3D Printing of Tunable Zero-Order Release Printlets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Drug-Loaded Filaments by Hot Melt Extrusion (HME)
2.2. FDM 3D Printing
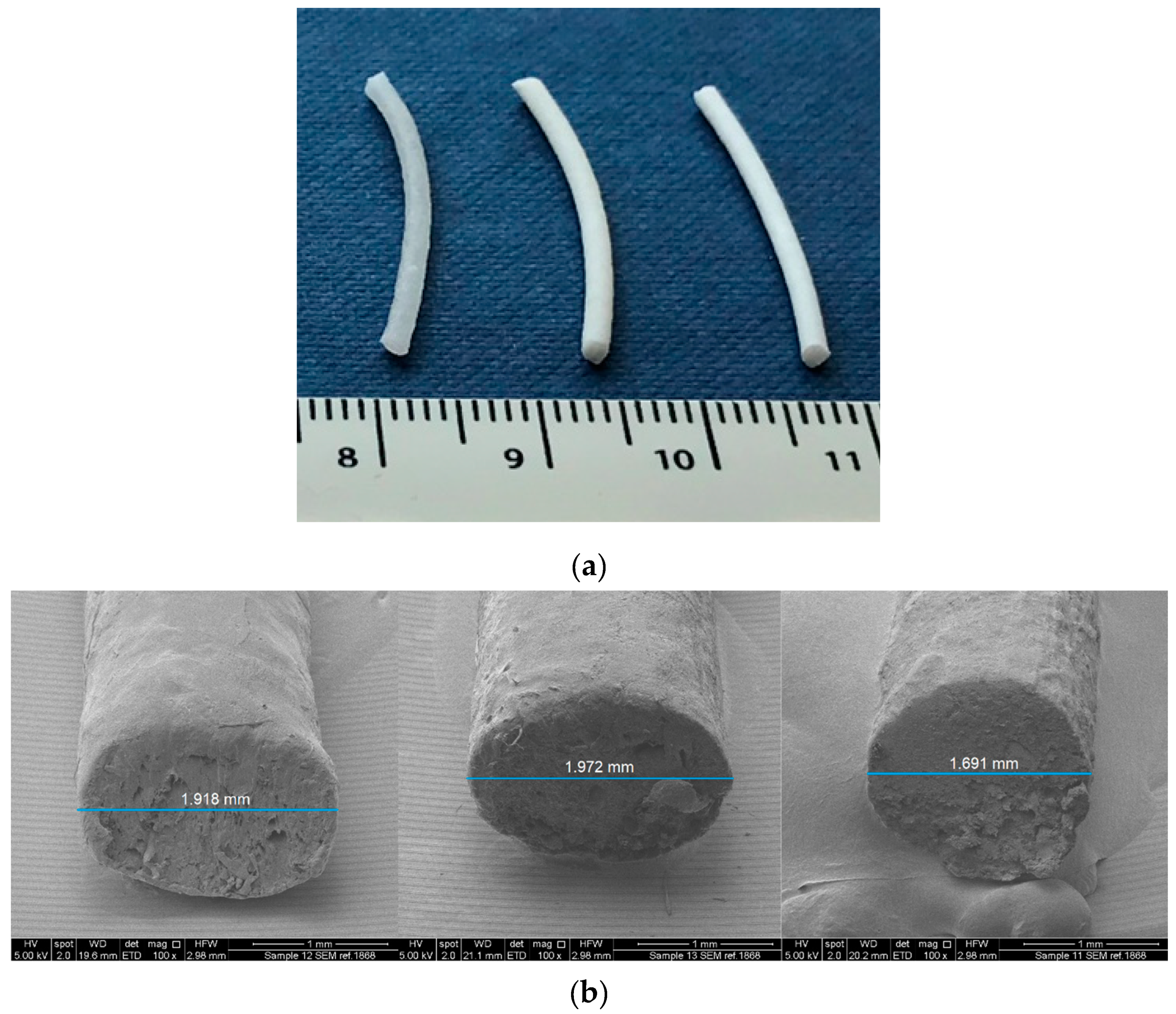
2.3. Mechanical Characterization of Filaments
2.3.1. Tensile Test
2.3.2. 3-Point Bending Test
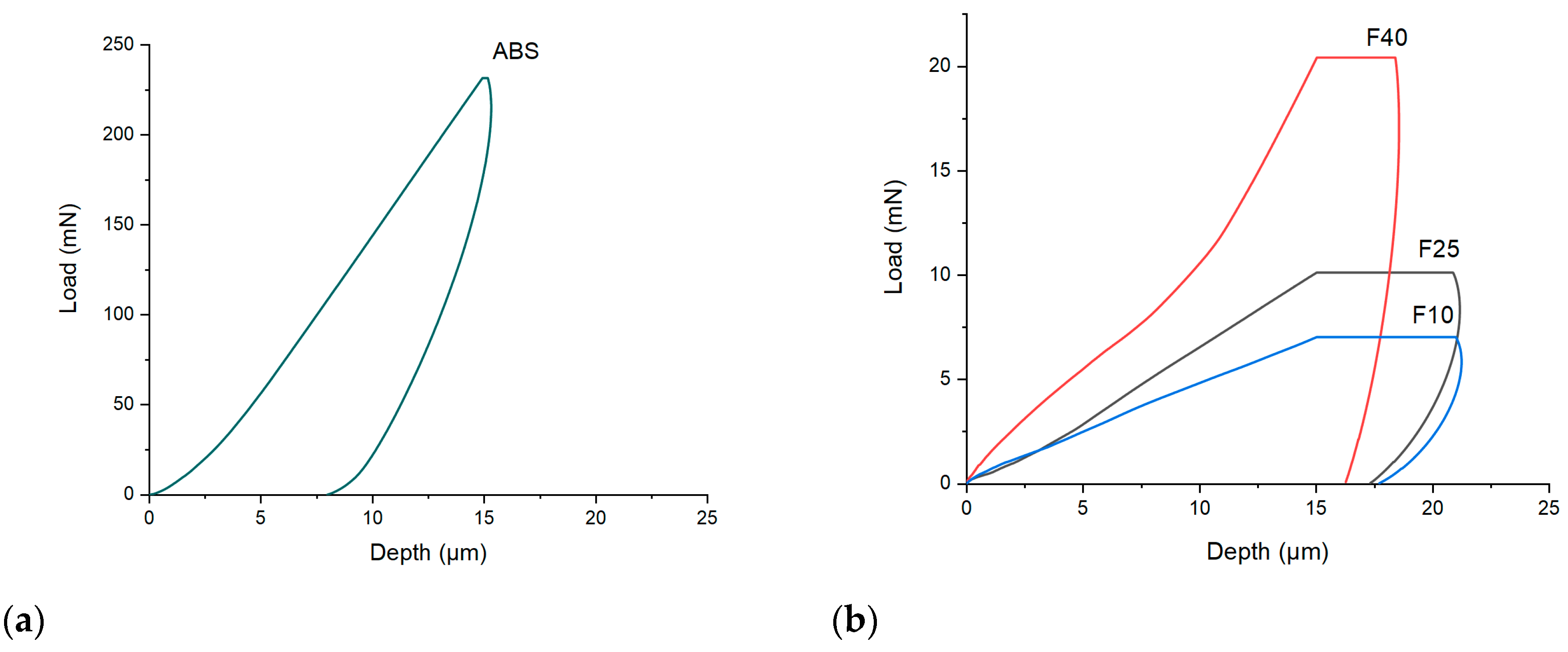
2.3.3. Nanoindentation
2.4. Mechanical Characterization of the Printlets
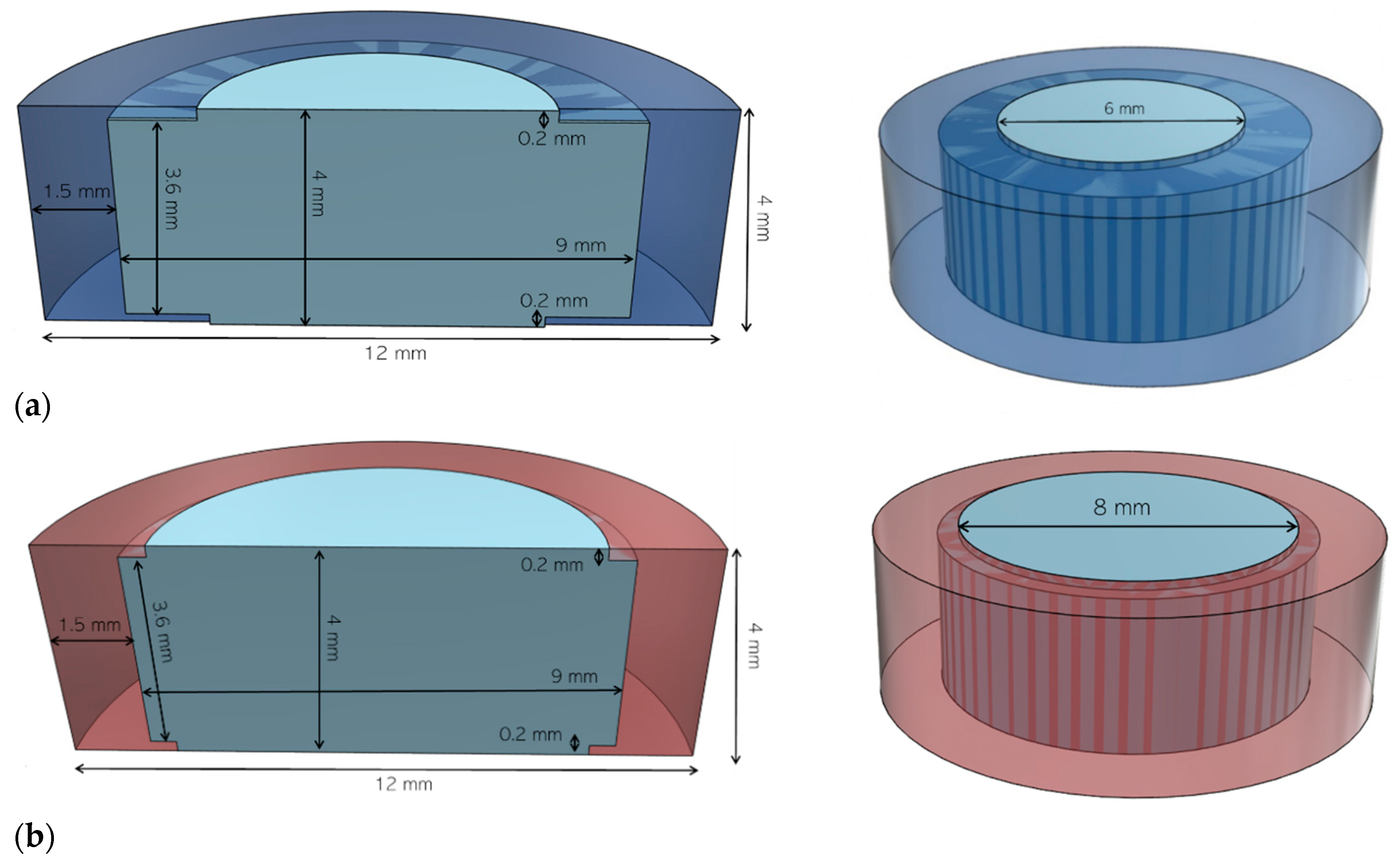
2.4.1. Determination of Printlet Strength
2.4.2. Determination of Printlet Friability
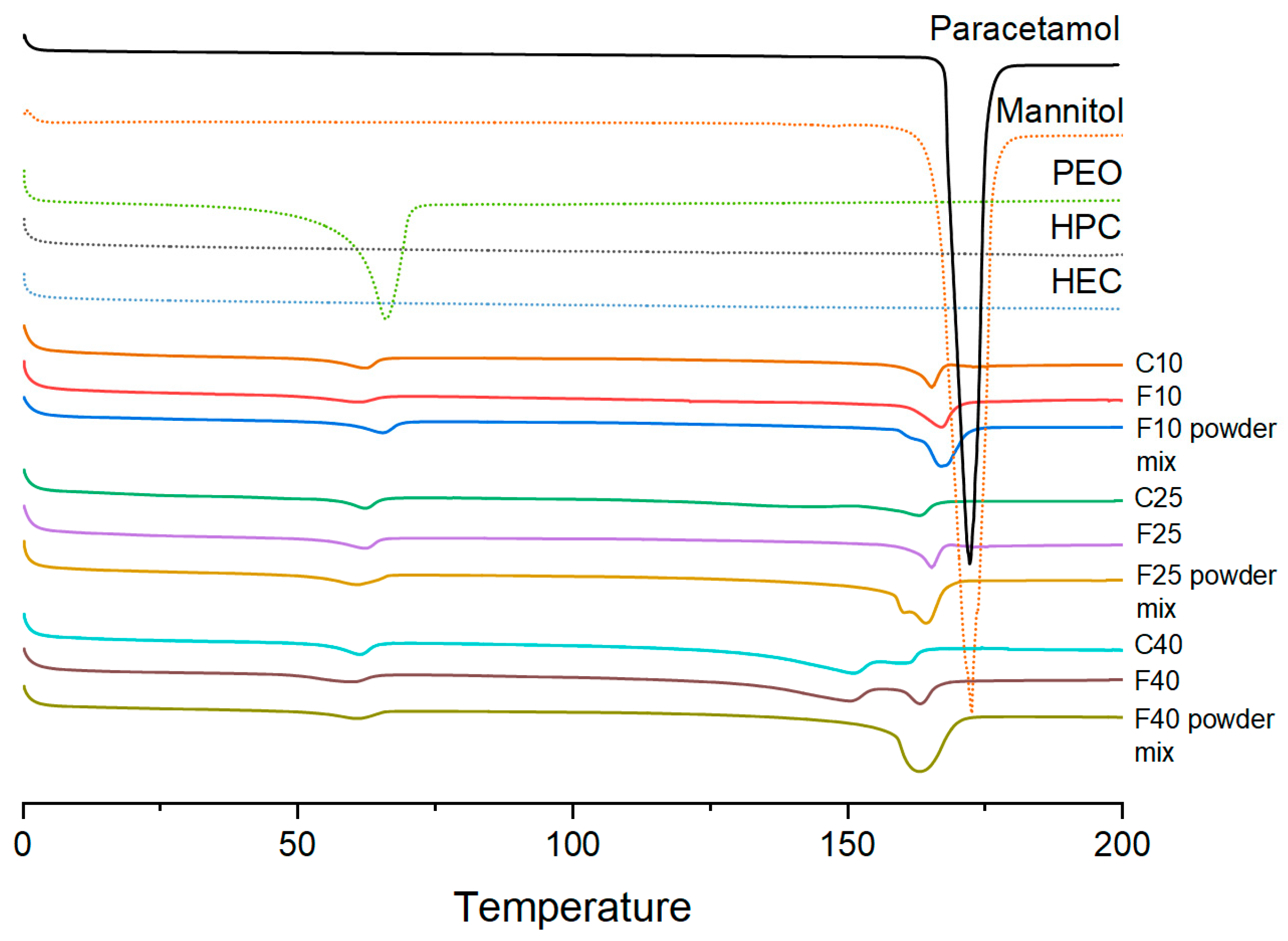
2.5. Thermal Analysis
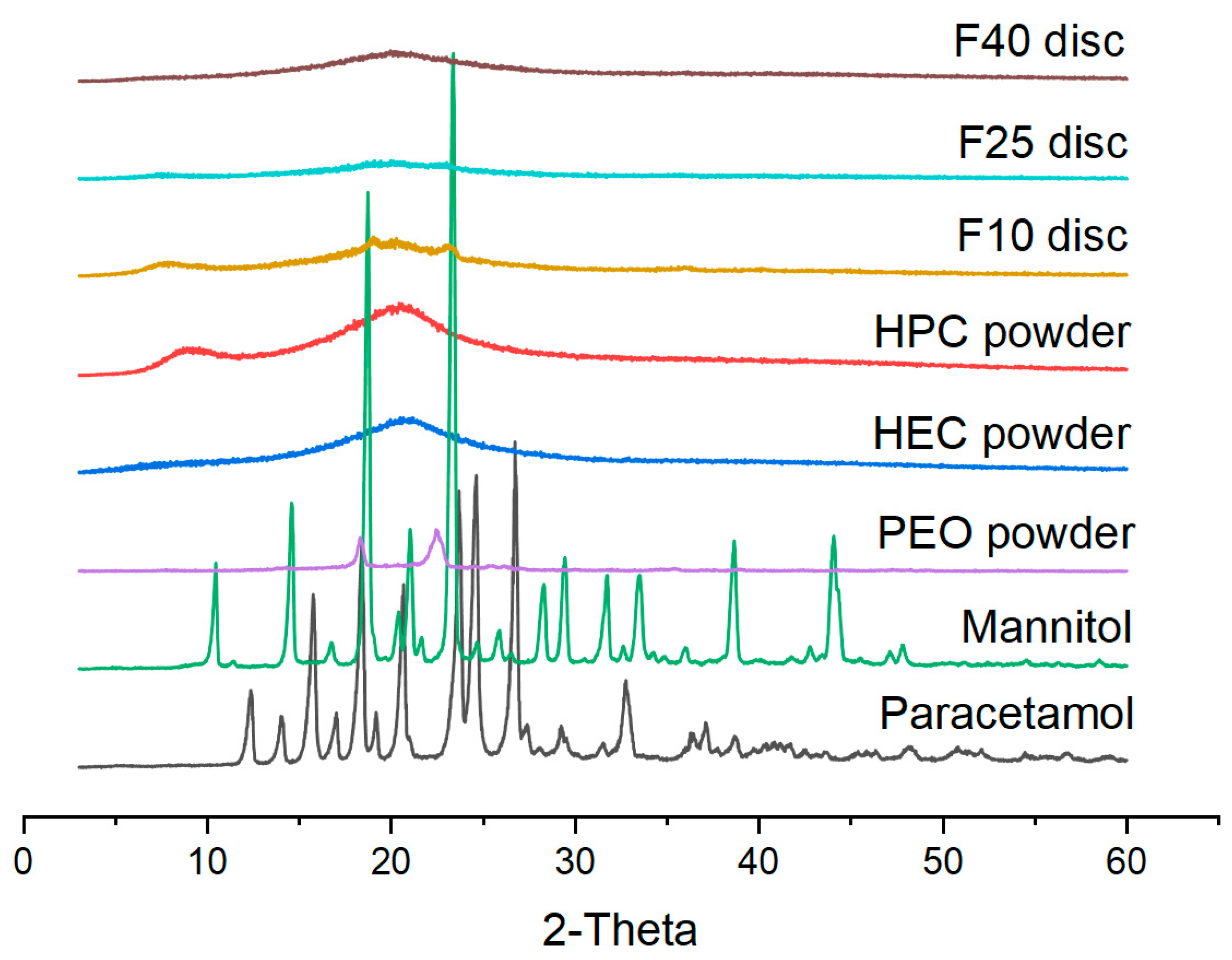
2.6. X-ray Powder Diffraction (XRPD)
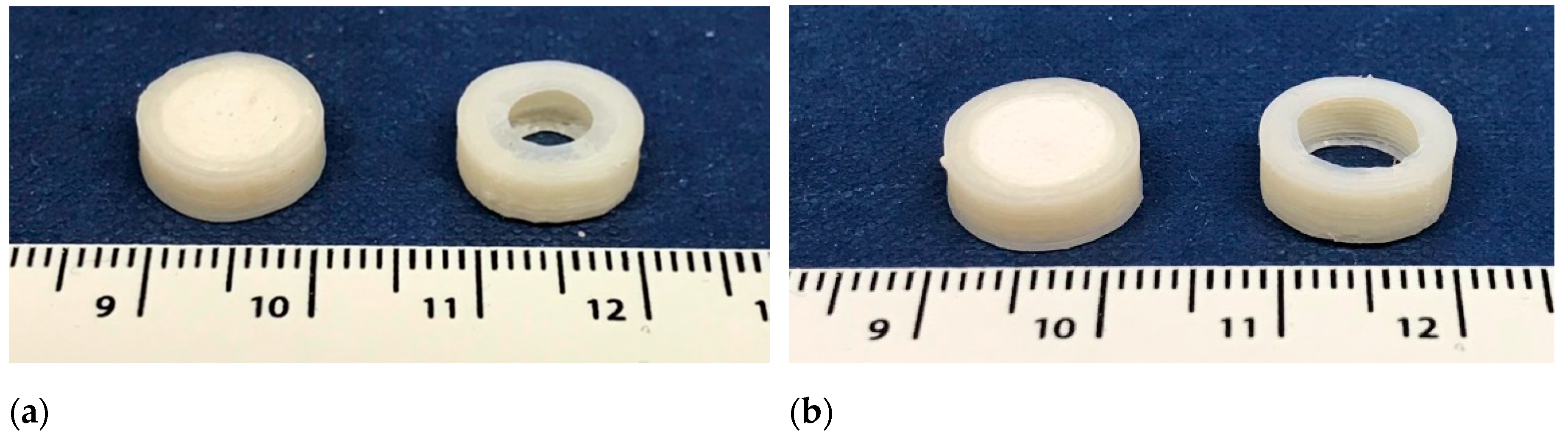
2.7. Morphology Characterization
2.8. Scanning Electron Microscopy (SEM)
2.9. Determination of Drug Loading
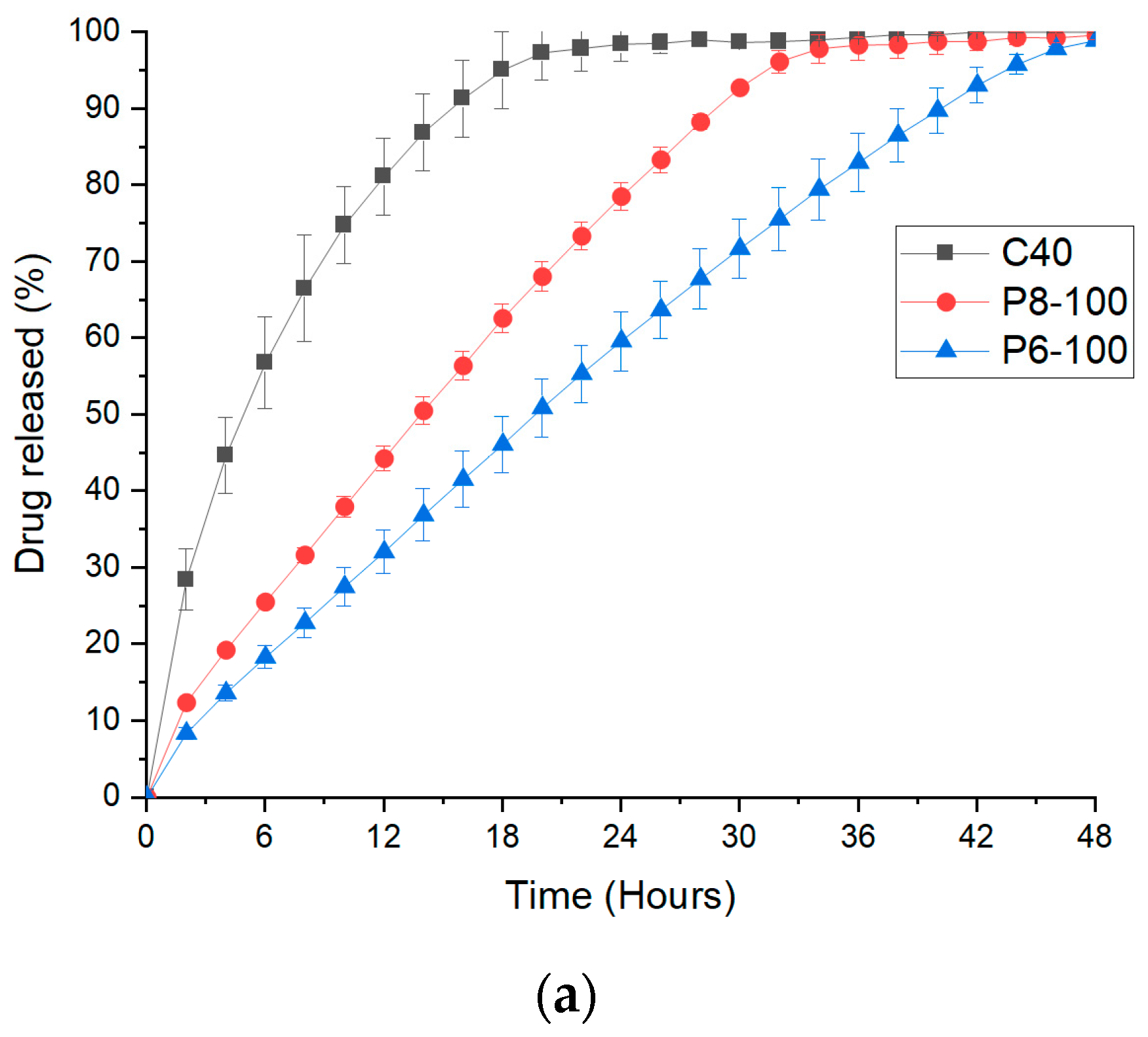
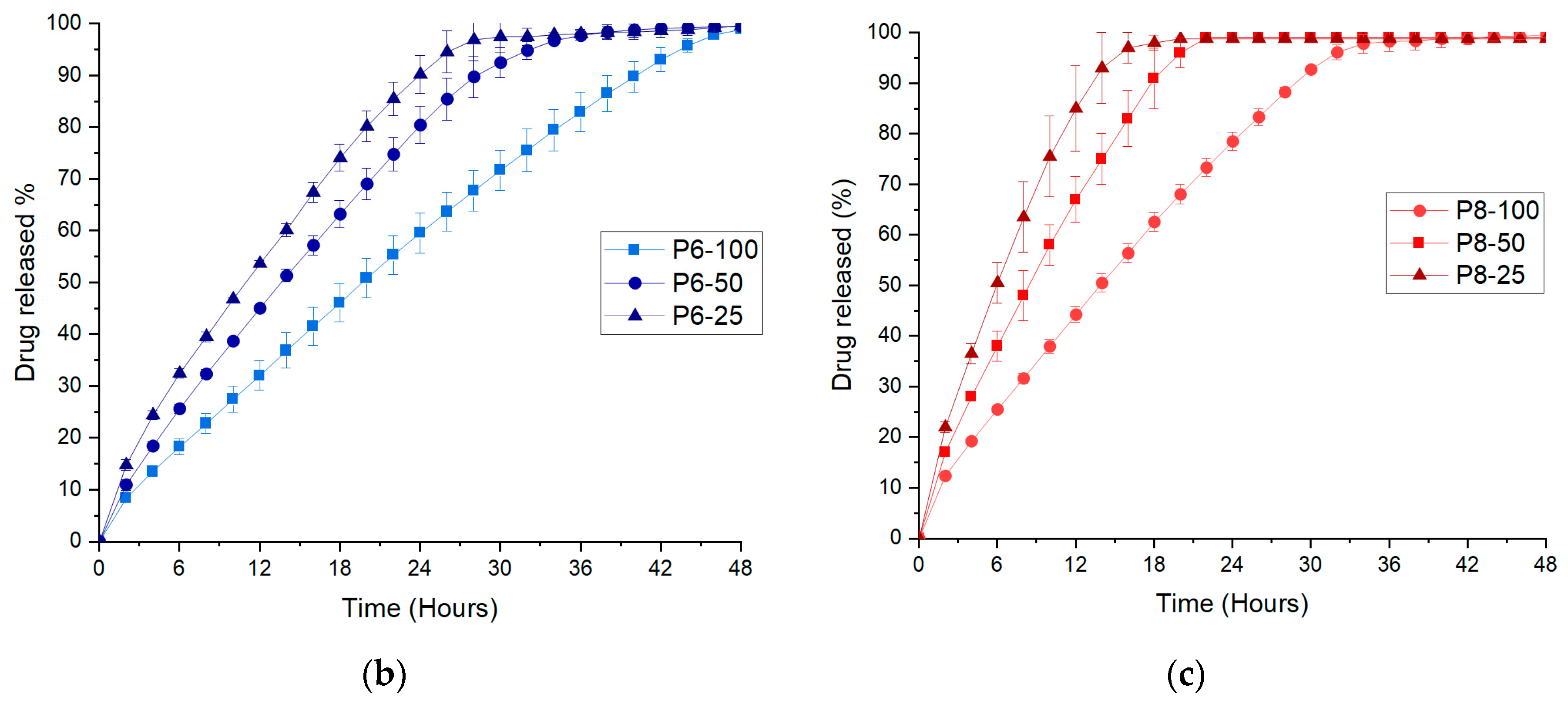
2.10. Dissolution Studies
3. Results
3.1. Mechanical Characterization of the Filaments
3.1.1. Pulling Tensile Strength
3.1.2. Flexural Tensile Strength
3.1.3. Indentation Hardness
3.2. Physical Characterization
3.3. FDM 3D Printing and In Vitro Dissolution Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, C.C.; Tejwani Motwani, M.R.; Roach, W.J.; Kay, J.L.; Yoo, J.; Surprenant, H.L.; Monkhouse, D.C.; Pryor, T.J. Development of near zero-order release dosage forms using three-dimensional printing (3-dp) technology. Drug Dev. Ind. Pharm. 2006, 32, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jasti, B. Osmotic controlled drug delivery systems. In Design of Controlled Release of Drug Delivery Systems; McGraw Hill: New York, NY, USA, 2006; pp. 203–229. [Google Scholar]
- Sundy, E.; Danckwerts, M.P. A novel compression-coated doughnut-shaped tablet design for zero-order sustained release. Eur. J. Pharm. Sci. 2004, 22, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-J. Compressed donut-shaped tablets with zero-order release kinetics. Pharm. Res. 1995, 12, 1045–1048. [Google Scholar] [CrossRef] [PubMed]
- Danckwerts, M.; Watt, J.V.D.; Moodley, I. Zero-order release of theophylline from a core-in-cup tablet in sequenced simulated gastric and intestinal fluid. Drug Dev. Ind. Pharm. 1998, 24, 163–167. [Google Scholar] [CrossRef]
- Cheng, K.; Zhu, J.; Song, X.; Sun, L.; Zhang, J. Studies of hydroxypropyl methylcellulose donut-shaped tablets. Drug Dev. Ind. Pharm. 1999, 25, 1067–1071. [Google Scholar] [CrossRef]
- Liu, L.; Khang, G.; Rhee, M.J.; Lee, B.H. Monolithic osmotic tablet system for nifedipine delivery. J. Control. Release 2000, 67, 309–322. [Google Scholar] [CrossRef]
