Surface Thermo-Dynamic Characterization of Poly (Vinylidene Chloride-Co-Acrylonitrile) (P(VDC-co-AN)) Using Inverse-Gas Chromatography and Investigation of Visual Traits Using Computer Vision Image Processing Algorithms
Abstract
1. Introduction
1.1. Purpose of Study
1.2. Inverse Gas Chromatography
1.3. Computer Vision and Image Processing
- Surface thermo-dynamic characterization of the polymer (P(VDC-co-AN)) has been carried. IGC attributes, such as London dispersive surface energy, Gibbs free energy, and Guttman Lewis acid-base parameters, were estimated.
- Visual traits, such as intricate patterns, surface morphology, texture/roughness, particle area distribution (), directionality (), mean average particle area (), and mean average particle standard deviation (), were investigated using CVIP techniques on SEM images of the polymer in its purest form.
2. Experimental Setup
2.1. IGC Experimental Setup
2.2. SEM Image Acquisition
3. IGC Surface Thermo-Dynamic Characterization
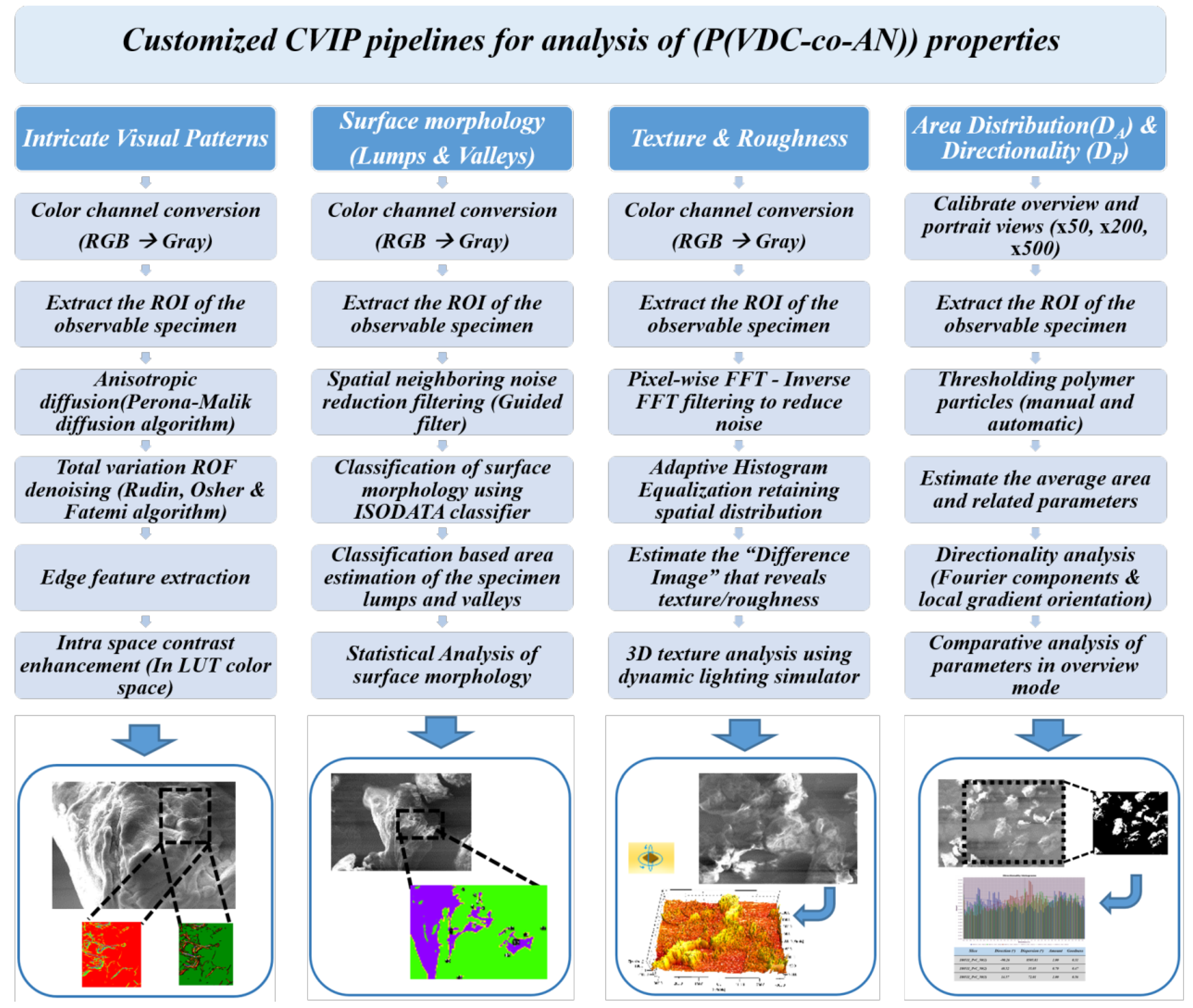
4. Image Analysis of (P(VDC-co-AN)) Visual Traits Using CVIP Techniques
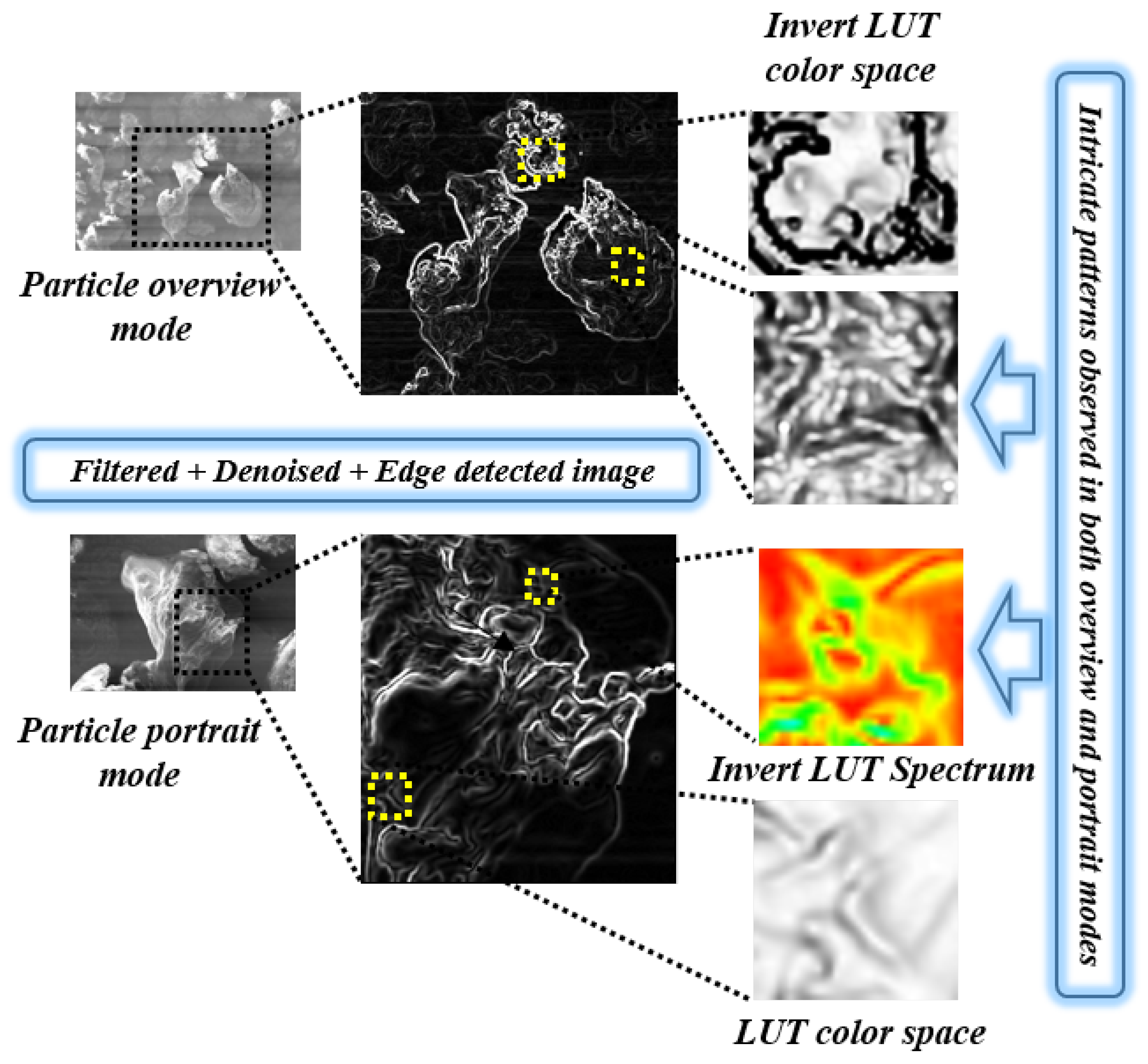
4.1. Intricate Visual Patterns
4.2. Surface Morphology-Lumps and Valleys
4.3. Texture and Roughness
4.4. Area Distribution (DA) and Particle Directionality (DP)
5. Results and Discussions
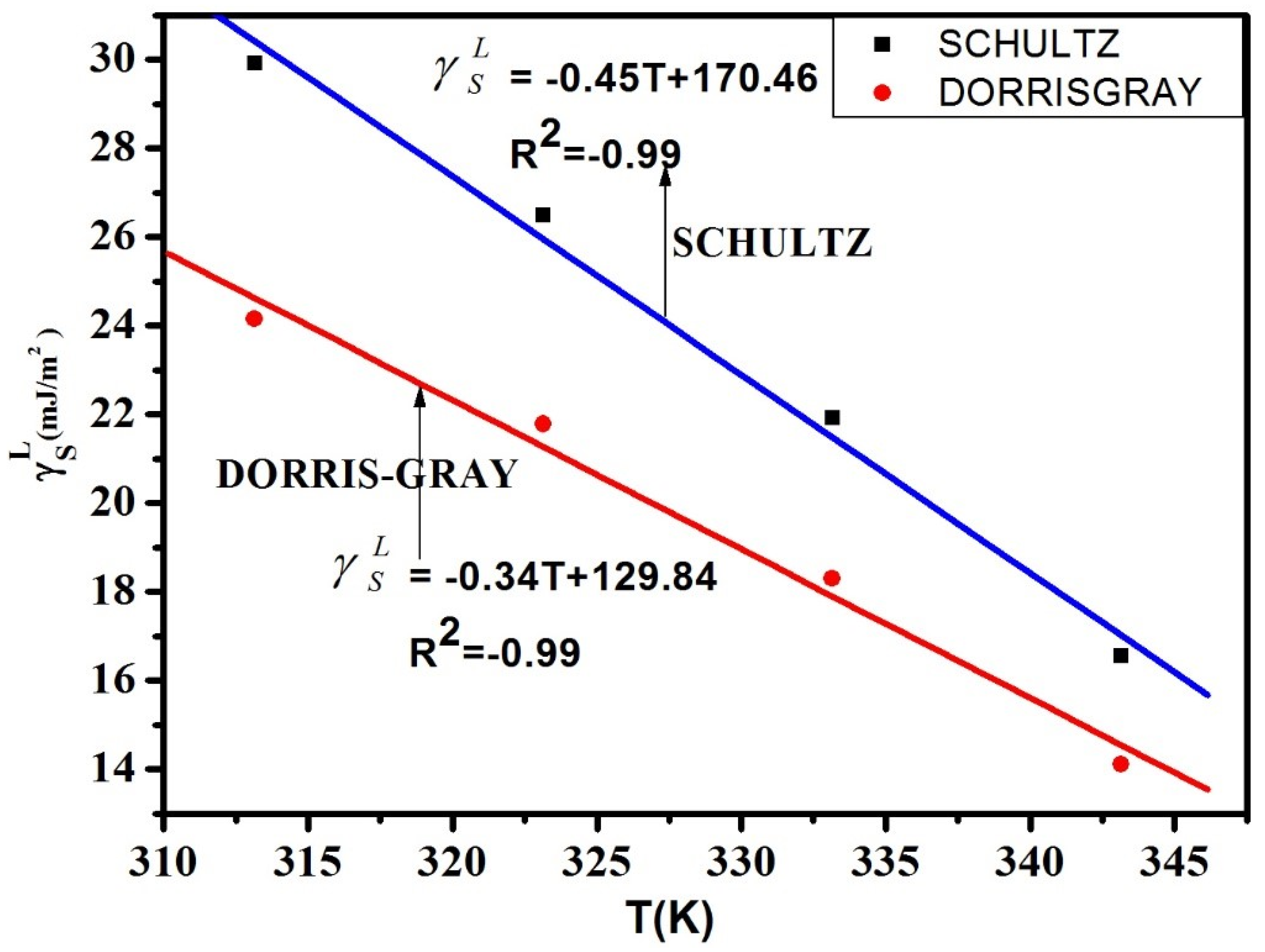
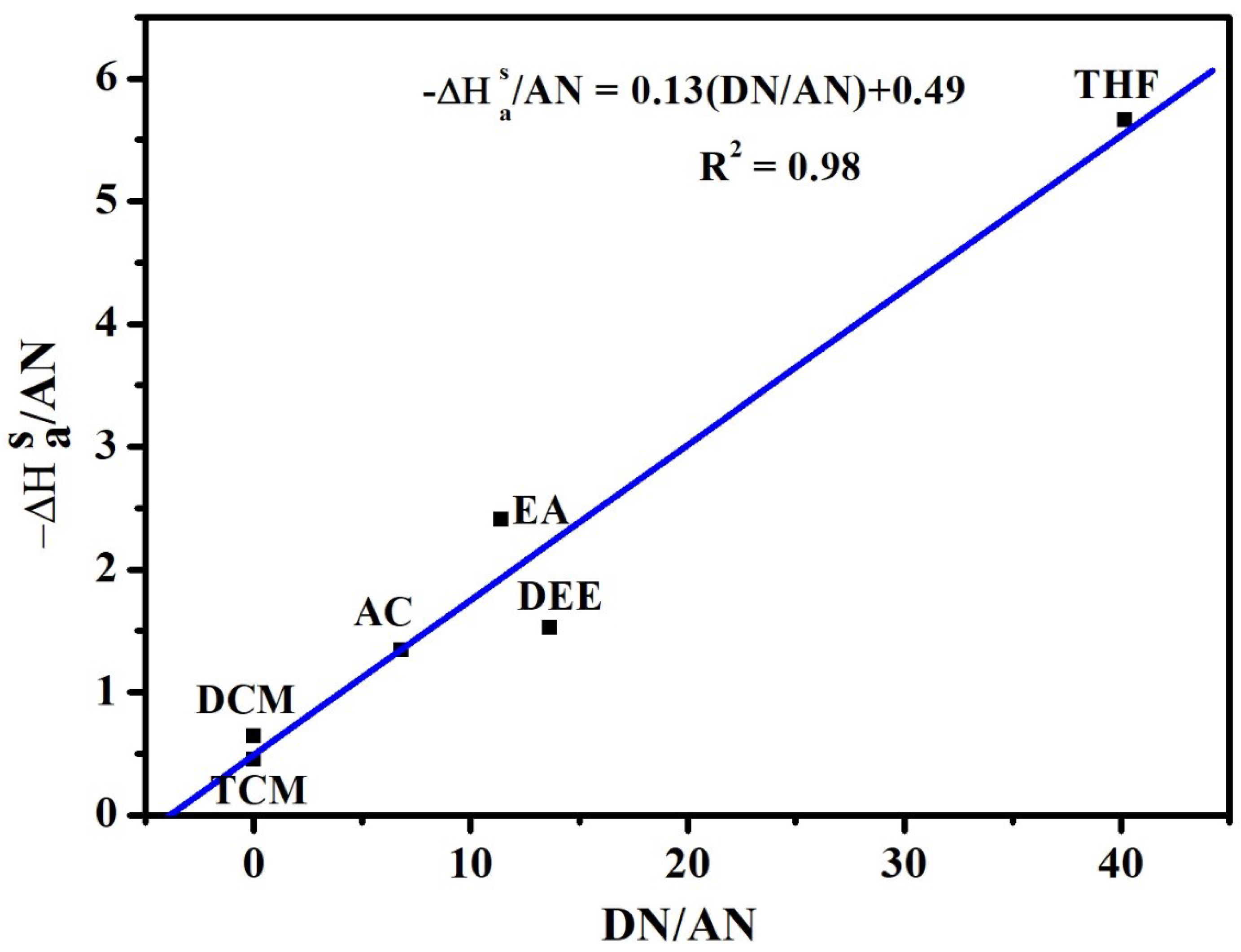
5.1. IGC Study on Polymer Surface Characterization
5.2. CVIP Study on Polymer Visual Traits
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. IGC Appendix
| Solute | a | |||
|---|---|---|---|---|
| n-Hexane | 2.21 | 0.515 | - | - |
| n-Heptane | 2.57 | 0.570 | - | - |
| n-Octane | 2.91 | 0.630 | - | - |
| n-Nonane | 3.29 | 0.690 | - | - |
| n-Decane | 3.63 | 0.750 | - | - |
| Acetone | 1.73 | 0.425 | 10.5 | 71.4 |
| Diethyl ether | 1.82 | 0.470 | 5.88 | 80.6 |
| Trichloromethane | 2.24 | 0.440 | 22.7 | 0 |
