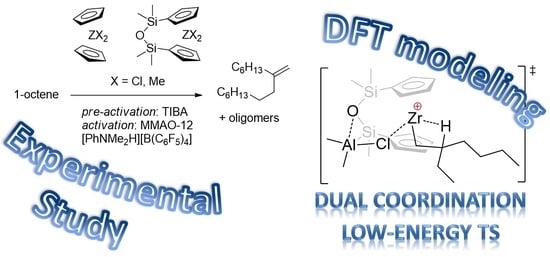
Experimental and Theoretical Study of Zirconocene-Catalyzed Oligomerization of 1-Octene
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimentsl Remarks
2.2. Synthesis of Zirconocene 2′
2.3. Oligomerization Experiments
2.4. DFT Calculations
3. Results and Discussion
3.1. Oligomerization Experiments and End-Group Analysis
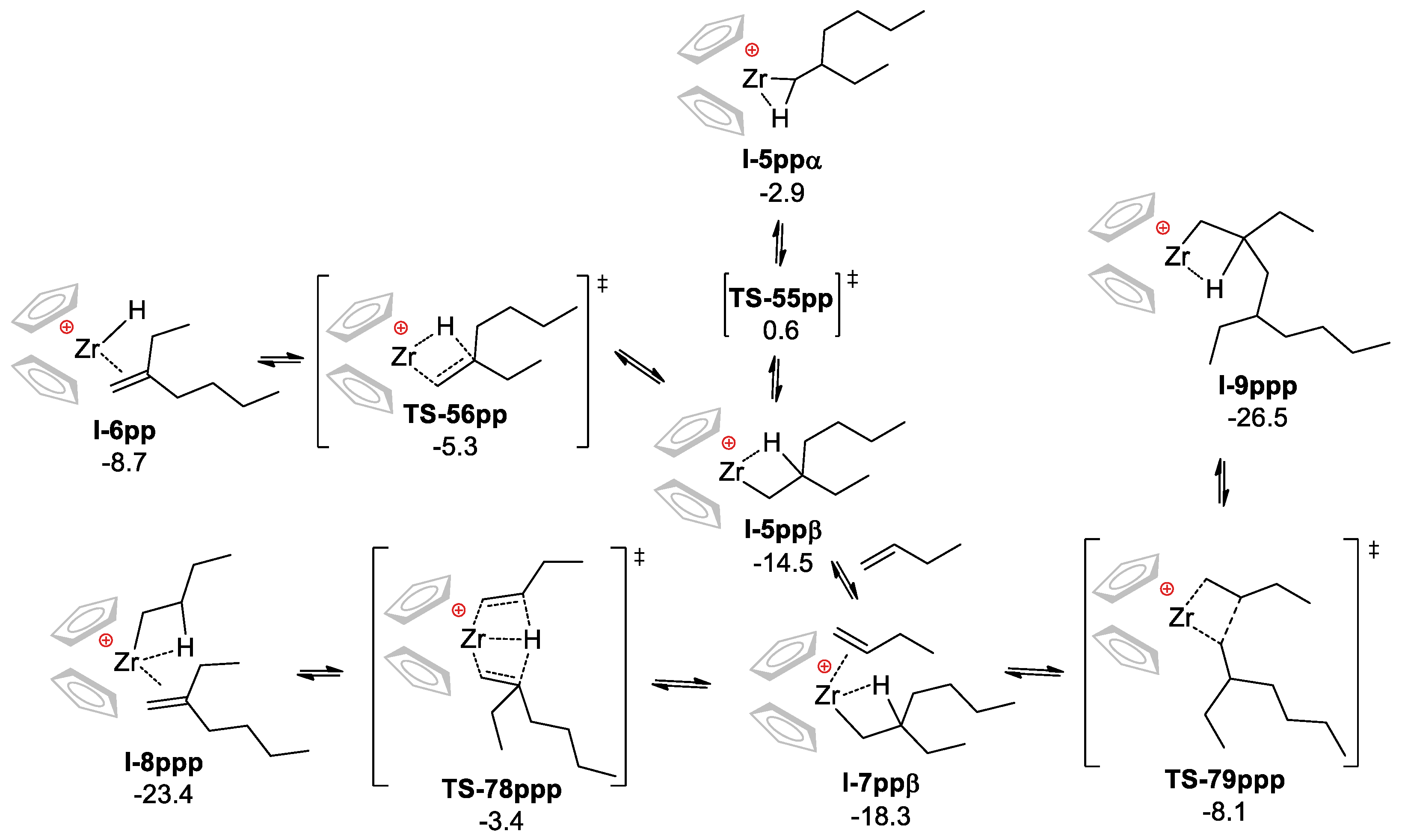
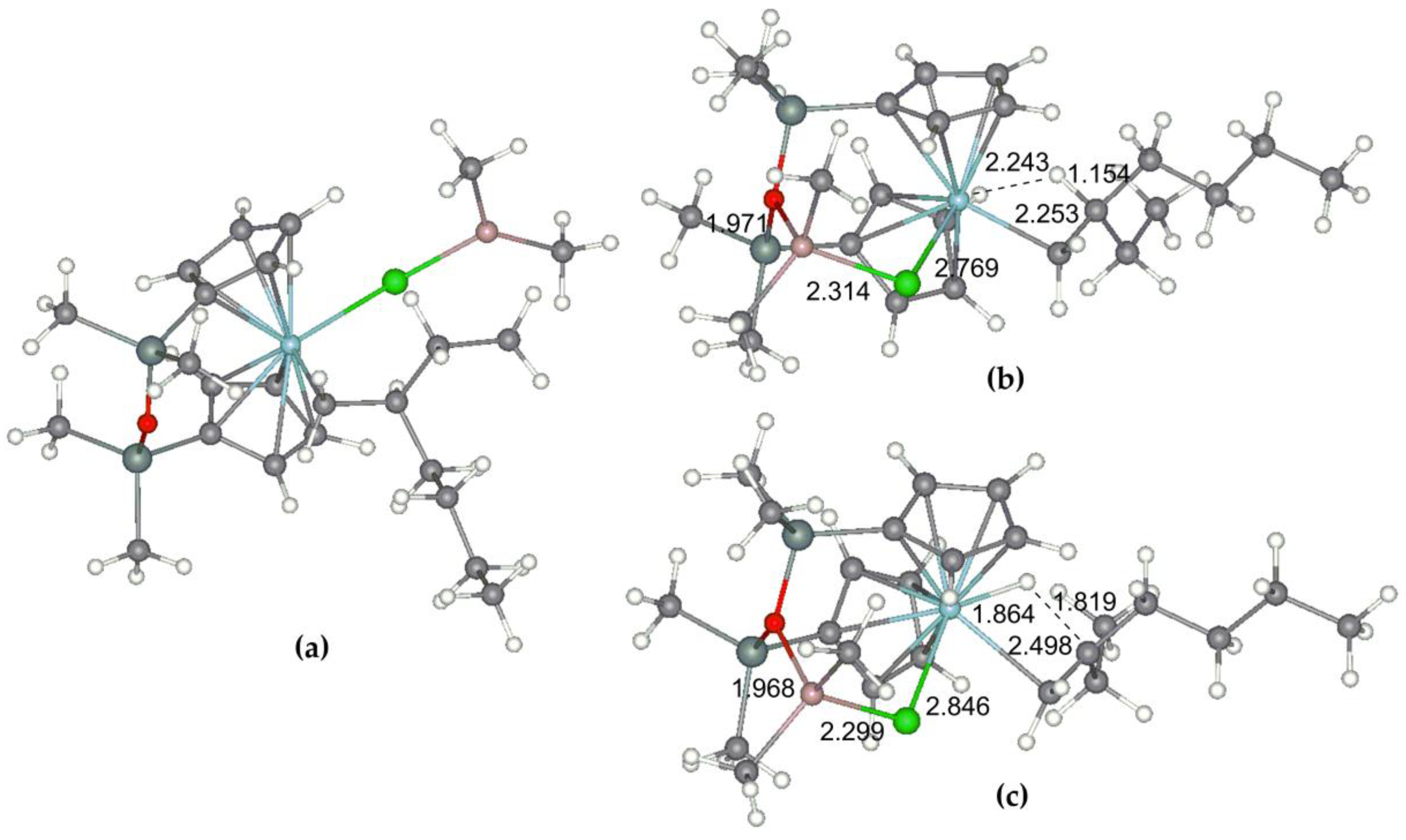
3.2. DFT Modeling of the Reaction Pathways for (η5-C5H5)2Zr-Based Catalytic Species
3.2.1. Mononuclear Reaction Mechanism
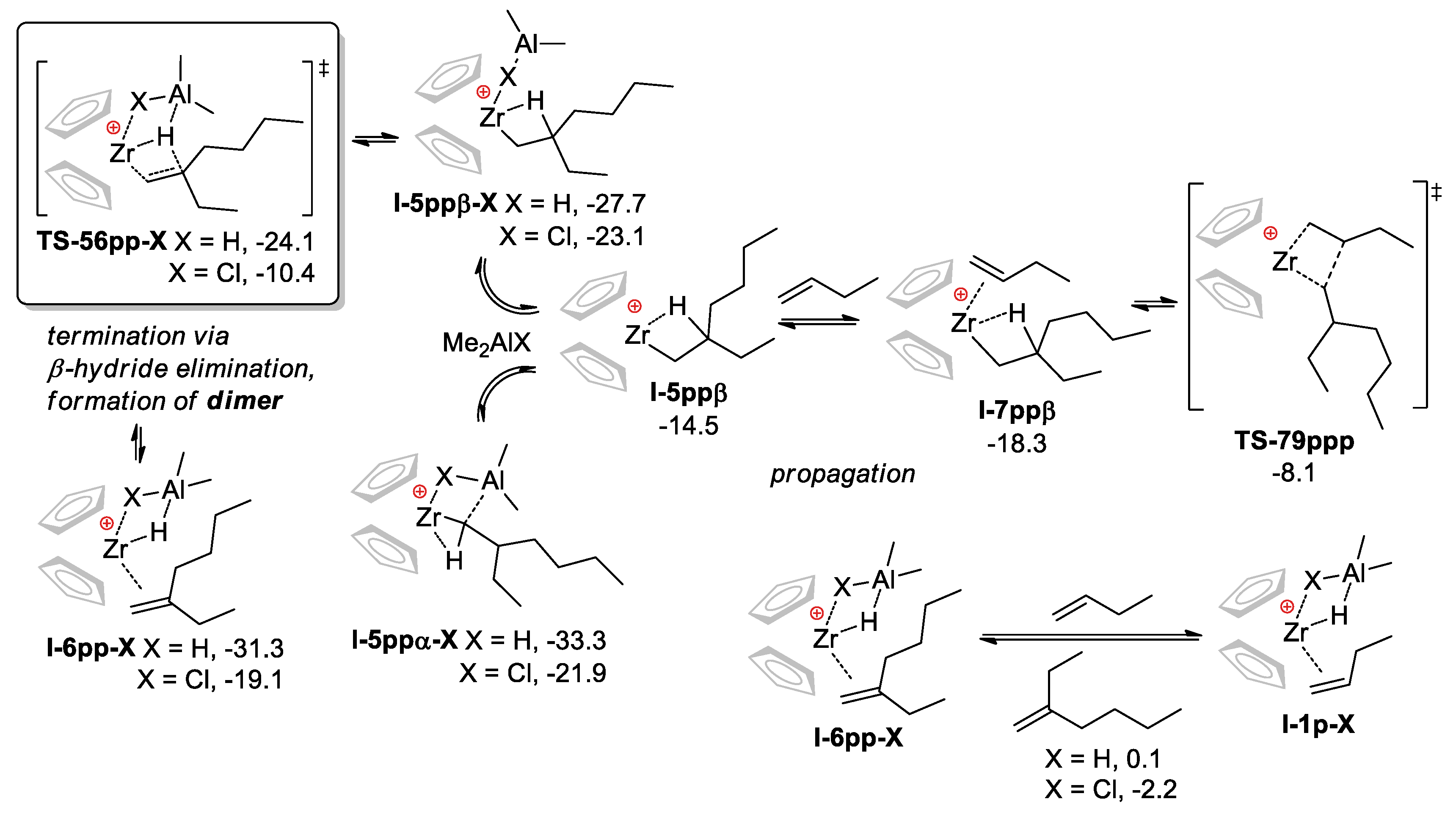
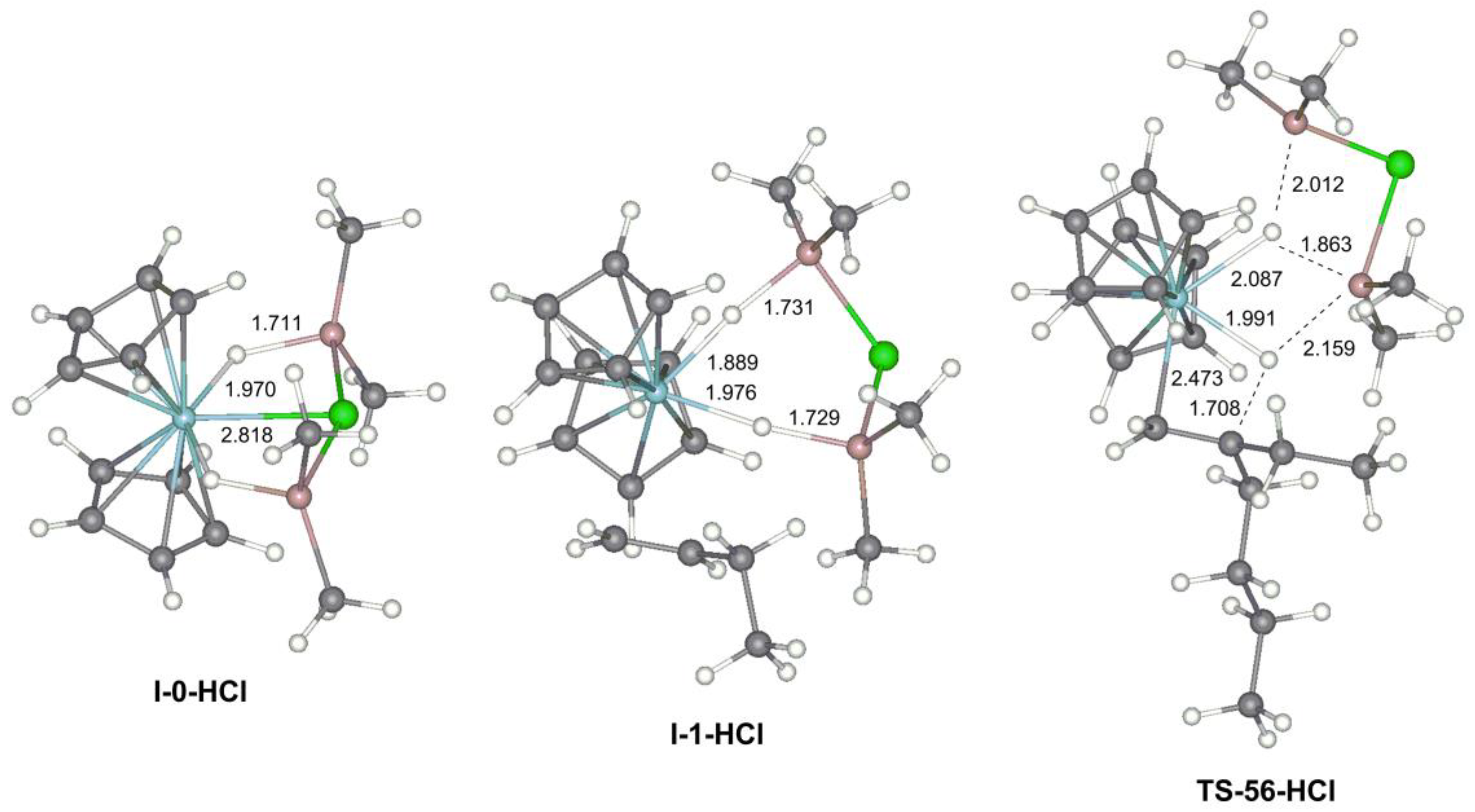
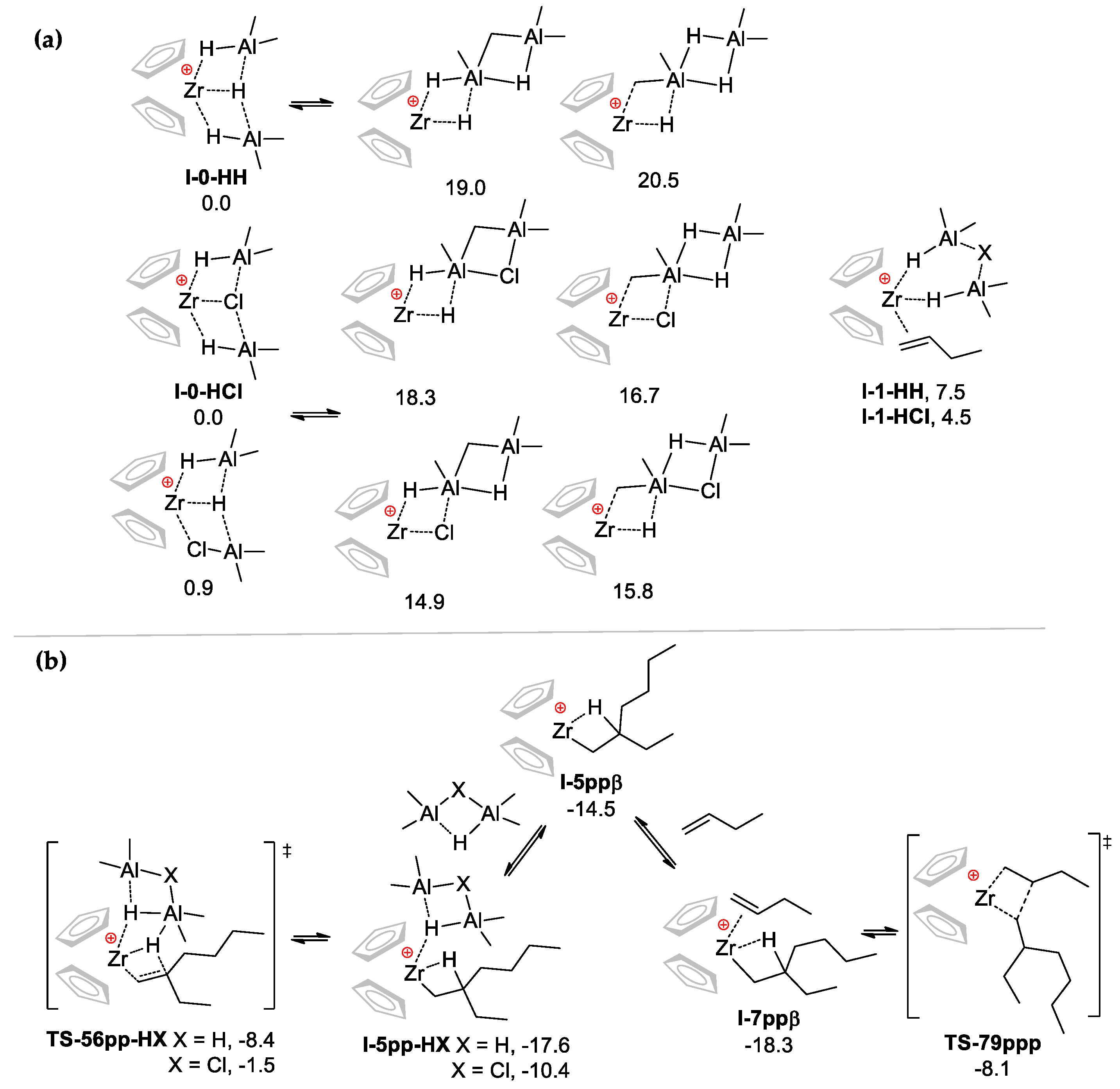
3.2.2. The Effect of the Formation of Zr-Al1 Species on the Reaction Pathway
3.2.3. Theoretical Analysis of the Possible Participation of Zr-Al2 Species
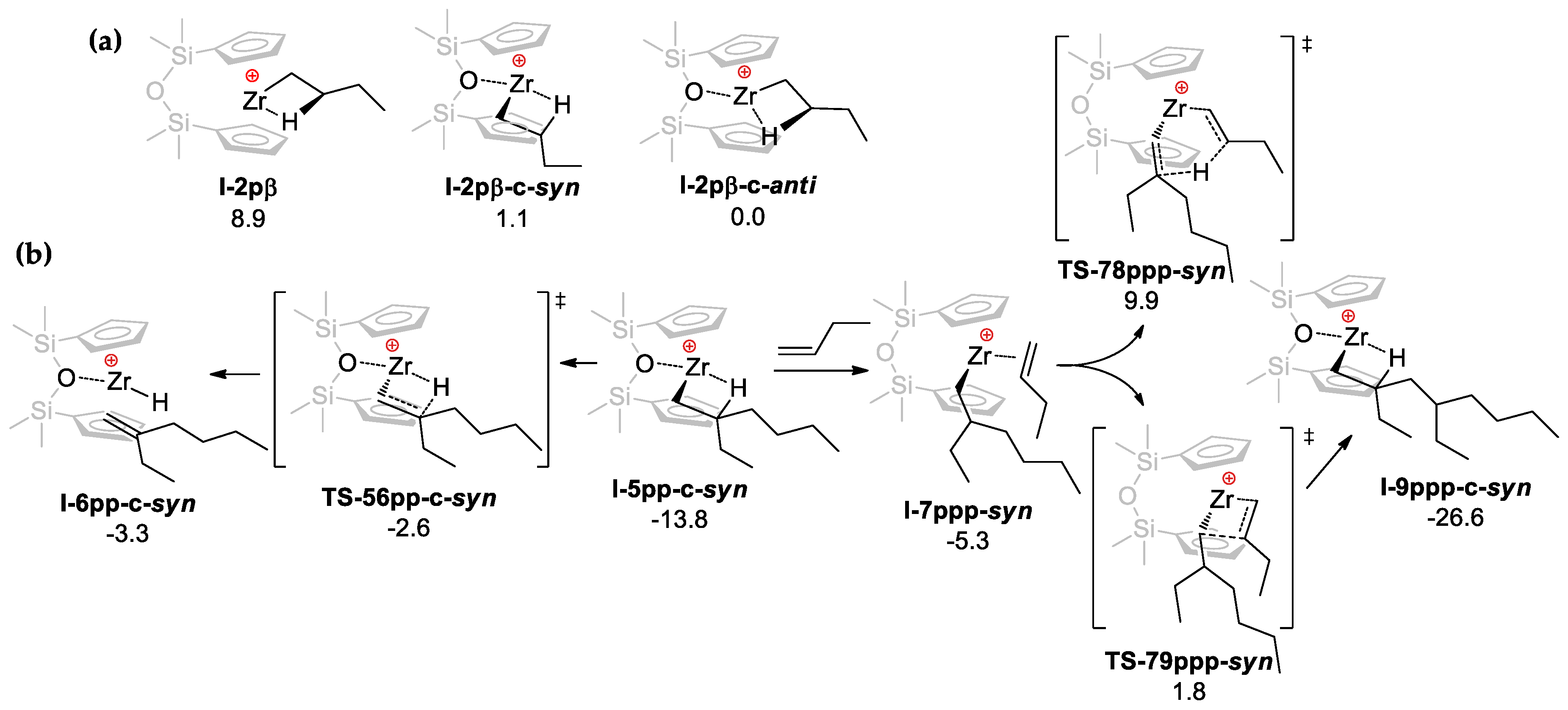
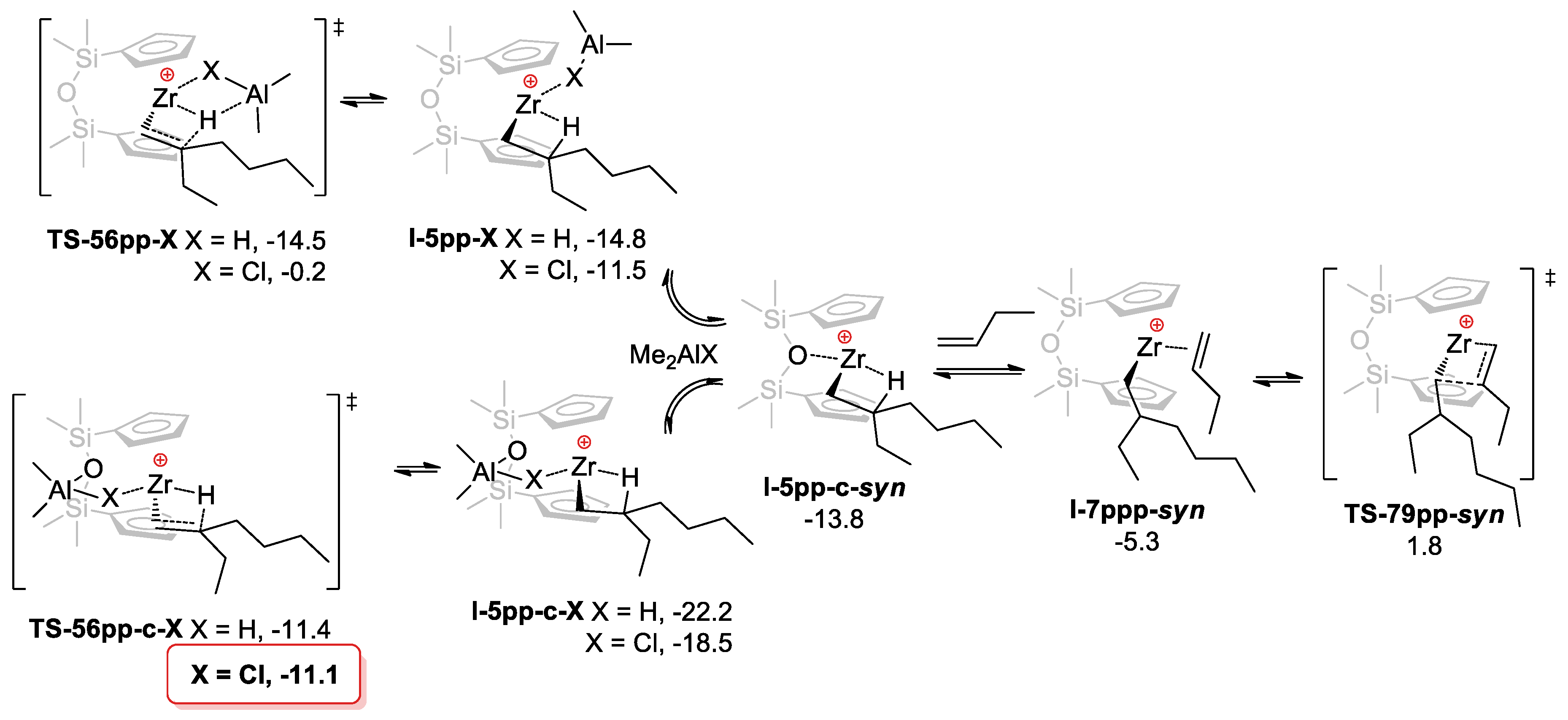
3.3. DFT Modeling of the Reaction Pathways for O[SiMe2(η5-C5H4)]2Zr-Based Catalytic Species
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
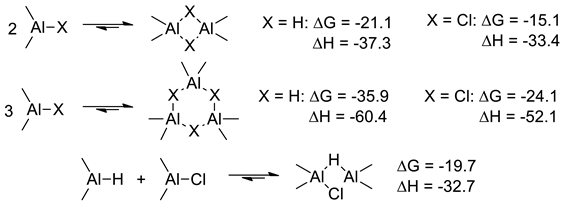
References
- Mohring, P.C.; Coville, N.J. Group 4 metallocene polymerisation catalysts: Quantification of ring substituent steric effects. Coord. Chem. Rev. 2006, 250, 18–35. [Google Scholar] [CrossRef]
- Chirik, P.J. Group 4 transition metal sandwich complexes: Still fresh after almost 60 years. Organometallics 2010, 29, 1500–1517. [Google Scholar] [CrossRef]
- Brintzinger, H.H.; Fischer, D. Development of ansa-metallocene catalysts for isotactic olefin polymerization. In Polyolefins: 50 Years after Ziegler and Natta; Kaminsky, W., Ed.; Book Series Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2013; Volume 258, pp. 29–42. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F. Single site metallorganic polymerization catalysis as a method to probe the properties of polyolefins. Polym. Chem. 2011, 2, 2155–2168. [Google Scholar] [CrossRef]
- Collins, R.A.; Russell, A.F.; Mountford, P. Group 4 metal complexes for homogeneous olefin polymerisation: A short tutorial review. Appl. Petrochem. Res. 2015, 5, 153–171. [Google Scholar] [CrossRef]
- Stürzel, M.; Mihan, S.; Mülhaupt, R. From multisite polymerization catalysis to sustainable materials and all-polyolefin composites. Chem. Rev. 2016, 116, 1398–1433. [Google Scholar] [CrossRef] [PubMed]
- Desert, X.; Carpentier, J.-F.; Kirillov, E. Quantification of active sites in single-site group 4 metal olefin polymerization catalysis. Coord. Chem. Rev. 2019, 386, 50–68. [Google Scholar] [CrossRef]
- Castro, L.; Kirillov, E.; Miserque, O.; Welle, A.; Haspeslagh, L.; Carpentier, J.-F.; Maron, L. Are solvent and dispersion effects crucial in olefin polymerization DFT calculations? Some insights from propylene coordination and insertion reactions with group 3 and 4 metallocenes. ACS Catal. 2015, 5, 416–425. [Google Scholar] [CrossRef]
- Desert, X.; Proutiere, F.; Welle, A.; Den Dauw, K.; Vantomme, A.; Miserque, O.; Brusson, J.-M.; Carpentier, J.-F.; Kirillov, E. Zirconocene-catalyzed polymerization of α-olefins: When intrinsic higher activity is flawed by rapid deactivation. Organometallics 2019, 38, 2664–2673. [Google Scholar] [CrossRef]
- Kissin, Y.V. Oligomerization reactions of 1-hexene with metallocene catalysts: Detailed data on reaction chemistry and kinetics. Mol. Catal. 2019, 463, 87–93. [Google Scholar] [CrossRef]
