh, S.K.; Xu, Q.; Misra, A.; Pandey, D.S. Fluorescent Zinc(II) Complex Exhibiting “On-Off-On” Switching Toward Cu2+and Ag+Ions. Inorg. Chem. 2011, 50, 3189–3197. [Google Scholar] [CrossRef]
- Anbu, S.; Ravishankaran, R.; da Silva, M.F.C.G.; Karande, A.A.; Pombeiro, A.J.L. Differentially Selective Chemosensor with Fluorescence Off–On Responses on Cu2+and Zn2+Ions in Aqueous Media and Applications in Pyrophosphate Sensing, Live Cell Imaging, and Cytotoxicity. Inorg. Chem. 2014, 53, 6655–6664. [Google Scholar] [CrossRef]
- Iervolino, G.; Vaiano, V.; Pepe, G.; Campiglia, P.; Palma, V. Degradation of Acid Orange 7 Azo Dye in Aqueous Solution by a Catalytic-Assisted, Non-Thermal Plasma Process. Catalysts 2020, 10, 888. [Google Scholar] [CrossRef]
- Coughlin, M.F.; Kinkle, B.K.; Bishop, P.L. Degradation of acid orange 7 in an aerobic biofilm. Chemosphere 2002, 46, 11–19. [Google Scholar] [CrossRef]
- Zheng, J.; Gao, Z.; He, H.; Yang, S.; Sun, C. Efficient degradation of Acid Orange 7 in aqueous solution by iron ore tailing Fenton-like process. Chemosphere 2016, 150, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Dong, C.; Zhang, J.; Lan, Y. Biogenic synthetic schwertmannite photocatalytic degradation of acid orange 7 (AO7) assisted by citric acid. Sep. Purif. Technol. 2015, 143, 27–31. [Google Scholar] [CrossRef]
- Liu, C.-X.; Zhang, W.-H.; Wang, N.; Guo, P.; Muhler, M.; Wang, Y.; Lin, S.; Chen, Z.; Yang, G. High;y efficient phoyocatalytic degradation of dyes bt a novel copper–triazolate metal–organic framework. Chem. Eur. J. 2018, 24, 16804–16813. [Google Scholar] [CrossRef]
- Hasan, Z.; Jhung, S.H. Removal of hazardous organics from water using metal-organic frameworks (MOFs): Plausible mechanisms for selective adsorptions. J. Hazard. Mater. 2015, 283, 329–339. [Google Scholar] [CrossRef]
- Dias, E.M.; Petit, C. Towards the use of metal–organic frameworks for water reuse: A review of the recent advances in the field of organic pollutants removal and degradation and the next steps in the field. J. Mater. Chem. A 2015, 3, 22484–22506. [Google Scholar] [CrossRef]
- Chen, C.; Ma, W.; Zhao, J. Semiconductor-mediated photodegradation of pollutants under visible-light irradiation. Chem. Soc. Rev. 2010, 39, 4206–4219. [Google Scholar] [CrossRef]
- Wang, C.-C.; Li, J.-R.; Lv, X.-L.; Zhang, Y.-Q.; Guo, G. Photocatalytic organic pollutants degradation in metal–organic frameworks. Energy Environ. Sci. 2014, 7, 2831–2867. [Google Scholar] [CrossRef]
- Du, P.-Y.; Li, H.; Fu, X.; Gu, W.; Liu, X. A 1D anionic lanthanide coordination polymer as an adsorbent material for the selective uptake of cationic dyes from aqueous solutions. Dalton Trans. 2015, 44, 13752–13759. [Google Scholar] [CrossRef]
