High Acid Biochar-Based Solid Acid Catalyst from Corn Stalk for Lignin Hydrothermal Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
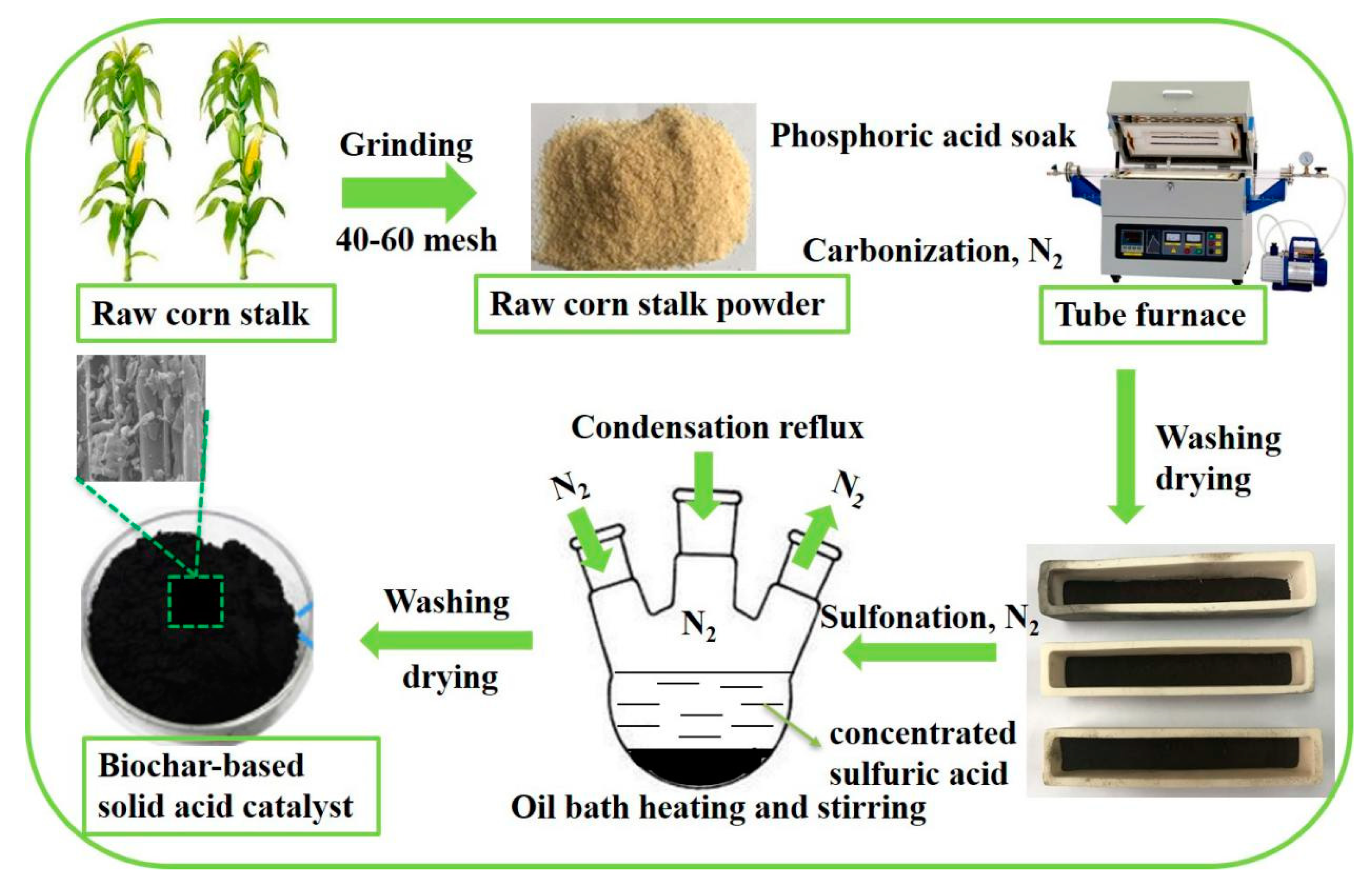
2.2. Synthesis of Biochar-Based Solid Acid Catalysts
2.3. Characterization of Biochar-Based Solid Acid Catalysts
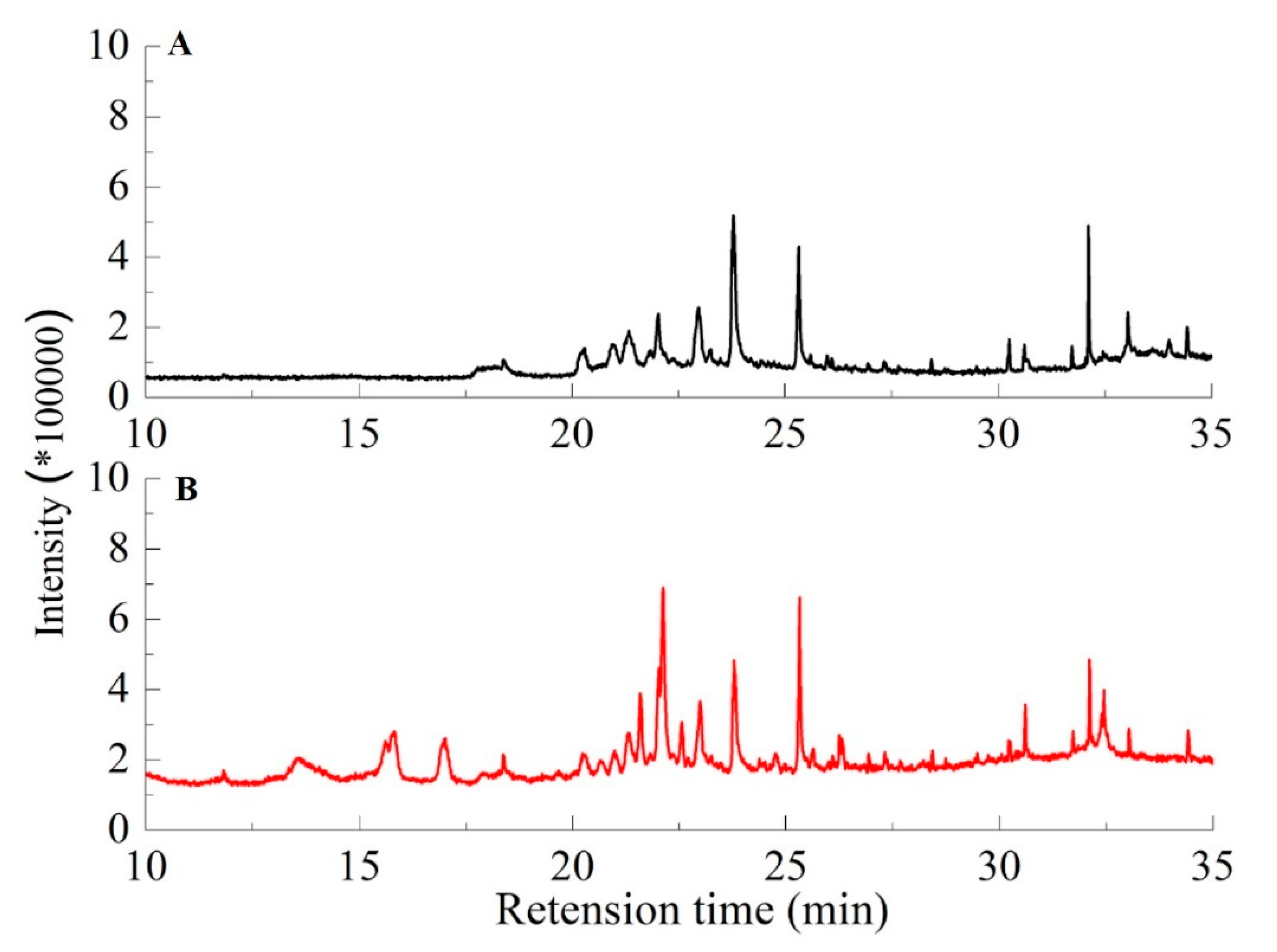
2.4. Degradation of Lignin Using Biochar-Based Solid Acid Catalysts
3. Results and Discussion
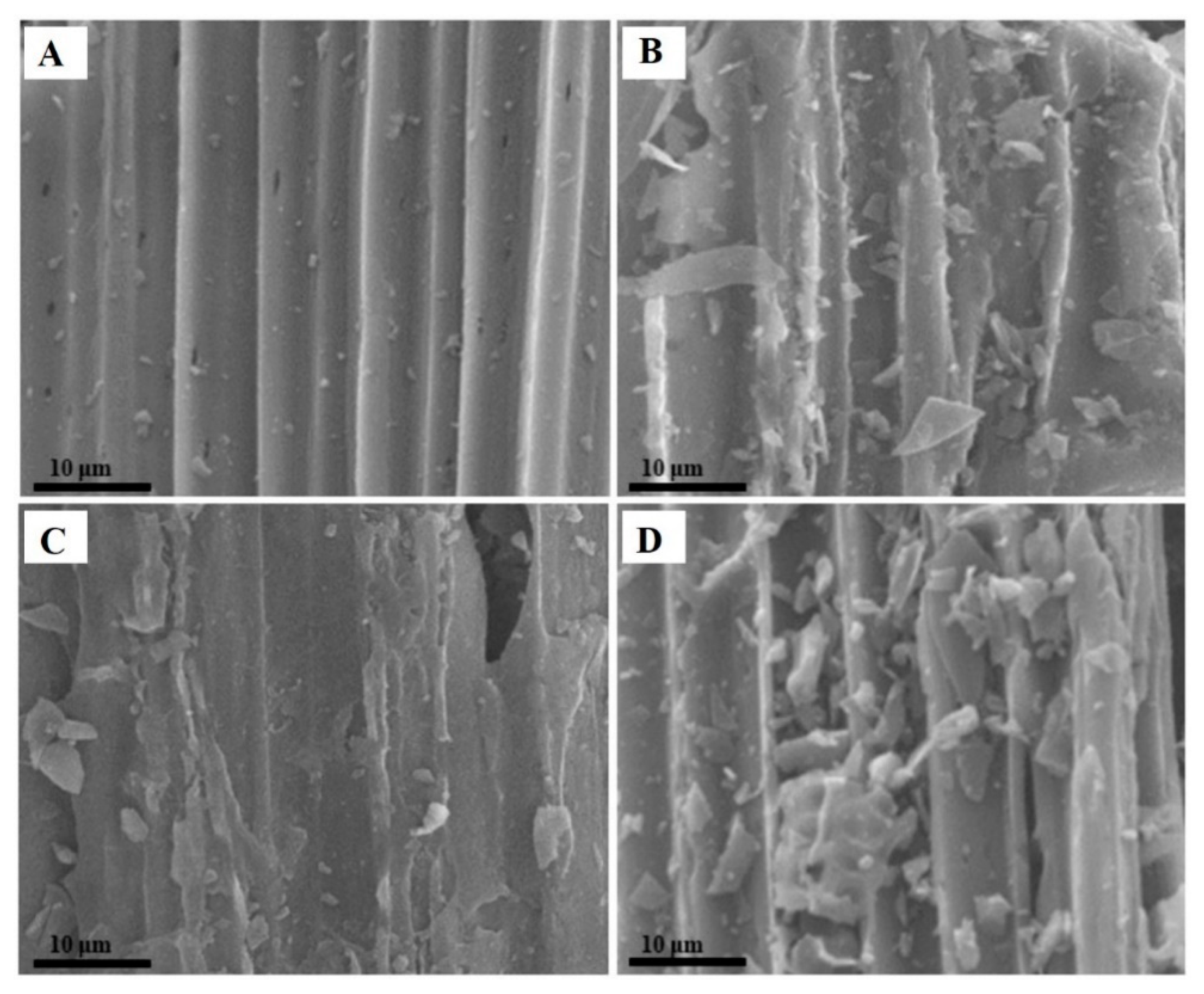
3.1. Surface Morphology Analysis
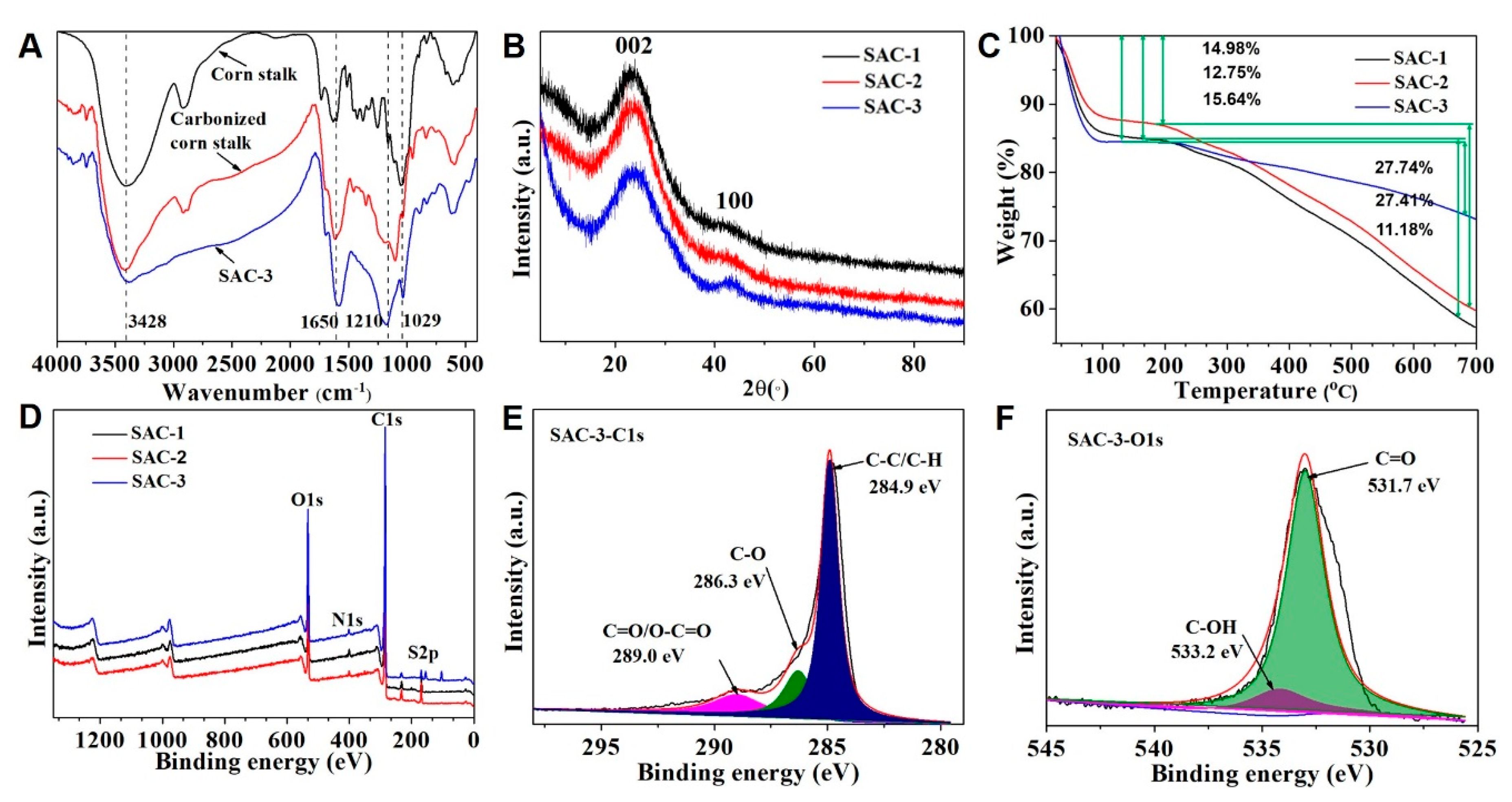
3.2. Acid Amount and Specific Surface Area Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, H.; Lei, Z.; Liu, C.; Zhang, Z.; Lu, B. Photocatalytic degradation of lignin on synthesized Ag–AgCl/ZnO nanorods under solar light and preliminary trials for methane fermentation. Bioresour. Technol. 2015, 175, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Yang, J. Synthesis of a Novel Carbon Based Strong Acid Catalyst through Hydrothermal Carbonization. Catal. Letters 2009, 132, 460–463. [Google Scholar] [CrossRef]
- Barbier, J.; Charon, N.; Dupassieux, N.; Loppinet-Serani, A.; Mahé, L.; Ponthus, J.; Courtiade, M.; Ducrozet, A.; Quoineaud, A.A.; Cansell, F. Hydrothermal conversion of lignin compounds. A detailed study of fragmentation and condensation reaction pathways. Biomass Bioenergy 2012, 46, 479–491. [Google Scholar] [CrossRef]