- Martínez-Araya, J.I. Explaining some anomalies in catalytic activity values in some zirconocene methyl cations: Local hyper-softness. J. Phys. Chem. C 2013, 117, 24773–24786. [Google Scholar] [CrossRef]
- Bravo, I.; Alonso-Moreno, C.; Carrillo-Hermosilla, F.; López-Solera, I.; Antiñolo, A.; Albaladejo, J. Toward the prediction of activity in the ethylene polymerisation of ansa-Bis(indenyl) zirconocenes: Effect of the stereochemistry and hydrogenation of the indenyl moiety. ChemPlusChem 2015, 80, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Falivene, L.; Cavallo, L.; Talarico, G. Buried volume analysis for propene polymerization catalysis promoted by group 4 metals: A tool for molecular mass prediction. ACS Catal. 2015, 5, 6815–6822. [Google Scholar] [CrossRef]
- Zaccaria, F.; Cipullo, R.; Budzelaar, P.H.M.; Busico, V.; Ehm, C. Backbone rearrangement during olefin capture as the rate limiting step in molecular olefin polymerization catalysis and its effect on comonomer affinity. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 2807–2814. [Google Scholar] [CrossRef]
- Zaccaria, F.; Ehm, C.; Budzelaar, P.H.M.; Busico, V. Accurate prediction of copolymerization statistics in molecular olefin polymerization catalysis: The role of entropic, electronic, and steric effects in catalyst comonomer affinity. ACS Catal. 2017, 7, 1512–1519. [Google Scholar] [CrossRef]
- Parveen, R.; Cundari, T.R.; Younker, J.M.; Rodriguez, G.; McCullough, L. DFT and QSAR studies of ethylene polymerization by zirconocene catalysts. ACS Catal. 2019, 9, 9339–9349. [Google Scholar] [CrossRef]
- Cossee, P. Ziegler-Natta catalysis I. Mechanism of polymerization of α-olefins with Ziegler-Natta catalysts. J. Catal. 1964, 3, 80–88. [Google Scholar] [CrossRef]
- Arlman, E.J. Ziegler-Natta catalysis II. Surface structure of layer-lattice transition metal chlorides. J. Catal. 1964, 3, 89–98. [Google Scholar] [CrossRef]
- Arlman, E.J.; Cossee, P. Ziegler-Natta catalysis III. Stereospecific polymerization of propene with the catalyst system TiCl3-AlEt3. J. Catal. 1964, 3, 99–104. [Google Scholar] [CrossRef]
- Borrelli, M.; Busico, V.; Cipullo, R.; Ronca, S.; Budzelaar, P.H.M. Selectivity of metallocene-catalyzed olefin polymerization: A combined experimental and quantum mechanical study. 1. Nonchiral bis(cyclopentadienyl) systems. Macromolecules 2002, 35, 2835–2844. [Google Scholar] [CrossRef]
- Moscardi, G.; Resconi, L.; Cavallo, L. Propene polymerization with the isospecific, highly regioselective rac-Me2C(3-t-Bu-1-Ind)2ZrCl2/MAO catalyst. 2. Combined DFT/MM analysis of chain propagation and chain release reactions. Organometallics 2001, 20, 1918–1931. [Google Scholar] [CrossRef]
- Silanes, I.; Ugalde, J.M. Comparative study of various mechanisms for metallocene-catalyzed α-olefin polymerization. Organometallics 2005, 24, 3233–3246. [Google Scholar] [CrossRef]
- Chan, M.S.W.; Vanka, K.; Pye, C.C.; Ziegler, T. Density functional study on activation and ion-pair formation in group IV metallocene and related olefin polymerization catalysts. Organometallics 1999, 18, 4624–4636. [Google Scholar] [CrossRef]
- Woo, T.K.; Fan, L.; Ziegler, T. A density functional study of chain growing and chain terminating steps in olefin polymerization by metallocene and constrained geometry catalysts. Organometallics 1994, 13, 2252–2261. [Google Scholar] [CrossRef]
