Xylan-Derived Light Conversion Nanocomposite Film
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CDs
2.3. Preparation of Carboxymethylated Xylan (CX)
2.4. The Degree of Substitution (DS) of Carboxyl Group in CX
2.5. Preparation of CX/PVA-CDs Composite Films
2.6. Characterizations
3. Results and Discussion
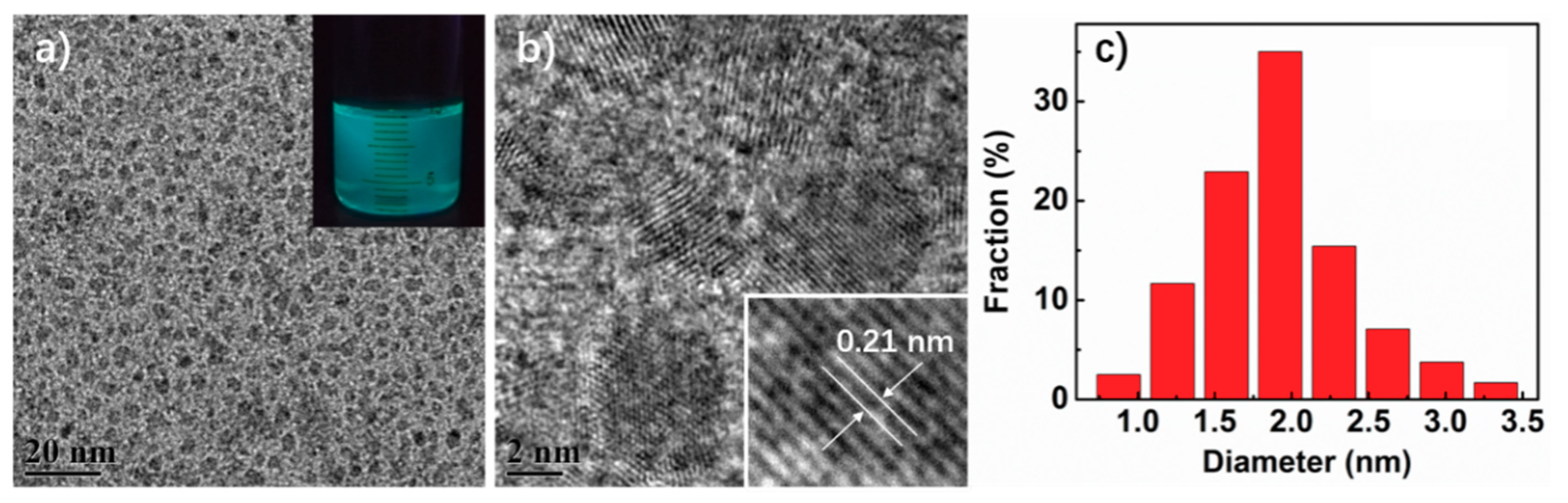
3.1. Surface Morphology of the Composite Films
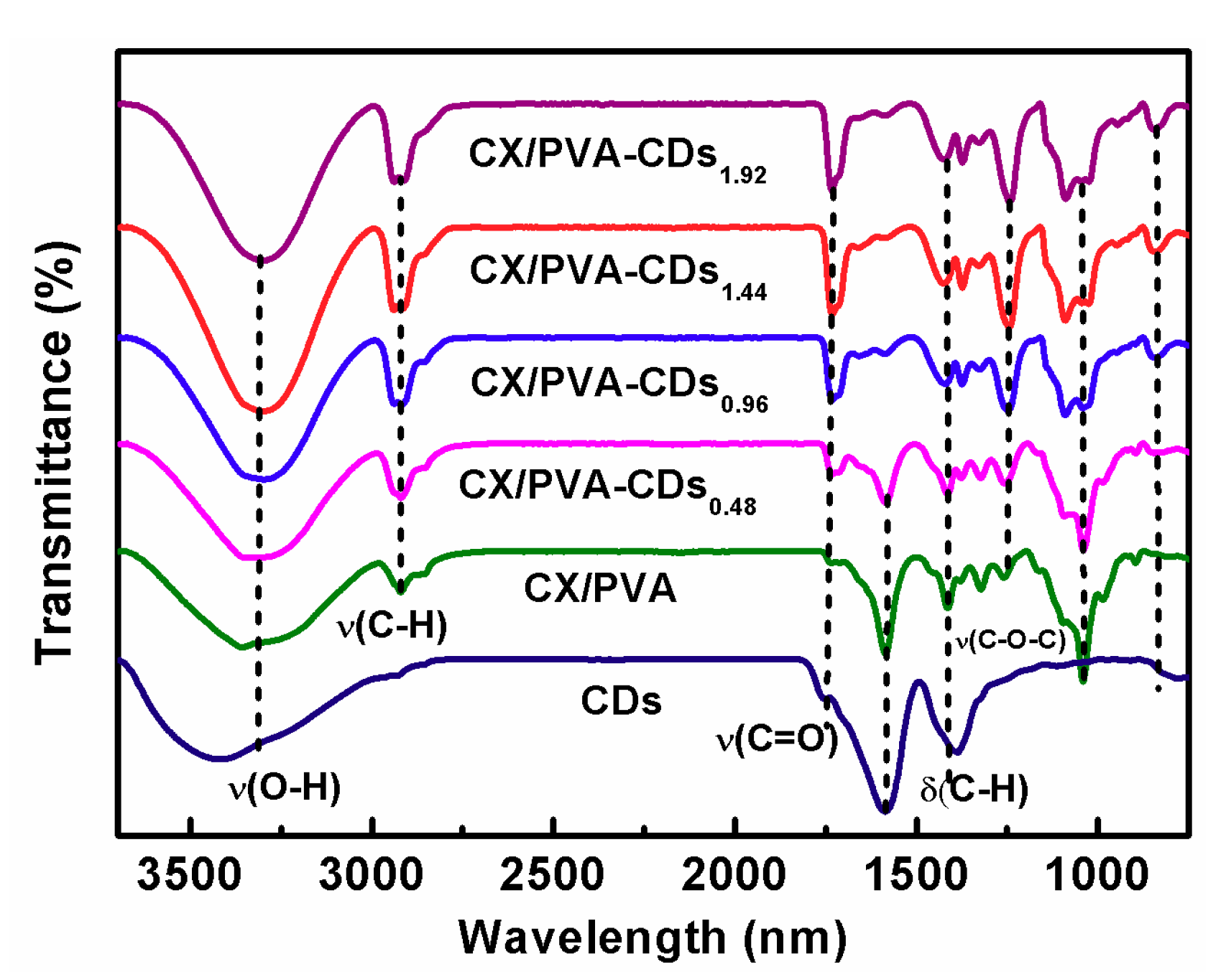
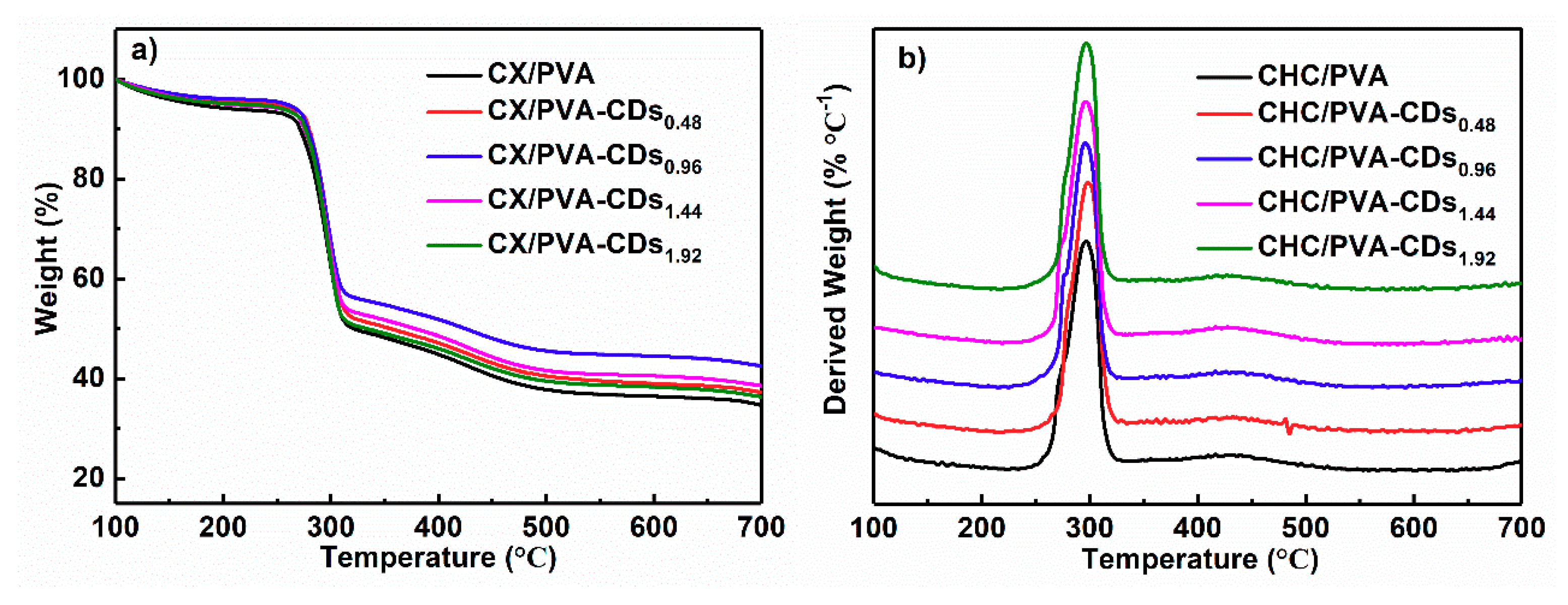
3.2. FT-IR and TG Analyzations
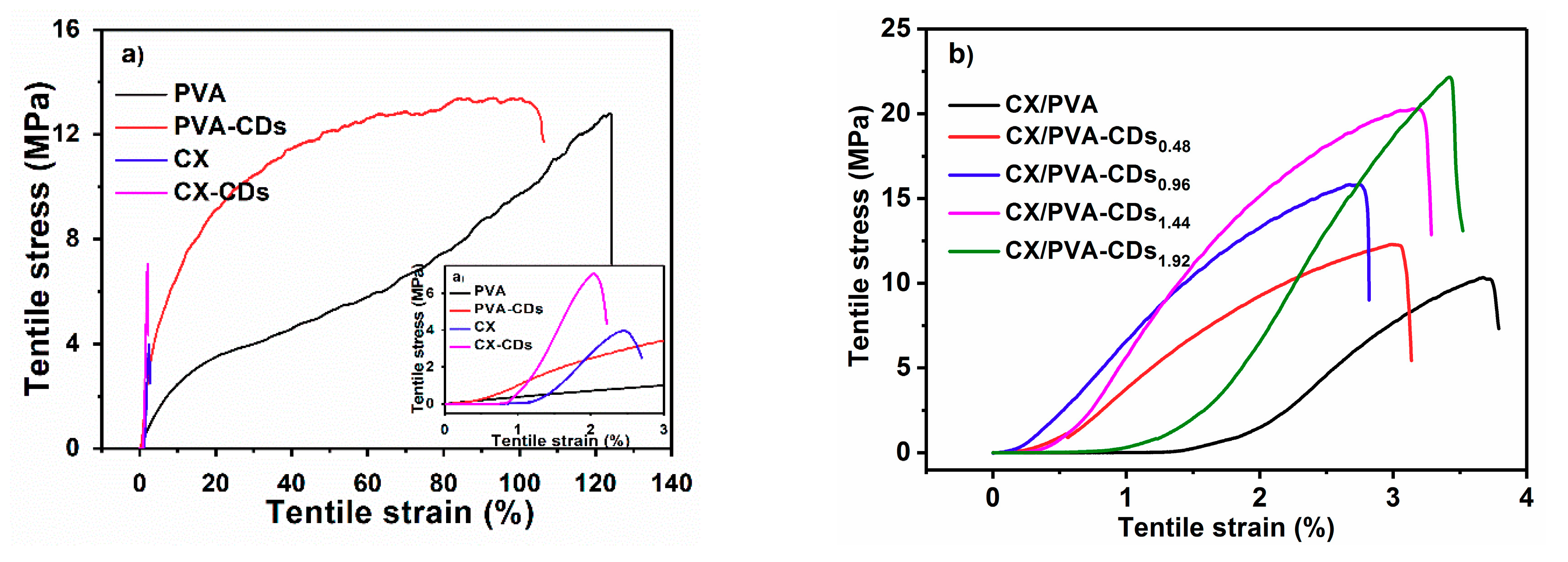
3.3. Mechanical Performances
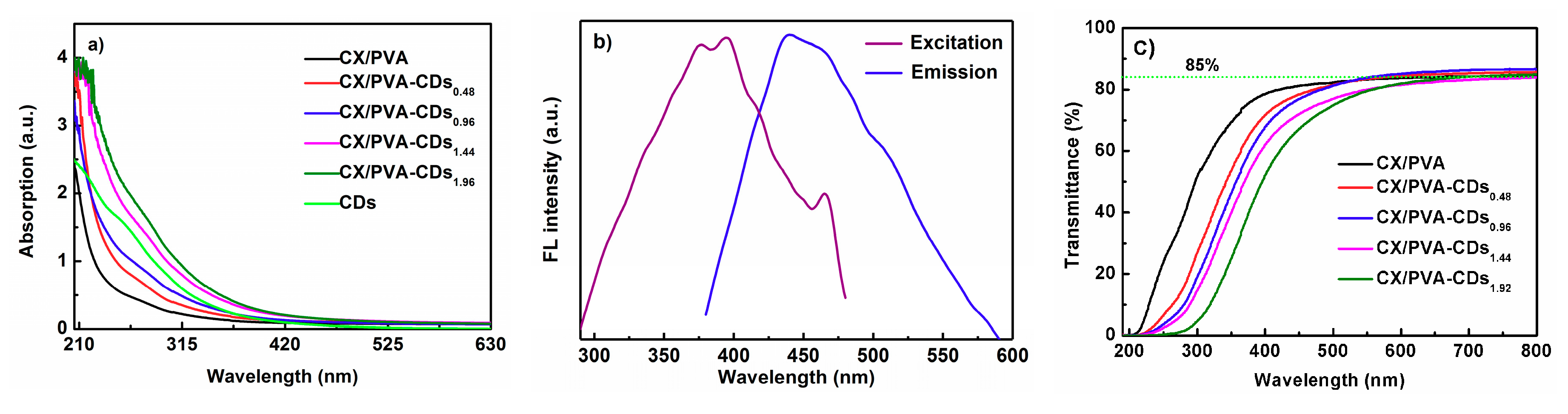
3.4. Optical Performance of CX/PVA-CDs Composite Films
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- You, Y.; Zhang, H.; Liu, Y.; Lei, B. Transparent sunlight conversion film based on carboxymethyl cellulose and carbon dots. Carbohydr. Polym. 2016, 151, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Poudel, P.R.; Kataoka, I.; Mochioka, R. Effect of red- and blue-light-emitting diodes on growth and morphogenesis of grapes. Plant Cell Tiss. Org. Cult. 2007, 92, 147–153. [Google Scholar] [CrossRef]
- Mestdagh, F.; Meulenaer, B.D.; Clippeleer, J.D.; Devlieghere, F.; Huyghebaert, A. Protective Influence of Several Packaging Materials on Light Oxidation of Milk. Packag. Technol. Sci. 2005, 32, 9. [Google Scholar] [CrossRef]
- Su, X.; Chen, B. Transparent, UV-proof and mechanically strong montmorillonite/alginate/Ca2+ nanocomposite hydrogel films with solvent sensitivity. Appl. Clay Sci. 2018, 165, 223–233. [Google Scholar] [CrossRef]