- Pronyk, C.; Mazza, G.; Tamaki, Y. Production of Carbohydrates, Lignins, and Minor Components from Triticale Straw by Hydrothermal Treatment. J. Agric. Food Chem. 2011, 59, 3788–3796. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Marshall, M.N.; Geib, S.M.; Ming, T.; Richard, T.L. Effects of laccase on lignin depolymerization and enzymatic hydrolysis of ensiled corn stover. Bioresour. Technol. 2012, 117, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.L.; Yuan, H.Y.; Yin, X.L.; Wu, C.Z.; Wu, S.B.; Zhou, Z.Q. Effects of chemical form of sodium on the product characteristics of alkali lignin pyrolysis. Bioresour. Technol. 2014, 152, 147–153. [Google Scholar] [CrossRef]
- Baktash, M.M.; Ahsan, L.; Ni, Y. Production of furfural from an industrial pre-hydrolysis liquor. Sep. Purif. Technol. 2015, 149, 407–412. [Google Scholar] [CrossRef]
- Jothiramalingam, R.; Ming, K.W. Review of Recent Developments in Solid Acid, Base, and Enzyme Catalysts (Heterogeneous) for Biodiesel Production via Transesterification. Ind. Eng. Chem. Res. 2009, 40, 6162–6172. [Google Scholar] [CrossRef]
- Shu, Q.; Nawaz, Z.; Gao, J.; Liao, Y.; Qiang, Z.; Wang, D.; Wang, J. Synthesis of biodiesel from a model waste oil feedstock using a carbon-based solid acid catalyst: Reaction and separation. Bioresour. Technol. 2010, 101, 5374–5384. [Google Scholar] [CrossRef]
- Yan, L.; Liu, N.; Yu, W.; Machida, H.; Qi, X. Production of 5-hydroxymethylfurfural from corn stalk catalyzed by corn stalk-derived carbonaceous solid acid catalyst. Bioresour. Technol. 2014, 173, 462–466. [Google Scholar] [CrossRef]
