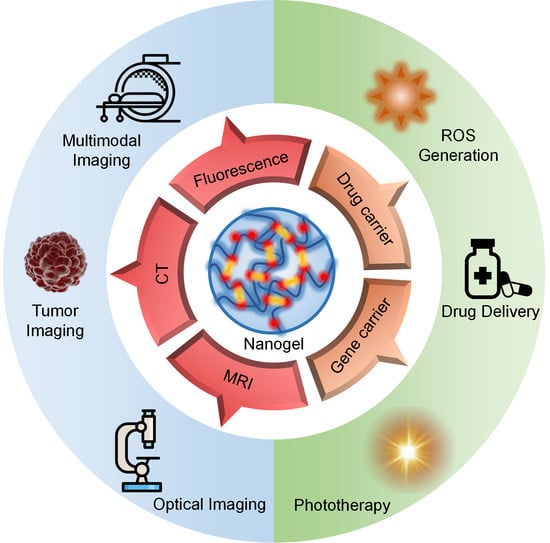
Recent Advances in Crosslinked Nanogel for Multimodal Imaging and Cancer Therapy
Abstract
1. Introduction
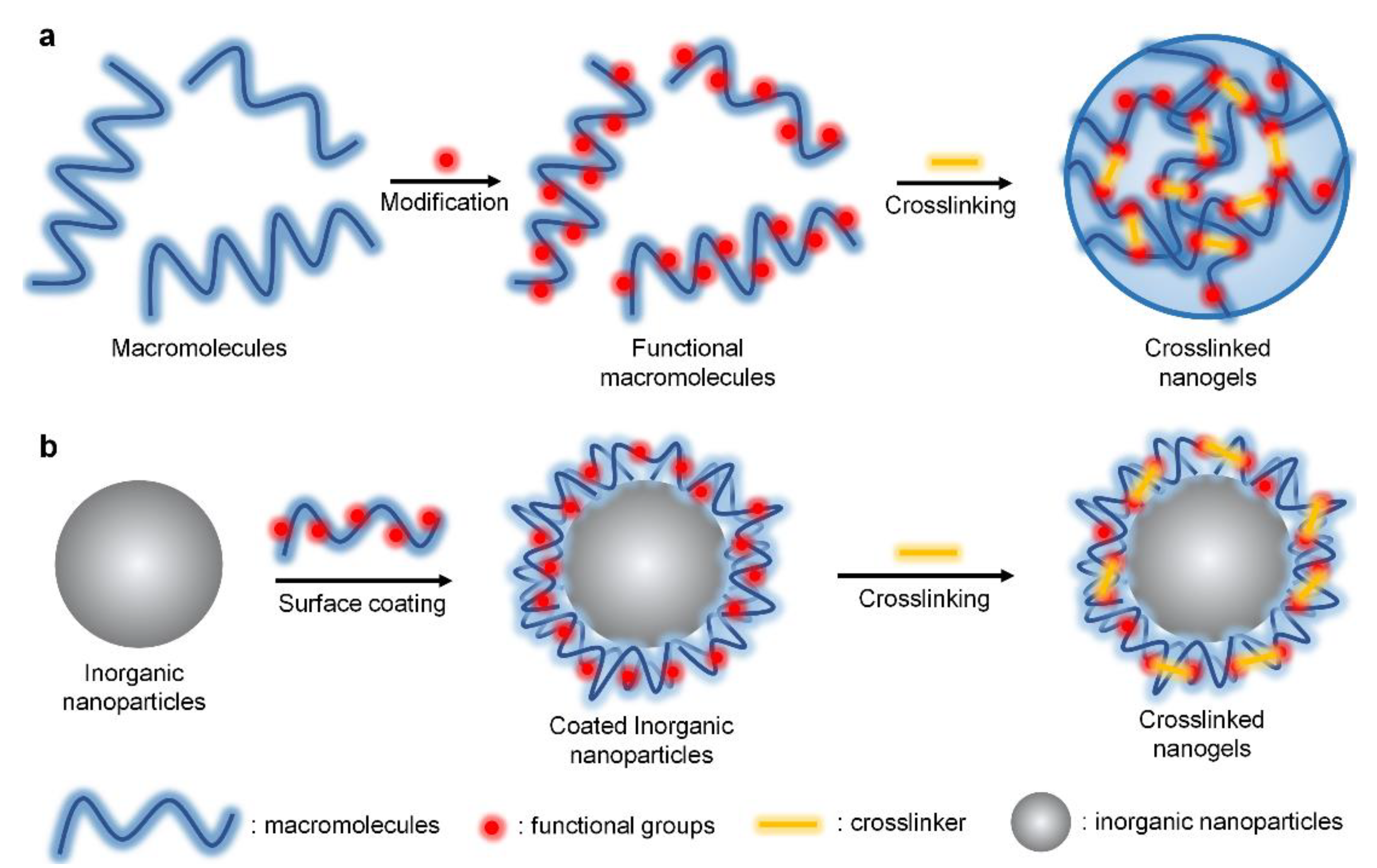
2. Preparation of Crosslinked Nanogels
3. Crosslinked Nanogels for Cancer Therapy
3.1. Chemotherapy
3.1.1. Environmentally Responsive Nanogels for Chemotherapy
3.1.2. Drug-Crosslinked Nanogels
3.2. Gene Therapy
3.2.1. Gene-Crosslinked Nanogels
3.2.2. Environmental Responsive Nanogels for Gene Therapy
3.3. Enzyme Dynamic Therapy
4. Crosslinked Nanogels for Cancer Diagnosis and Imaging-Guided Cancer Therapy
4.1. Cancer Imaging
4.2. Imaging-Guided Cancer Therapy
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Li, J.C.; Pu, K.Y. Development of organic semiconducting materials for deep-tissue optical imaging, phototherapy and photoactivation. Chem. Soc. Rev. 2019, 48, 38–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Zhang, W.Z.; Zhu, G.Z.; Xie, J.; Chen, X.Y. Rethinking cancer nanotheranostics. Nat. Rev. Mater. 2017, 2, 17024. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, C.; Feng, L.Z.; Yang, K.; Liu, Z. Functional Nanomaterials for Phototherapies of Cancer. Chem. Rev. 2014, 114, 10869–10939. [Google Scholar] [CrossRef]
- Ng, K.K.; Zheng, G. Molecular Interactions in Organic Nanoparticles for Phototheranostic Applications. Chem. Rev. 2015, 115, 11012–11042. [Google Scholar] [CrossRef]
- Ali, I.; Alsehli, M.; Scotti, L.; Scotti, M.T.; Tsai, S.T.; Yu, R.S.; Hsieh, M.F.; Chen, J.C. Progress in Polymeric Nano-Medicines for Theranostic Cancer Treatment. Polymers 2020, 12, 598. [Google Scholar] [CrossRef]
- Li, J.C.; Cui, D.; Huang, J.G.; He, S.S.; Yang, Z.B.; Zhang, Y.; Luo, Y.; Pu, K.Y. Organic Semiconducting Pro-nanostimulants for Near-Infrared Photoactivatable Cancer Immunotherapy. Angew. Chem. Int. Ed. 2019, 58, 12680–12687. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Pu, K.Y. Multimodal Biophotonics of Semiconducting Polymer Nanoparticles. Acc. Chem. Res. 2018, 51, 1840–1849. [Google Scholar] [CrossRef]
- Yin, C.; Zhen, X.; Fan, Q.L.; Huang, W.; Pu, K.Y. Degradable Semiconducting Oligomer Amphiphile for Ratiometric Photoacoustic Imaging of Hypochlorite. ACS Nano 2017, 11, 4174–4182. [Google Scholar] [CrossRef]
- Zhang, W.S.; Deng, W.X.; Zhang, H.; Sun, X.L.; Huang, T.; Wang, W.J.; Sun, P.F.; Fan, Q.L.; Huang, W. Bioorthogonal-targeted 1064 nm excitation theranostic nanoplatform for precise NIR-IIa fluorescence imaging guided efficient NIR-II photothermal therapy. Biomaterials 2020, 243, 119934. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, J.Z.; Geng, R.Y.; Cai, J.; Li, J.; Xie, C.; Tang, W.H.; Shen, Q.M.; Huang, W.; Fan, Q.L. High performance one-for-all phototheranostics: NIR-II fluorescence imaging guided mitochondria-targeting phototherapy with a single-dose injection and 808 nm laser irradiation. Biomaterials 2020, 231, 119671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Ning, L.L.; Huang, J.G.; Zhang, C.; Pu, K.Y. Activatable molecular agents for cancer theranostics. Chem. Sci. 2020, 11, 618–630. [Google Scholar] [CrossRef]
- Zhen, X.; Zhang, J.J.; Huang, J.G.; Xie, C.; Miao, Q.Q.; Pu, K.Y. Macrotheranostic Probe with Disease-Activated Near-Infrared Fluorescence, Photoacoustic, and Photothermal Signals for Imaging-Guided Therapy. Angew. Chem. Int. Ed. 2018, 57, 7804–7808. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Huang, J.G.; Zhen, X.; Li, J.C.; Jiang, Y.Y.; Pu, K.Y. A Semiconducting Polymer Nano-prodrug for Hypoxia-Activated Photodynamic Cancer Therapy. Angew. Chem. Int. Ed. 2019, 58, 5920–5924. [Google Scholar] [CrossRef]