| Tetrahydrofuran | 2.13 | 0.450 | 2.1 | 84.4 |
| Ethyl acetate | 1.95 | 0.480 | 6.3 | 71.8 |
Appendix B. CVIP Appendix

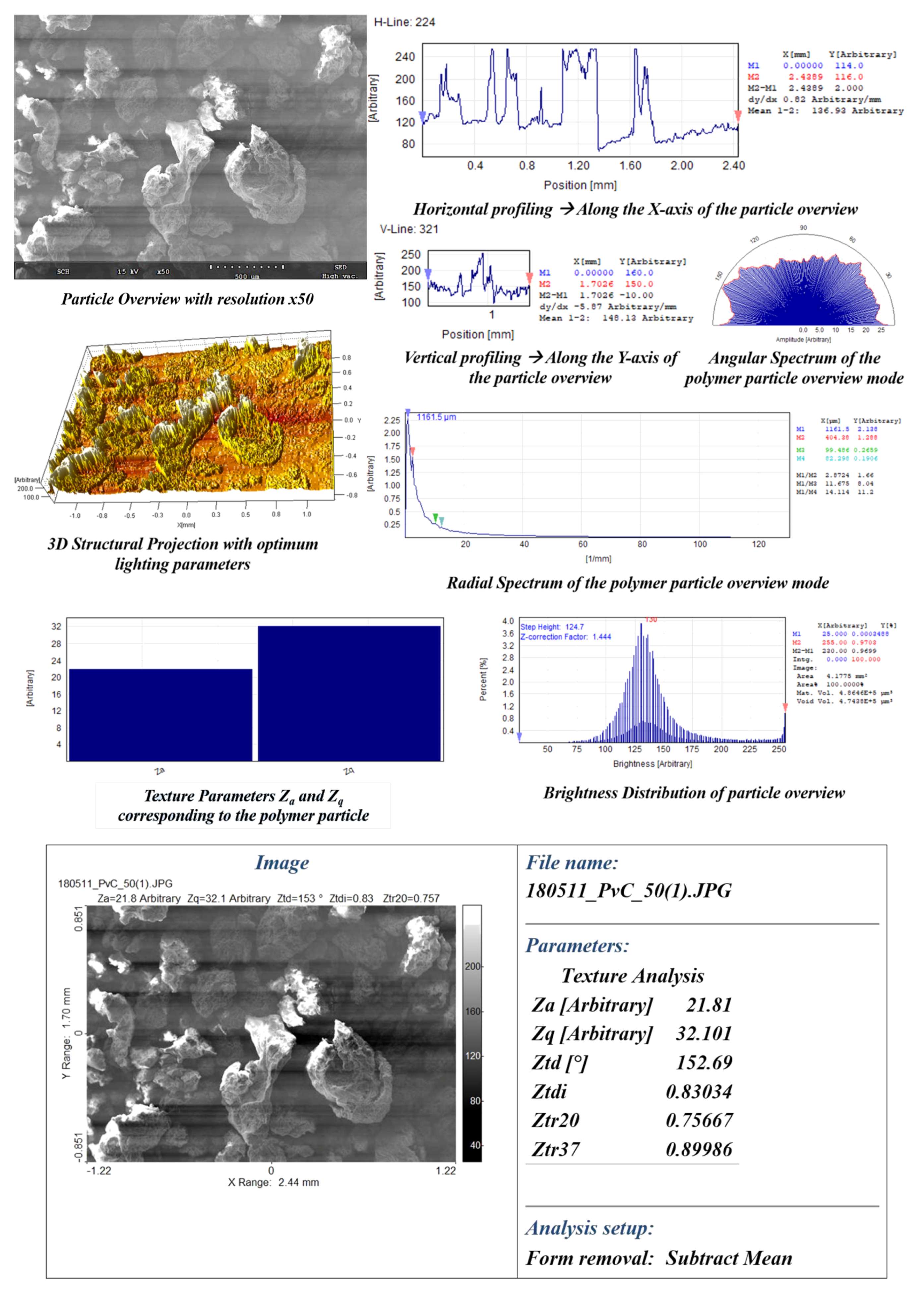
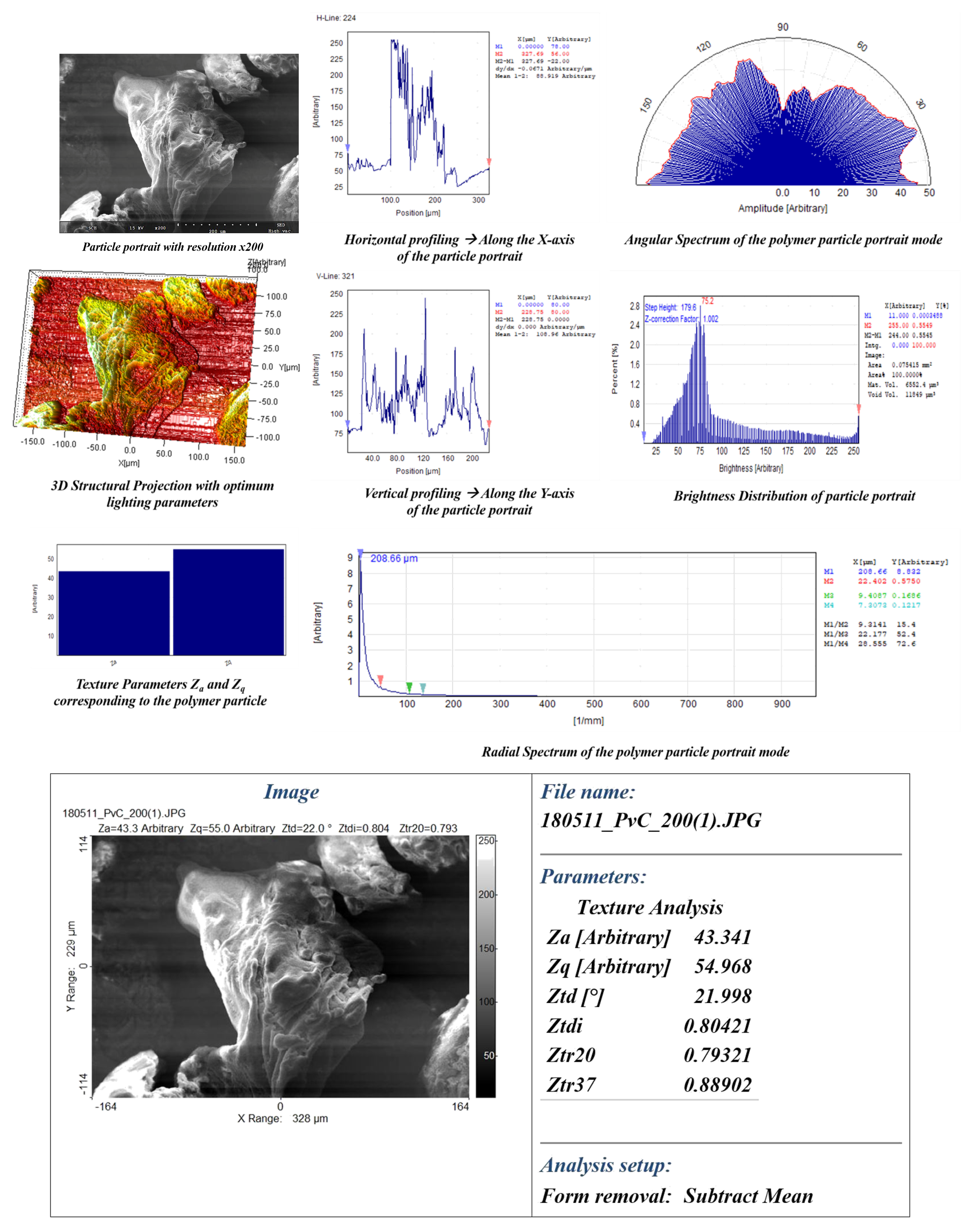
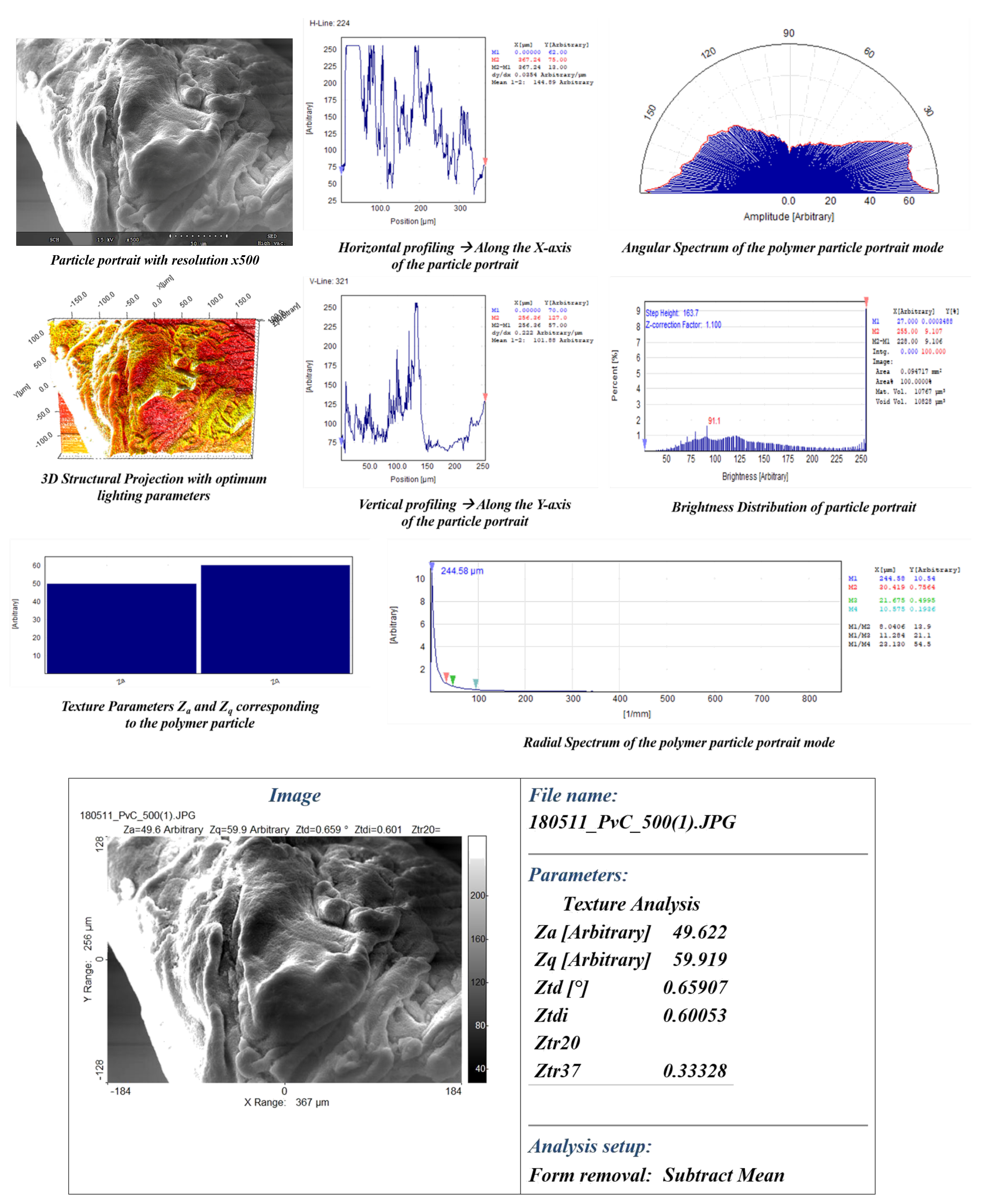
References
- Titow, W.V. PVC Plastics: Properties, Processing, and Applications; Springer Science & Business Media: Heidelberg, Germany, 2012. [Google Scholar]
- Leblanc, J.L. Filled Polymers: Science and Industrial Applications; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Chanda, M.; Roy, S.K. Industrial Polymers, Specialty Polymers, and Their Applications; CRC Press: Boca Raton, FL, USA, 2008; Volume 74. [Google Scholar]
- Pleşa, I.; Noţingher, P.V.; Schlögl, S.; Sumereder, C.; Muhr, M. Properties of polymer composites used in high-voltage applications. Polymers 2016, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, K.O.; Ju, Y.M. Polyethylene oxide additive-entrapped polyvinyl chloride as a new blood bag material. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. 1999, 48, 328–334. [Google Scholar] [CrossRef]
- Joshi, G.M.; Deshmukh, K. Optimized quality factor of graphene oxide-reinforced PVC nanocomposite. J. Electron. Mater. 2014, 43, 1161–1165. [Google Scholar] [CrossRef]
- Luzio, A.; Canesi, E.V.; Bertarelli, C.; Caironi, M. Electrospun polymer fibers for electronic applications. Materials 2014, 7, 906–947. [Google Scholar] [CrossRef]
- Shi, H.; Liu, X.; Zhang, Y. Fabrication of novel antimicrobial poly (vinyl chloride) plastic for automobile interior applications. Iran. Polym. J. 2014, 23, 297–305. [Google Scholar] [CrossRef]
- Schmid, M.; Wollecke, F.; Levy, G. Long-term Durability of SLS Polymer Components under Automotive Application Environment. In Proceedings of the 23th Annual International Solid Freeform Fabrication (SFF) Symposium, Austin, TX, USA, 6–8 August 2012. [Google Scholar]
