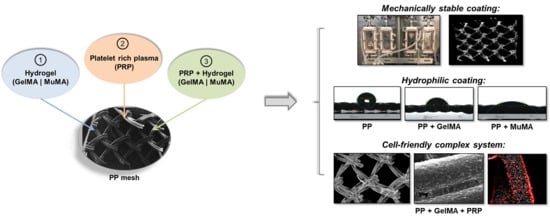
Bioinspired Hydrogel Coating Based on Methacryloyl Gelatin Bioactivates Polypropylene Meshes for Abdominal Wall Repair
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Hydrogel Coatings
2.2.2. Characterization of Coating
Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
Contact Angle (CA) Measurements
Stability in Physiologically Simulated Conditions
2.2.3. PRP Preparation
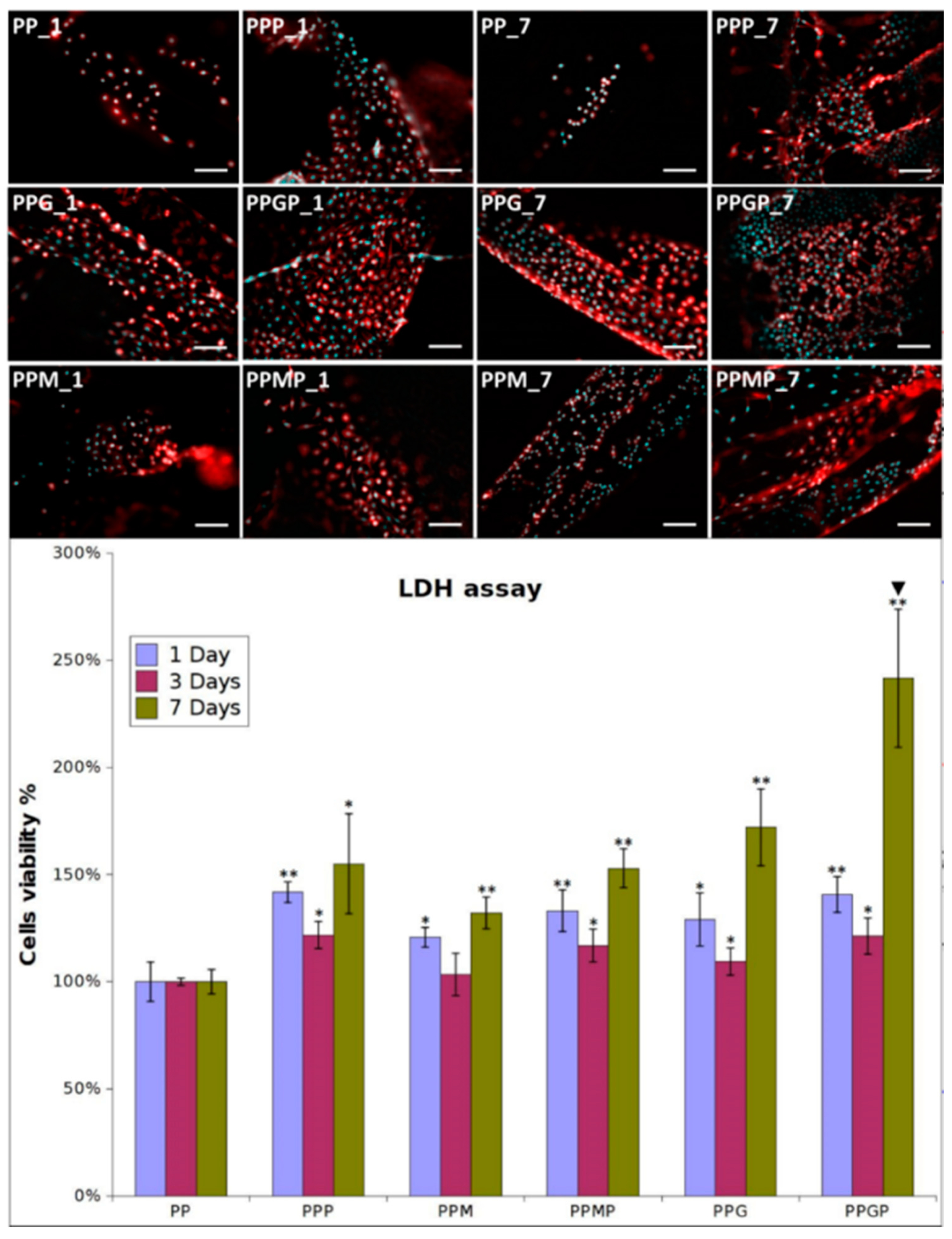
2.2.4. In Vitro Biocompatibility
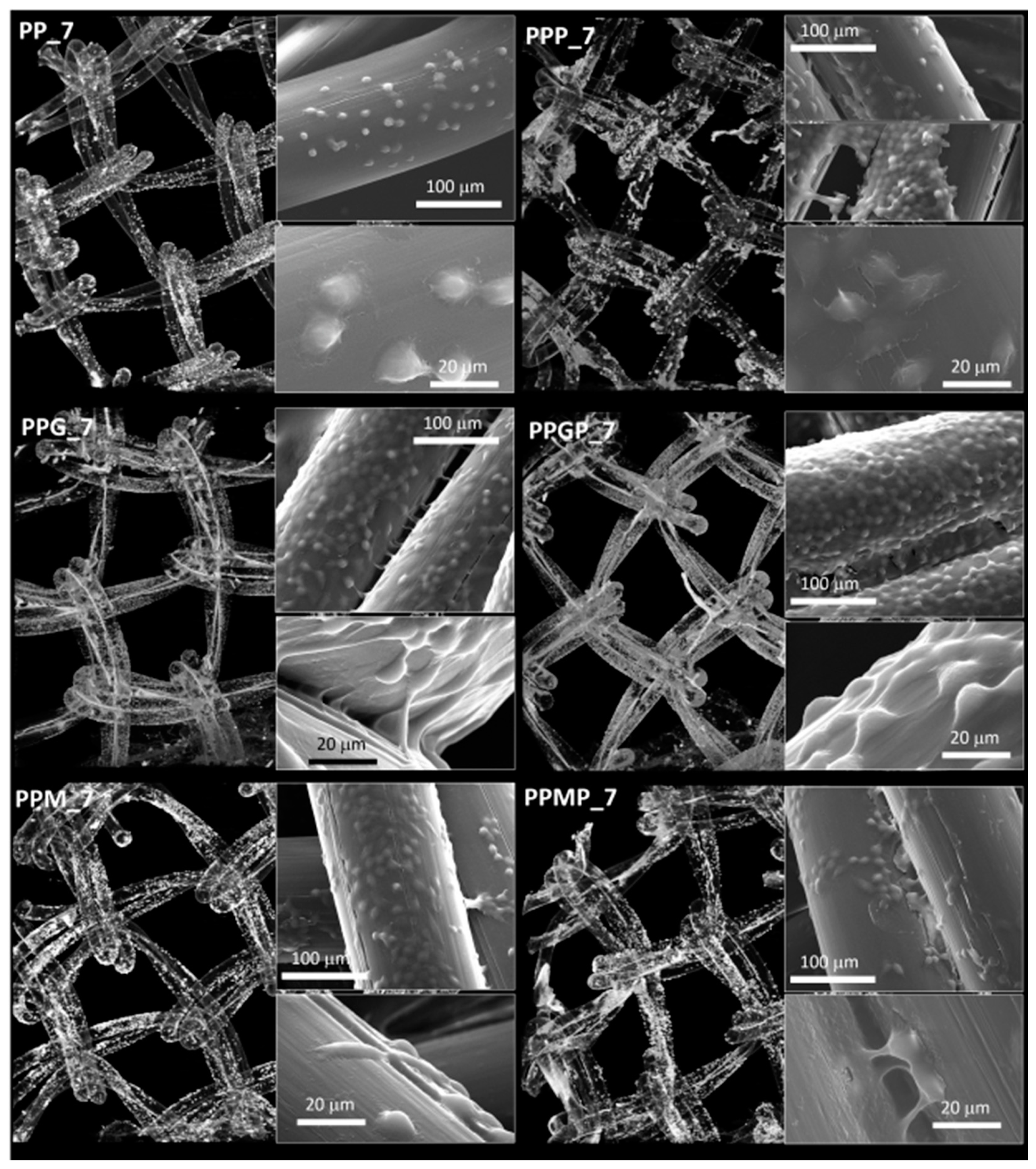
2.2.5. Scanning Electron Microscopy (SEM)
2.2.6. Micro-Computed Tomography (micro-CT)
3. Results
3.1. Characterization of Hydrogel Coatings
3.2. Effect of Approached Route on PP Bioactivation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Brown, C.N.; Finch, J.G. Which mesh for hernia repair? Ann. R. Coll. Surg. Engl. 2010, 92, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Kalaba, S.; Gerhard, E.; Winder, J.S.; Pauli, E.M.; Haluck, R.S.; Yang, J. Design strategies and applications of biomaterials and devices for Hernia repair. Bioact. Mater. 2016, 1, 2–17. [Google Scholar] [CrossRef]
- Guillaume, O.; Pérez-Tanoira, R.; Fortelny, R.; Redl, H.; Moriarty, T.F.; Richards, R.G.; Eglin, D.; Puchner, A.P. Infections associated with mesh repairs of abdominal wall hernias: Are antimicrobial biomaterials the longed-for solution? Biomaterials 2018, 167, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Bilsel, Y.; Abci, I. The