- Silanes, I.; Mercero, J.M.; Ugalde, J.M. Comparison of Ti, Zr, and Hf as cations for metallocene-catalyzed olefin polymerization. Organometallics 2006, 25, 4483–4490. [Google Scholar] [CrossRef]
- Laine, A.; Linnolahti, M.; Pakkanen, T.A.; Severn, J.R.; Kokko, E.; Pakkanen, A. Comparative theoretical study on homopolymerization of α-olefins by bis(cyclopentadienyl) zirconocene and hafnocene: Elemental propagation and termination reactions between monomers and metals. Organometallics 2010, 29, 1541–1550. [Google Scholar] [CrossRef]
- Slaugh, L.H.; Schoenthal, G.W. Vinylidene Olefin Process. U.S. Patent 4658078, 14 April 1987. [Google Scholar]
- Christoffers, J.; Bergman, R.G. Catalytic dimerization reactions of α-olefins and α,ω-dienes with Cp2ZrCl2/poly(methylalumoxane): Formation of dimers, carbocycles, and oligomers. J. Am. Chem. Soc. 1996, 118, 4715–4716. [Google Scholar] [CrossRef]
- Christoffers, J.; Bergman, R.G. Zirconocene-alumoxane (1:1)—A catalyst for the selective dimerization of α-olefins. Inorg. Chim. Acta 1998, 270, 20–27. [Google Scholar] [CrossRef]
- Takeuchi, K.; Fujikawa, S. Base Oil for Oil Drilling Fluid and Oil Drilling Fluid Composition. U.S. Patent 2011251445, 13 October 2011. [Google Scholar]
- Kissin, Y.V.; Schwab, F.C. Post-oligomerization of alpha-olefin oligomers: A route to single-component and multicomponent synthetic lubricating oils. J. Appl. Polym. Sci. 2009, 111, 273–280. [Google Scholar] [CrossRef]
- Heilman, W.J.; Jois, Y.H.; De Kraker, A.R.; Song, W. Hydrocarbon Compositions Useful as Lubricants. U.S. Patent 2011178348, 1 May 2011. [Google Scholar]
- Fujikawa, S.; Yokota, K.; Okano, M.; Tsuji, M. Method for producing α-olefin oligomers and lubricating oil compositions. U.S. Patent Application 2011207977, 25 August 2011. [Google Scholar]
- Janiak, C.; Blank, F. Metallocene catalysts for olefin oligomerization. Macromol. Symp. 2006, 236, 14–22. [Google Scholar] [CrossRef]
- Janiak, C. Metallocene and related catalysts for olefin, alkyne and silane dimerization and oligomerization. Coord. Chem. Rev. 2006, 250, 66–94. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Ivchenko, P.V. Zirconocene-catalyzed dimerization of 1-hexene: Two-stage activation and structure–catalytic performance relationship. Catal. Commun. 2016, 79, 6–10. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Bezzubov, S.I.; Ivchenko, P.V. Catalytic oligomerization of α-olefins in the presence of two-stage activated zirconocene catalyst based on 6,6-dimethylfulvene ‘dimer’. Mendeleev Commun. 2017, 27, 35–37. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Sedov, I.V.; Dorokhov, V.G.; Lyadov, A.S.; Ivchenko, P.V. Structurally uniform 1-hexene, 1-octene, and 1-decene oligomers: Zirconocene/MAO-catalyzed preparation, characterization, and prospects of their use as low-viscosity low-temperature oil base stocks. Appl. Catal. A Gen. 2018, 549, 40–50. [Google Scholar] [CrossRef]
- Fujikawa, S.; Okamoto, T.; Yokota, K. Process for Producing Unsaturated Hydrocarbon Compound. U.S. Patent 8119850, 21 February 2012. [Google Scholar]
- Nifant’ev, I.; Ivchenko, P.; Tavtorkin, A.; Vinogradov, A.; Vinogradov, A. Non-traditional Ziegler-Natta catalysis in α-olefin transformations: Reaction mechanisms and product design. Pure Appl. Chem. 2017, 89, 1017–1032. [Google Scholar] [CrossRef]