- Ma, F.; Ma, H.L.; Zhang, Z.Q.; Gao, J.M.; Du, H.T. Solid-acid catalyst made from corn stalk and study on catalytic synthesis of biodiesel technology. J. Northwest F Univ. 2010, 38, 69–74. [Google Scholar]
- Liang, X.; Zeng, M.; Qi, C. One-step synthesis of carbon functionalized with sulfonic acid groups using hydrothermal carbonization. Carbon 2010, 48, 1844–1848. [Google Scholar] [CrossRef]
- Bai, Y.Y.; Xiao, L.P.; Sun, R.C. Efficient hydrolyzation of cellulose in ionic liquid by novel sulfonated biomass-based catalysts. Cellulose 2014, 21, 2327–2336. [Google Scholar] [CrossRef]
- Li, X.Q.; Lei, T.Z.; Wang, Z.W.; Li, X.Y.; Wen, M.Y.; Yang, M.; Chen, G.F.; He, X.F.; Xu, H.Y.; Guan, Q.; et al. Catalytic pyrolysis of corn straw with magnetic solid acid catalyst to prepare levulinic acid by response surface methodology. Ind. Crops Prod. 2018, 116, 73–80. [Google Scholar] [CrossRef]
- Zhou, M.; Sharma, B.K.; Li, J.; Zhao, J.; Xu, J.; Jiang, J. Catalytic valorization of lignin to liquid fuels over solid acid catalyst assisted by microwave heating. Fuel 2019, 239, 239–244. [Google Scholar] [CrossRef]
- Castro, J.B.; Bonelli, P.R.; Cerrella, E.G.; Cukierman, A.L. Phosphoric Acid Activation of Agricultural Residues and Bagasse from Sugar Cane: Influence of the Experimental Conditions on Adsorption Characteristics of Activated Carbons. Ind. Eng. Chem. Res. 2016, 39, 4166–4172. [Google Scholar] [CrossRef]