search for ideal hernia repair; mesh materials and types. Int. J. Surg. 2012, 10, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Schreinemacher, M.H.F.; Van Barneveld, K.W.Y.; Dikmans, R.E.G.; Gijbels, M.J.J.; Greve, J.W.M.; Bouvy, N.D. Coated meshes for hernia repair provide comparable intraperitoneal adhesion prevention. Surg. Endosc. Other Interv. Tech. 2013, 27, 4202–4209. [Google Scholar] [CrossRef]
- Wolf, M.T.; Carruthers, C.A.; Dearth, C.L.; Peter, M.; Huber, A.; Burnsed, O.A.; Londono, R.; Johnson, S.A.; Daly, K.A.; Stahl, E.C.; et al. Polypropylene Surgical Mesh Coated with Extracellular Matrix Mitigates the Host Foreign Body Response. J. Biomed. Mater. Res. Part A 2014, 102, 234–246. [Google Scholar] [CrossRef]
- Houshyar, S.; Sarker, A.; Jadhav, A.; Kumar, G.S.; Bhattacharyya, A.; Nayak, R.; Shanks, R.A.; Saha, T.; Rifai, A.; Padhye, R.; et al. Polypropylene-nanodiamond composite for hernia mesh. Mater. Sci. Eng. C 2020, 111, 110780. [Google Scholar] [CrossRef]
- Baylón, K.; Rodríguez-Camarillo, P.; Elías-Zúñiga, A.; Díaz-Elizondo, J.A.; Gilkerson, R.; Lozano, K. Past, present and future of surgical meshes: A review. Membranes 2017, 7, 1–23. [Google Scholar]
- He, M.; Jiang, H.; Wang, R.; Xie, Y.; Zhao, W.; Zhao, C. A versatile approach towards multi-functional surfaces via covalently attaching hydrogel thin layers. J. Colloid Interface Sci. 2016, 484, 60–69. [Google Scholar] [CrossRef]