- Sirerol, J.A.; Feddi, F.; Mena, S.; Rodriguez, M.L.; Sirera, P.; Aupí, M.; Pérez, S.; Asensi, M.; Ortega, A.; Estrela, J.M. Topical treatment with pterostilbene, a natural phytoalexin, effectively protects hairless mice against UVB radiation-induced skin damage and carcinogenesis. Free Radic. Biol. Med. 2015, 85, 1–11. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, Y.; Yu, Y.; Liu, Z.; Zhang, Y.; Qi, Y.; Zhou, C. Exploring highly efficient light conversion agents for agricultural film based on aggregation induced emission effects. J. Mater. Chem. C 2016, 4, 11291–11297. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Y.; Liu, W.; Jia, X.; Ma, L.; Ren, L.; Xue, M.; Liu, X. Exploration of highly efficient light conversion agents for agricultural film based on the bay-substituted perylene diimides derivatives. Dye. Pigment. 2018, 159, 483–490. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Y.N.; Zhan, S.; Li, J.; Nie, G.; Hu, S.; Yan, C.; Wu, S.; Cheng, S.; Hu, J.; et al. Tracing of dye molecules in living plants through NaGdF4: Yb3+, Er3+ fluorescent nanoprobes. J. Rare Earths 2019, 37, 237–241. [Google Scholar] [CrossRef]
- Dawidczyk, T.J.; Walton, M.D.; Jang, W.-S.; Grunlan, J.C. Layer-by-Layer Assembly of UV-Resistant Poly(3,4-ethylenedioxythiophene) Thin Films. Langmuir 2008, 24, 5. [Google Scholar] [CrossRef]
