Synthesis and Structural Characterization of Sequential Structure and Crystallization Properties for Hydrophilic Modified Polyester
Abstract
:1. Introduction
2. Experimental
2.1. Materials
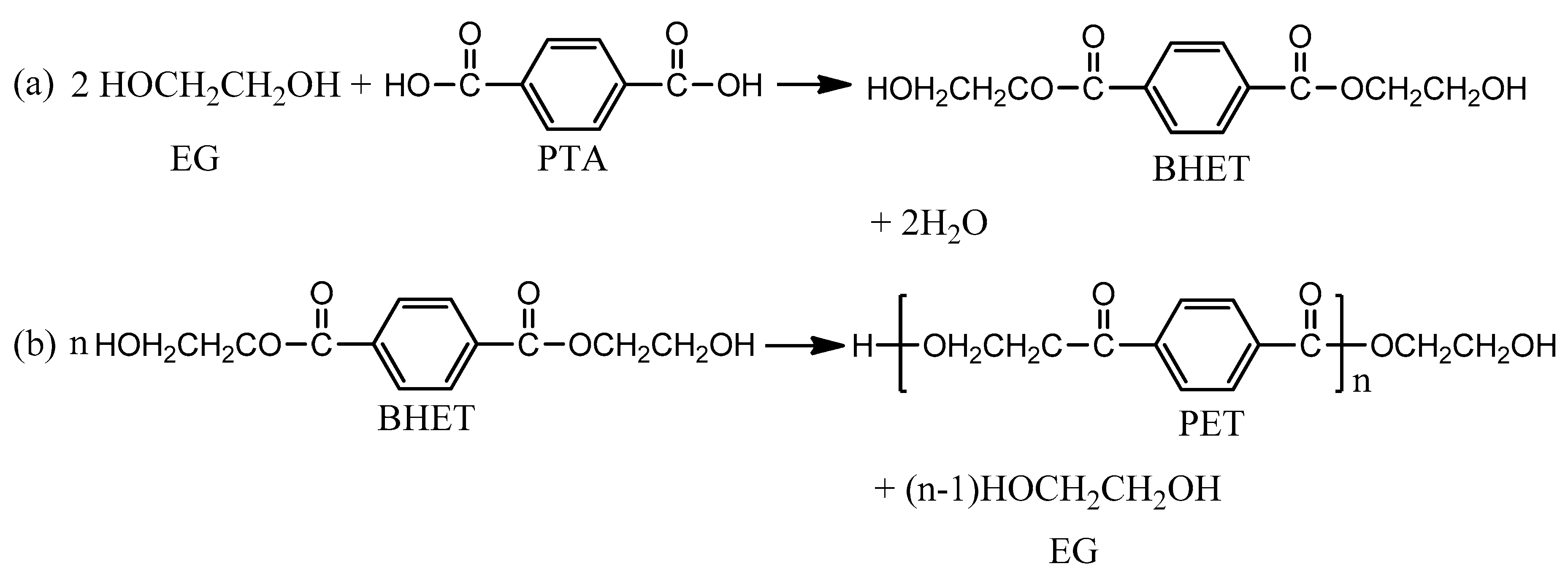
2.2. Synthesis of Copolyester
2.3. Characterization of Copolyester
3. Results and Discussion
3.1. Fourier Infrared Spectroscopy
3.2. Nuclear Magnetic Resonance (NMR) Analysis
3.3. 1H-NMR Analysis
3.4. 2D NMR HETCOR Spectra Analysis
3.5. 13C-NMR Analysis
3.6. DSC Analysis
3.7. TG Analysis
3.8. The Contact Angle
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wu, C.; Mao, X.; Zhang, X.; Lu, C.; Wang, X. Study on preparation and properties of hydrophilic copolyester of PET-co-PEA/Nano SiO2: Proceedings of Chinese Materials Conference 2018. In Physics and Techniques of Ceramic and Polymeric Materials; Springer: Berlin/Heidelberg, Germany, 2019; pp. 155–163. [Google Scholar]
- Xiao, W.; Yu, H.; Han, K.; Yu, M. Study on PET fiber modified by nanomaterials: Improvement of dimensional thermal stability of PET fiber by forming PET/MMT nanocomposites. J. Appl. Polym. Sci. 2005, 96, 2247–2252. [Google Scholar] [CrossRef]
- Gao, Z.H.; Tang, H.Y.; Fan, X.H.; Chen, X.F.; Zhou, Q.F. The synthesis and characterization of polyesters containing selane in the molecular main-chain. Acta Polym. Sin. 2006, 6, 368–370. [Google Scholar] [CrossRef]
- Chen, B.; Gu, L. Isothermal crystallization and melting behavior of 2-methyl-1,3-propanediol substituted sulfonated poly(ethylene terephthalate) copolyesters. J. Appl. Polym. Sci. 2010, 117, 2454–2463. [Google Scholar] [CrossRef]
- Rwei, S.P.; Lin, W.P. Synthesis and characterization of adipic acid/polyethylene glycol/poly(ethylene terephthalate) copolyester fiber. Text. Res. J. 2015, 85, 0040517515573406. [Google Scholar] [CrossRef]
- Huang, X.; Guo, R.; Lan, J. Synthesis and characterization of biodegradable hexabasic aliphatic-aromatic copolyester. Polym. Sci. 2014, 56, 744–752. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, F.; Wang, Y.; Ren, X.; Huang, T.S. Antibacterial and hydrophilic modification of PET fabrics by Electron beam irradiation process. Fibers Polym. 2020, 21, 1023–1031. [Google Scholar] [CrossRef]
- Sawada, K.; Sugimoto, M.; Ueda, M.; Chan, H.P. Hydrophilic treatment of polyester surfaces using TiO2 photocatalytic reactions. Text. Res. J. 2003, 73, 819–822. [Google Scholar] [CrossRef]