- He, S.S.; Xie, C.; Jiang, Y.Y.; Pu, K.Y. An Organic Afterglow Protheranostic Nanoassembly. Adv. Mater. 2019, 31, 1902672. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, S.; Deng, D.; Xiao, Z.; Dong, Z.; Wang, Z.; Lei, Q.; Gao, S.; Huang, G.; Zhang, E.; et al. Fluorinated Chitosan To Enhance Transmucosal Delivery of Sonosensitizer-Conjugated Catalase for Sonodynamic Bladder Cancer Treatment Post-intravesical Instillation. ACS Nano 2020, 14, 1586–1599. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.Y.; Knox, H.J.; Reinhardt, C.J.; Partipilo, G.; Nilges, M.J.; Chan, J. Near-Infrared Photoactivatable Nitric Oxide Donors with Integrated Photoacoustic Monitoring. J. Am. Chem. Soc. 2018, 140, 11686–11697. [Google Scholar] [CrossRef]
- Huang, J.G.; Pu, K.Y. Activatable Molecular Probes for Second Near-Infrared Fluorescence, Chemiluminescence, and Photoacoustic Imaging. Angew. Chem. Int. Ed. 2020, 59, 11717–11731. [Google Scholar] [CrossRef]
- Hu, X.M.; Tang, Y.F.; Hu, Y.X.; Lu, F.; Lu, X.M.; Wang, Y.Q.; Li, J.; Li, Y.Y.; Ji, Y.; Wang, W.J.; et al. Gadolinium-Chelated Conjugated Polymer-Based Nanotheranostics for Photoacoustic/Magnetic Resonance/NIR-II Fluorescence Imaging-Guided Cancer Photothermal Therapy. Theranostics 2019, 9, 4168–4181. [Google Scholar] [CrossRef]
- Wang, Q.; Dai, Y.N.; Xu, J.Z.; Cai, J.; Niu, X.R.; Zhang, L.; Chen, R.F.; Shen, Q.M.; Huang, W.; Fan, Q.L. All-in-One Phototheranostics: Single Laser Triggers NIR-II Fluorescence/Photoacoustic Imaging Guided Photothermal/Photodynamic/Chemo Combination Therapy. Adv. Funct. Mater. 2019, 29, 1901480. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, X.Y. Semiconducting Perylene Diimide Nanostructure: Multifunctional Phototheranostic Nanoplatform. Acc. Chem. Res. 2019, 52, 1245–1254. [Google Scholar] [CrossRef]
- Xie, C.; Upputuri, P.K.; Zhen, X.; Pramanik, M.; Pu, K. Self-quenched semiconducting polymer nanoparticles for amplified in vivo photoacoustic imaging. Biomaterials 2016, 119, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, R.C.; Wang, Q.; Li, X.; Hu, X.M.; Yuan, Y.; Lu, X.M.; Wang, W.J.; Huang, W.; Fan, Q.L. Semiconducting polymer nanotheranostics for NIR-II/Photoacoustic imaging-guided photothermal initiated nitric oxide/photothermal therapy. Biomaterials 2019, 217, 119304. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Z.; Yin, Z.; Zhang, H.; Gao, Y.; Huo, G.; Wu, A.; Zeng, L. Amplified Photoacoustic Signal and Enhanced Photothermal Conversion of Polydopamine-Coated Gold Nanobipyramids for Phototheranostics and Synergistic Chemotherapy. ACS Appl. Mater. Interfaces 2020, 12, 14866–14875. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Fan, W.P.; Zhang, W.Z.; Yang, Z.; Li, L.; Wang, Z.T.; Chiang, Y.L.; Liu, Y.J.; Deng, L.M.; He, L.C.; et al. Wet/Sono-Chemical Synthesis of Enzymatic Two-Dimensional MnO2 Nanosheets for Synergistic Catalysis-Enhanced Phototheranostics. Adv. Mater. 2019, 31, 19000401. [Google Scholar] [CrossRef]
- Wang, X.W.; Zhong, X.Y.; Lei, H.L.; Geng, Y.H.; Zhao, Q.; Gong, F.; Yang, Z.J.; Dong, Z.L.; Liu, Z.; Cheng, L. Hollow Cu2Se Nanozymes for Tumor Photothermal-Catalytic Therapy. Chem. Mater. 2019, 31, 6174–6186. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, X.W.; Gong, F.; Liu, T.; Liu, Z. 2D Nanomaterials for Cancer Theranostic Applications. Adv. Mater. 2020, 32, 1902333. [Google Scholar] [CrossRef]
- Wang, C.; Xiao, Y.; Zhu, W.; Chu, J.; Xu, J.; Zhao, H.; Shen, F.; Peng, R.; Liu, Z. Photosensitizer-Modified MnO2 Nanoparticles to Enhance Photodynamic Treatment of Abscesses and Boost Immune Protection for Treated Mice. Small 2020, 16, 2000589. [Google Scholar] [CrossRef]
- Lin, Z.X.; Jiang, B.P.; Liang, J.Z.; Wen, C.C.; Shen, X.C. Phycocyanin functionalized single-walled carbon nanohorns hybrid for near-infrared light-mediated cancer phototheranostics. Carbon 2019, 143, 814–827. [Google Scholar] [CrossRef]
