Metal-Organic Decomposition-Mediated Nanoparticulate Vanadium Oxide Hole Transporting Buffer Layer for Polymer Bulk-Heterojunction Solar Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of Devices
2.2. Characterization
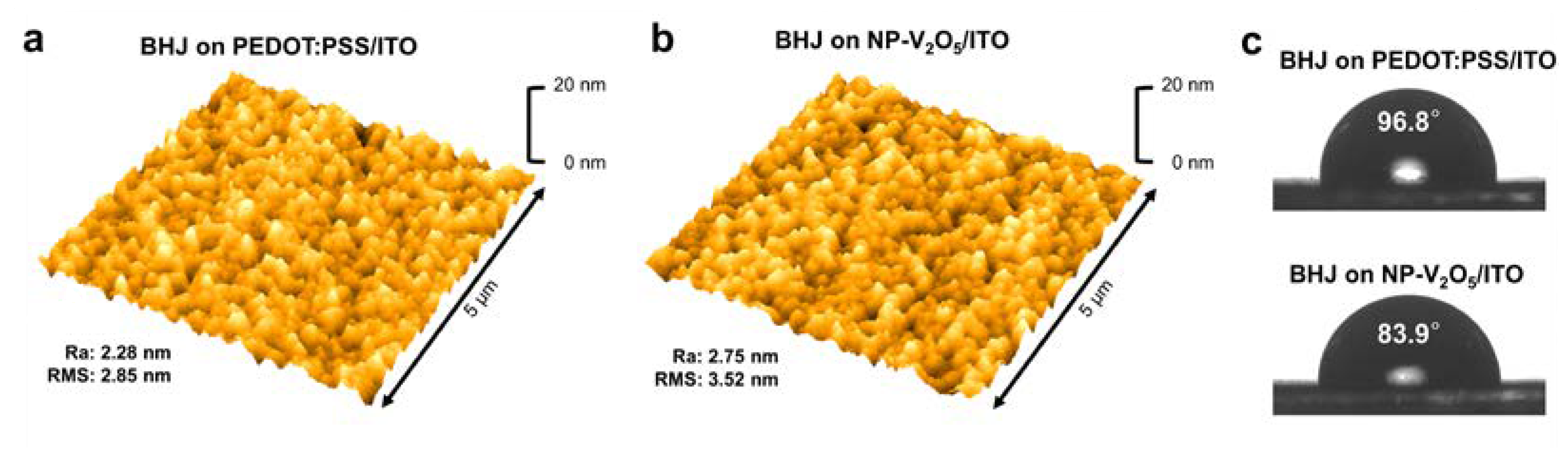
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, G.; Zhu, R.; Yang, Y. Polymer solar cells. Nat. Photonics 2012, 6, 153–161. [Google Scholar] [CrossRef]
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer photovoltaic cells: Enhanced efficiencies via a network of internal donor-acceptor heterojunctions. Science 1995, 270, 1789–1791. [Google Scholar] [CrossRef]
- Gu, X.; Zhou, Y.; Gu, K.; Kurosawa, T.; Guo, Y.; Li, Y.; Lin, H.; Schroeder, B.C.; Yan, H.; Molina-Lopez, F.; et al. Roll-to-roll printed large-area all-polymer solar cells with 5% efficiency based on a low crystallinity conjugated polymer blend. Adv. Energy Mater. 2017, 7, 1602742. [Google Scholar] [CrossRef]
- Yin, C.; Pieper, B.; Stiller, B.; Kietzke, T.; Neher, D. Charge carrier generation and electron blocking at interlayers in polymer solar cells. Appl. Phys. Lett. 2007, 90, 133502. [Google Scholar] [CrossRef]
- Chang, Y.; Lau, T.-K.; Chow, P.C.Y.; Wu, N.; Su, D.; Zhang, W.; Meng, H.; Ma, C.; Liu, T.; Li, K.; et al. A 16.4% efficiency organic photovoltaic cell enabled using two donor polymers with their side-chains oriented differently by a ternary strategy. J. Mater. Chem. A 2020, 8, 3676–3685. [Google Scholar] [CrossRef]
- Wang, D.H.; Kim, J.K.; Seo, J.H.; Park, I.; Hong, B.H.; Park, J.H.; Heeger, A.J. Transferable graphene oxide by stamping nanotechnology: Electron-transport layer for efficient bulk-heterojunction solar cells. Angew. Chem. Int. Ed. 2013, 52, 2874–2880. [Google Scholar] [CrossRef] [PubMed]
- Menke, S.M.; Ran, N.A.; Bazan, G.C.; Friend, R.H. Understanding energy loss in organic solar cells: Toward a new efficiency regime. Joule 2018, 2, 25–35. [Google Scholar] [CrossRef]
- Hou, C.; Yu, H. Modifying the nanostructures of pedot:Pss/ti3c2tx composite hole transport layers for highly efficient polymer solar cells. J. Mater. Chem. C 2020, 8, 4169–4180. [Google Scholar] [CrossRef]
- McClain, W.E.; Florence, P.R.; Shu, A.; Kahn, A.; Schwartz, J. Surface dipole engineering for conducting polymers. Org. Electron. 2013, 14, 411–415. [Google Scholar] [CrossRef]
- Khodabakhsh, S.; Poplavskyy, D.; Heutz, S.; Nelson, J.; Bradley, D.D.C.; Murata, H.; Jones, T.S. Using self-assembling dipole molecules to improve hole injection in conjugated polymers. Adv. Funct. Mater. 2004, 14, 1205–1210. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, I.; Kim, W.; Wang, D.H.; Choi, D.-G.; Choi, Y.S.; Park, J.H. Enhanced performance and stability of polymer bhj photovoltaic devices from dry transfer of pedot:Pss. ChemSusChem 2014, 7, 1957–1963. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ruderer, M.A.; Metwalli, E.; Guo, S.; Herzig, E.M.; Perlich, J.; Müller-Buschbaum, P. Effect of methanol addition on the resistivity and morphology of pedot:Pss layers on top of carbon nanotubes for use as flexible electrodes. ACS Appl. Mater. Interfaces 2015, 7, 8789–8797. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Zhang, Y.; Wan, X.; Li, C.; Zhang, X.; Wang, Y.; Ke, X.; Xiao, Z.; Ding, L.; Xia, R.; et al. Organic and solution-processed tandem solar cells with 17.3% efficiency. Science 2018, 361, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yao, H.; Zhang, J.; Zhang, T.; Wang, Y.; Hong, L.; Xian, K.; Xu, B.; Zhang, S.; Peng, J.; et al. Over 16% efficiency organic photovoltaic cells enabled by a chlorinated acceptor with increased open-circuit voltages. Nat. Commun. 2019, 10, 2515. [Google Scholar] [CrossRef]
- Xu, X.; Feng, K.; Bi, Z.; Ma, W.; Zhang, G.; Peng, Q. Single-junction polymer solar cells with 16.35% efficiency enabled by a platinum(ii) complexation strategy. Adv. Mater. 2019, 31, 1901872. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, H.S.; Rhee, D.K.; Ham, S.-J.; Lee, K.-J.; Yoo, P.J.; Park, J.H. Ultrathin nanoclay films with tunable thickness as barrier layers in organic light emitting devices. J. Mater. Chem. 2012, 22, 7718–7723. [Google Scholar] [CrossRef]
- Lee, T.-W.; Chung, Y. Control of the surface composition of a conducting-polymer complex film to tune the work function. Adv. Funct. Mater. 2008, 18, 2246–2252. [Google Scholar] [CrossRef]
- Peng, B.; Guo, X.; Cui, C.; Zou, Y.; Pan, C.; Li, Y. Performance improvement of polymer solar cells by using a solvent-treated poly(3,4-ethylenedioxythiophene):Poly(styrenesulfonate) buffer layer. Appl. Phys. Lett. 2011, 98, 243308. [Google Scholar] [CrossRef]
- Gu, C.; Chen, Y.; Zhang, Z.; Xue, S.; Sun, S.; Zhong, C.; Zhang, H.; Lv, Y.; Li, F.; Huang, F.; et al. Achieving high efficiency of ptb7-based polymer solar cells via integrated optimization of both anode and cathode interlayers. Adv. Energy Mater. 2014, 4, 1301771. [Google Scholar] [CrossRef]
- Syed, A.A.; Poon, C.Y.; Li, H.W.; Zhu, F. A sodium citrate-modified-pedot:Pss hole transporting layer for performance enhancement in inverted planar perovskite solar cells. J. Mater. Chem. C 2019, 7, 5260–5266. [Google Scholar] [CrossRef]
- Li, S.-H.; Xu, Z.; Yang, G.; Ma, L.; Yang, Y. Solution-processed poly(3-hexylthiophene) vertical organic transistor. Appl. Phys. Lett. 2008, 93, 213301. [Google Scholar] [CrossRef]
- Arbab, E.A.A.; Mola, G.T. V2o5 thin film deposition for application in organic solar cells. Appl. Phys. A 2016, 122, 405. [Google Scholar] [CrossRef]
- Terán-Escobar, G.; Pampel, J.; Caicedo, J.M.; Lira-Cantú, M. Low-temperature, solution-processed, layered v2o5 hydrate as the hole-transport layer for stable organic solar cells. Energy Environ. Sci. 2013, 6, 3088–3098. [Google Scholar] [CrossRef]
- Pan, J.; Li, P.; Cai, L.; Hu, Y.; Zhang, Y. All-solution processed double-decked PEDOT:PSS/V2O5 nanowires as buffer layer of high performance polymer photovoltaic cells. Sol. Energy Mater. Sol. Cells 2016, 144, 616–622. [Google Scholar] [CrossRef]