- Köhl, M.; Jorgensen, G.; Brunold, S.; Carlsson, B.; Heck, M.; Möller, K. Durability of polymeric glazing materials for solar applications. Sol. Energy 2005, 79, 618–623. [Google Scholar] [CrossRef]
- Subbu, C.; Rajendran, S.; Kesavan, K.; Mathew, C. Lithium ion conduction in PVdC-co-AN based polymer blend electrolytes doped with different lithium salts. Int. Polym. Process. 2015, 30, 476–486. [Google Scholar] [CrossRef]
- Gonzalez, A.; Iriarte, M.; Iriondo, P.; Iruin, J. Miscibility and carbon dioxide transport properties of blends of bacterial poly (3-hydroxybutyrate) and a poly (vinylidene chloride-co-acrylonitrile) copolymer. Polymer 2002, 43, 6205–6211. [Google Scholar] [CrossRef]
- Subbu, C.; Mathew, C.M.; Kesavan, K.; Rajendran, S. Electrochemical, structural and optical studies on poly (vinylidene chloride-co-acrylonitrile) based polymer blend membranes. Int. J. Electrochem. Sci. 2014, 9, 4944–4958. [Google Scholar]
- Subbu, C.; Rajendran, S.; Kesavan, K.; Premila, R. The physical and electrochemical properties of poly (vinylidene chloride-co-acrylonitrile)-based polymer electrolytes prepared with different plasticizers. Ionics 2016, 22, 229–240. [Google Scholar] [CrossRef]
- Hwang, T.; Pu, L.; Kim, S.W.; Oh, Y.S.; Nam, J.D. Synthesis and barrier properties of poly (vinylidene chloride-co-acrylonitrile)/SiO2 hybrid composites by sol–gel process. J. Membr. Sci. 2009, 345, 90–96. [Google Scholar] [CrossRef]
- Tanaka, H.; Hayashi, T.; Nishi, T. Application of digital image analysis to pattern formation in polymer systems. J. Appl. Phys. 1986, 59, 3627–3643. [Google Scholar] [CrossRef]
- Pouteau, C.; Baumberger, S.; Cathala, B.; Dole, P. Lignin–polymer blends: Evaluation of compatibility by image analysis. Comptes Rendus Biol. 2004, 327, 935–943. [Google Scholar] [CrossRef]
- Gleeson, J.; Larson, R.; Mead, D.; Kiss, G.; Cladis, P. Image analysis of shear-induced textures in liquid-crystalline polymers. Liq. Cryst. 1992, 11, 341–364. [Google Scholar] [CrossRef]