- Li, X.F.; Li, P.Y.; Ding, D.J.; Chen, K.; Zhang, L.; Xie, Y. One-step preparation of kraft lignin derived mesoporous carbon as solid acid catalyst for fructose conversion to 5-hydroxymethylfurfural. Bioresources 2018, 13, 4428–4439. [Google Scholar] [CrossRef]
- Wisniewska, M.; Nosal-wiercinska, A.; Dabrowska, I.; Szewczuk-karpisz, K. Effect of the solid pore size on the structure of polymer film at the metal oxide/polyacrylic acid solution interface: Temperature impact. Micropor. Mesopor. Mat. 2013, 175, 92–98. [Google Scholar] [CrossRef]
- Shen, D.; Zhao, J.; Xiao, R. Catalytic transformation of lignin to aromatic hydrocarbons over solid-acid catalyst: Effect of lignin sources and catalyst species. Energ Convers Manage 2016, 124, 61–72. [Google Scholar] [CrossRef]
- Li, H.L.; Deng, A.J.; Ren, J.L.; Liu, C.Y.; Lu, Q.; Zhong, L.J.; Peng, F.; Sun, R.C. Catalytic hydrothermal pretreatment of corncob into xylose and furfural via solid acid catalyst. Bioresour. Technol. 2014, 158, 313–320. [Google Scholar] [CrossRef]
- Hasegawa, I.; Inoue, Y.; Muranaka, Y.; Yasukawa, T.; Mae, K. Selective Production of Organic Acids and Depolymerization of Lignin by Hydrothermal Oxidation with Diluted Hydrogen Peroxide. Energ. Fuel. 2011, 25, 791–796. [Google Scholar] [CrossRef]