- Liu, L.; Ku, J.; Khang, G.; Lee, B.; Rhee, M.J.; Lee, B.H. Nifedipine controlled delivery by sandwiched osmotic tablet system. J. Control. Release 2000, 68, 145–156. [Google Scholar] [CrossRef]
- Waterman, K.C.; MacDonald, B.C.; Roy, M.C. Extrudable core system: Development of a single-layer osmotic controlled-release tablet. J. Control. Release 2009, 134, 201–206. [Google Scholar] [CrossRef]
- Thombre, A.G.; Appel, L.E.; Chidlaw, M.B.; Daugherity, P.D.; Dumont, F.; Evans, L.A.F.; Sutton, S.C. Osmotic drug delivery using swellable-core technology. J. Control. Release 2004, 94, 75–89. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Awad, A.; Madla, C.M.; Hatton, G.B.; Firth, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Shaping the future: Recent advances of 3D printing in drug delivery and healthcare. Expert Opin. Drug Deliv. 2019, 16, 1081–1094. [Google Scholar] [CrossRef]
- Fina, F.; Madla, C.M.; Goyanes, A.; Zhang, J.; Gaisford, S.; Basit, A.W. Fabricating 3D printed orally disintegrating printlets using selective laser sintering. Int. J. Pharm. 2018, 541, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Sadia, M.; Arafat, B.; Ahmed, W.; Forbes, R.T.; Alhnan, M.A. Channelled tablets: An innovative approach to accelerating drug release from 3D printed tablets. J. Control. Release 2018, 269, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Fina, F.; Goyanes, A.; Madla, C.M.; Awad, A.; Trenfield, S.J.; Kuek, J.M.; Patel, P.; Gaisford, S.; Basit, A.W. 3D printing of drug-loaded gyroid lattices using selective laser sintering. Int. J. Pharm. 2018, 547, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Isreb, A.; Baj, K.; Wojsz, M.; Isreb, M.; Peak, M.; Alhnan, M.A. 3D printed oral theophylline doses with innovative ‘radiator-like’ design: Impact of polyethylene oxide (peo) molecular weight. Int. J. Pharm. 2019, 564, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Rycerz, K.; Stepien, K.A.; Czapiewska, M.; Arafat, B.T.; Habashy, R.; Isreb, A.; Peak, M.; Alhnan, M.A. Embedded 3D printing of novel bespoke soft dosage form concept for pediatrics. Pharmaceutics 2019, 11, 630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Robles-Martinez, P.; Madla, C.M.; Joubert, F.; Goyanes, A.; Basit, A.W.; Gaisford, S. Stereolithography (sla) 3D printing of an antihypertensive polyprintlet: Case study of an unexpected photopolymer-drug reaction. Addit. Manuf. 2020, 33, 101071. [Google Scholar] [CrossRef]
- Pereira, B.C.; Isreb, A.; Forbes, R.T.; Dores, F.; Habashy, R.; Petit, J.B.; Alhnan, M.A.; Oga, E.F. ‘Temporary plasticiser’: A novel solution to fabricate 3D printed patient-centred cardiovascular ‘polypill’ architectures. Eur. J. Pharm. Biopharm. 2019, 135, 94–103. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J. Control. Release 2015, 217, 308–314. [Google Scholar] [CrossRef]