- Jing, S.; Zhao, Y.; Sun, R.-C.; Zhong, L.; Peng, X. Facile and High-Yield Synthesis of Carbon Quantum Dots from Biomass-Derived Carbons at Mild Condition. ACS Sustain. Chem. Eng. 2019, 7, 7833–7843. [Google Scholar] [CrossRef]
- Yuan, M.; Zhong, R.; Gao, H.; Li, W.; Yun, X.; Liu, J.; Zhao, X.; Zhao, G.; Zhang, F. One-step, green, and economic synthesis of water-soluble photoluminescent carbon dots by hydrothermal treatment of wheat straw, and their bio-applications in labeling, imaging, and sensing. Appl. Surf. Sci. 2015, 355, 1136–1144. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.; Wang, L.; Zhang, Y.; Qin, X.; Luo, Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Hydrothermal Treatment of Grass: A Low-Cost, Green Route to Nitrogen-Doped, Carbon-Rich, Photoluminescent Polymer Nanodots as an Effective Fluorescent Sensing Platform for Label-Free Detection of Cu(II) Ions. Adv. Mater. 2012, 24, 2037–2041. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.L.; Zhang, P.; Gao, M.X.; Liu, C.F.; Wang, W.; Leng, F.; Huang, C.Z. One-pot hydrothermal synthesis of highly luminescent nitrogen-doped amphoteric carbon dots for bioimaging from Bombyx mori silk–natural proteins. J. Mater. Chem. B 2013, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Q.; Ma, D.-K.; Zhuang, Y.; Zhang, X.; Chen, W.; Hong, L.-L.; Yan, Q.-X.; Yu, K.; Huang, S.-M. One-pot synthesis of N-doped carbon dots with tunable luminescence properties. J. Mater. Chem. 2012, 22. [Google Scholar] [CrossRef]