- Yang, J.-M.; Ying, R.-J.; Han, C.-X.; Hu, Q.-T.; Xu, H.-M.; Li, J.-H.; Wang, Q.; Zhang, W. Adsorptive removal of organic dyes from aqueous solution by a Zr-based metal–organic framework: Effects of Ce(iii) doping. Dalton Trans. 2018, 47, 3913–3920. [Google Scholar] [CrossRef]
- Huang, L.; He, M.; Chen, B.; Hu, B. Magnetic Zr-MOFs nanocomposites for rapid removal of heavy metal ions and dyes from water. Chemosphere 2018, 199, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.-W.; Su, Y.-T.; Wu, J.-Y. Ligand dissociation/recoordination in fluorescent ionic zinc–salicylideneimine compounds: Synthesis, characterization, photophysical properties, and 1H NMR studies. Dalton Trans. 2013, 42, 15169–15182. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-Y.; Tsai, C.-J.; Chang, C.-Y.; Wu, Y.-Y. Metal-ion exchange induced structural transformation as a way of forming novel Ni(II)− and Cu(II)−salicylaldimine structures. J. Solid State Chem. 2017, 246, 23–28. [Google Scholar] [CrossRef]
- Tsai, M.-J.; Wu, J.-Y. Insight into the influence of framework metal ion of analogous metal–organic frameworks on the adsorptive removal performances of dyes from water. J. Taiwan Inst. Chem. Eng. 2019, 102, 73–84. [Google Scholar] [CrossRef]
- Tsai, M.-J.; Luo, J.-H.; Wu, J.-Y. Two-fold 2D + 2D → 2D interweaved rhombus (4,4) grid: Synthesis, structure, and dye removal properties in darkness and in daylight. Dalton Trans. 2019, 48, 1095–1107. [Google Scholar] [CrossRef]
- Tsai, M.-J.; Wu, J.-Y. Synthesis, Structure, and Dye Adsorption Properties of a Nickel(II) Coordination Layer Built from d-Camphorate and Bispyridyl Ligands. Polymers 2017, 9, 661. [Google Scholar] [CrossRef]
- Tsai, M.-J.; Wu, J.-Y. Synthesis, characterization, and dye capture of a 3D Cd(II)–carboxylate pcu network. Polyhedron 2017, 122, 124–130. [Google Scholar] [CrossRef]
- Williams, A.B.; Weiser, P.T.; Hanson, R.N.; Gunther, J.R.; Katzenellenbogen, J.A. Synthesis of Biphenyl Proteomimetics as Estrogen Receptor-α Coactivator Binding Inhibitors. Org. Lett. 2009, 11, 5370–5373. [Google Scholar] [CrossRef][Green Version]
- Song, F.; Wang, C.; Falkowski, J.M.; Ma, L.; Lin, W. Isoreticular Chiral Metal–Organic Frameworks for Asymmetric Alkene Epoxidation: Tuning Catalytic Activity by Controlling Framework Catenation and Varying Open Channel Sizes. J. Am. Chem. Soc. 2010, 132, 15390–15398. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Brandenburg, K.; Putz, H. Diamond; Crystal Impact GbR: Bonn, Germany, 1999. [Google Scholar]
- Etter, M.C. Encoding and decoding hydrogen-bond patterns of organic compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Addison, C.C.; Logan, N.; Wallwork, S.C.; Garner, C.D. Structural Aspects of Co-ordinated Nitrate Groups. Q. Rev. Chem. Soc. 1971, 25, 289–322. [Google Scholar] [CrossRef]