- Sasov, A.Y.; Ermakova, T.; Lotmentsev, Y.M. Quantitative analysis of images depicting structure of polymers and polymer composites. Polym. Sci. USSR 1991, 33, 597–602. [Google Scholar] [CrossRef]
- Ruskin, R.S.; Yu, Z.; Grigorieff, N. Quantitative characterization of electron detectors for transmission electron microscopy. J. Struct. Biol. 2013, 184, 385–393. [Google Scholar] [CrossRef]
- Uhlemann, S.; Müller, H.; Hartel, P.; Zach, J.; Haider, M. Thermal magnetic field noise limits resolution in transmission electron microscopy. Phys. Rev. Lett. 2013, 111, 046101. [Google Scholar] [CrossRef]
- Misell, D. Conventional and scanning transmission electron microscopy: Image contrast and radiation damage. J. Phys. D Appl. Phys. 1977, 10, 1085. [Google Scholar] [CrossRef]
- Kushwaha, H.S.; Tanwar, S.; Rathore, K.; Srivastava, S. De-noising filters for tem (transmission electron microscopy) image of nanomaterials. In Proceedings of the 2012 Second International Conference on Advanced Computing & Communication Technologies, Rohtak, India, 7–8 January 2012; pp. 276–281. [Google Scholar]
- Lloyd, D.R.; Ward, T.C.; Schreiber, H.P. Inverse Gas Chromatography; Technical Report; American Chemical Society: Washington, DC, USA, 1989. [Google Scholar]
- Voelkel, A. Inverse gas chromatography: Characterization of polymers, fibers, modified silicas, and surfactants. Crit. Rev. Anal. Chem. 1991, 22, 411–439. [Google Scholar] [CrossRef]
- Mohammadi-Jam, S.; Waters, K. Inverse gas chromatography applications: A review. Adv. Colloid Interface Sci. 2014, 212, 21–44. [Google Scholar] [CrossRef]
- Jennings, W.; Mittlefehldt, E.; Stremple, P. Analytical Gas Chromatography; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Panzer, U.; Schreiber, H.P. On the evaluation of surface interactions by inverse gas chromatography. Macromolecules 1992, 25, 3633–3637. [Google Scholar] [CrossRef]