- Hu, D.R.; Chen, L.J.; Qu, Y.; Peng, J.R.; Chu, B.Y.; Shi, K.; Hao, Y.; Zhong, L.; Wang, M.Y.; Qian, Z.Y. Oxygen-generating Hybrid Polymeric Nanoparticles with Encapsulated Doxorubicin and Chlorin e6 for Trimodal Imaging-Guided Combined Chemo-Photodynamic Therapy. Theranostics 2018, 8, 1558–1574. [Google Scholar] [CrossRef]
- Fusco, L.; Gazzi, A.; Peng, G.T.; Shin, Y.; Vranic, S.; Bedognetti, D.; Vitale, F.; Yilmazer, A.; Feng, X.; Fadeel, B.; et al. Graphene and other 2D materials: A multidisciplinary analysis to uncover the hidden potential as cancer theranostics. Theranostics 2020, 10, 5435–5488. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Li, X.; Wen, G.; Yang, B.; Zhang, Y.; Chen, X.; Zhao, P.; Li, S.; Li, R.; Wang, L.; et al. Organic semiconducting polymer amphiphile for near-infrared-II light-triggered phototheranostics. Biomaterials 2020, 232, 119684. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Fan, W.P.; Tang, W.; Shen, Z.Y.; Dai, Y.L.; Song, J.B.; Wang, Z.T.; Liu, Y.; Lin, L.S.; Shan, L.L.; et al. Near-Infrared Semiconducting Polymer Brush and pH/GSH-Responsive Polyoxometalate Cluster Hybrid Platform for Enhanced Tumor-Specific Phototheranostics. Angew. Chem. Int. Ed. 2018, 57, 14101–14105. [Google Scholar] [CrossRef]
- Senthilkumar, T.; Zhou, L.Y.; Gu, Q.; Liu, L.B.; Lv, F.T.; Wang, S. Conjugated Polymer Nanoparticles with Appended Photo-Responsive Units for Controlled Drug Delivery, Release, and Imaging. Angew. Chem. Int. Ed. 2018, 57, 13114–13119. [Google Scholar] [CrossRef]
- Zhen, X.; Xie, C.; Pu, K.Y. Temperature-Correlated Afterglow of a Semiconducting Polymer Nanococktail for Imaging-Guided Photothermal Therapy. Angew. Chem. Int. Ed. 2018, 57, 3938–3942. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, Z.L.; Shen, Y.Q.; Radosz, M. Fabrication of micellar nanoparticles for drug delivery through the self-assembly of block copolymers. Prog. Polym. Sci. 2010, 35, 1128–1143. [Google Scholar] [CrossRef]
- Owen, S.C.; Chan, D.P.Y.; Shoichet, M.S. Polymeric micelle stability. Nano Today 2012, 7, 53–65. [Google Scholar] [CrossRef]
- Kang, N.; Perron, M.E.; Prud’homme, R.E.; Zhang, Y.B.; Gaucher, G.; Leroux, J.C. Stereocomplex block copolymer micelles: Core-shell nanostructures with enhanced stability. Nano Lett. 2005, 5, 315–319. [Google Scholar] [CrossRef]
- O’Reilly, R.K.; Hawker, C.J.; Wooley, K.L. Cross-linked block copolymer micelles: Functional nanostructures of great potential and versatility. Chem. Soc. Rev. 2006, 35, 1068–1083. [Google Scholar] [CrossRef]
- Huang, H.Y.; Remsen, E.E.; Kowalewski, T.; Wooley, K.L. Nanocages derived from shell cross-linked micelle templates. J. Am. Chem. Soc. 1999, 121, 3805–3806. [Google Scholar] [CrossRef]
- Thurmond, K.B.; Kowalewski, T.; Wooley, K.L. Water-soluble knedel-like structures: The preparation of shell-cross-linked small particles. J. Am. Chem. Soc. 1996, 118, 7239–7240. [Google Scholar] [CrossRef]
- Tian, S.; Liu, G.; Wang, X.; Zhang, G.; Hu, J. pH-Responsive Tumor-Targetable Theranostic Nanovectors Based on Core Crosslinked (CCL) Micelles with Fluorescence and Magnetic Resonance (MR) Dual Imaging Modalities and Drug Delivery Performance. Polymers 2016, 8, 226. [Google Scholar] [CrossRef] [PubMed]
- Garcia, F.P.; Rippe, M.; Companhoni, M.V.P.; Stefanello, T.F.; Louage, B.; Van Herck, S.; Sancey, L.; Coll, J.L.; De Geest, B.G.; Vataru Nakamura, C.; et al. A versatile method for the selective core-crosslinking of hyaluronic acid nanogels via ketone-hydrazide chemistry: From chemical characterization to in vivo biodistribution. Biomater. Sci. 2018, 6, 1754–1763. [Google Scholar] [CrossRef] [PubMed]