- Melocchi, A.; Uboldi, M.; Parietti, F.; Cerea, M.; Foppoli, A.; Palugan, L.; Gazzaniga, A.; Maroni, A.; Zema, L. Lego-inspired capsular devices for the development of personalized dietary supplements: Proof of concept with multimodal release of caffeine. J. Pharm. Sci. 2020, 109, 1990–1999. [Google Scholar] [CrossRef]
- Maroni, A.; Melocchi, A.; Parietti, F.; Foppoli, A.; Zema, L.; Gazzaniga, A. 3D printed multi-compartment capsular devices for two-pulse oral drug delivery. J. Control. Release 2017, 268, 10–18. [Google Scholar] [CrossRef]
- Melocchi, A.; Parietti, F.; Maccagnan, S.; Ortenzi, M.A.; Antenucci, S.; Briatico-Vangosa, F.; Maroni, A.; Gazzaniga, A.; Zema, L. Industrial development of a 3D-printed nutraceutical delivery platform in the form of a multicompartment hpc capsule. AAPS PharmSciTech 2018, 19, 3343–3354. [Google Scholar] [CrossRef] [PubMed]
- Okwuosa, T.C.; Soares, C.; Gollwitzer, V.; Habashy, R.; Timmins, P.; Alhnan, M.A. On demand manufacturing of patient-specific liquid capsules via co-ordinated 3D printing and liquid dispensing. Eur. J. Pharm. Sci. 2018, 118, 134–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, K.; Carmone, S.; Brambilla, D.; Leroux, J.-C. 3D printing of a wearable personalized oral delivery device: A first-in-human study. Sci. Adv. 2018, 4, eaat2544. [Google Scholar] [CrossRef] [Green Version]
- Goyanes, A.; Det-Amornrat, U.; Wang, J.; Basit, A.W.; Gaisford, S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J. Control. Release 2016, 234, 41–48. [Google Scholar] [CrossRef]
- Economidou, S.N.; Pere, C.P.P.; Reid, A.; Uddin, M.J.; Windmill, J.F.; Lamprou, D.A.; Douroumis, D. 3D printed microneedle patches using stereolithography (sla) for intradermal insulin delivery. Mater. Sci. Eng. C 2019, 102, 743–755. [Google Scholar] [CrossRef]
- Melocchi, A.; Inverardi, N.; Uboldi, M.; Baldi, F.; Maroni, A.; Pandini, S.; Briatico-Vangosa, F.; Zema, L.; Gazzaniga, A. Retentive device for intravesical drug delivery based on water-induced shape memory response of poly(vinyl alcohol): Design concept and 4d printing feasibility. Int. J. Pharm. 2019, 559, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Armin, G.; Mohsen, J.; Amir Sanati, N.; Kibret, M. Scalable microfabrication of drug-loaded core-shell tablets from a single erodible polymer with adjustable release profiles. Biofabrication 2020, 12, 045007. [Google Scholar]
- Tan, Y.J.N.; Yong, W.P.; Kochhar, J.S.; Khanolkar, J.; Yao, X.; Sun, Y.; Ao, C.K.; Soh, S. On-demand fully customizable drug tablets via 3D printing technology for personalized medicine. J. Control. Release 2020, 322, 42–52. [Google Scholar] [CrossRef]