- Schultz, J.; Lavielle, L.; Martin, C. The role of the interface in carbon fibre-epoxy composites. J. Adhes. 1987, 23, 45–60. [Google Scholar] [CrossRef]
- Dorris, G.M.; Gray, D.G. Adsorption of n-alkanes at zero surface coverage on cellulose paper and wood fibers. J. Colloid Interface Sci. 1980, 77, 353–362. [Google Scholar] [CrossRef]
- Shi, B.; Wang, Y.; Jia, L. Comparison of Dorris–Gray and Schultz methods for the calculation of surface dispersive free energy by inverse gas chromatography. J. Chromatogr. A 2011, 1218, 860–862. [Google Scholar] [CrossRef] [PubMed]
- Umbaugh, S.E. Computer Vision and Image Processing: A Practical Approach Using Cviptools with Cdrom; Prentice Hall PTR: Upper Saddle River, NJ, USA, 1997. [Google Scholar]
- Qahwaji, R.; Qahwaji, R.; Green, R.; Hines, E.L. Applied Signal and Image Processing: Multidisciplinary Advancements; IGI Publishing: Hershey, PA, USA, 2011. [Google Scholar]
- Rosenfeld, A. From image analysis to computer vision: An annotated bibliography, 1955–1979. Comput. Vis. Image Underst. 2001, 84, 298–324. [Google Scholar] [CrossRef]
- Kraut, R.; Egido, C.; Galegher, J. Patterns of contact and communication in scientific research collaboration. In Proceedings of the 1988 ACM Conference on Computer-Supported Cooperative Work, Portland, OR, USA, 26–29 September 1988; pp. 1–12. [Google Scholar]
- Kakani, V.; Nguyen, V.H.; Kumar, B.P.; Kim, H.; Pasupuleti, V.R. A critical review on computer vision and artificial intelligence in food industry. J. Agric. Food Res. 2020, 2, 100033. [Google Scholar] [CrossRef]
- Kakani, V.; Kim, H.; Kumbham, M.; Park, D.; Jin, C.B.; Nguyen, V.H. Feasible Self-Calibration of Larger Field-of-View (FOV) Camera Sensors for the Advanced Driver-Assistance System (ADAS). Sensors 2019, 19, 3369. [Google Scholar] [CrossRef]