- Romanò, C.L.; Malizos, K.; Capuano, N.; Mezzoprete, R.; D’Arienzo, M.; Van Der Straeten, C.; Scarponi, S.; Drago, L. Does an Antibiotic-Loaded Hydrogel Coating Reduce Early Post-Surgical Infection after Joint Arthroplasty? J. Bone Jt. Infect. 2016, 1, 34–41. [Google Scholar]
- Hajj, F.E.; Hasan, A.; Nakhleh, J.; Osta, M.; Darwish, G.; Karam, P.; Nassereddine, M. Nanosilver loaded GelMA hydrogel for antimicrobial coating of biomedical implants. In Proceedings of the 2015 International Conference on Advances in Biomedical Engineering (ICABME), Beirut, Lebanon, 16–18 September 2015; pp. 189–192. [Google Scholar]
- Matsusaki, M.; Sakaguchi, H.; Serizawa, T.; Akashi, M. Controlled release of vascular endothelial growth factor from alginate hydrogels nano-coated with polyelectrolyte multilayer films. J. Biomater. Sci. Polym. Ed. 2007, 18, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Ataabadi, M.; Mostafavi-pour, Z.; Vojdani, Z.; Sani, M.; Latifi, M.; Talaei-Khozani, T. Fabrication and characterization of platelet-rich plasma scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2017, 71, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J. Staining proteins in gels with silver nitrate. CSH Protoc. 2007, 2007, prot4727. [Google Scholar] [CrossRef] [PubMed]
- Diana-Maria, D.; Van Vlierberghe, S.; Dubruel, P.; Dierick, M.; Van Hoorebeke, L.; Declercq, H.A.; Cornelissen, M.M.; Izabela-Cristina, S. Novel gelatin–PHEMA porous scaffolds for tissue engineering applications. Soft Matter. 2012, 8, 9589–9602. [Google Scholar]