- Ma, H.; Li, J.B.; Liu, W.W.; Cheng, B.J.; Cao, X.Y.; Mao, J.D.; Zhu, S.W. Hydrothermal preparation and characterization of novel corncob-derived solid acid catalysts. J. Agric. Food Chem. 2014, 62, 5345–5353. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhuang, X.; Zhao, Q.F.; Wan, Y.Y. Self-assembly synthesis of a high-content sulfonic acid group functionalized ordered mesoporous polymer-based solid as a stable and highly active acid catalyst. J. Mater. Chem. 2012, 22, 15874–15886. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Q.; Wang, B.B.; Yang, G.H.; Lucia, L.A.; Chen, J.C. Hydrothermal Carbonization of Corncob Residues for Hydrochar Production. Energy Fuels 2015, 29, 872–876. [Google Scholar] [CrossRef]
- Xing, R.; Liu, N.; Liu, Y.; Wu, H.; Jiang, Y.; Chen, L.; He, M.; Wu, P. Novel Solid Acid Catalysts: Sulfonic Acid Group-Functionalized Mesostructured Polymers. Adv. Funct. Mater. 2010, 17, 2455–2461. [Google Scholar] [CrossRef]
- Kamwilaisak, K.; Wright, P.C. Investigating Laccase and Titanium Dioxide for Lignin Degradation. Energy Fuels 2012, 26, 2400–2406. [Google Scholar] [CrossRef]
- Zhang, X.H.; Wang, T.J.; Ma, L.L.; Zhang, Q.; Huang, X.M.; Yu, Y.X. Production of cyclohexane from lignin degradation compounds over Ni/ZrO2-SiO2 catalysts. Appl. Energy 2013, 112, 533–538. [Google Scholar] [CrossRef]
| Samples | T1 | t1 | T2 | t2 | Sulfuric Acid Concentration (g/mL) |
|---|---|---|---|---|---|
| SAC-1 | 500 | 5 | 150 | 8 | 1.84 |
| SAC-2 | 500 | 6 | 150 | 8 | 1.84 |