- Kakani, V.; Kim, H.; Lee, J.; Ryu, C.; Kumbham, M. Automatic Distortion Rectification of Wide-Angle Images Using Outlier Refinement for Streamlining Vision Tasks. Sensors 2020, 20, 894. [Google Scholar] [CrossRef]
- Ayache, N. Medical computer vision, virtual reality and robotics. Image Vis. Comput. 1995, 13, 295–313. [Google Scholar] [CrossRef]
- Capitán-Vallvey, L.F.; Lopez-Ruiz, N.; Martinez-Olmos, A.; Erenas, M.M.; Palma, A.J. Recent developments in computer vision-based analytical chemistry: A tutorial review. Anal. Chim. Acta 2015, 899, 23–56. [Google Scholar] [CrossRef]
- Kaehler, A.; Bradski, G. Learning OpenCV 3: Computer Vision in C++ with the OpenCV Library; O’Reilly Media, Inc.: Sebastopol, CA, USA, 2016. [Google Scholar]
- Higham, D.J.; Higham, N.J. MATLAB Guide; SIAM: Philadelphia, PA, USA, 2016. [Google Scholar]
- Maeder, R.E. Programming in Mathematica; Addison-Wesley Longman Publishing Co., Inc.: Boston, MA, USA, 1991. [Google Scholar]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Silly, F. A robust method for processing scanning probe microscopy images and determining nanoobject position and dimensions. J. Microsc. 2009, 236, 211–218. [Google Scholar] [CrossRef]
- Hua-jun, L. Application of Origin7. 0 to Chemical Engineering Calculation. J. Anhui Univ. Sci. Technol. Nat. Sci. 2005, 1, 1–5. [Google Scholar]
- Basivi, P.K.; Pasupuleti, V.R.; Seella, R.; Tukiakula, M.R.; Kalluru, S.R.; Park, S.J. Inverse gas chromatography study on London dispersive surface free energy and electron acceptor–donor of fluconazole drug. J. Chem. Eng. Data 2017, 62, 2090–2094. [Google Scholar] [CrossRef]
- Perdigao, J.; Lambrechts, P.; Vanherle, G. Microscopy investigations: Techniques, results, limitations. Am. J. Dent. 2000, 13, 3D18D. [Google Scholar]
- Weickert, J. Anisotropic Diffusion In Image Processing; Teubner Stuttgart: Copenhagen, Denmark, 1998; Volume 1. [Google Scholar]