- Jianmin, L.; Huaqianga, Z.; Yugenb, Z.; Jinhua, C.; Yanxiong, K.; Quanming, W.; Xintao, W. The Crystal Structure of [La(NO3)6{Cu(2,2′-bipy)2}2][La(NO3)6Cu(2,2′-bipy)2]·CH3CN with the Most Profuse Modes of Nitrate Coordination. Cryst. Res. Technol. 1999, 34, 925–928. [Google Scholar] [CrossRef]
- Banu, K.S.; Ghosh, T.; Guha, A.; Chattopadhyay, T.; Das, D.; Zangrando, E. Structure and luminescence of a nitrate-bridged heterotrinuclear Cu2-Pr complex with compartmental Schiff base ligand. J. Coord. Chem. 2010, 63, 3714–3723. [Google Scholar] [CrossRef]
- Ling, J.; Ozga, M.; Stoffer, M.; Burns, P.C. Uranyl peroxide pyrophosphate cage clusters with oxalate and nitrate bridges. Dalton Trans. 2012, 41, 7278–7284. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Joarder, B.; Rivière, E.; Rogez, G.; Ghosh, S.K. Nitrate-Bridged “Pseudo-Double-Propeller”-Type Lanthanide(III)−Copper(II) Heterometallic Clusters: Syntheses, Structures, and Magnetic Properties. Inorg. Chem. 2012, 51, 9159–9161. [Google Scholar] [CrossRef]
- She, S.; Chen, Y.; Zaworotko, M.J.; Liu, W.; Cao, Y.; Wu, J.; Li, Y. Synthesis, structures and magnetic properties of a family of nitrate-bridged octanuclear [Na2Ln6] (Ln = Dy, Tb, Gd, Sm) complexes. Dalton Trans. 2013, 42, 10433–10438. [Google Scholar] [CrossRef]
- Morozov, I.V.; Serezhkin, V.N.; Troyanov, S.I. Modes of coordination and stereochemistry of the NO3− anions in inorganic nitrates. Russ. Chem. Bull. Int. Ed. 2008, 57, 439–450. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Goswami, R.; Smith, V.J.; Bajaj, H.C.; Neogi, S. Pore Wall-Functionalized Luminescent Cd(II) Framework for Selective CO2 Adsorption, Highly Specific 2,4,6-Trinitrophenol Detection, and Colorimetric Sensing of Cu2+Ions. ACS Sustain. Chem. Eng. 2018, 6, 10295–10306. [Google Scholar] [CrossRef]
- Udhayakumari, D.; Velmathi, S.; Sung, Y.-M.; Wu, S.-P. Highly fluorescent probe for copper (II) ion based on commercially available compounds and live cell imaging. Sens. Actuators B Chem. 2014, 198, 285–293. [Google Scholar] [CrossRef]
- Yang, H.; He, X.-W.; Wang, F.; Kang, Y.; Zhang, J. Doping copper into ZIF-67 for enhancing gas uptake capacity and visible-light-driven photocatalytic degradation of organic dye. J. Mater. Chem. 2012, 22, 21849–21851. [Google Scholar] [CrossRef]
- Wen, T.; Zhang, D.-X.; Zhang, J. Two-Dimensional Copper(I) Coordination Polymer Materials as Photocatalysts for the Degradation of Organic Dyes. Inorg. Chem. 2013, 52, 12–14. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, J.; Fu, Z. Polyoxometalate Cluster Sensitized with Copper-Viologen Framework for Efficient Degradation of Organic Dye in Ultraviolet, Visible, and Near-Infrared Light. ACS Appl. Mater. Interfaces 2018, 10, 35671–35675. [Google Scholar] [CrossRef]
- Peng, Y.-F.; Qian, L.-L.; Ding, J.-G.; Zheng, T.-R.; Zhang, Y.-Q.; Li, B.-L. Syntheses, structures and photocatalytic degradation of organic dyes for two isostructural copper coordination polymers involving in situ hydroxylation reaction. J. Coord. Chem. 2018, 71, 1392–1402. [Google Scholar] [CrossRef]