- Seok, H.Y.; Sanoj Rejinold, N.; Lekshmi, K.M.; Cherukula, K.; Park, I.K.; Kim, Y.C. CD44 targeting biocompatible and biodegradable hyaluronic acid cross-linked zein nanogels for curcumin delivery to cancer cells: In vitro and in vivo evaluation. J. Control. Release 2018, 280, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.Q.; Pu, K.Y. Organic Semiconducting Agents for Deep-Tissue Molecular Imaging: Second Near-Infrared Fluorescence, Self-Luminescence, and Photoacoustics. Adv. Mater. 2018, 30, 1801778. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.J.; Xie, C.; Chen, P.; Pu, K.Y. Organic Nanotheranostics for Photoacoustic Imaging-Guided Phototherapy. Curr. Med. Chem. 2019, 26, 1389–1405. [Google Scholar] [CrossRef]
- Cheng, P.; Pu, K. Activatable Phototheranostic Materials for Imaging-Guided Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 5286–5299. [Google Scholar] [CrossRef]
- Xie, C.; Yang, C.C.; Zhang, P.; Zhang, J.L.; Wu, W.; Jiang, X.Q. Synthesis of drug-crosslinked polymer nanoparticles. Polym. Chem. 2015, 6, 1703–1713. [Google Scholar] [CrossRef]
- Qian, Q.H.; Shi, L.L.; Gao, X.H.; Ma, Y.; Yang, J.P.; Zhang, Z.H.; Qian, J.W.; Zhu, X.Y. A Paclitaxel-Based Mucoadhesive Nanogel with Multivalent Interactions for Cervical Cancer Therapy. Small 2019, 15, 1903208. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, J.J.; Wang, J.J.; Wang, X.; Wu, C.; Chen, M.W.; Wu, Q.; Lesniak, M.S.; Mi, Y.L.; Cheng, Y.; et al. A Neutrophil-Inspired Supramolecular Nanogel for Magnetocaloric-Enzymatic Tandem Therapy. Angew. Chem. Int. Ed. 2020, 59, 3732–3738. [Google Scholar] [CrossRef]
- Ding, F.; Mou, Q.B.; Ma, Y.; Pan, G.F.; Guo, Y.Y.; Tong, G.S.; Choi, C.H.J.; Zhu, X.Y.; Zhang, C. A Crosslinked Nucleic Acid Nanogel for Effective siRNA Delivery and Antitumor Therapy. Angew. Chem. Int. Ed. 2018, 57, 3064–3068. [Google Scholar] [CrossRef]
- Qian, H.Q.; Wang, X.; Yuan, K.J.; Xie, C.; Wu, W.; Jiang, X.Q.; Hu, L.J. Delivery of doxorubicin in vitro and in vivo using bio-reductive cellulose nanogels. Biomater. Sci. 2014, 2, 220–232. [Google Scholar] [CrossRef]
- Wu, Q.; He, Z.G.; Wang, X.; Zhang, Q.; Wei, Q.C.; Ma, S.Q.; Ma, C.; Li, J.; Wang, Q.G. Cascade enzymes within self-assembled hybrid nanogel mimicked neutrophil lysosomes for singlet oxygen elevated cancer therapy. Nat. Commun. 2019, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.S.; Gao, L.N.; Zhu, X.N.; Zhang, Y.; Zhang, C.N.; Xu, D.; Cui, Y.L. Co-delivery of glycyrrhizin and doxorubicin by alginate nanogel particles attenuates the activation of macrophage and enhances the therapeutic efficacy for hepatocellular carcinoma. Theranostics 2019, 9, 6239–6255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Andren, O.C.J.; Nordstrom, R.; Fan, Y.M.; Malmsten, M.; Mongkhontreerat, S.; Malkoch, M. Off-Stoichiometric Thiol-Ene Chemistry to Dendritic Nanogel Therapeutics. Adv. Funct. Mater. 2019, 29, 1806693. [Google Scholar] [CrossRef]
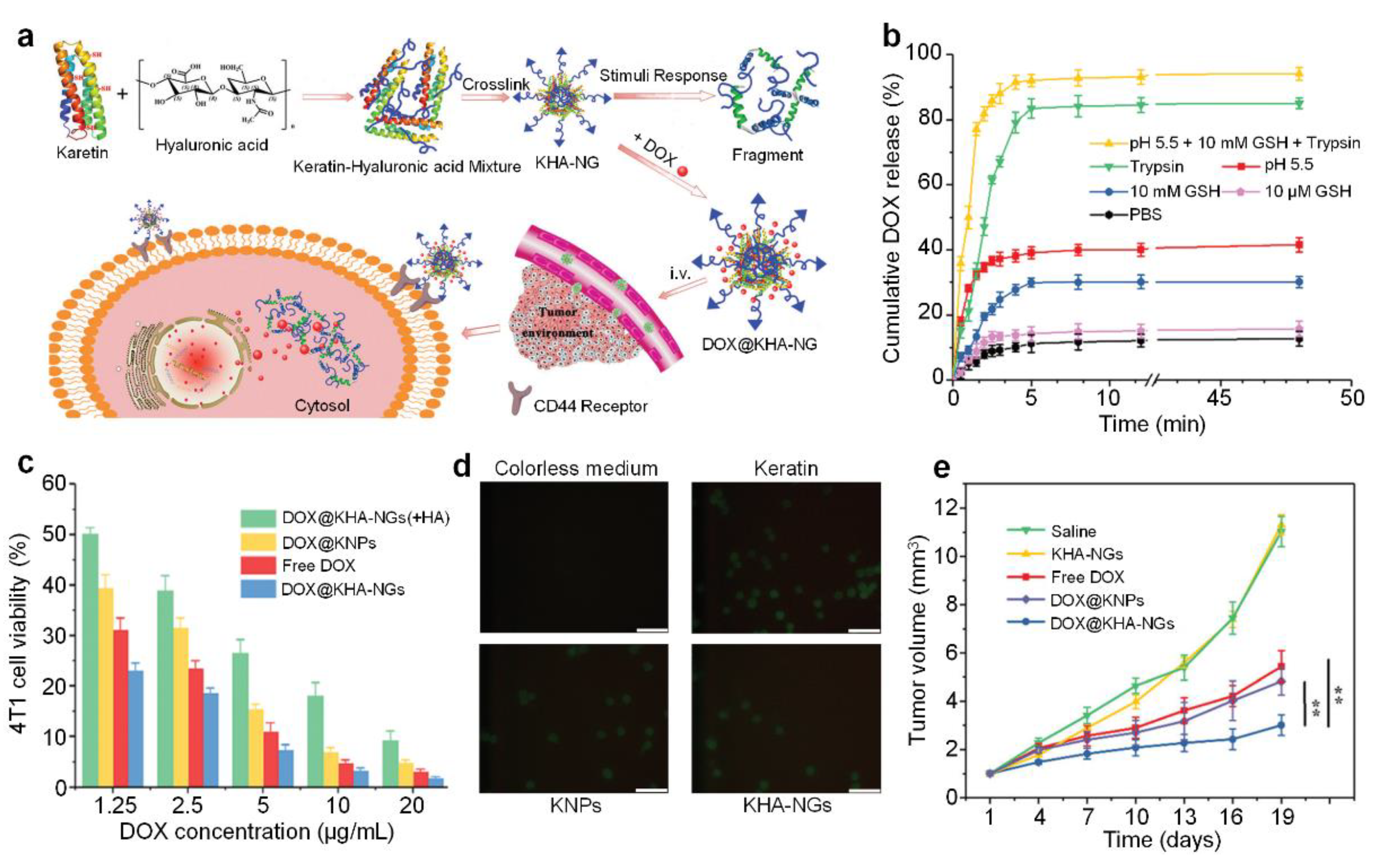
- Sun, Z.; Yi, Z.; Cui, X.X.; Chen, X.Y.; Su, W.; Ren, X.X.; Li, X.D. Tumor-targeted and nitric oxide-generated nanogels of keratin and hyaluronan for enhanced cancer therapy. Nanoscale 2018, 10, 12109–12122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Li, Y.J.; Wan, J.X.; Long, P.H.; Guo, J.; Chen, G.S.; Wang, C.C. Preparation of Pt(IV)-crosslinked polymer nanoparticles with an anti-detoxifying effect for enhanced anticancer therapy. Polym. Chem. 2017, 8, 2410–2422. [Google Scholar] [CrossRef]
- Guo, D.B.; Xu, S.T.; Yasen, W.; Zhang, C.; Shen, J.; Huang, Y.; Chen, D.; Zhu, X.Y. Tirapazamine-embedded polyplatinum(iv) complex: A prodrug combo for hypoxia-activated synergistic chemotherapy. Biomater. Sci. 2020, 8, 694–701. [Google Scholar] [CrossRef]
- Ding, F.; Gao, X.; Huang, X.; Ge, H.; Xie, M.; Qian, J.; Song, J.; Li, Y.; Zhu, X.; Zhang, C. Polydopamine-coated nucleic acid nanogel for siRNA-mediated low-temperature photothermal therapy. Biomaterials 2020, 245, 119976. [Google Scholar] [CrossRef]