- Vogel, C.R.; Oman, M.E. Iterative methods for total variation denoising. SIAM J. Sci. Comput. 1996, 17, 227–238. [Google Scholar] [CrossRef]
- Li, S.; Kang, X.; Hu, J. Image fusion with guided filtering. IEEE Trans. Image Process. 2013, 22, 2864–2875. [Google Scholar]
- Buijs, H.; Pomerleau, A.; Fournier, M.; Tam, W. Implementation of a fast Fourier transform (FFT) for image processing applications. IEEE Trans. Acoust. Speech Signal Process. 1974, 22, 420–424. [Google Scholar] [CrossRef]
- Perona, P.; Shiota, T.; Malik, J. Anisotropic diffusion. In Geometry-Driven Diffusion in Computer Vision; Springer: Heidelberg, Germany, 1994; pp. 73–92. [Google Scholar]
- He, K.; Sun, J.; Tang, X. Guided image filtering. In Proceedings of the European Conference on Computer Vision, Crete, Greece, 5–11 September 2010; pp. 1–14. [Google Scholar]
- Ball, G.H.; Hall, D.J. ISODATA, A Novel Method of Data Analysis and Pattern Classification; Technical Report; Stanford Research Institute: Menlo Park, CA, USA, 1965. [Google Scholar]
- Liu, Z.Q. Scale space approach to directional analysis of images. Appl. Opt. 1991, 30, 1369–1373. [Google Scholar] [CrossRef]
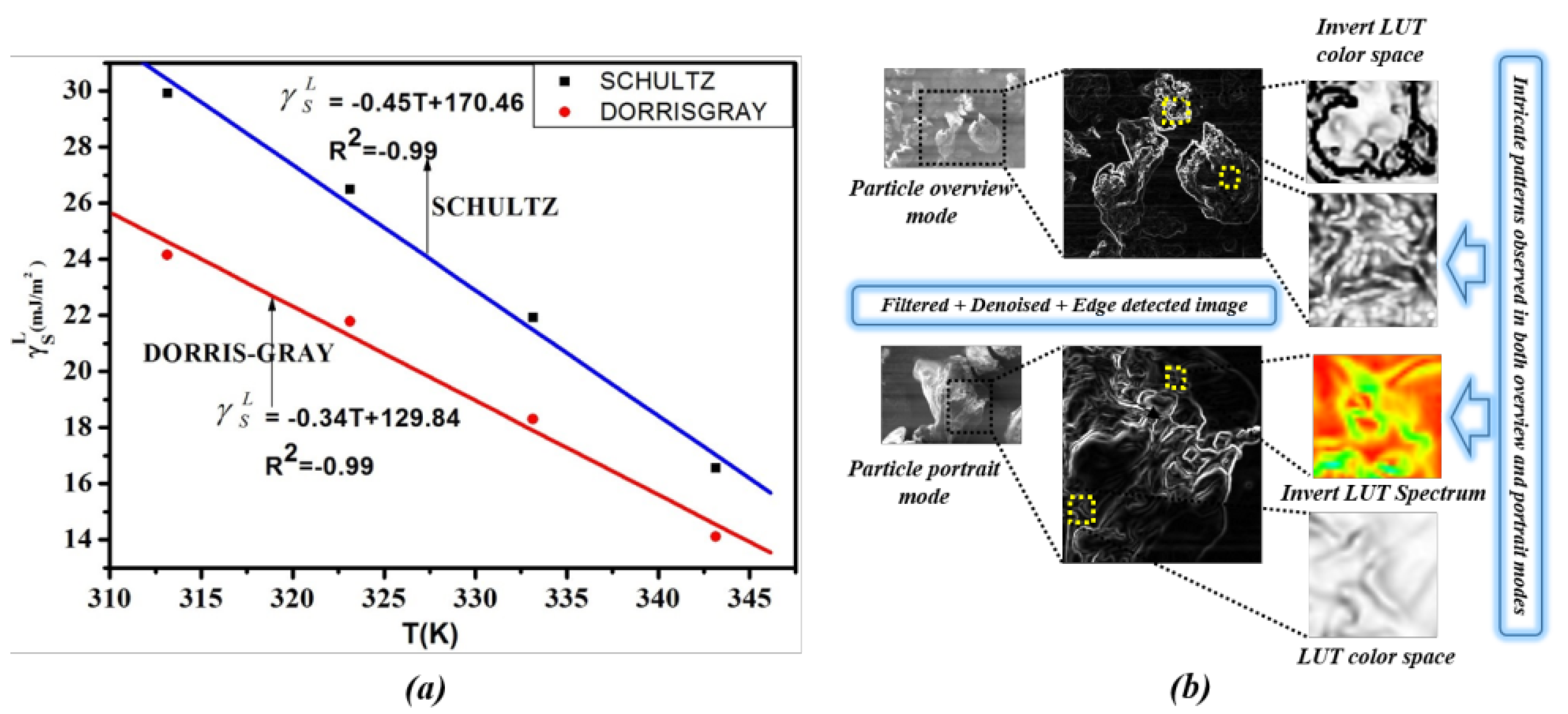






| Solutes | 313.15 K | 323.15 K | 333.15 K | 343.15 K |
|---|---|---|---|---|
| n-Pentane | 7.40 | 7.61 | 7.41 | – |
| n-Hexane | 9.51 | 10.02 | 9.37 | 9.27 |
| n-Heptane | 10.92 | 11.50 | 12.22 | 10.42 |
| n-Octane | 12.48 | 13.10 | 13.84 | 11.82 |
| n-Nonane | 16.03 | 15.16 | 15.51 | 13.22 |
| n-Decane | 18.11 | 17.21 | 16.94 | 14.91 |
| Acetone | 17.88 | 18.48 | 18.61 | 16.28 |
| Di-ethyl ether | 17.70 | 17.87 | 17.84 | 16.02 |
| Dichloromethane | 17.26 | 17.51 | 17.56 | 15.61 |
| Trichloromethane | 19.82 | 19.62 | 19.27 | 16.66 |
| Tetrahydrofuran | 19.11 | 19.05 | 18.74 | 17.04 |
| Ethyl acetate | 19.32 | 18.45 | 18.37 | 16.98 |
| (Schultz) | (Dorris-Gray) | |
|---|---|---|
| 313.15 | 29.93 | 24.15 |
| 323.15 | 26.49 | 21.78 |