- Wang, L.; Li, B.; Xu, F.; Shi, X.; Feng, D.; Wei, D.; Li, Y.; Feng, Y.; Wang, Y.; Jia, D.; et al. High-yield synthesis of strong photoluminescent N-doped carbon nanodots derived from hydrosoluble chitosan for mercury ion sensing via smartphone APP. Biosens. Bioelectron. 2016, 79, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wan, X.; Su, Y.; Zeng, X.; Tang, J. Acidophilic S-doped carbon quantum dots derived from cellulose fibers and their fluorescence sensing performance for metal ions in an extremely strong acid environment. J. Mater. Chem. A 2016, 4, 12841–12849. [Google Scholar] [CrossRef]
- Chen, W.; Hu, C.; Yang, Y.; Cui, J.; Liu, Y. Rapid Synthesis of Carbon Dots by Hydrothermal Treatment of Lignin. Materials 2016, 9, 184. [Google Scholar] [CrossRef]
- Zhao, Y.; Jing, S.; Peng, X.; Chen, Z.; Hu, Y.; Zhuo, H.; Sun, R.; Zhong, L. Synthesizing green carbon dots with exceptionally high yield from biomass hydrothermal carbon. Cellu 2019, 27, 415–428. [Google Scholar] [CrossRef]
- Romani, V.P.; Martins, V.G.; Goddard, J.M. Radical scavenging polyethylene films as antioxidant active packaging materials. Food Control 2020, 109. [Google Scholar] [CrossRef]
- Ren, J.; Wang, S.; Gao, C.; Chen, X.; Li, W.; Peng, F. TiO2-containing PVA/xylan composite films with enhanced mechanical properties, high hydrophobicity and UV shielding performance. Cellulose 2014, 22, 593–602. [Google Scholar] [CrossRef]
- Bilgen, S.; Kaygusuz, K.; Sari, A. Renewable energy for a clean and sustainable future. Energy Sour. 2004, 26, 11. [Google Scholar] [CrossRef]