- Serafim, A.; Tucureanu, C.; Daniela Geta, P.; Diana Maria, D.; Salageanu, A.; Van Vlierberghe, S.; Dubruel, P.; Izabela Cristina, S. One-pot synthesis of superabsorbent hybrid hydrogels based on methacrylamide gelatin and polyacrylamide. Effortless control of hydrogel properties through composition design. New J. Chem. 2014, 38, 3112. [Google Scholar] [CrossRef]
- Serafim, A.; Olaret, E.; Cecoltan, S.; Butac, L.M.; Balanuca, B.; Vasile, E. Bicomponent Hydrogels Based on Methacryloyl Derivatives of Gelatin and Mucin with Potential Wound Dressing Applications. Mater. Plast. 2018, 55, 68. [Google Scholar] [CrossRef]
- Duffy, C.V.; David, L.; Crouzier, T. Covalently-crosslinked mucin biopolymer hydrogels for sustained drug delivery. Acta Biomater. 2015, 20, 51–59. [Google Scholar] [CrossRef]
- Hai-Yin, Y.; Xiao-Chun, H.; Lan-Qin, L.; Jia-Shan, G.; Xian-Wen, W. Surface modification of polypropylene microporous membrane to improve its antifouling property in MBR: CO2 plasma treatment. J. Memb. Sci. 2005, 254, 219–227. [Google Scholar]
- Elango, S.; Perumalsamy, S.; Ramachandran, K.; Vadodaria, K. Mesh materials and hernia repair. BioMedicine 2017, 7, 16. [Google Scholar] [CrossRef]
- Food and Drug Administration. Hernia Surgical Mesh Implants, (n.d.). Available online: https://www.fda.gov/medical-devices/implants-and-prosthetics/hernia-surgical-mesh-implants (accessed on 9 July 2020).