| 333.15 | 21.92 | 18.30 |
| 343.15 | 16.84 | 14.11 |
| Solutes | (kJ mol) | (kJ mol) | r |
|---|---|---|---|
| Acetone | 14.07 | −0.04 | 0.95 |
| Di-ethyl ether | 9.00 | −0.02 | 0.96 |
| Dichloromethane | 7.49 | −0.01 | 0.99 |
| Trichloromethane | 14.67 | −0.04 | 0.99 |
| Tetrahydrofuran | 11.89 | −0.03 | 0.99 |
| Ethyl acetate | 15.19 | −0.03 | 0.99 |
| Method | S | |||
|---|---|---|---|---|
| Schultz et al. | 0.13 | 0.49 | 3.77 | 0.98 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakani, V.; Kim, H.; Basivi, P.K.; Pasupuleti, V.R. Surface Thermo-Dynamic Characterization of Poly (Vinylidene Chloride-Co-Acrylonitrile) (P(VDC-co-AN)) Using Inverse-Gas Chromatography and Investigation of Visual Traits Using Computer Vision Image Processing Algorithms. Polymers 2020, 12, 1631. https://doi.org/10.3390/polym12081631
Kakani V, Kim H, Basivi PK, Pasupuleti VR. Surface Thermo-Dynamic Characterization of Poly (Vinylidene Chloride-Co-Acrylonitrile) (P(VDC-co-AN)) Using Inverse-Gas Chromatography and Investigation of Visual Traits Using Computer Vision Image Processing Algorithms. Polymers. 2020; 12(8):1631. https://doi.org/10.3390/polym12081631
Chicago/Turabian StyleKakani, Vijay, Hakil Kim, Praveen Kumar Basivi, and Visweswara Rao Pasupuleti. 2020. "Surface Thermo-Dynamic Characterization of Poly (Vinylidene Chloride-Co-Acrylonitrile) (P(VDC-co-AN)) Using Inverse-Gas Chromatography and Investigation of Visual Traits Using Computer Vision Image Processing Algorithms" Polymers 12, no. 8: 1631. https://doi.org/10.3390/polym12081631
APA StyleKakani, V., Kim, H., Basivi, P. K., & Pasupuleti, V. R. (2020). Surface Thermo-Dynamic Characterization of Poly (Vinylidene Chloride-Co-Acrylonitrile) (P(VDC-co-AN)) Using Inverse-Gas Chromatography and Investigation of Visual Traits Using Computer Vision Image Processing Algorithms. Polymers, 12(8), 1631. https://doi.org/10.3390/polym12081631