- Hou, Y.-L.; Sun, R.W.-Y.; Zhou, X.-P.; Wang, J.-H.; Li, D. A copper(I)/copper(II)–salen coordination polymer as a bimetallic catalyst for three-component Strecker reactions and degradation of organic dyes. Chem. Commun. 2014, 50, 2295–2297. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Qiu, J. Degradation of Acid Orange 7 in aqueous solution by a novel electro/Fe2+/peroxydisulfate process. J. Hazard. Mater. 2012, 215–216, 138–145. [Google Scholar] [CrossRef]
- Piumetti, M.; Freyria, F.S.; Armandi, M.; Saracco, G.; Garrone, E.; Gonzalez, G.E.; Bonelli, B. Catalytic degradation of Acid Orange 7 by H2O2 as promoted by either bare or V-loaded titania under UV light, in dark conditions, and after incubating the catalysts in ascorbic acid. Catal. Struct. React. 2015, 1, 183–191. [Google Scholar] [CrossRef]
- Ji, P.; Zhang, J.; Chen, F.; Anpo, M. Study of adsorption and degradation of Acid Orange 7 on the surface of CeO2 under visible light irradiation. Appl. Catal. B 2009, 85, 148–154. [Google Scholar] [CrossRef]
- Elías, V.; Vaschetto, E.; Sapag, K.; Oliva, M.; Casuscelli, S.; Eimer, G. MCM-41-based materials for the photo-catalytic degradation of Acid Orange 7. Catal. Today 2011, 172, 58–65. [Google Scholar] [CrossRef]
- Yin, C.; Zhao, C.; Xu, X.-J. Crystal structure and photocatalytic degradation properties of a new two-dimensional zinc coordination polymer based on 4,4′-oxy-bis(benzoic acid). Z. Naturforsch. 2019, 74, 861–864. [Google Scholar] [CrossRef]
- Wang, F.; Li, F.-L.; Xu, M.-M.; Yu, H.; Zhang, J.-G.; Xia, H.-T.; Lang, J.-P. Facile synthesis of a Ag(I)-doped coordination polymer with enhanced catalytic performance in the photodegradation of azo dyes in water. J. Mater. Chem. A 2015, 3, 5908–5916. [Google Scholar] [CrossRef]
- Li, J.; Pham, A.N.; Dai, R.; Wang, Z.; Waite, T.D. Recent advances in Cu-Fenton systems for the treatment of industrial wastewaters: Role of Cu complexes and Cu composites. J. Hazard. Mater. 2020, 392, 122261. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, P.; Yang, J.; Xu, G.; Yang, H.; Shi, Z.; Hu, Q.; Dong, B.; Guo, Z. Photocatalytic degradation of organic dye and phytohormone by a Cu(II) complex powder catalyst with added H2O2. Colloids Surf. A 2020, 603, 125147. [Google Scholar] [CrossRef]
- Carvalho, S.S.F.; Rodrigues, A.C.C.; Lima, J.F.; Carvalho, N.M.F. Photocatalytic degradation of dyes by mononuclear copper(II) complexes from bis-(2-pyridylmethyl)amine NNN-derivative ligands. Inorg. Chim. Acta 2020, 512, 119924. [Google Scholar] [CrossRef]



| Complex | 1 | 2 |
|---|---|---|
| Empirical formula | C18H19ClCuN2O3 | C18H19CuN3O6 |
| Mw | 410.34 | 436.90 |
| Crystal system | Triclinic | Monoclinic |
| Space group | P21/c | |