- Lee, K.J.; Kang, A.; Delfino, J.J.; West, T.G.; Chetty, D.; Monkhouse, D.C.; Yoo, J. Evaluation of critical formulation factors in the development of a rapidly dispersing captopril oral dosage form. Drug Dev. Ind. Pharm. 2003, 29, 967–979. [Google Scholar] [CrossRef]
- Yu, D.G.; Yang, X.L.; Huang, W.D.; Liu, J.; Wang, Y.G.; Xu, H. Tablets with material gradients fabricated by three-dimensional printing. J. Pharm. Sci. 2007, 96, 2446–2456. [Google Scholar] [CrossRef]
- Yu, D.G.; Branford-White, C.; Ma, Z.H.; Zhu, L.M.; Li, X.Y.; Yang, X.L. Novel drug delivery devices for providing linear release profiles fabricated by 3Dp. Int. J. Pharm. 2009, 370, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of tablets containing multiple drugs with defined release profiles. Int. J. Pharm. 2015, 494, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Algahtani, M.S.; Mohammed, A.A.; Ahmad, J.; Saleh, E. Development of a 3D printed coating shell to control the drug release of encapsulated immediate-release tablets. Polymers 2020, 12, 1395. [Google Scholar] [CrossRef]
- Goyanes, A.; Madla, C.M.; Umerji, A.; Duran Pineiro, G.; Giraldez Montero, J.M.; Lamas Diaz, M.J.; Gonzalez Barcia, M.; Taherali, F.; Sanchez-Pintos, P.; Couce, M.L.; et al. Automated therapy preparation of isoleucine formulations using 3D printing for the treatment of msud: First single-centre, prospective, crossover study in patients. Int. J. Pharm. 2019, 567, 118497. [Google Scholar] [CrossRef] [PubMed]
- Vithani, K.; Goyanes, A.; Jannin, V.; Basit, A.W.; Gaisford, S.; Boyd, B.J. A proof of concept for 3D printing of solid lipid-based formulations of poorly water-soluble drugs to control formulation dispersion kinetics. Pharm. Res. 2019, 36, 102. [Google Scholar] [CrossRef]
- Cui, M.; Li, Y.; Wang, S.; Chai, Y.; Lou, J.; Chen, F.; Li, Q.; Pan, W.; Ding, P. Exploration and preparation of a dose-flexible regulation system for levetiracetam tablets via novel semi-solid extrusion three-dimensional printing. J. Pharm. Sci. 2019, 108, 977–986. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Cui, M.; Zhu, Z.; Chen, K.; Wen, H.; Jia, D.; Hou, J.; Xu, W.; Yang, X.; et al. Preparation and investigation of novel gastro-floating tablets with 3D extrusion-based printing. Int. J. Pharm. 2018, 535, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.L.; Modica de Mohac, L.; Tolulope Raimi-Abraham, B. 3D-printed solid dispersion drug products. Pharmaceutics 2019, 11, 672. [Google Scholar] [CrossRef] [Green Version]
- Goyanes, A.; Buanz, A.B.; Basit, A.W.; Gaisford, S. Fused-filament 3D printing (3Dp) for fabrication of tablets. Int. J. Pharm. 2014, 476, 88–92. [Google Scholar] [CrossRef]