- Cho, J.Y.; Hong, C.J.; Choi, H.M. Microwave-assisted glycolysis for PET with highly hydrophilic surface. Ind. Eng. Chem. Res. 2013, 52, 2309–2315. [Google Scholar] [CrossRef]
- Bandelli, D.; Helbing, C.; Weber, C.; Seifert, M.; Muljajew, I.; Jandt, K.D.; Schubert, U.S. Maintaining the hydrophilic–hydrophobic balance of polyesters with adjustable crystallinity for tailor-made nanoparticles. Macromolecules 2018. [Google Scholar] [CrossRef]
- Zhang, J.C.; Zhou, R.; Zhao, H.; Wang, X.L.; Yu, J.Y.; Liu, L.F. College of Textiles, Donghua University; Research Institute, Donghua University. Synthesis and properties of copolyester modified by neopentyl glycol. J. Donghua Univ. 2016, 42, 185–190. [Google Scholar]
- Tankhiwale, S.; Gupta, M.C.; Viswanath, S.G. Crystallization studies of crystalline–amorphous blends: Polyethylene terephthalate (PET)–polystyrene (PS). Polym. Plast. Technol. Eng. 2002, 41, 171–181. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, X.; Yu, J. Preparation and characterization of poly(ethylene terephthalate) copolyesters and fibers modified with sodium-5-sulfo-bis-(hydroxyethyl)-isophthalate and poly(ethylene glycol). J. Text. Inst. Proc. Abstr. 2016, 107, 1284–1295. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, C.S.; Wang, H.P.; Jiang, Z.L.; Liu, J.L. Preparation and characterization of the hydrophilic copolyester. Adv. Mater. Res. 1969, 535–537, 1499–1502. [Google Scholar] [CrossRef]
- Ji, P.; Shi, Y.; Liu, H.F.; Wang, Q.; Wang, C.S.; Wang, H.P. Preparation and properties of hydrophilic polyester. Int. Fiber J. 2015, 30, 34–38. [Google Scholar]
- Zhou, R.; Wang, X.; Huang, L.; Li, F.; Liu, S.; Yu, J. Poly(ethylene terephthalate) copolyesters and fibers modified with NPG and SIPE for improved hydrophilicity and dyeability. J. Text. Inst. 2017, 108, 1949–1956. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, J.; Wang, X.; Shi, L.; Zeng, Z. Synthesis and properties of the hydrophilic modified copolyester. J. Donghua Univ. Nat. Sci. Ed. 2018, 44, 889. [Google Scholar]
- Zeng, F.R.; Xu, J.; Sun, L.H.; Ma, J.; Jiang, H.; Li, Z.L. Copolymers of ε-caprolactone and ε-caprolactam via polyesterification: Towards sequence-controlled poly(ester amide) s. Polym. Chem. 2020, 11, 1211–1219. [Google Scholar] [CrossRef]
- Bech, L.; Meylheuc, T.; Lepoittevin, B.; Roger, P. Chemical surface modification of poly(ethylene terephthalate) fibers by aminolysis and grafting of carbohydrates. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 2172–2183. [Google Scholar] [CrossRef]