| a (Å) | 8.2742 (7) | 13.8487 (7) |
| b (Å) | 8.5837 (7) | 8.2653 (4) |
| c (Å) | 12.6658 (9) | 16.2531 (8) |
| α (°) | 99.124 (6) | 90 |
| β (°) | 90.116 (6) | 97.386 (2) |
| γ (°) | 102.508 (7) | 90 |
| V (Å3) | 866.49 (12) | 1844.95 (16) |
| Z | 2 | 4 |
| T (K) | 150(2) | 150 (2) |
| λ (Å) | 0.71073 | 0.71073 |
| Dcalc (g cm−3) | 1.573 | 1.573 |
| F000 | 422 | 900 |
| μ (mm−1) | 1.434 | 1.226 |
| θmin,θmax (deg) | 2.952, 29.356 | 2.966, 26.431 |
| R1a (I > 2σ (I)) | 0.0327 | 0.0411 |
| wR2b (I > 2σ (I)) | 0.0738 | 0.1061 |
| R1a (all data) | 0.0398 | 0.0563 |
| wR2b (all data) | 0.0780 | 0.1250 |
| GOF on F2 | 1.046 | 1.169 |
| 1 | |||
| Cu1–Cl2 | 2.2299 (6) | Cu1–O1 | 1.8857 (14) |
| Cu1–N1 | 1.9376 (17) | Cu1–N2 | 1.9971 (17) |
| O1–Cu1–N1 | 92.00 (6) | O1–Cu1–N2 | 165.35 (7) |
| N1–Cu1–N2 | 82.99 (7) | O1–Cu1–Cl2 | 90.81 (5) |
| N1–Cu1–Cl2 | 164.08 (6) | N2–Cu1–Cl2 | 97.79 (5) |
| 2 | |||
| Cu1–O1 | 1.892 (2) | Cu1–N1 | 1.931 (2) |
| Cu1–N2 | 1.994 (3) | Cu1–O4 | 2.019 (2) |
| Cu1–O5#1 | 2.437 (3) | ||
| O1–Cu1–N1 | 93.00 (9) | O1–Cu1–N2 | 176.25 (9) |
| N1–Cu1–N2 | 83.45 (10) | O1–Cu1–O4 | 89.81 (9) |
| N1–Cu1–O4 | 168.19 (10) | N2–Cu1–O4 | 93.43 (10) |
| O1–Cu1–O5#1 | 95.63 (9) | N1–Cu1–O5#1 | 120.20 (9) |
| N2–Cu1–O5#1 | 87.21 (10) | O4–Cu1–O5#1 | 70.84 (8) |
| aSymmetry code: For 2, #1, −x, y + 1/2, −z + 1/2. | |||
| D–H∙∙∙A | d(D–H) (Å) | d(H∙∙∙A) (Å) | d(D∙∙∙A) (Å) | ∠(D–H∙∙∙A) (°) |
|---|---|---|---|---|
| 1 | ||||
| O3–H3∙∙∙O2#1 | 0.81 (2) | 1.84 (2) | 2.637 (2) | 168 (3) |
| C9–H9B∙∙∙Cl2#2 | 0.97 | 2.70 | 3.567 (3) | 150 |
| 2 | ||||
| O3–H3∙∙∙O2#2 | 0.82 (3) | 1.81 (3) | 2.633 (3) | 174 (4) |
| aSymmetry code: 1, #1, −1 − x, −y, −z; #2, −1 + x, y, z. 2, #1, 1 − x, 2 − y, −z. | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, M.-J.; Tsai, C.-J.; Lin, K.; Wu, J.-Y. Anion-Dominated Copper Salicyaldimine Complexes—Structures, Coordination Mode of Nitrate and Decolorization Properties toward Acid Orange 7 Dye. Polymers 2020, 12, 1910. https://doi.org/10.3390/polym12091910
Tsai M-J, Tsai C-J, Lin K, Wu J-Y. Anion-Dominated Copper Salicyaldimine Complexes—Structures, Coordination Mode of Nitrate and Decolorization Properties toward Acid Orange 7 Dye. Polymers. 2020; 12(9):1910. https://doi.org/10.3390/polym12091910
Chicago/Turabian StyleTsai, Meng-Jung, Chi-Jou Tsai, Ken Lin, and Jing-Yun Wu. 2020. "Anion-Dominated Copper Salicyaldimine Complexes—Structures, Coordination Mode of Nitrate and Decolorization Properties toward Acid Orange 7 Dye" Polymers 12, no. 9: 1910. https://doi.org/10.3390/polym12091910
APA StyleTsai, M.-J., Tsai, C.-J., Lin, K., & Wu, J.-Y. (2020). Anion-Dominated Copper Salicyaldimine Complexes—Structures, Coordination Mode of Nitrate and Decolorization Properties toward Acid Orange 7 Dye. Polymers, 12(9), 1910. https://doi.org/10.3390/polym12091910