- Li, H.P.; Yang, X.; Gao, F.; Qian, C.G.; Li, C.Z.; Oupicky, D.; Sun, M.J. Bioreduction-ruptured nanogel for switch on/off release of Bcl2 siRNA in breast tumor therapy. J. Control. Release 2018, 292, 78–90. [Google Scholar] [CrossRef]
- Si, X.H.; Ma, S.; Xu, Y.; Zhang, D.; Shen, N.; Yu, H.Y.; Zhang, Y.; Song, W.T.; Tang, Z.H.; Chen, X. Hypoxia-sensitive supramolecular nanogels for the cytosolic delivery of ribonuclease A as a breast cancer therapeutic. J. Control. Release 2020, 320, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Aktan, B.; Chambre, L.; Sanyal, R.; Sanyal, A. “Clickable” Nanogels via Thermally Driven Self-Assembly of Polymers: Facile Access to Targeted Imaging Platforms using Thiol—Maleimide Conjugation. Biomacromolecules 2017, 18, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.X.; Jia, H.R.; Chen, Z.; Wu, F.G. Photosensitizer (PS)/polyhedral oligomeric silsesquioxane (POSS)-crosslinked nanohybrids for enhanced imaging-guided photodynamic cancer therapy. Nanoscale 2017, 9, 12874–12884. [Google Scholar] [CrossRef]
- Peng, S.J.; Wang, H.; Zhao, W.; Xin, Y.J.; Liu, Y.; Yu, X.R.; Zhan, M.X.; Shen, S.; Lu, L.G. Zwitterionic Polysulfamide Drug Nanogels with Microwave Augmented Tumor Accumulation and On-Demand Drug Release for Enhanced Cancer Therapy. Adv. Funct. Mater. 2020, 30, 20001832. [Google Scholar] [CrossRef]
- Zhai, Y.H.; Ran, W.; Su, J.H.; Lang, T.Q.; Meng, J.; Wang, G.R.; Zhang, P.C.; Li, Y.P. Traceable Bioinspired Nanoparticle for the Treatment of Metastatic Breast Cancer via NIR-Trigged Intracellular Delivery of Methylene Blue and Cisplatin. Adv. Mater. 2018, 30, 1802378. [Google Scholar] [CrossRef]
- Xue, Y.A.; Xia, X.Y.; Yu, B.; Tao, L.J.; Wang, Q.; Huang, S.W.; Yu, F.Q. Selenylsulfide Bond-Launched Reduction-Responsive Superparamagnetic Nanogel Combined of Acid-Responsiveness for Achievement of Efficient Therapy with Low Side Effect. ACS Appl. Mater. Interfaces 2017, 9, 30253–30257. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, W.; Zhang, J.; Zhou, Y.; Shen, M.; Peng, C.; Shi, X. Facile Formation of Gold-Nanoparticle-Loaded γ-Polyglutamic Acid Nanogels for Tumor Computed Tomography Imaging. Bioconju. Chem. 2017, 28, 2692–2697. [Google Scholar] [CrossRef]
- Sun, W.J.; Zhang, J.L.; Zhang, C.C.; Wang, P.; Peng, C.; Shen, M.W.; Shi, X.Y. Construction of Hybrid Alginate Nanogels Loaded with Manganese Oxide Nanoparticles for Enhanced Tumor Magnetic Resonance Imaging. ACS Macro Lett. 2018, 7, 137–142. [Google Scholar] [CrossRef]
- Sun, W.J.; Zhang, J.L.; Zhang, C.C.; Zhou, Y.W.; Zhu, J.Z.; Peng, C.; Shen, M.W.; Shi, X.Y. A unique nanogel-based platform for enhanced dual mode tumor MR/CT imaging. J. Mater. Chem. B 2018, 6, 4835–4842. [Google Scholar] [CrossRef]
- Li, Q.; Plao, X.K.; Wang, F.C.; Li, X.J.; Yang, J.; Liu, Y.; Shi, L.Q.; Liu, D.B. Encapsulating a Single Nanoprobe in a Multifunctional Nanogel for High-Fidelity Imaging of Caspase Activity in Vivo. Anal. Chem. 2019, 91, 13633–13638. [Google Scholar] [CrossRef]
- Xiang, H.; Xue, F.; Yi, T.; Tham, H.P.; Liu, J.G.; Zhao, Y. Cu2−xS Nanocrystals Cross-Linked with Chlorin e6-Functionalized Polyethylenimine for Synergistic Photodynamic and Photothermal Therapy of Cancer. ACS Appl. Mater. Interfaces 2018, 10, 16344–16351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.C.; Sun, W.J.; Wang, Y.; Xu, F.; Qu, J.; Xia, J.D.; Shen, M.W.; Shi, X.Y. Gd-/CuS-Loaded Functional Nanogels for MR/PA Imaging-Guided Tumor-Targeted Photothermal Therapy. ACS Appl. Mater. Interfaces 2020, 12, 9107–9117. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Li, D.; Wang, Y.; Ouyang, Z.J.; Peng, Y.C.; Tomas, H.; Xia, J.D.; Rodrigues, J.; Shen, M.W.; Shi, X.Y. Polyethylenimine Nanogels Incorporated with Ultrasmall Iron Oxide Nanoparticles and Doxorubicin for MR Imaging-Guided Chemotherapy of Tumors. Bioconju. Chem. 2020, 31, 907–915. [Google Scholar] [CrossRef]