- Zou, H.T.; Jiang, J.M.; Yang, S.L.; Li, G. The composition, sequence analysis, and crystallization characterization of poly(trimethylene-co-butylene terephthalate) copolymer. J. Macromol. Sci. Part B-Phys. 2006, 45, 581–592. [Google Scholar] [CrossRef]
- Li, W.; Peng, W.; Ren, S.; He, A. Synthesis and characterization of TBIR with different fraction and chain sequence distribution and its influence on the properties of NR/TBIR/CB composites. Ind. Eng. Chem. Res. 2019, 58, 10609–10617. [Google Scholar] [CrossRef]
- Zhang, D.; Qi, D.; Wang, J.; Yu, S.; Deng, M. Effects of ethyl-L-glutamated and phenylalanine ratio/sequence on the secondary structure and gelation properties of their PEGylated copolymers. Polymer 2020, 191, 122276. [Google Scholar] [CrossRef]
- Kim, J.H.; Lyoo, W.S.; Ha, W.S. Sequence analysis of poly(ethylene terephthalate)/poly(butylene terephthalate) copolymer prepared by ester-interchange reactions. J. Appl. Polym. Sci. 2001, 82, 159–168. [Google Scholar] [CrossRef]
- Ilarduya, A.M.D.; Kint, D.P.R.; Muñoz-Guerra, S. Sequence Analysis of Poly(ethylene terephthalate-co-isophthalate) Copolymers by13C NMR. Macromolecules 2000, 33, 4596–4598. [Google Scholar] [CrossRef]
- Zhou, X.M.; Jiang, Z.H. Sequence analysis of poly (ether sulfone) copolymers by 13C NMR. J. Polym. Sci. Part B 2005, 43, 1624–1630. [Google Scholar] [CrossRef]
- Matsuda, H.; Nagasaka, B.; Asakura, T. Sequence analysis of poly(ethylene/1,4-cyclohexanedimethylene terephthalate) copolymer using 1H and 13C NMR. Polymer 2003, 44, 4681–4687. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, M.; Zhang, Q.; Wang, R.; Tong, X.; Xue, S.; Zhou, G. Biobased copolyesters: Synthesis, sequence distribution, crystal structure, thermal and mechanical properties of poly(butylene sebacate-co-butylene furandicarboxylate). Polym. Degrad. Stab. 2017, 143, 1–8, S0141391017301507. [Google Scholar] [CrossRef]
- Matsuda, H.; Asakura, T.; Miki, T. Triad Sequence Analysis of Poly(ethylene/butylene terephthalate) Copolymer Using 1H NMR. Macromolecules 2002, 35, 4664–4668. [Google Scholar] [CrossRef]
- Shyr, T.W.; Lo, C.M.; Ye, S.R.; Bian, J. Sequence distribution and crystal structure of poly(ethylene/butylene terephthalate) copolyesters compared with poly(ethylene/trimethylene terephthalate) copolyesters. J. Polym. Sci. Part B 2007, 45, 405–419. [Google Scholar] [CrossRef]