- Goyanes, A.; Buanz, A.B.; Hatton, G.B.; Gaisford, S.; Basit, A.W. 3D printing of modified-release aminosalicylate (4-asa and 5-asa) tablets. Eur. J. Pharm. Biopharm. 2015, 89, 157–162. [Google Scholar] [CrossRef]
- Cerda, J.R.; Arifi, T.; Ayyoubi, S.; Knief, P.; Ballesteros, M.P.; Keeble, W.; Barbu, E.; Healy, A.M.; Lalatsa, A.; Serrano, D.R. Personalised 3D printed medicines: Optimising material properties for successful passive diffusion loading of filaments for fused deposition modelling of solid dosage forms. Pharmaceutics 2020, 12, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagami, T.; Kuwata, E.; Sakai, N.; Ozeki, T. Drug incorporation into polymer filament using simple soaking method for tablet preparation using fused deposition modeling. Biol. Pharm. Bull. 2019, 42, 1753–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trenfield, S.J.; Xian Tan, H.; Awad, A.; Buanz, A.; Gaisford, S.; Basit, A.W.; Goyanes, A. Track-and-trace: Novel anti-counterfeit measures for 3D printed personalized drug products using smart material inks. Int. J. Pharm. 2019, 567, 118443. [Google Scholar] [CrossRef]
- Kollamaram, G.; Croker, D.M.; Walker, G.M.; Goyanes, A.; Basit, A.W.; Gaisford, S. Low temperature fused deposition modeling (fdm) 3D printing of thermolabile drugs. Int. J. Pharm. 2018, 545, 144–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, C.; Solanki, N.G.; Vasoya, J.M.; Shah, A.V.; Serajuddin, A.T.M. Development of 3D printed tablets by fused deposition modeling using polyvinyl alcohol as polymeric matrix for rapid drug release. J. Pharm. Sci. 2020, 109, 1558–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arafat, B.; Qinna, N.; Cieszynska, M.; Forbes, R.T.; Alhnan, M.A. Tailored on demand anti-coagulant dosing: An in vitro and in vivo evaluation of 3D printed purpose-designed oral dosage forms. Eur. J. Pharm. Biopharm. 2018, 128, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Feng, X.; Patil, H.; Tiwari, R.V.; Repka, M.A. Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets. Int. J. Pharm. 2017, 519, 186–197. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, W.; Vo, A.Q.; Feng, X.; Ye, X.; Kim, D.W.; Repka, M.A. Hydroxypropyl methylcellulose-based controlled release dosage by melt extrusion and 3D printing: Structure and drug release correlation. Carbohydr. Polym. 2017, 177, 49–57. [Google Scholar] [CrossRef]
- Oblom, H.; Zhang, J.; Pimparade, M.; Speer, I.; Preis, M.; Repka, M.; Sandler, N. 3D-printed isoniazid tablets for the treatment and prevention of tuberculosis-personalized dosing and drug release. AAPS PharmSciTech 2019, 20, 52. [Google Scholar] [CrossRef] [Green Version]