| SAC-3 | 800 | 6 | 150 | 8 | 1.84 |
| Samples | BET Specific Surface Area (m2/g) | Acid Amount a (mmol/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|---|
| Raw corn stalk | 13.9 ± 2 | - | 0.1 | 10 |
| SAC-1 | 1120 ± 5 | 1.2 ± 0.1 | 0.685 | 38 |
| SAC-2 | 1268 ± 5 | 1.8 ± 0.1 | 0.832 | 46 |
| SAC-3 | 1640 ± 5 | 2.4 ± 0.1 | 1.019 | 55 |
| Samples | C (wt.%) | H (wt.%) | O (wt.%) | N (wt.%) | S (wt.%) | –SO3H a (mmol/g) |
|---|---|---|---|---|---|---|
| Raw corn stalk | 72.27 | 3.41 | 23.59 | 0.22 | 0.16 | - |
| SAC-1 | 70.92 | 2.45 | 24.82 | 0.24 | 0.96 | 0.25 ± 0.05 |
| SAC-2 | 66.18 | 2.10 | 28.26 | 0.22 | 1.24 | 0.34 ± 0.05 |
| SAC-3 | 64.73 | 1.86 | 31.52 | 0.23 | 1.64 | 0.46 ± 0.05 |
| Products with/without Solid Acid Catalyst | Aromatic Compound (wt. %) | Furfural (wt. %) | Ester (wt. %) | Acetate (wt. %) | Benzene (wt. %) | Toluene (wt. %) | Heterocyclic Compounds (wt. %) | Long-Chain Alkanes (wt. %) |
|---|---|---|---|---|---|---|---|---|
| with | 65.6 ± 0.5 | 6.2 ± 0.5 | 2.4 ± 0.2 | 1.6 ± 0.2 | 2.0 ± 0.2 | 1.8 ± 0.2 | 14.2 ± 0.5 | 6.2 ± 0.2 |
| without | 40.5 ± 0.5 | 34.7 ± 0.5 | 0.6 ± 0.2 | 1.4 ± 0.2 | 0.4 ± 0.2 | 0.5 ± 0.2 | 11.7 ± 0.5 | 10.2 ± 0.2 |
| Number of Recycling | BET Surface Area (m2/g) | Sulfonate Group (mmol/g) | Yield of Reaction (%) |
|---|---|---|---|
| 0 | 1640 ± 5 | 2.4 ± 0.1 | -- |
| 1 | 1605 ± 10 | 2.0 ± 0.1 | 96 ± 1 |
| 2 | 1565 ± 12 | 1.7 ± 0.1 | 92 ± 1 |