- Kissin, Y.V. Detailed kinetics of 1-hexene oligomerization reaction with (n-Bu-Cp)2ZrCl2–MAO catalyst. Macromol. Chem. Phys. 2009, 210, 1241–1246. [Google Scholar] [CrossRef]
- Graeper, J.; Paolucci, G.; Fischer, R.D. Zirconocenophane dichlorides with di- and trisiloxane-bridged ring ligands: Crystal structure of rac-[1,1,3,3-tetramethyldisiloxane-diyl-bis(3-tert-butyl-η5-cyclopentadienyl)zirconium(IV) dichloride. J. Organomet. Chem. 1995, 501, 211–218. [Google Scholar] [CrossRef]
- Samuel, E.; Rausch, M.D. π-Cyclopentadienyl and π-indenyl compounds of titanium, zirconium, and hafnium containing σ-bonded organic substituents. J. Am. Chem. Soc. 1973, 95, 6263–6267. [Google Scholar] [CrossRef]
- Laikov, D.N.; Ustynyuk, Y.A. PRIRODA-04: A quantum-chemical program suite. New possibilities in the study of molecular systems with the application of parallel computing. Russ. Chem. Bull. 2005, 54, 820–826. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision, A.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other fun. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Godbout, N.; Salahub, D.R.; Andzelm, J.; Wimmer, E. Optimization of Gaussian-type basis sets for local spin density functional calculations. Part, I. Boron through neon, optimization technique and validation. Can. J. Chem. 1992, 70, 560–571. [Google Scholar] [CrossRef]
- Sosa, C.; Andzelm, J.; Elkin, B.C.; Wimmer, E.; Dobbs, K.D.; Dixon, D.A. A local density functional study of the structure and vibrational frequencies of molecular transition-metal compounds. J. Phys. Chem. 1992, 96, 6630–6636. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, H. Performance of density functionals for activation energies of Zr-mediated reactions. J. Chem. Theory Comput. 2013, 9, 4735–4743. [Google Scholar] [CrossRef] [PubMed]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Churakov, A.V.; Bagrov, V.V.; Kashulin, I.A.; Roznyatovsky, V.A.; Grishin, Y.K.; Ivchenko, P.V. The catalytic behavior of heterocenes activated by TIBA and MMAO under a low Al/Zr ratios in 1-octene polymerization. Appl. Catal. A Gen. 2019, 571, 12–24. [Google Scholar] [CrossRef]
- Kawahara, N.; Saito, J.; Matsuo, S.; Kaneko, H.; Matsugi, T.; Toda, Y.; Kashiwa, N. Study on unsaturated structures of polyhexene, poly(4-methylpentene) and poly(3-methylpentene) prepared with metallocene catalysts. Polymer 2007, 48, 425–428. [Google Scholar] [CrossRef]
- Kim, I.; Zhou, J.-M.; Chung, H. Higher α-olefin polymerizations catalyzed by rac-Me2Si(1-C5H2-2-CH3-4-tBu)2Zr(NMe2)2/Al(iBu)3/[Ph3C][B(C6F5)4]. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 1687–1697. [Google Scholar] [CrossRef]
- Babu, G.N.; Newmark, R.A.; Chien, J.C.W. Microstructure of poly(1-hexene) produced by ansa-zirconocenium catalysis. Macromolecules 1994, 27, 3383–3388. [Google Scholar] [CrossRef]
- Grumel, V.; Brüll, R.; Pasch, H.; Raubenheimer, H.G.; Sanderson, R.; Wahner, U.M. Homopolymerization of higher 1-olefins with metallocene/MAO catalysts. Macromol. Mater. Eng. 2001, 286, 480–487. [Google Scholar] [CrossRef]
- Babushkin, D.E.; Panchenko, V.N.; Brintzinger, H.H. Zirconium allyl complexes as participants in zirconocene-catalyzed alpha-olefin polymerizations. Angew. Chem. Int. Ed. 2014, 53, 9645–9649. [Google Scholar] [CrossRef]