- Gan, W.; Ding, B. Preparing sodium isophthalate-5-sulfonate involves preparing raw material comprising dimethyl isophthalate-5-sulfonate, 1,3-propanediol, and catalyst, adding dimethyl isophthalate-5-sulfonate and 1,3-propanediol to reaction kettle. CN109796379-A, 24 May 2019. C07C-309/58 201948, p. 4. (In Chinese). [Google Scholar]
- Fonseca, F.M.C.; Patricio, P.S.O.; Souza, S.D.; Orefice, R.L. Prodegradant effect of titanium dioxide nanoparticulates on polypropylene-polyhydroxybutyrate blends. J. Appl. Polym. Sci. 2018, 135, 8. [Google Scholar] [CrossRef]
- Tengfei, Z.; Ludan, S.; Hongmei, H.; Yu, W.; Xueli, W.; Jianyong, Y.; University, Dnghua. Synthesis and characterization of bio-based polyamide 56 oligomer modified polyester. J. Text. Res. 2019, 40, 1–7. [Google Scholar]










| Numbers | Samples | TiO2 Proportion (%) |
|---|---|---|
| PET-0 | PET | - |
| PET-1 | PET | 0.03 |
| PET-2 | PET | 0.24 |
| ENCDP-0 | ENCDP | - |
| ENCDP-1 | ENCDP | 0.03 |
| ENCDP-2 | ENCDP | 0.24 |
| Samples | [η] (dL∙g−1) | Mη (g∙mol−1) |
|---|---|---|
| PET-0 | 0.676 | 18,900 |
| PET-1 | 0.671 | 18,800 |
| PET-2 | 0.647 | 18,000 |
| ENCDP-0 | 0.594 | 16,200 |
| ENCDP-1 | 0.586 | 15,900 |
| ENCDP-2 | 0.568 | 15,300 |
| Wave Numbers (cm−1) | Vibrational Conformation |
|---|---|
| 3450 cm−1 | Stretching vibration peaks for –OH |
| 2853 cm−1 | Stretching vibration peaks for –CH2– in PEG |
| 2892 cm−1 | feature peaks for –CH2– |
| 1740 cm−1 | The asymmetric stretching of –C=O for the aliphatic-aromatic ester units. |
| 1578–1504 cm−1 | Stretching vibration peaks for Benzene |
| 1372 cm−1 | Symmetrical deformation vibration peaks for C–H in NPG |
| 1101,1117 cm−1 | Absorbing vibration peaks for (C–O–C) |
| 727 cm−1 | Vibration peaks generated by the conjugation of benzene and –C=O |
| 630 cm−1 | Stretching vibration peaks for S–C in SIPE |
| Samples | Feed Ratio | Actual Reaction Yield | ||||
|---|---|---|---|---|---|---|
| SIPE/PTA (mol%) | PEG/PTA (mol%) | NPG/PTA (mol%) | SIPE/PTA (mol%) | PEG/PTA (mol%) | NPG/PTA (mol%) | |
| ENCDP-0 | 1.72 | 0.61 | 3.46 | 98.28 | 76.25 | 80.46 |
| ENCDP-1 | 1.74 | 0.58 | 3.21 | 99.43 | 72.50 | 74.65 |
| ENCDP-2 | 1.73 | 0.63 | 3.52 | 98.85 | 78.75 | 81.86 |
| Results | Triads Sequence (mol%) | |||||||
|---|---|---|---|---|---|---|---|---|
| BBB | BBS | SBS | BBN | NBN | SBN | SSS | NNN | |
| Theoretical values | 90.44 | 2.96 | 0.03 | 5.94 | 0.10 | 0.05 | - | - |
| Experimental values | 89.71 | 2.21 | 0.03 | 5.73 | 0.12 | 0.07 | - | - |