- Balasubramani, V.; Chandrasekaran, J.; Marnadu, R.; Vivek, P.; Maruthamuthu, S.; Rajesh, S. Impact of annealing temperature on spin coated v2o5 thin films as interfacial layer in cu/v2o5/n-si structured schottky barrier diodes. J. Inorg. Organomet. Polym. 2019, 29, 1533–1547. [Google Scholar] [CrossRef]
- Almora, O.; Gerling, L.G.; Voz, C.; Alcubilla, R.; Puigdollers, J.; Garcia-Belmonte, G. Superior performance of v2o5 as hole selective contact over other transition metal oxides in silicon heterojunction solar cells. Sol. Energy Mater. Sol. Cells 2017, 168, 221–226. [Google Scholar] [CrossRef]
- Gerling, L.G.; Mahato, S.; Morales-Vilches, A.; Masmitja, G.; Ortega, P.; Voz, C.; Alcubilla, R.; Puigdollers, J. Transition metal oxides as hole-selective contacts in silicon heterojunctions solar cells. Sol. Energy Mater. Sol. Cells 2016, 145, 109–115. [Google Scholar] [CrossRef]
- Cho, S.-P.; Yeo, J.-S.; Kim, D.-Y.; Na, S.-i.; Kim, S.-S. Brush painted v2o5 hole transport layer for efficient and air-stable polymer solar cells. Sol. Energy Mater. Sol. Cells 2015, 132, 196–203. [Google Scholar] [CrossRef]
- Wang, D.; Elumalai, N.K.; Mahmud, M.A.; Wright, M.; Upama, M.B.; Chan, K.H.; Xu, C.; Haque, F.; Conibeer, G.; Uddin, A. V2o5 -pedot: Pss bilayer as hole transport layer for highly efficient and stable perovskite solar cells. Org. Electron. 2018, 53, 66–73. [Google Scholar] [CrossRef]
- Lee, S.J.; Pil Kim, H.; Mohd Yusoff, A.R.b.; Jang, J. Organic photovoltaic with pedot:Pss and v2o5 mixture as hole transport layer. Sol. Energy Mater. Sol. Cells 2014, 120, 238–243. [Google Scholar] [CrossRef]
- Kim, J.K.; Shin, K.; Cho, S.M.; Lee, T.-W.; Park, J.H. Synthesis of transparent mesoporous tungsten trioxide films with enhanced photoelectrochemical response: Application to unassisted solar water splitting. Energy Environ. Sci. 2011, 4, 1465–1470. [Google Scholar] [CrossRef]
- Oh, H.J.; Freeman, B.D.; McGrath, J.E.; Lee, C.H.; Paul, D.R. Thermal analysis of disulfonated poly(arylene ether sulfone) plasticized with poly(ethylene glycol) for membrane formation. Polymer 2014, 55, 235–247. [Google Scholar] [CrossRef]
- Friedel, B.; Keivanidis, P.E.; Brenner, T.J.K.; Abrusci, A.; McNeill, C.R.; Friend, R.H.; Greenham, N.C. Effects of layer thickness and annealing of pedot:Pss layers in organic photodetectors. Macromolecules 2009, 42, 6741–6747. [Google Scholar] [CrossRef]
- Reese, M.O.; Gevorgyan, S.A.; Jørgensen, M.; Bundgaard, E.; Kurtz, S.R.; Ginley, D.S.; Olson, D.C.; Lloyd, M.T.; Morvillo, P.; Katz, E.A.; et al. Consensus stability testing protocols for organic photovoltaic materials and devices. Sol. Energy Mater. Sol. Cells 2011, 95, 1253–1267. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Li, Y.; Wang, D.; Ren, L.; Zou, K. Effect of annealing temperature on the structure and properties of vanadium oxide films. Opt. Mater. Express 2016, 6, 1552–1560. [Google Scholar] [CrossRef]