- Goyanes, A.; Allahham, N.; Trenfield, S.J.; Stoyanov, E.; Gaisford, S.; Basit, A.W. Direct powder extrusion 3D printing: Fabrication of drug products using a novel single-step process. Int. J. Pharm. 2019, 567, 118471. [Google Scholar] [CrossRef]
- Fanous, M.; Gold, S.; Muller, S.; Hirsch, S.; Ogorka, J.; Imanidis, G. Simplification of fused deposition modeling 3D-printing paradigm: Feasibility of 1-step direct powder printing for immediate release dosage form production. Int. J. Pharm. 2020, 578, 119124. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.J.; Awad, A.; Martorana, A.; Gaisford, S.; Stoyanov, E.; Basit, A.W.; Goyanes, A. 3D printed opioid medicines with alcohol-resistant and abuse-deterrent properties. Int. J. Pharm. 2020, 579, 119169. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Chia, S.M.; Kang, L.; Yap, K.Y. Three-dimensional printing of carbamazepine sustained-release scaffold. J. Pharm. Sci. 2016, 105, 2155–2163. [Google Scholar] [CrossRef] [PubMed]
- Gioumouxouzis, C.I.; Katsamenis, O.L.; Bouropoulos, N.; Fatouros, D.G. 3D printed oral solid dosage forms containing hydrochlorothiazide for controlled drug delivery. J. Drug Deliv. Sci. Technol. 2017, 40, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Kimura, S.I.; Ishikawa, T.; Iwao, Y.; Itai, S.; Kondo, H. Fabrication of zero-order sustained-release floating tablets via fused depositing modeling 3D printer. Chem. Pharm. Bull. 2019, 67, 992–999. [Google Scholar] [CrossRef] [Green Version]
- Huanbutta, K.; Sangnim, T. Design and development of zero-order drug release gastroretentive floating tablets fabricated by 3D printing technology. J. Drug Deliv. Sci. Technol. 2019, 52, 831–837. [Google Scholar] [CrossRef]
- Novak, M.; Boleslavska, T.; Grof, Z.; Wanek, A.; Zadrazil, A.; Beranek, J.; Kovacik, P.; Stepanek, F. Virtual prototyping and parametric design of 3D-printed tablets based on the solution of inverse problem. AAPS PharmSciTech 2018, 19, 3414–3424. [Google Scholar] [CrossRef]
- Homaee Borujeni, S.; Mirdamadian, S.Z.; Varshosaz, J.; Taheri, A. Three-dimensional (3D) printed tablets using ethyl cellulose and hydroxypropyl cellulose to achieve zero order sustained release profile. Cellulose 2020, 27, 1573–1589. [Google Scholar] [CrossRef]
- Yalkowsky, S.H.; He, Y. Handbook of Aqueous Solubility Data; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Selective laser sintering (sls) 3D printing of medicines. Int. J. Pharm. 2017, 529, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Goyanes, A.; Fina, F.; Martorana, A.; Sedough, D.; Gaisford, S.; Basit, A.W. Development of modified release 3D printed tablets (printlets) with pharmaceutical excipients using additive manufacturing. Int. J. Pharm. 2017, 527, 21–30. [Google Scholar] [CrossRef]