- Dar, A.A.; Garai, A.; Das, A.R.; Ghosh, S. Rheological and Fluorescence Investigation of Interaction between Hexadecyltrimethylammonium Bromide and Methylcellulose in the Presence of Hydrophobic Salts. J. Phys. Chem. A 2010, 114, 9. [Google Scholar] [CrossRef] [PubMed]
- Srinivasa, P.C.; Tharanathan, R.N. Chitin/Chitosan-Safe, Ecofriendly Packaging Materials with Multiple Potential Uses. Food Rev. Int. 2007, 23, 20. [Google Scholar] [CrossRef]
- Yu, S.; Ding, L.; Lin, H.; Wu, W.; Huang, J. A novel optical fiber glucose biosensor based on carbon quantum dots-glucose oxidase/cellulose acetate complex sensitive film. Biosens. Bioelectron. 2019, 146. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Tao, Y.; Xue, J.; Kong, Y.; Dai, J.; Deng, L. Electrochemical enantiorecognition of tryptophan enantiomers based on graphene quantum dots–chitosan composite film. Electrochem. Commun. 2015, 57, 5–9. [Google Scholar] [CrossRef]
- Cuevas, A.; Campos, B.B.; Romero, R.; Algarra, M.; Vázquez, M.I.; Benavente, J. Eco-friendly modification of a regenerated cellulose based film by silicon, carbon and N-doped carbon quantum dots. Carbohydr. Polym. 2019, 206, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Gao, Y.; Sun, J.; Gao, F. Carbon nanodots–chitosan composite film: A platform for protein immobilization, direct electrochemistry and bioelectrocatalysis. Biosens. Bioelectron. 2014, 58, 351–358. [Google Scholar] [CrossRef]