- Brant, P.; Jiang, P.; Lovell, J.; Crowther, D. Termination events in sterically hindered metallocene-catalyzed olefin oligomerizations: Vinyl chain ends in oligooctenes. Organometallics 2016, 35, 2836–2839. [Google Scholar] [CrossRef]
- Wu, M.M.; Pafford, B.J.; Stavens, K.B. Polyalphaolefins by Oligomerization and Isomerization. U.S. Patent application 2014323665, 30 October 2014. [Google Scholar]
- Park, J.H.; Jang, Y.E.; Jeon, J.Y.; Go, M.J.; Lee, J.; Kim, S.K.; Lee, S.-I.; Lee, B.Y. Preparation of ansa-metallocenes for production of poly(α-olefin) lubricants. Dalton Trans. 2014, 43, 10132–10138. [Google Scholar] [CrossRef]
- Wu, M.M.; Hagemeister, M.P.; Yang, N. Process to Produce Polyalphaolefins. U.S. Patent 8513478, 20 August 2013. [Google Scholar]
- Welle, A.; Wassenaar, J.; Slawinski, M. Use of a Metallocene Catalyst to Produce a Polyalpha-Olefin. U.S. Patent 9688792, 27 June 2017. [Google Scholar]
- Shimizu, H.; Katayama, K.; Noda, H.; Okano, M. 1-Octene, 1-decene, 1-dodecene ternary copolymer and lubricants therewith. U.S. Patent 2014256997, 11 September 2014. [Google Scholar]
- Patil, A.O.; Bodige, S. Synthetic Libricant Basestocks and Methods of Preparation Thereof. U.S. Patent 2014046878, 23 August 2016. [Google Scholar]
- Patil, A.O.; Bodige, S.; Luo, S.; Chu, J.W.; Stavens, K.; Harrrington, B.A. Ultra High Viscosity Synthetic Base Stocks and Process for Preparing Same. U.S. Patent 2014213834, 31 July 2014. [Google Scholar]
- Wu, M.M.; Rucker, S.P.; Canich, A.M. Process for Producing Novel Synthetic Basestocks. U.S. Patent 9701595, 11 July 2017. [Google Scholar]
- Nifant’ev, I.; Vinogradov, A.; Vinogradov, A.; Karchevsky, S.; Ivchenko, P. Zirconocene-catalyzed dimerization of α-olefins: DFT modeling of the Zr-Al binuclear reaction mechanism. Molecules 2019, 24, 3565. [Google Scholar] [CrossRef] [PubMed]
- Janiak, C.; Lange, K.C.H.; Marquardt, P. Alkyl-substituted cyclopentadienyl- and phospholyl-zirconium/MAO catalysts for propene and 1-hexene oligomerization. J. Mol. Catal. A Chem. 2002, 180, 43–58. [Google Scholar] [CrossRef]
- Janiak, C.; Lange, K.C.H.; Marquardt, P.; Krüger, R.-P.; Hanselmann, R. Analyses of propene and 1-hexene oligomers from zirconocene/MAO catalysts—Mechanistic implications by NMR, SEC, and MALDI-TOF MS. Macromol. Chem. Phys. 2002, 203, 129–138. [Google Scholar] [CrossRef]
- Marks, T.J.; Yang, X. Homogeneous Alpha-Olefin Dimerization Catalysts. U.S. Patent 5500398, 19 March 1996. [Google Scholar]
- Margl, P.M.; Woo, T.K.; Ziegler, T. Potential catalyst deactivation Reaction in homogeneous Ziegler−Natta polymerization of olefins: Formation of an allyl intermediate. Organometallics 1998, 17, 4997–5002. [Google Scholar] [CrossRef]
- Lieber, S.; Prosenc, M.-H.; Brintzinger, H.-H. Zirconocene allyl complexes: Dynamics in solution, reaction with aluminum alkyls, B(C6F5)3-induced propene insertion, and density-functional calculations on possible formation and reaction pathways. Organometallics 2000, 19, 377–387. [Google Scholar] [CrossRef]
- Landis, C.R.; Christianson, M.D. Metallocene-catalyzed alkene polymerization and the observation of Zr-allyls. Proc. Natl. Acad. Sci. USA 2006, 103, 15349–15354. [Google Scholar] [CrossRef] [PubMed]
- Vatamanu, M. Synthesis, structures, and dynamic features of d0 zirconocene–allyl complexes. Organometallics 2014, 33, 3683–3694. [Google Scholar] [CrossRef]