- Jing, X.; Zhi, Z.; Jin, L.; Wang, F.; Wu, Y.; Wang, D.; Yan, K.; Shao, Y.; Meng, L. pH/redox dual-stimuli-responsive cross-linked polyphosphazene nanoparticles for multimodal imaging-guided chemo-photodynamic therapy. Nanoscale 2019, 11, 9457–9467. [Google Scholar] [CrossRef]
- Hubbell, J.A.; Chilkoti, A. Nanomaterials for Drug Delivery. Science 2012, 337, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, S.S.; Goncalves, C.; David, L.; Gama, M. A Novel Crosslinked Hyaluronic Acid Nanogel for Drug Delivery. Macromol. Biosci. 2014, 14, 1556–1568. [Google Scholar] [CrossRef]
- Chacko, R.T.; Ventura, J.; Zhuang, J.M.; Thayumanavan, S. Polymer nanogels: A versatile nanoscopic drug delivery platform. Adv. Drug Deliv. Rev. 2012, 64, 836–851. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, S.; Zhang, Z.; Shi, D. Nanomaterials for Cancer Precision Medicine. Adv. Mater. 2018, 30, 1705660. [Google Scholar] [CrossRef]
- Elsabahy, M.; Wooley, K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef]
- Saito, G.; Swanson, J.A.; Lee, K.D. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: Role and site of cellular reducing activities. Adv. Drug Deliv. Rev. 2003, 55, 199–215. [Google Scholar] [CrossRef]
- Tang, M.L.; Zhou, M.L.; Huang, Y.A.; Zhong, J.J.; Zhou, Z.; Luo, K. Dual-sensitive and biodegradable core-crosslinked HPMA copolymer-doxorubicin conjugate-based nanoparticles for cancer therapy. Polym. Chem. 2017, 8, 2370–2380. [Google Scholar] [CrossRef]
- Zhou, Z.X.; Liu, X.R.; Zhu, D.C.; Wang, Y.; Zhang, Z.; Zhou, X.F.; Qiu, N.S.; Chen, X.S.; Shen, Y.Q. Nonviral cancer gene therapy: Delivery cascade and vector nanoproperty integration. Adv. Drug Deliv. Rev. 2017, 115, 115–154. [Google Scholar] [CrossRef] [PubMed]
- Kay, M.A. State-of-the-art gene-based therapies: The road ahead. Nat. Rev. Genet. 2011, 12, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Liu, Y.; Bu, W.B.; Cheng, C.; Zuo, C.J.; Xiao, Q.F.; Sun, Y.; Ni, D.L.; Zhang, C.; Liu, J.A.; et al. Hypoxia Induced by Upconversion-Based Photodynamic Therapy: Towards Highly Effective Synergistic Bioreductive Therapy in Tumors. Angew. Chem. Int. Ed. 2015, 54, 8105–8109. [Google Scholar] [CrossRef]
- Yang, S.C.; Tang, Z.H.; Hu, C.Y.; Zhang, D.W.; Shen, N.; Yu, H.Y.; Chen, X.S. Selectively Potentiating Hypoxia Levels by Combretastatin A4 Nanomedicine: Toward Highly Enhanced Hypoxia-Activated Prodrug Tirapazamine Therapy for Metastatic Tumors. Adv. Mater. 2019, 31, 1805955. [Google Scholar] [CrossRef]
- Zabernigg, A.; Gamper, E.M.; Giesinger, J.M.; Rumpold, G.; Kemmler, G.; Gattringer, K.; Sperner-Unterweger, B.; Holzner, B. Taste alterations in cancer patients receiving chemotherapy: A neglected side effect? Oncologist 2010, 15, 913–920. [Google Scholar] [CrossRef]
- Tang, Z.M.; Liu, Y.Y.; He, M.Y.; Bu, W.B. Chemodynamic Therapy: Tumour Microenvironment-Mediated Fenton and Fenton-like Reactions. Angew. Chem. Int. Ed. 2019, 58, 946–956. [Google Scholar] [CrossRef]
- Ding, B.B.; Shao, S.; Jiang, F.; Dang, P.P.; Sun, C.Q.; Huang, S.S.; Ma, P.A.; Jin, D.Y.; Al Kheraif, A.A.; Lin, J. MnO2-Disguised Upconversion Hybrid Nanocomposite: An Ideal Architecture for Tumor Microenvironment-Triggered UCL/MR Bioimaging and Enhanced Chemodynamic Therapy. Chem. Mater. 2019, 31, 2651–2660. [Google Scholar] [CrossRef]
- Fang, C.; Deng, Z.; Cao, G.D.; Chu, Q.; Wu, Y.L.; Li, X.; Peng, X.S.; Han, G.R. Co-Ferrocene MOF/Glucose Oxidase as Cascade Nanozyme for Effective Tumor Therapy. Adv. Funct. Mater. 2020, 30, 1910085. [Google Scholar] [CrossRef]