- Zeng, J.; Yan, L. Metal-free transparent luminescent cellulose films. Cellu 2014, 22, 729–736. [Google Scholar] [CrossRef]
- Peng, X.-W.; Ren, J.-L.; Zhong, L.-X.; Sun, R.-C. Nanocomposite Films Based on Xylan-Rich Hemicelluloses and Cellulose Nanofibers with Enhanced Mechanical Properties. Biomacromolecules 2011, 12, 3321–3329. [Google Scholar] [CrossRef]
- Zhong, L.-X.; Peng, X.-W.; Yang, D.; Cao, X.-F.; Sun, R.-C. Long-Chain Anhydride Modification: A New Strategy for Preparing Xylan Films. J. Agric. Food. Chem. 2013, 61, 655–661. [Google Scholar] [CrossRef]
- Yang, Y.; Mei, X.; Hu, Y.; Su, L.; Bian, J.; Li, M.; Peng, F.; Sun, R. Fabrication of antimicrobial composite films based on xylan from pulping process for food packaging. Int. J. Biol. Macromol. 2019, 134, 9. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Ma, J.; Shan, X.; Feng, H.; Shao, L.; Chen, J. Highly Luminescent N-Doped Carbon Quantum Dots as an Effective Multifunctional Fluorescence Sensing Platform. Chem.-Eur. J. 2014, 20, 2254–2263. [Google Scholar] [CrossRef] [PubMed]
- Luís, Â.; Pereira, L.; Domingues, F.; Ramos, A. Development of a carboxymethyl xylan film containing licorice essential oil with antioxidant properties to inhibit the growth of foodborne pathogens. LWT 2019, 111, 218–225. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, Z.; Chen, M.; Chen, W.; Zhou, X. Microwave-assisted synthesis of xylan-derived carbon quantum dots for tetracycline sensing. Opt. Mater. 2018, 85, 329–336. [Google Scholar] [CrossRef]