- Gorgieva, S.; Modic, M.; Dovgan, B.; Kaisersberger-Vincek, M.; Kokol, V. Plasma-activated polypropylene mesh-gelatin scaffold composite as potential implant for bioactive hernia treatment. Plasma Process. Polym. 2015, 12, 237–251. [Google Scholar] [CrossRef]
- Emans, P.J.; Schreinemacher, M.H.F.; Gijbels, M.J.J.; Beets, G.L.; Greve, J.W.M.; Koole, L.H.; Bouvy, N.D. Polypropylene meshes to prevent abdominal herniation. Can stable coatings prevent adhesions in the long term? Ann. Biomed. Eng. 2009, 37, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Hersant, B.; La Padula, S.; SidAhmed-Mezi, M.; Rodriguez, A.M.; Meningaud, J.P. Use of platelet-rich plasma (PRP) in microsurgery. J. Stomatol. Oral Maxillofac. Surg. 2017, 118, 236–237. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.R.; Udpa, N.; Gao, Y. Chitosan selectively promotes adhesion of myoblasts over fibroblasts. J. Biomed. Mater. Res. A 2015, 103, 1899–1906. [Google Scholar] [CrossRef]
- Pascual, G.; Sotomayor, S.; Rodriguez, M.; Bayon, Y.; Bellon, J.M. Behaviour of a new composite mesh for the repair of full-thickness abdominal wall defects in a rabbit model. PLoS ONE 2013, 8, e80647. [Google Scholar] [CrossRef] [PubMed]
- van’t Riet, M.; Burger, J.W.A.; Bonthuis, F.; Jeekel, J.; Bonjer, H.J. Prevention of adhesion formation to polypropylene mesh by collagen coating: A randomized controlled study in a rat model of ventral hernia repair. Surg. Endosc. 2004, 18, 681–685. [Google Scholar]
- Faulk, D.M.; Londono, R.; Wolf, M.T.; Ranallo, C.A.; Carruthers, C.A.; Wildemann, J.D.; Dearth, C.L.; Badylak, S.F. ECM hydrogel coating mitigates the chronic inflammatory response to polypropylene mesh. Biomaterials 2014, 35, 8585–8595. [Google Scholar] [CrossRef]
- Hu, W.; Lu, S.; Ma, Y.; Ren, P.; Ma, X.; Zhou, N.; Zhang, T.; Ji, Z. Poly(dopamine)-inspired surface functionalization of polypropylene tissue mesh for prevention of intra-peritoneal adhesion formation. J. Mater. Chem. B 2017, 5, 575–585. [Google Scholar] [CrossRef]
- Sanbhal, N.; Mao, Y.; Sun, G.; Xu, R.F.; Zhang, Q.; Wang, L. Surface modification of polypropylene mesh devices with cyclodextrin via cold plasma for hernia repair: Characterization and antibacterial properties. Appl. Surf. Sci. 2018, 439, 749–759. [Google Scholar] [CrossRef]
- Labay, C.; Canal, J.M.; Modic, M.; Cvelbar, U.; Quiles, M.; Armengol, M.; Arbos, M.A.; Gil, F.J.; Canal, C. Antibiotic-loaded polypropylene surgical meshes with suitable biological behaviour by plasma functionalization and polymerization. Biomaterials 2015, 71, 132–144. [Google Scholar] [CrossRef]
- Badiou, W.; Lavigne, J.P.; Bousquet, P.J.; O’Callaghan, D.; Mares, P.; de Tayrac, R. In vitro and in vivo assessment of silver-coated polypropylene mesh to prevent infection in a rat model. Int. Urogynecol. J. 2011, 22, 265–272. [Google Scholar] [CrossRef]
- Parizzi, N.G.; Rubini, O.A.; de Almeida, S.H.M.; Ireno, L.C.; Tashiro, R.M.; de Carvalho, V.H.T. Effect of platelet-rich plasma on polypropylene meshes implanted in the rabbit vagina: Histological analysis. Int. Braz J. Urol. 2017, 43, 746–752. [Google Scholar] [CrossRef]