| 3 | 1514 ± 15 | 1.2 ± 0.1 | 89 ± 1 |
| 4 | 1410 ± 15 | 0.4 ± 0.1 | 85 ± 1 |
| Number of Recycling | SAC-1 | SAC-2 | ||
|---|---|---|---|---|
| Sulfonate Group (mmol/g) | Yield of Reaction (%) | Sulfonate Group (mmol/g) | Yield of Reaction (%) | |
| 0 | 1.2 ± 0.1 | - | 1.8 ± 0.1 | - |
| 1 | 1.0 ± 0.1 | 95 ± 1 | 1.5 ± 0.1 | 95 ± 1 |
| 2 | 0.7 ± 0.1 | 90 ± 1 | 1.1 ± 0.1 | 91 ± 1 |
| 3 | 0.4 ± 0.1 | 86 ± 1 | 0.6 ± 0.1 | 87 ± 1 |
| 4 | 0.3 ± 0.1 | 82 ± 1 | 0.4 ± 0.1 | 84 ± 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Q.; Yang, G.; Kong, F.; Fatehi, P.; Wang, X. High Acid Biochar-Based Solid Acid Catalyst from Corn Stalk for Lignin Hydrothermal Degradation. Polymers 2020, 12, 1623. https://doi.org/10.3390/polym12071623
Jiang Q, Yang G, Kong F, Fatehi P, Wang X. High Acid Biochar-Based Solid Acid Catalyst from Corn Stalk for Lignin Hydrothermal Degradation. Polymers. 2020; 12(7):1623. https://doi.org/10.3390/polym12071623
Chicago/Turabian StyleJiang, Qimeng, Guihua Yang, Fangong Kong, Pedram Fatehi, and Xiaoying Wang. 2020. "High Acid Biochar-Based Solid Acid Catalyst from Corn Stalk for Lignin Hydrothermal Degradation" Polymers 12, no. 7: 1623. https://doi.org/10.3390/polym12071623
APA StyleJiang, Q., Yang, G., Kong, F., Fatehi, P., & Wang, X. (2020). High Acid Biochar-Based Solid Acid Catalyst from Corn Stalk for Lignin Hydrothermal Degradation. Polymers, 12(7), 1623. https://doi.org/10.3390/polym12071623