- Jiang, X.; Huang, J.; Chen, T.; Zhao, Q.; Xu, F.; Zhang, X. Synthesis of hemicellulose/deep eutectic solvent based carbon quantum dots for ultrasensitive detection of Ag+ and L-cysteine with “off-on” pattern. Int. J. Biol. Macromol. 2020, 153, 412–420. [Google Scholar] [CrossRef]

| Samples | Breaking Strength (MPa) | Breaking Elongation (%) | Modulus of Elasticity (MPa) |
|---|---|---|---|
| CX | 3.97 | 2.45 | 416.45 |
| CX-CDs | 7.06 | 2.04 | 719.31 |
| PVA | 12.81 | 124.22 | 24.19 |
| PVA-CDs | 13.11 | 103.6 | 35.07 |
| CX/PVA | 10.34 | 3.67 | 714.70 |
| CX/PVA-CDs0.48 | 12.29 | 2.99 | 690.33 |
| CX/PVA-CDs0.96 | 15.83 | 2.75 | 869.12 |
| CX/PVA-CDs1.44 | 20.28 | 3.15 | 1204.75 |
| CX/PVA-CDs1.92 | 22.16 | 3.42 | 1363.11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhao, Y.; Hu, Y.; Peng, X.; Zhong, L. Xylan-Derived Light Conversion Nanocomposite Film. Polymers 2020, 12, 1779. https://doi.org/10.3390/polym12081779
Yang Y, Zhao Y, Hu Y, Peng X, Zhong L. Xylan-Derived Light Conversion Nanocomposite Film. Polymers. 2020; 12(8):1779. https://doi.org/10.3390/polym12081779
Chicago/Turabian StyleYang, Yunyi, Yushuang Zhao, Yijie Hu, Xinwen Peng, and Linxin Zhong. 2020. "Xylan-Derived Light Conversion Nanocomposite Film" Polymers 12, no. 8: 1779. https://doi.org/10.3390/polym12081779
APA StyleYang, Y., Zhao, Y., Hu, Y., Peng, X., & Zhong, L. (2020). Xylan-Derived Light Conversion Nanocomposite Film. Polymers, 12(8), 1779. https://doi.org/10.3390/polym12081779