| Result | Av Ls | Randomness BS (%) | Av LN | Randomness BN (%) | ||
|---|---|---|---|---|---|---|
| LB | LS | LB | LN | |||
| Theoretical values | 64.72 | 1.02 | 99.50 | 31.45 | 1.04 | 99.35 |
| Experimental values | 75.45 | 1.03 | 99.17 | 30.61 | 1.04 | 99.45 |
| Sample | Tg (°C) | Tc (°C) | Tcc (°C) | Tm (°C) | △Hm (J/g) | Xc (%) |
|---|---|---|---|---|---|---|
| PET-0 | 70.17 | 137.69 | 163.18 | 245.59 | 35.82 | 26.38 |
| PET-1 | 72.16 | 136.84 | 184.54 | 250.24 | 39.03 | 28.74 |
| PET-2 | 72.65 | 134.00 | 196.8 | 251.55 | 40.08 | 29.51 |
| ENCDP-0 | 64.00 | 154.37 | - | 227.94 | 26.70 | 19.66 |
| ENCDP-1 | 67.67 | 160.19 | - | 234.12 | 27.98 | 20.60 |
| ENCDP-2 | 67.78 | 156.87 | 148.35 | 234.94 | 28.50 | 20.99 |
| Samples | Tc (°C) | t1/2 (s) | n | Zc (min−1) |
|---|---|---|---|---|
| PET-0 | 137.69 | 74.4 | 2.96 | 0.57 |
| PET-1 | 136.84 | 67.2 | 3.28 | 0.79 |
| PET-2 | 134.00 | 58.6 | 3.76 | 0.91 |
| ENCDP-0 | 154.37 | 145.7 | 2.75 | 0.41 |
| ENCDP-1 | 160.19 | 142.8 | 3.01 | 0.43 |
| ENCDP-2 | 156.87 | 138.2 | 3.26 | 0.46 |
| Samples | Td (5%) | Tdm |
|---|---|---|
| PET-0 | 425.48 | 460.89 |
| PET-1 | 419.60 | 455.36 |
| PET-2 | 417.33 | 450.61 |
| ENCDP-0 | 411.48 | 449.74 |
| ENCDP-1 | 410.25 | 448.95 |
| ENCDP-2 | 410.14 | 448.09 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Huang, L.; Wang, X.; Hu, H.; Guo, J.; Zhu, R.; He, S. Synthesis and Structural Characterization of Sequential Structure and Crystallization Properties for Hydrophilic Modified Polyester. Polymers 2020, 12, 1733. https://doi.org/10.3390/polym12081733
Sun L, Huang L, Wang X, Hu H, Guo J, Zhu R, He S. Synthesis and Structural Characterization of Sequential Structure and Crystallization Properties for Hydrophilic Modified Polyester. Polymers. 2020; 12(8):1733. https://doi.org/10.3390/polym12081733
Chicago/Turabian StyleSun, Lina, Liqian Huang, Xueli Wang, Hongmei Hu, Juanzi Guo, Ruishu Zhu, and Shuang He. 2020. "Synthesis and Structural Characterization of Sequential Structure and Crystallization Properties for Hydrophilic Modified Polyester" Polymers 12, no. 8: 1733. https://doi.org/10.3390/polym12081733
APA StyleSun, L., Huang, L., Wang, X., Hu, H., Guo, J., Zhu, R., & He, S. (2020). Synthesis and Structural Characterization of Sequential Structure and Crystallization Properties for Hydrophilic Modified Polyester. Polymers, 12(8), 1733. https://doi.org/10.3390/polym12081733