- Marinaro, F.; Sanchez-Margallo, F.M.; Alvarez, V.; Lopez, E.; Tarazona, R.; Brun, M.V.; Blazquez, R.; Casado, J.G. Meshes in a mess: Mesenchymal stem cell-based therapies for soft tissue reinforcement. Acta Biomater. 2019, 85, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, I.F.; Oliveira, J.M.; Reis, R.L. Micro-CT–a digital 3D microstructural voyage into scaffolds: A systematic review of the reported methods and results. Biomater. Res. 2018, 22, 1–11. [Google Scholar]
- Saito, E.; Suarez-Gonzalez, D.; Rao, R.R.; Stegemann, J.P.; Murphy, W.L.; Hollister, S.J. Use of micro-computed tomography to nondestructively characterize biomineral coatings on solid freeform fabricated poly (L-Lactic Acid) and poly (ε-Caprolactone) scaffolds in vitro and in vivo. Tissue Eng. Part C Methods 2013, 19, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Segvich, S.; Smith, H.C.; Luong, L.N.; Kohn, D.H. Uniform Deposition of Protein Incorporated Mineral Layer on Three-Dimensional Porous Polymer Scaffolds. J. Biomed. Mater. Res. Part B Appl. Mater. 2008, 2, 340–349. [Google Scholar] [CrossRef]
- Castejón, D.; Alba-Tercedor, J.; Rotllant, G.; Ribes, E.; Durfort, M.; Guerao, G. Micro-computed tomography and histology to explore internal morphology in decapod larvae. Sci. Rep. 2018, 8, 14399. [Google Scholar]
- Shepherd, V.D.; Shepherd, J.H.; Best, S.M.; Cameron, R.E. 3D imaging of cells in scaffolds: Direct labelling for micro CT. J. Mater. Sci. Mater. Med. 2018, 29, 86. [Google Scholar] [CrossRef]
- Farini, A.; Villa, C.; Belicchi, M.; Meregalli, M.; Torrente, Y. Micro-CT technique for three-dimensional visualization of human stem cells. Methods Mol. Biol. 2013, 1052, 143–152. [Google Scholar]
- Medel, S.; Alarab, M.; Kufaishi, H.; Drutz, H.; Shynlova, O. Attachment of Primary Vaginal Fibroblasts to Absorbable and Nonabsorbable Implant Materials Coated with Platelet-Rich Plasma: Potential Application in Pelvic Organ Prolapse Surgery. Female Pelvic Med. Reconstr. Surg. 2015, 21, 190–197. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serafim, A.; Cecoltan, S.; Olăreț, E.; Dragusin, D.-M.; Vasile, E.; Popescu, V.; Manolescu Mastalier, B.S.; Iovu, H.; Stancu, I.-C. Bioinspired Hydrogel Coating Based on Methacryloyl Gelatin Bioactivates Polypropylene Meshes for Abdominal Wall Repair. Polymers 2020, 12, 1677. https://doi.org/10.3390/polym12081677
Serafim A, Cecoltan S, Olăreț E, Dragusin D-M, Vasile E, Popescu V, Manolescu Mastalier BS, Iovu H, Stancu I-C. Bioinspired Hydrogel Coating Based on Methacryloyl Gelatin Bioactivates Polypropylene Meshes for Abdominal Wall Repair. Polymers. 2020; 12(8):1677. https://doi.org/10.3390/polym12081677
Chicago/Turabian StyleSerafim, Andrada, Sergiu Cecoltan, Elena Olăreț, Diana-Maria Dragusin, Eugeniu Vasile, Valentin Popescu, Bogdan Stelian Manolescu Mastalier, Horia Iovu, and Izabela-Cristina Stancu. 2020. "Bioinspired Hydrogel Coating Based on Methacryloyl Gelatin Bioactivates Polypropylene Meshes for Abdominal Wall Repair" Polymers 12, no. 8: 1677. https://doi.org/10.3390/polym12081677
APA StyleSerafim, A., Cecoltan, S., Olăreț, E., Dragusin, D.-M., Vasile, E., Popescu, V., Manolescu Mastalier, B. S., Iovu, H., & Stancu, I.-C. (2020). Bioinspired Hydrogel Coating Based on Methacryloyl Gelatin Bioactivates Polypropylene Meshes for Abdominal Wall Repair. Polymers, 12(8), 1677. https://doi.org/10.3390/polym12081677