- Babushkin, D.E.; Panchenko, V.N.; Brintzinger, H.-H. Zirconium-allyl complexes as resting states in zirconocene-catalyzed a-olefin polymerization. Macromol. Rapid Commun. 2015, 36, 249–253. [Google Scholar] [CrossRef]
- Vatamanu, M. Observation of zirconium allyl species formed during zirconocene-catalyzed propene polymerization and mechanistic insights. J. Catal. 2015, 323, 112–120. [Google Scholar] [CrossRef]
- Panchenko, V.N.; Babushkin, D.E.; Bercaw, J.E.; Brintzinger, H.H. Catalyst speciation during ansa-zirconocene-catalyzed polymerization of 1-hexene studied by UV-vis spectroscopy—Formation and partial re-activation of Zr-Allyl intermediates. Polymers 2019, 11, 936. [Google Scholar] [CrossRef]
- Götz, C.; Rau, A.; Luft, G. Ternary metallocene catalyst systems based on metallocene dichlorides and AlBu3i/[PhNMe2H][B(C6F5)4]: NMR investigations of the influence of Al/Zr ratios on alkylation and on formation of the precursor of the active metallocene species. J. Mol. Catal. A Chem. 2002, 174, 95–110. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Vil’danova, R.F.; Pechatkina, S.V.; Khalilov, L.M.; Dzhemilev, U.M. New effective reagent [Cp2ZrH2···ClAlEt2]2 for alkene hydrometallation. J. Organomet. Chem. 2007, 692, 3424–3429. [Google Scholar] [CrossRef]
- Sizov, A.I.; Zvukova, T.M.; Belsky, V.K.; Bulychev, B.M. Aluminium zirconium (+3 and +4) heterometallic hydrido complexes of compositions [(η5-C5H5)2Zr(μ-H)]2(μ-H)AlCl2 and [(η5-C5H5)2ZrH(μ-H)2]3Al. J. Organomet. Chem. 2001, 619, 36–42. [Google Scholar] [CrossRef]
- Wehmschulte, R.J.; Power, P.P. Reaction of cyclopentadienyl zirconium derivatives with sterically encumbered arylaluminum hydrides: X-ray crystal structure of (η5-C5H5)2Zr(μ2-H)2AlC6H2-2,4,6-But3. Polyhedron 1999, 18, 1885–1888. [Google Scholar] [CrossRef]
- Babushkin, D.E.; Panchenko, V.N.; Timofeeva, M.N.; Zakharov, V.A.; Brintzinger, H.H. Novel zirconocene hydride complexes in homogeneous and in SiO2-supported olefin-polymerization catalysts modified with diisobutylaluminium hydride or triisobutylaluminum. Macromol. Chem. Phys. 2008, 209, 1210–1219. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Khalilov, L.M.; Dzhemilev, U.M. Mechanisms of reactions of organoaluminium compounds with alkenes and alkynes catalyzed by Zr complexes. Russ. Chem. Rev. 2012, 81, 524–548. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Balaev, A.V.; Gubaidullin, I.M.; Abzalilova, L.R.; Pechatkina, S.V.; Khalilov, L.M.; Spivak, S.I.; Dzhemilev, U.M. Kinetic model of olefin hydroalumination by HAlBui2 and AlBui3 in the presence of Cp2ZrCl2 catalyst. Int. J. Chem. Kinet. 2007, 39, 333–339. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Kovyazin, P.V.; Nifant’ev, I.E.; Khalilov, L.M.; Dzhemilev, U.M. Role of Zr,Al hydride intermediate structure and dynamics in alkene hydroalumination with XAlBui2 (X = H, Cl, Bui), catalyzed by Zr η5 complexes. Organometallics 2015, 34, 3559–3570. [Google Scholar] [CrossRef]
- Pankratyev, E.Y.; Tyumkina, T.V.; Parfenova, L.V.; Khalilov, L.M.; Khursan, S.L.; Dzhemilev, U.M. DFT study on mechanism of olefin hydroalumination by XAlBui2 in the presence of Cp2ZrCl2 Catalyst. I. Simulation of intermediate formation in reaction of HAlBui2 with Cp2ZrCl2. Organometallics 2009, 28, 968–977. [Google Scholar] [CrossRef]