- Kumar, R.; Han, J.; Lim, H.J.; Ren, W.X.; Lim, J.Y.; Kim, J.H.; Kim, J.S. Mitochondrial Induced and Self-Monitored Intrinsic Apoptosis by Antitumor Theranostic Prodrug: In Vivo Imaging and Precise Cancer Treatment. J. Am. Chem. Soc. 2014, 136, 17836–17843. [Google Scholar] [CrossRef]
- Bi, W.L.; Hosny, A.; Schabath, M.B.; Giger, M.L.; Birkbak, N.J.; Mehrtash, A.; Allison, T.; Arnaout, O.; Abbosh, C.; Dunn, I.F.; et al. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA Cancer J. Clin. 2019, 69, 127–157. [Google Scholar] [CrossRef] [PubMed]
- Satoh, Y.; Imai, M.; Ikegawa, C.; Arai, T. Dedicated breast PET versus whole-body PET/CT: A comparative study. J. Nucl. Med. 2018, 59, 1582. [Google Scholar]
- Zhou, W.; Chen, Y.; Zhang, Y.T.; Xin, X.Y.; Li, R.T.; Xie, C.; Fan, Q.L. Iodine-Rich Semiconducting Polymer Nanoparticles for CT/Fluorescence Dual-Modal Imaging-Guided Enhanced Photodynamic Therapy. Small 2020, 16, 1905641. [Google Scholar] [CrossRef] [PubMed]
- Ni, D.L.; Ehlerding, E.B.; Cai, W.B. Multimodality Imaging Agents with PET as the Fundamental Pillar. Angew. Chem. Int. Ed. 2019, 58, 2570–2579. [Google Scholar] [CrossRef]
- Wang, C.; Fan, W.P.; Zhang, Z.J.; Wen, Y.; Xiong, L.; Chen, X.Y. Advanced Nanotechnology Leading the Way to Multimodal Imaging-Guided Precision Surgical Therapy. Adv. Mater. 2019, 31, 1904329. [Google Scholar] [CrossRef]
- Miao, Q.Q.; Xie, C.; Zhen, X.; Lyu, Y.; Duan, H.W.; Liu, X.G.; Jokerst, J.V.; Pu, K.Y. Molecular afterglow imaging with bright, biodegradable polymer nanoparticles. Nat. Biotechnol. 2017, 35, 1102–1110. [Google Scholar] [CrossRef]
- Xie, C.; Lyu, Y.; Zhen, X.; Miao, Q.; Pu, K. Activatable Semiconducting Oligomer Amphiphile for Near-Infrared Luminescence Imaging of Biothiols. ACS Appl. Bio. Mater. 2018, 1, 1147–1153. [Google Scholar] [CrossRef]
- Park, S.M.; Aalipour, A.; Vermesh, O.; Yu, J.H.; Gambhir, S.S. Towards clinically translatable in vivo nanodiagnostics. Nat. Rev. Mater. 2017, 2, 17014. [Google Scholar] [CrossRef]
- Li, J.C.; Zhen, X.; Lyu, Y.; Jiang, Y.Y.; Huang, J.G.; Pu, K.Y. Cell Membrane Coated Semiconducting Polymer Nanoparticles for Enhanced Multimodal Cancer Phototheranostics. ACS Nano 2018, 12, 8520–8530. [Google Scholar] [CrossRef]
- Zhen, X.; Cheng, P.H.; Pu, K.Y. Recent Advances in Cell Membrane-Camouflaged Nanoparticles for Cancer Phototherapy. Small 2019, 15, 1804105. [Google Scholar] [CrossRef]
- An, H.W.; Li, L.L.; Wang, Y.; Wang, Z.Q.; Hou, D.Y.; Lin, Y.X.; Qiao, S.L.; Wang, M.D.; Yang, C.; Cong, Y.; et al. A tumour-selective cascade activatable self-detained system for drug delivery and cancer imaging. Nat. Commun. 2019, 10, 4861. [Google Scholar] [CrossRef]
- Lyu, Y.; Fang, Y.; Miao, Q.Q.; Zhen, X.; Ding, D.; Pu, K.Y. Intraparticle molecular orbital engineering of semiconducting polymer nanoparticles as amplified theranostics for in vivo photoacoustic imaging and photothermal therapy. ACS Nano 2016, 10, 4472–4481. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Wu, Y.X.; Chen, J.T.; Wan, J.L.; Xiao, C.; Guan, J.K.; Song, X.L.; Li, S.Y.; Zhang, M.M.; Cui, H.C.; et al. A Simple Glutathione-Responsive Turn-On Theranostic Nanoparticle for Dual-Modal Imaging and Chemo-Photothermal Combination Therapy. Nano Lett. 2019, 19, 5806–5817. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, D.; Hui, Y.; Middelberg, A.P.J.; Zhao, C.X. Interfacial engineering for silica nanocapsules. Adv. Colloid Interfac. 2016, 236, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Miro, M.; Chen, S.X.; Gonzaga, Z.J.; Evert, B.; Wibowo, D.; Rehm, B.H.A. Polyester as Antigen Carrier toward Particulate Vaccines. Biomacromolecules 2019, 20, 3213–3232. [Google Scholar] [CrossRef]
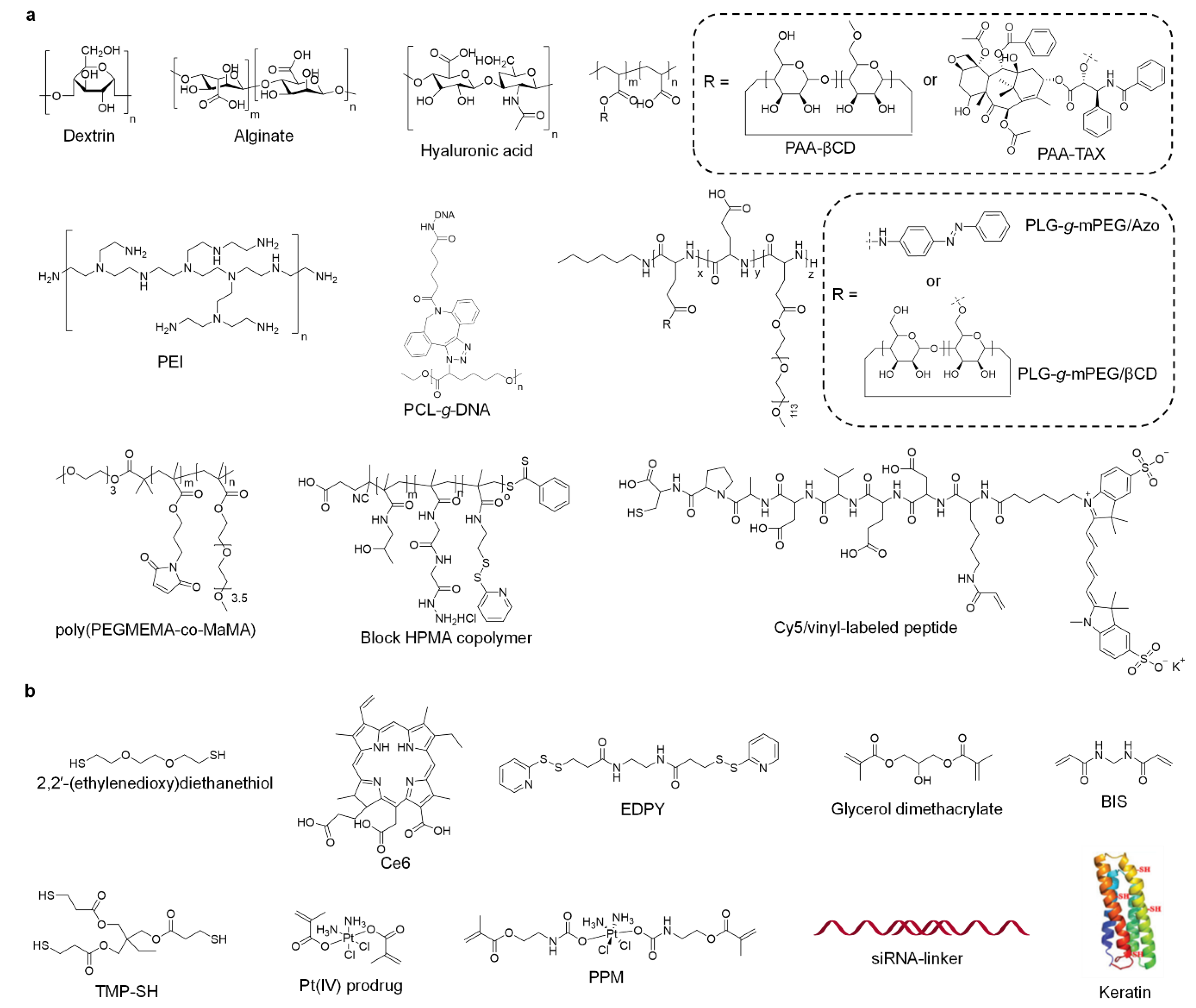



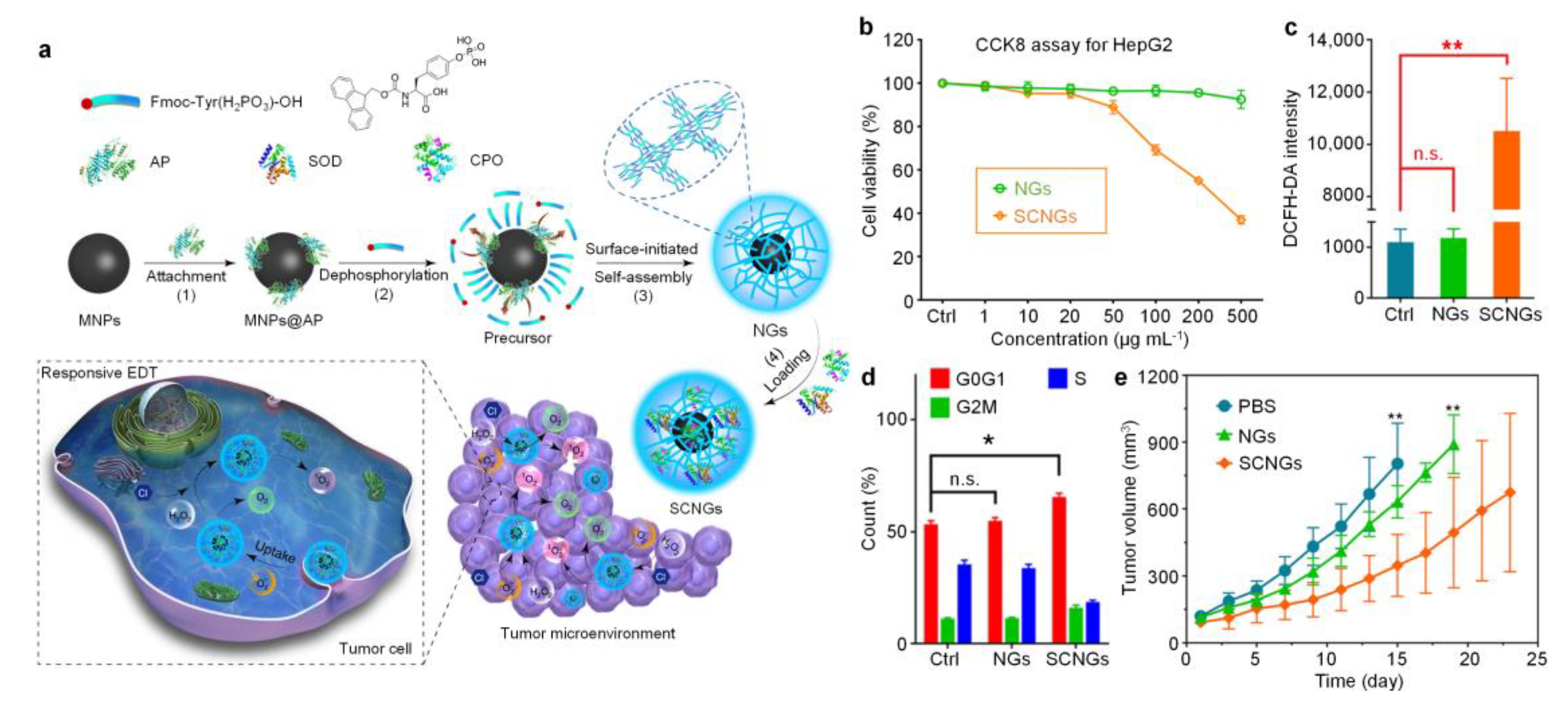
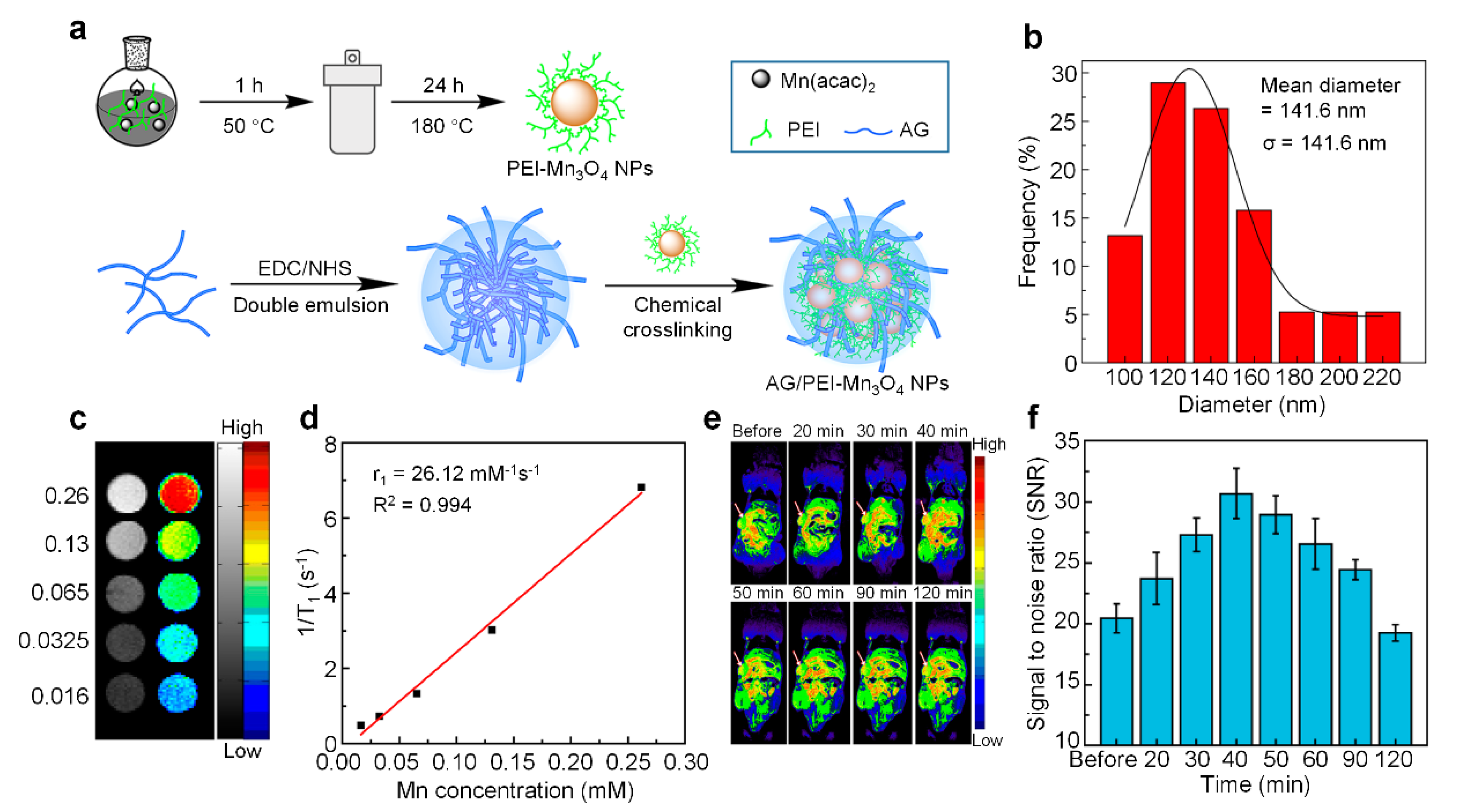

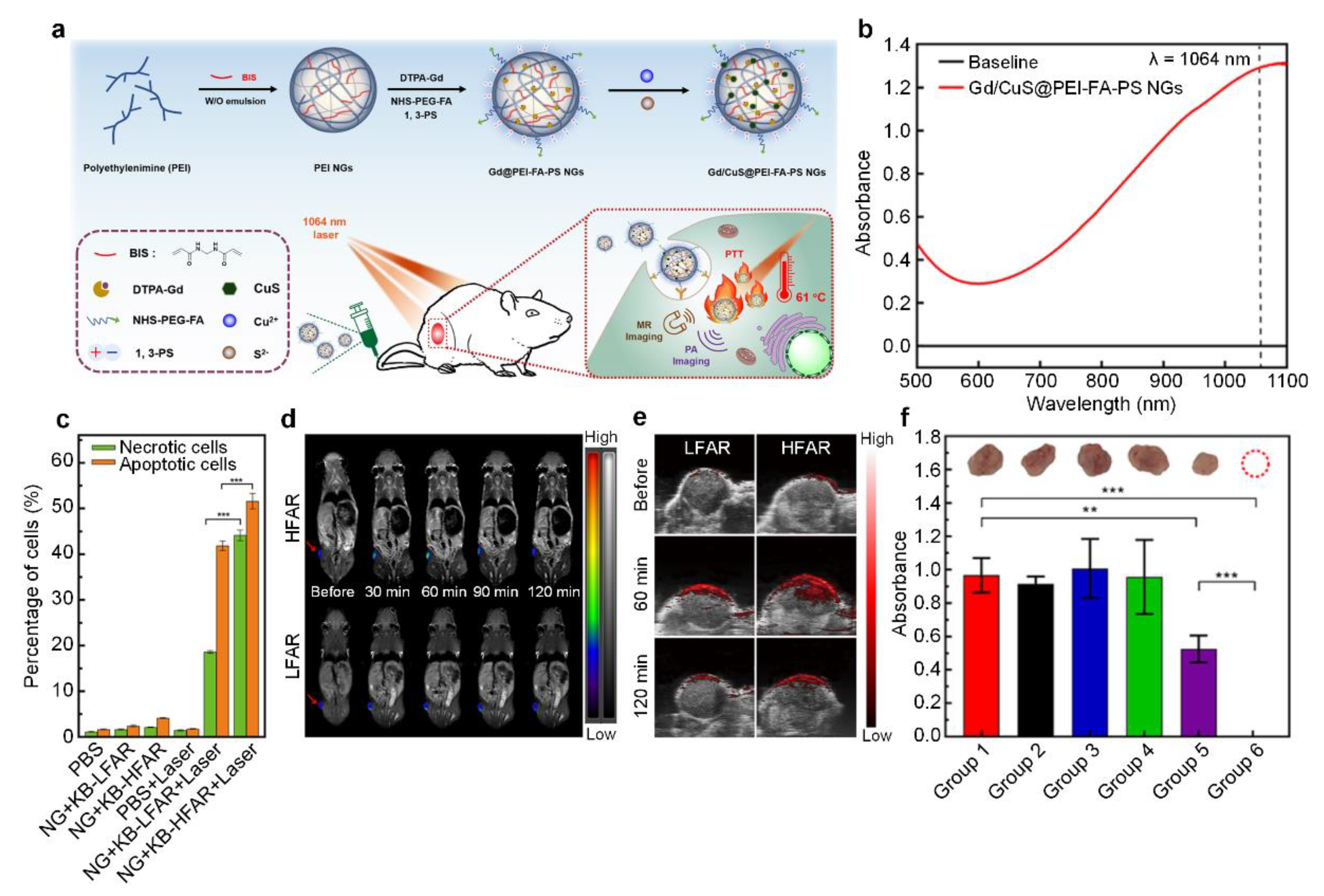