- Singh, M.; Saini, S.K.; Kumar, P.; Sharma, R.K.; Reddy, G.B. Plasma assisted facile synthesis of vanadium oxide (v3o7) nanostructured thin films. AIP Conf. Proc. 2018, 1953, 030176. [Google Scholar] [CrossRef]
- Le, T.K.; Kang, M.; Kim, S.W. A review on the optical characterization of v2o5 micro-nanostructures. Ceram. Int. 2019, 45, 15781–15798. [Google Scholar] [CrossRef]
- Silversmit, G.; Depla, D.; Poelman, H.; Marin, G.B.; De Gryse, R. An xps study on the surface reduction of v2o5(001) induced by ar+ ion bombardment. Surf. Sci. 2006, 600, 3512–3517. [Google Scholar] [CrossRef]
- Berenguer, R.; Guerrero-Pérez, M.O.; Guzmán, I.; Rodríguez-Mirasol, J.; Cordero, T. Synthesis of vanadium oxide nanofibers with variable crystallinity and v5+/v4+ ratios. ACS Omega 2017, 2, 7739–7745. [Google Scholar] [CrossRef]
- Surnev, S.; Ramsey, M.G.; Netzer, F.P. Vanadium oxide surface studies. Prog. Surf. Sci. 2003, 73, 117–165. [Google Scholar] [CrossRef]
- Proctor, C.M.; Nguyen, T.-Q. Effect of leakage current and shunt resistance on the light intensity dependence of organic solar cells. Appl. Phys. Lett. 2015, 106, 083301. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, S.J.; Park, M.J.; Bae, S.; Cho, S.-P.; Du, Q.G.; Wang, D.H.; Park, J.H.; Hong, B.H. Surface-engineered graphene quantum dots incorporated into polymer layers for high performance organic photovoltaics. Sci. Rep. 2015, 5, 14276. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Yan, C.; Li, Y.; Ma, W.; Zhan, X. Diluting concentrated solution: A general, simple and effective approach to enhance efficiency of polymer solar cells. Energy Environ. Sci. 2015, 8, 2357–2364. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, C.; Hong, W.T.; Kim, Y.E.; Choe, W.-S.; Kim, D.-H.; Kim, J.K. Metal-Organic Decomposition-Mediated Nanoparticulate Vanadium Oxide Hole Transporting Buffer Layer for Polymer Bulk-Heterojunction Solar Cells. Polymers 2020, 12, 1791. https://doi.org/10.3390/polym12081791
Xia C, Hong WT, Kim YE, Choe W-S, Kim D-H, Kim JK. Metal-Organic Decomposition-Mediated Nanoparticulate Vanadium Oxide Hole Transporting Buffer Layer for Polymer Bulk-Heterojunction Solar Cells. Polymers. 2020; 12(8):1791. https://doi.org/10.3390/polym12081791
Chicago/Turabian StyleXia, Chengkai, Won Tae Hong, Young Eun Kim, Woo-Seok Choe, Dong-Hwan Kim, and Jung Kyu Kim. 2020. "Metal-Organic Decomposition-Mediated Nanoparticulate Vanadium Oxide Hole Transporting Buffer Layer for Polymer Bulk-Heterojunction Solar Cells" Polymers 12, no. 8: 1791. https://doi.org/10.3390/polym12081791
APA StyleXia, C., Hong, W. T., Kim, Y. E., Choe, W.-S., Kim, D.-H., & Kim, J. K. (2020). Metal-Organic Decomposition-Mediated Nanoparticulate Vanadium Oxide Hole Transporting Buffer Layer for Polymer Bulk-Heterojunction Solar Cells. Polymers, 12(8), 1791. https://doi.org/10.3390/polym12081791