- Pankratyev, E.Y.; Tyumkina, T.V.; Parfenova, L.V.; Khalilov, L.M.; Khursan, S.L.; Dzhemilev, U.M. DFT and Ab initio study on mechanism of olefin hydroalumination by XAlBui2 in the presence of Cp2ZrCl2 catalyst. II. Olefin interaction with catalytically active centers. Organometallics 2011, 30, 6078–6089. [Google Scholar] [CrossRef]
- Baldwin, S.M.; Bercaw, J.E.; Brintzinger, H.H. Alkylaluminum-complexed zirconocene hydrides: Identification of hydride-bridged species by NMR spectroscopy. J. Am. Chem. Soc. 2008, 130, 17423–17433. [Google Scholar] [CrossRef] [PubMed]
- Parfenova, L.V.; Kovyazin, P.V.; Tyumkina, T.V.; Islamov, D.N.; Lyapina, A.R.; Karchevsky, S.G.; Ivchenko, P.V. Reactions of bimetallic Zr, Al-hydride complexes with methylaluminoxane: NMR and DFT study. J. Organomet. Chem. 2017, 851, 30–39. [Google Scholar] [CrossRef]
- Van der Heijden, H.; Hessen, B.; Orpen, A.G. A zwitterionic zirconocene alkyl complex as a single-component α-olefin dimerization catalyst. J. Am. Chem. Soc. 1998, 120, 1112–1113. [Google Scholar] [CrossRef][Green Version]
- Bochmann, M.; Lancaster, S.J. Monomer–dimer equilibria in homo- and heterodinuclear cationic alkylzirconium complexes and their role in polymerization catalysis. Angew. Chem. Int. Ed. 1994, 33, 1634–1637. [Google Scholar] [CrossRef]
- Babushkin, D.E.; Semikolenova, N.V.; Zakharov, V.A.; Talsi, E.P. Mechanism of dimethylzirconocene activation with methylaluminoxane: NMR monitoring of intermediates at high Al/Zr ratios. Macromol. Chem. Phys. 2000, 201, 558–567. [Google Scholar] [CrossRef]
- Babushkin, D.E.; Brintzinger, H.-H. Activation of dimethyl zirconocene by methylaluminoxane (MAO)-size estimate for Me-MAO– anions by pulsed field-gradient NMR. J. Am. Chem. Soc. 2002, 124, 12869–12873. [Google Scholar] [CrossRef]
- Theurkauff, G.; Bader, M.; Marquet, N.; Bondon, A.; Roisnel, T.; Guegan, J.-P.; Amar, A.; Boucekkine, A.; Carpentier, J.-F.; Kirillov, E. Discrete ionic complexes of highly isoselective zirconocenes. solution dynamics, trimethylaluminum adducts, and implications in propylene polymerization. Organometallics 2016, 35, 258–276. [Google Scholar] [CrossRef]
- Theurkauff, G.; Bondon, A.; Dorcet, V.; Carpentier, J.-F.; Kirillov, E. Heterobi- and -trimetallic ion pairs of zirconocene-based isoselective olefin polymerization catalysts with AlMe3. Angew. Chem. Int. Ed. 2015, 54, 6343–6346. [Google Scholar] [CrossRef]
- Ehm, C.; Cipullo, R.; Budzelaar, P.H.M.; Busico, V. Role of TMA in polymerization. Dalton Trans. 2016, 45, 6847–6855. [Google Scholar] [CrossRef]
- Guo, Y.; He, F.; Zhang, Z.; Khan, A.; Fu, Z.; Xu, J.; Fan, Z. Influence of trimethylaluminum on kinetics of rac-Et(Ind)ZrCl2/aluminoxane catalyzed ethylene polymerization. J. Organomet. Chem. 2016, 808, 109–116. [Google Scholar] [CrossRef]
- Collins, S.; Linnolahti, M.; Garcia Zamora, M.; Zijlstra, H.S.; Rodríguez Hernández, M.T.; Perez-Camacho, O. Activation of Cp2ZrX2 (X = Me, Cl) by methylaluminoxane as studied by electrospray ionization mass spectrometry: Relationship to polymerization catalysis. Macromolecules 2017, 50, 8871–8884. [Google Scholar] [CrossRef]
- Baldwin, S.M.; Bercaw, J.E.; Brintzinger, H.H. Cationic alkylaluminum-complexed zirconocene hydrides as participants in olefin polymerization Catalysis. J. Am. Chem. Soc. 2010, 132, 13969–13971. [Google Scholar] [CrossRef] [PubMed]