| Type | Inorganic Core | Materials | Crosslinker | Crosslinking Interaction | References |
|---|---|---|---|---|---|
| Organic | Alginate | Ca ion | Electrostatic interaction | [54] | |
| Dendritic blocks | TMP-SH | Covalent bond | [55] | ||
| Hyaluronic acid | Keratin | Hydrogen bond | [56] | ||
| PAA-βCD | PAA-TAX | Supramolecular interaction | [49] | ||
| - | Pt(IV) prodrug | Covalent bond | [57] | ||
| - | PPM | Covalent bond | [58] | ||
| DNA-g-PCL | siRNA | Electrostatic interaction | [51] | ||
| DNA-g-PCL | siRNA | Electrostatic interaction | [59] | ||
| Dextrin-SH | PEI-SH | Covalent bond | [60] | ||
| PLG-g-mPEG/βCD | PLG-g-mPEG/Azo | Supramolecular interaction | [61] | ||
| poly(PEGMEMA-co-MaMA) | 2,2′-(ethylenedioxy)diethanethiol | Covalent bond | [62] | ||
| POSS | Ce6 | Covalent bond | [63] | ||
| MEDAPA | EGDMA/BAC | Covalent bond | [64] | ||
| Gelatin | glutaraldehyde | Covalent bond | [65] | ||
| Inorganic | MNP-NH2 | Alginate | - | Covalent bond | [66] |
| MNPs | Tyrosine | - | π-π interaction | [53] | |
| MNPs | Tyrosine | - | π-π interaction | [50] | |
| AuNPs | γ-PGA | PEI | Covalent bond | [67] | |
| Mn3O4 NPs | Alginate | PEI | Covalent bond | [68] | |
| AuNPs | Alginate | PEI | Covalent bond | [69] | |
| AuNPs | Cy5/vinyl-labeled peptide | Glycerol dimethacrylate | Covalent bond | [70] | |
| Cu2−xS NPs | PEI | Ce6 | Covalent bond | [71] | |
| CuS NPs | PEI | BIS | Covalent bond | [72] | |
| Fe3O4 NPs | PEI | BIS | Covalent bond | [73] | |
| Fe3O4 NPs | HCCP | CUR/HPS | Covalent bond | [74] |
| Type | Loaded Drug | Loading Capacity | Responsiveness | Animal Study | References |
|---|---|---|---|---|---|
| Chemotherapy | DOX/GL | 1.2% (DOX) | pH | Yes | [54] |
| DOX | 5.7% | - | No | [55] | |
| DOX | 54.1% | pH/GSH/trypsin | Yes | [56] | |
| DOX | 18.2% | pH/GSH | Yes | [66] | |
| TAX | 20–30% | pH/esterase | Yes | [49] | |
| Pt(IV) | 60.8% | GSH/ascorbic acid | Yes | [57] | |
| Pt(IV)/TPZ | 8.06% (Pt)/9.12% (TPZ) | GSH | Yes | [58] | |
| Gene therapy | siRNA | - | RNase H | Yes | [51] |
| siRNA | - | pH/RNase H | Yes | [59] | |
| siBcl2 | - | DTT | Yes | [60] | |
| RNase | 23.5% | NTR | Yes | [61] | |
| Enzyme dynamic therapy | - | - | ·O2−/H2O2 | Yes | [53] |
| - | - | H2O2 | Yes | [50] |
| Type | Imaging Modality | Therapeutic Method | Responsiveness | Animal Study | References |
|---|---|---|---|---|---|
| Imaging | CT | - | - | Yes | [67] |
| T1-MRI | - | - | Yes | [68] | |
| T1-MRI/CT | - | - | Yes | [69] | |
| FL | - | - | No | [62] | |
| FL | - | pH/caspases | Yes | [70] | |
| Imaging-guided therapy | FL | PDT/PTT | - | Yes | [71] |
| FL | PDT | - | Yes | [63] | |
| FL | Chemotherapy | Temperature | Yes | [64] | |
| T1-MRI/PA | PTT | - | Yes | [72] | |
| T1-MRI | Chemotherapy | pH | Yes | [73] | |
| PA/FL/PT | PDT/chemotherapy | Laser | Yes | [65] | |
| Fluorescence/T2-MRI | PDT/chemotherapy | pH/GSH | Yes | [74] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Yang, G.; Ni, X.; Diao, S.; Xie, C.; Fan, Q. Recent Advances in Crosslinked Nanogel for Multimodal Imaging and Cancer Therapy. Polymers 2020, 12, 1902. https://doi.org/10.3390/polym12091902
Zhou W, Yang G, Ni X, Diao S, Xie C, Fan Q. Recent Advances in Crosslinked Nanogel for Multimodal Imaging and Cancer Therapy. Polymers. 2020; 12(9):1902. https://doi.org/10.3390/polym12091902
Chicago/Turabian StyleZhou, Wen, Guangzhao Yang, Xiaoyue Ni, Shanchao Diao, Chen Xie, and Quli Fan. 2020. "Recent Advances in Crosslinked Nanogel for Multimodal Imaging and Cancer Therapy" Polymers 12, no. 9: 1902. https://doi.org/10.3390/polym12091902
APA StyleZhou, W., Yang, G., Ni, X., Diao, S., Xie, C., & Fan, Q. (2020). Recent Advances in Crosslinked Nanogel for Multimodal Imaging and Cancer Therapy. Polymers, 12(9), 1902. https://doi.org/10.3390/polym12091902