- Bruschi, M.L. 5-Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Cambridge, UK, 2015; pp. 63–86. [Google Scholar]
- Wójcik-Pastuszka, D.; Krzak, J.; Macikowski, B.; Berkowski, R.; Osiński, B.; Musiał, W. Evaluation of the release kinetics of a pharmacologically active substance from model intra-articular implants replacing the cruciate ligaments of the knee. Materials 2019, 12, 1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahoo, J.; Murthy, P.N.; Biswal, S.; Sahoo, S.K.; Mahapatra, A.K. Comparative study of propranolol hydrochloride release from matrix tablets with kollidonsr or hydroxy propyl methyl cellulose. AAPS PharmSciTech 2008, 9, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Ashland. Physical and chemical properties. In Klucel Hydroxypropylcellulose; Ashland Brochure; Ashland: Covington, KY, USA, 2019. [Google Scholar]
- Goyanes, A.; Scarpa, M.; Kamlow, M.; Gaisford, S.; Basit, A.W.; Orlu, M. Patient acceptability of 3D printed medicines. Int. J. Pharm. 2017, 530, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Palekar, S.; Nukala, P.K.; Mishra, S.M.; Kipping, T.; Patel, K. Application of 3D printing technology and quality by design approach for development of age-appropriate pediatric formulation of baclofen. Int. J. Pharm. 2019, 556, 106–116. [Google Scholar] [CrossRef]
- Gioumouxouzis, C.I.; Baklavaridis, A.; Katsamenis, O.L.; Markopoulou, C.K.; Bouropoulos, N.; Tzetzis, D.; Fatouros, D.G. A 3D printed bilayer oral solid dosage form combining metformin for prolonged and glimepiride for immediate drug delivery. Eur. J. Pharm. Sci. 2018, 120, 40–52. [Google Scholar] [CrossRef] [Green Version]
- Nasereddin, J.M.; Wellner, N.; Alhijjaj, M.; Belton, P.; Qi, S. Development of a simple mechanical screening method for predicting the feedability of a pharmaceutical fdm 3D printing filament. Pharm. Res. 2018, 35, 151. [Google Scholar] [CrossRef] [Green Version]
- Brian, N.T.; Strong, R.; Scott, A.G. A review of melt extrusion additive manufacturing processes: I. Process design and modeling. Rapid Prototyp. J. 2014, 20, 192–204. [Google Scholar]
- Goyanes, A.; Kobayashi, M.; Martinez-Pacheco, R.; Gaisford, S.; Basit, A.W. Fused-filament 3D printing of drug products: Microstructure analysis and drug release characteristics of pva-based caplets. Int. J. Pharm. 2016, 514, 290–295. [Google Scholar] [CrossRef]
- Rohringer, S. 3D Printer Filament Buyer’s Guide. Available online: https://all3Dp.com/1/3D-printer-filament-types-3D-printing-3D-filament/ (accessed on 15 April 2020).