- Kuklin, M.S.; Hirvi, J.T.; Bochmann, M.; Linnolahti, M. Toward controlling the metallocene/methylaluminoxane-catalyzed olefin polymerization process by a computational approach. Organometallics 2015, 34, 3586–3597. [Google Scholar] [CrossRef]
- Baldwin, S.M.; Bercaw, J.E.; Henling, L.M.; Day, M.W.; Brintzinger, H.H. Cationic alkylaluminum-complexed zirconocene hydrides: NMR-spectroscopic identification, crystallographic structure determination, and interconversion with other zirconocene cations. J. Am. Chem. Soc. 2011, 133, 1805–1813. [Google Scholar] [CrossRef]





| Run | Pre- cat. | TiBA/Zr Ratio | Activator | [Act]/ [Precat] Ratio | H2 | Conv. % | Dimer (C16), Trimer (C24), Tetramer (C32) and Pentamer (C40) wt. % in the Products | |||
|---|---|---|---|---|---|---|---|---|---|---|
| C16 | C24 | C32 | C40 | |||||||
| 1 | 1 | 20 | MMAO-12 | 10 | – | 85 | 88.8 | 9.2 | 2.1 | – |
| 2 | 1 | 20 | MMAO-12 +1 eq. Et2AlCl | 10 | – | 74 | 90.3 | 7.8 | 1.9 | – |
| 3 | 1 | 20 | MMAO-12 | 10 | 1 bar | 84 | 92.0 | 6.7 | 1.3 | – |
| 4 | 1 | 20 | MMAO-12 | 200 | – | 68 | 77.6 | 15.1 | 5.4 | 1.9 |
| 5 | 1 | 20 | NBF | 1 | – | 53 | 81.6 | 13.2 | 4.2 | 1.0 |
| 6 | 1 | 20 | NBF | 1 | 1 bar | 70 | 84.6 | 11.6 | 3.3 | 0.5 |
| 7 | 1′ | – | MMAO-12 | 10 | – | 47 | 88.7 | 8.6 | 2.6 | – |
| 8 | 1′ | – | MMAO-12 +1 eq. Et2AlCl | 10 | – | 60 | 93.4 | 5.6 | 1.0 | – |
| 9 | 1′ | – | NBF | 1 | – | 14 | 78.2 | 17.7 | 4.0 | – |
| 10 | 1′ | 20 | NBF | 1 | – | 8 | 75.2 | 18.9 | 5.8 | – |
| 11 | 1′ | – | NBF +1 eq. Et2AlCl | 1 | – | 7 | 90.7 | 8.2 | 1.0 | – |
| 12 | 1′ | 20 | NBF | 1 | 1 bar | 18 | 72.3 | 16.8 | 9.2 | 1.7 |
| 13 | 2 | 20 | MMAO-12 | 10 | – | 82 | 92.4 | 7.1 | 0.5 | – |
| 14 | 2 | 20 | MMAO-12 +1 eq. Et2AlCl | 10 | – | 79 | 96.3 | 3.5 | 0.2 | – |
| 15 | 2 | 20 | MMAO-12 | 10 | 1 bar | 86 | 92.3 | 7.2 | 0.5 | – |
| 16 | 2 | 20 | MMAO-12 | 200 | – | 70 | 78.1 | 15.8 | 5.7 | 0.4 |
| 17 | 2 | 20 | NBF | 1 | – | 68 | 67.7 | 23.3 | 7.6 | 1.4 |
| 18 | 2 | 20 | NBF | 1 | 1 bar | 85 | 82.2 | 13.6 | 3.4 | 0.8 |
| 19 | 2′ | – | MMAO-12 | 10 | – | 65 | 58.4 | 25.1 | 11.5 | 5.0 |
| 20 | 2′ | – | MMAO-12 +1 eq. Et2AlCl | 10 | – | 57 | 87.6 | 11.2 | 1.2 | – |
| 21 | 2′ | – | NBF | 1 | – | 60 | 25.2 | 25.2 | 22.9 | 26.7 |
| 22 | 2′ | 20 | NBF | 1 | – | 13 | 47.4 | 21.7 | 15.6 | 15.2 |
| 23 | 2′ | – | NBF +1 eq. Et2AlCl | 1 | – | 38 | 70.3 | 22.1 | 6.3 | 1.3 |
| 24 | 2′ | – | NBF | 1 | 1 bar | 73 | 48.3 | 26.0 | 14.6 | 11.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nifant’ev, I.; Vinogradov, A.; Vinogradov, A.; Karchevsky, S.; Ivchenko, P. Experimental and Theoretical Study of Zirconocene-Catalyzed Oligomerization of 1-Octene. Polymers 2020, 12, 1590. https://doi.org/10.3390/polym12071590
Nifant’ev I, Vinogradov A, Vinogradov A, Karchevsky S, Ivchenko P. Experimental and Theoretical Study of Zirconocene-Catalyzed Oligomerization of 1-Octene. Polymers. 2020; 12(7):1590. https://doi.org/10.3390/polym12071590
Chicago/Turabian StyleNifant’ev, Ilya, Alexander Vinogradov, Alexey Vinogradov, Stanislav Karchevsky, and Pavel Ivchenko. 2020. "Experimental and Theoretical Study of Zirconocene-Catalyzed Oligomerization of 1-Octene" Polymers 12, no. 7: 1590. https://doi.org/10.3390/polym12071590
APA StyleNifant’ev, I., Vinogradov, A., Vinogradov, A., Karchevsky, S., & Ivchenko, P. (2020). Experimental and Theoretical Study of Zirconocene-Catalyzed Oligomerization of 1-Octene. Polymers, 12(7), 1590. https://doi.org/10.3390/polym12071590