- Gioumouxouzis, C.I.; Tzimtzimis, E.; Katsamenis, O.L.; Dourou, A.; Markopoulou, C.; Bouropoulos, N.; Tzetzis, D.; Fatouros, D.G. Fabrication of an osmotic 3D printed solid dosage form for controlled release of active pharmaceutical ingredients. Eur. J. Pharm. Sci. 2020, 143, 105176. [Google Scholar] [CrossRef]
- Okwuosa, T.C.; Stefaniak, D.; Arafat, B.; Isreb, A.; Wan, K.W.; Alhnan, M.A. A lower temperature fdm 3D printing for the manufacture of patient-specific immediate release tablets. Pharm. Res. 2016, 33, 2704–2712. [Google Scholar] [CrossRef]
- Tiwari, S.B.; DiNunzio, J.; Rajabi-Siahboomi, A. Drug–polymer matrices for extended release. In Controlled Release in Oral Drug Delivery; Wilson, C.G., Crowley, P.J., Eds.; Springer US: Boston, MA, USA, 2011; pp. 131–159. [Google Scholar]
- Ma, L.; Deng, L.; Chen, J. Applications of poly(ethylene oxide) in controlled release tablet systems: A review. Drug Dev. Ind. Pharm. 2014, 40, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, G.; Samaro, A.; Grymonpre, W.; Vanhoorne, V.; Van Snick, B.; Boone, M.N.; Hellemans, T.; Van Hoorebeke, L.; Remon, J.P.; Vervaet, C. 3D printing of high drug loaded dosage forms using thermoplastic polyurethanes. Int. J. Pharm. 2018, 536, 318–325. [Google Scholar] [CrossRef] [PubMed]


| Filaments | HPC | HEC | PEO | Mannitol | Magnesium Stearate | Paracetamol | Extrusion Temperature (°C) |
|---|---|---|---|---|---|---|---|
| F10 | 62 | 5 | 10 | 10 | 3 | 10 | 110 |
| F25 | 47 | 5 | 10 | 10 | 3 | 25 | 120 |
| F40 | 32 | 5 | 10 | 10 | 3 | 40 | 120 |
| Filament | Maximum Load at Break (n) ** | Young Modulus (MPa) ** | Fracture Tensile Strength (MPa) ** | Hardness (MPa) |
|---|---|---|---|---|
| F10 | 10.7 ± 0.2 | 72.5 ± 14.0 | 4.4 ± 0.1 | 0.87 ± 0.07 |
| F25 | 11.55 ± 0.1 | 82.4 ± 9.9 | 4.6 ± 0.3 | 1.20 ± 0.09 |
| F40 | 14.4 ± 1.1 | 145.3 ± 11.9 | 5.8 ± 0.2 | 4.52 ± 0.12 |
| ABS | * | * | 49.1 ± 1.2 | 56.23 ± 4.6 |
| Printlets | Aperture Diameter (mm) | Infill (%) | Weight (mg ± SD) | Diameter (mm ± SD) |
|---|---|---|---|---|
| P6-100 | 6 | 100 | 473 ± 4.3 | 12.03 ± 0.04 |
| P6-50 | 6 | 50 | 393 ± 7.8 | 12.06 ± 0.07 |
| P6-25 | 6 | 25 | 333 ± 9.5 | 12.06 ± 0.15 |
| P8-100 | 8 | 100 | 475 ± 4.6 | 12.04 ± 0.14 |
| P8-50 | 8 | 50 | 395 ± 8.1 | 12.09 ± 0.12 |
| P8-25 | 8 | 25 | 335 ± 8.7 | 12.07 ± 0.12 |
| Printlet | Zero-Order R2 | First-Order R2 | Higuchi R2 | Korsmeyer–Peppas | |
|---|---|---|---|---|---|
| R2 | n | ||||
| Core only (C40) | 0.8541 | 0.9827 | 0.9806 | 0.9903 | 0.641 |
| P6-100 | 0.9945 | 0.9054 | 0.9626 | 0.9661 | 0.612 |
| P6-50 | 0.9882 | 0.9346 | 0.9664 | 0.9821 | 0.623 |
| P6-25 | 0.9812 | 0.9150 | 0.9758 | 0.9948 | 0.626 |
| P8-100 | 0.9918 | 0.9003 | 0.9649 | 0.9862 | 0.617 |
| P8-50 | 0.9869 | 0.9063 | 0.9664 | 0.9953 | 0.647 |
| P8-25 | 0.9691 | 0.9327 | 0.9755 | 0.9987 | 0.674 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fina, F.; Goyanes, A.; Rowland, M.; Gaisford, S.; Basit, A.W. 3D Printing of Tunable Zero-Order Release Printlets. Polymers 2020, 12, 1769. https://doi.org/10.3390/polym12081769
Fina F, Goyanes A, Rowland M, Gaisford S, Basit AW. 3D Printing of Tunable Zero-Order Release Printlets. Polymers. 2020; 12(8):1769. https://doi.org/10.3390/polym12081769
Chicago/Turabian StyleFina, Fabrizio, Alvaro Goyanes, Martin Rowland, Simon Gaisford, and Abdul W. Basit. 2020. "3D Printing of Tunable Zero-Order Release Printlets" Polymers 12, no. 8: 1769. https://doi.org/10.3390/polym12081769
APA StyleFina, F., Goyanes, A., Rowland, M., Gaisford, S., & Basit, A. W. (2020). 3D Printing of Tunable Zero-Order Release Printlets. Polymers, 12(8), 1769. https://doi.org/10.3390/polym12081769







