Crystallization Morphology Regulation on Enhancing Heat Resistance of Polylactic Acid
Abstract
1. Introduction
2. Experimental section
2.1. Materials and Sample Preparation
2.2. Heat Deflection Temperature (HDT)
2.3. Bending Modulus
2.4. Storage and Loss Moduli
2.5. Differential Scanning Calorimetric (DSC) Analysis
2.6. X-ray Diffraction (XRD) Characterization
2.7. Polarized Optical Microscopy (POM)
3. Results and Discussions
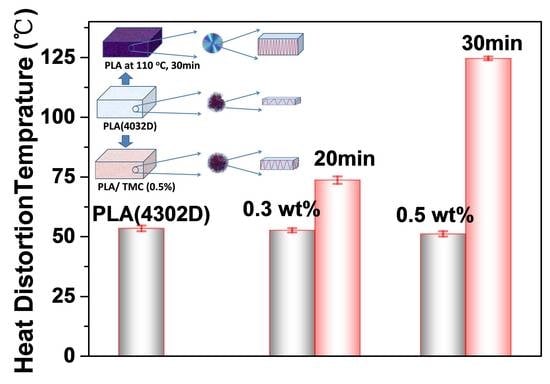
3.1. Heat Deflection Temperature (HDT) and Moduli
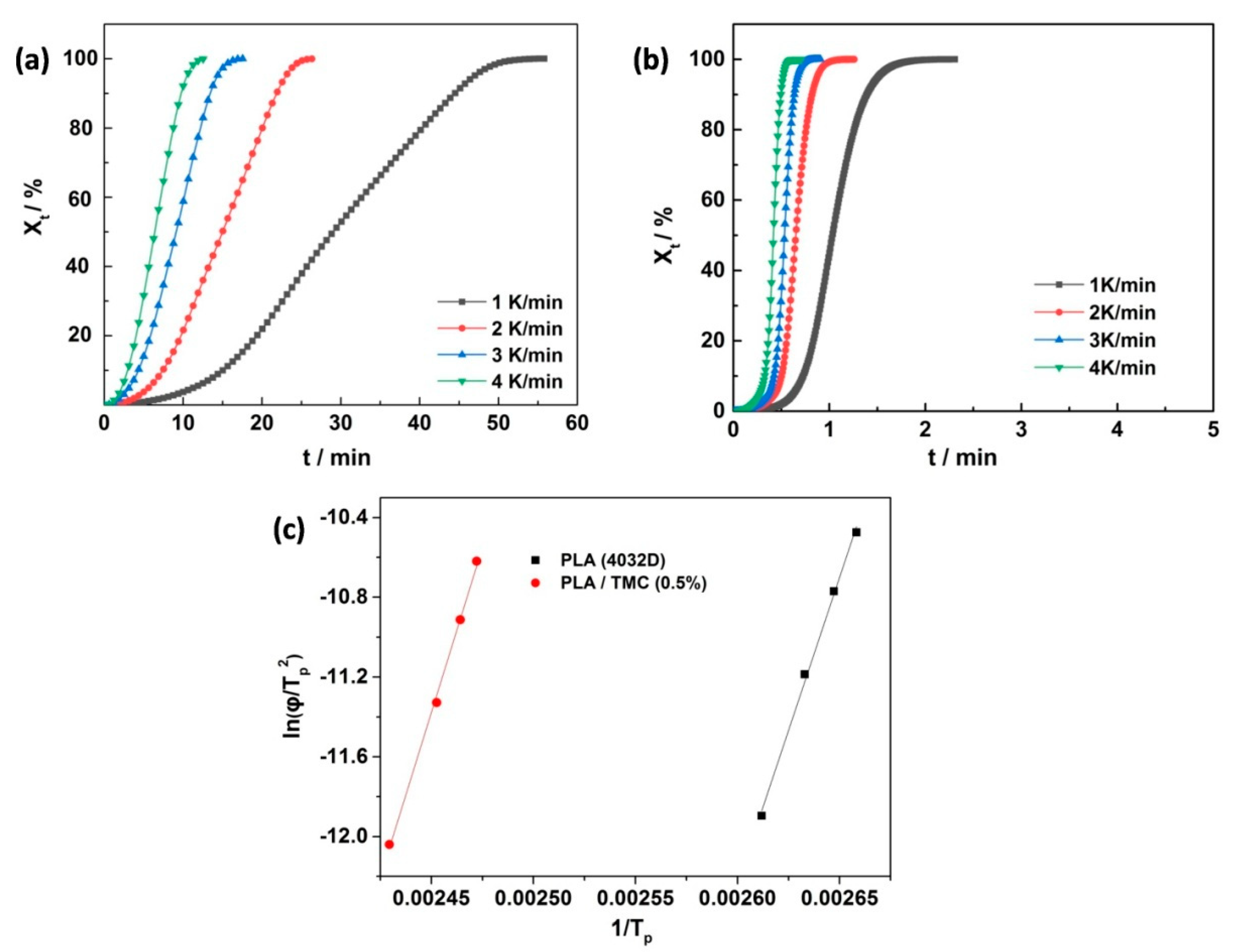
3.2. Differential Scanning Calorimetric (DSC)
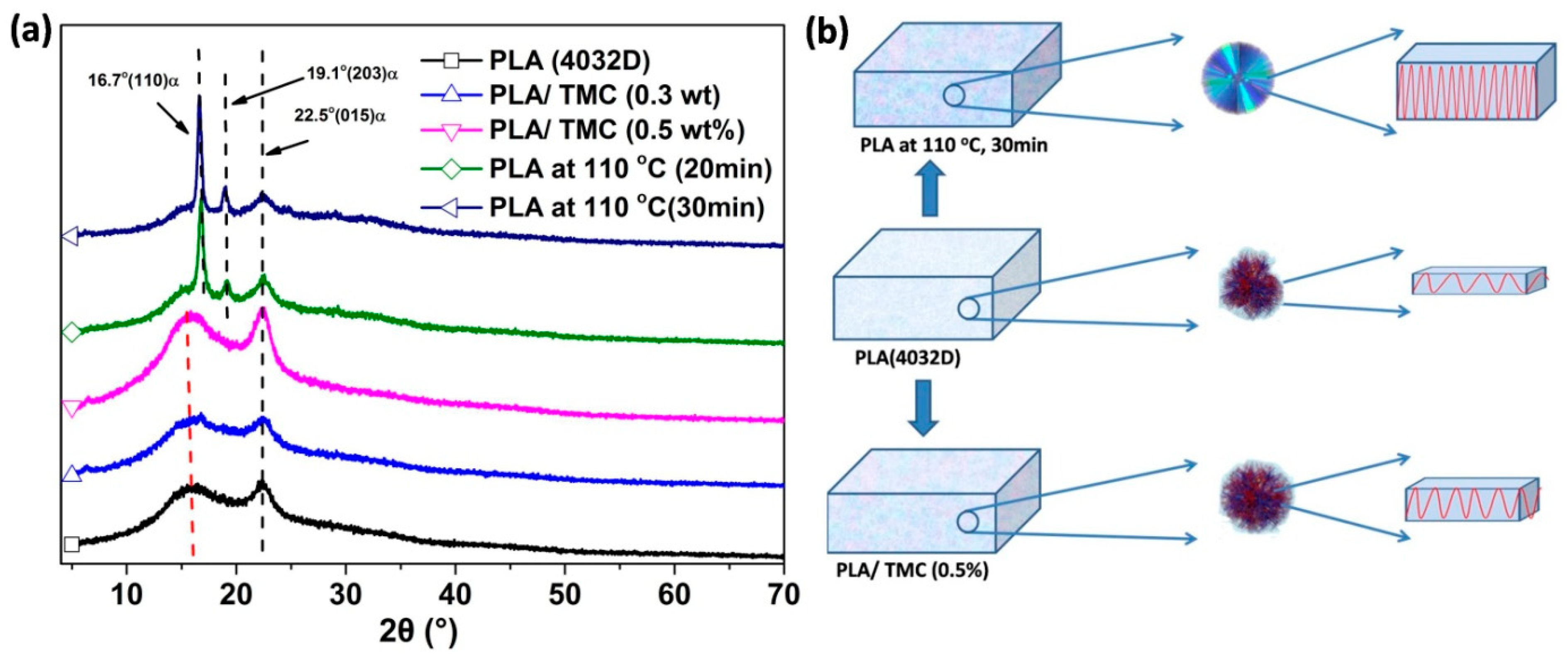
3.3. X-ray Diffraction (XRD) Characterization
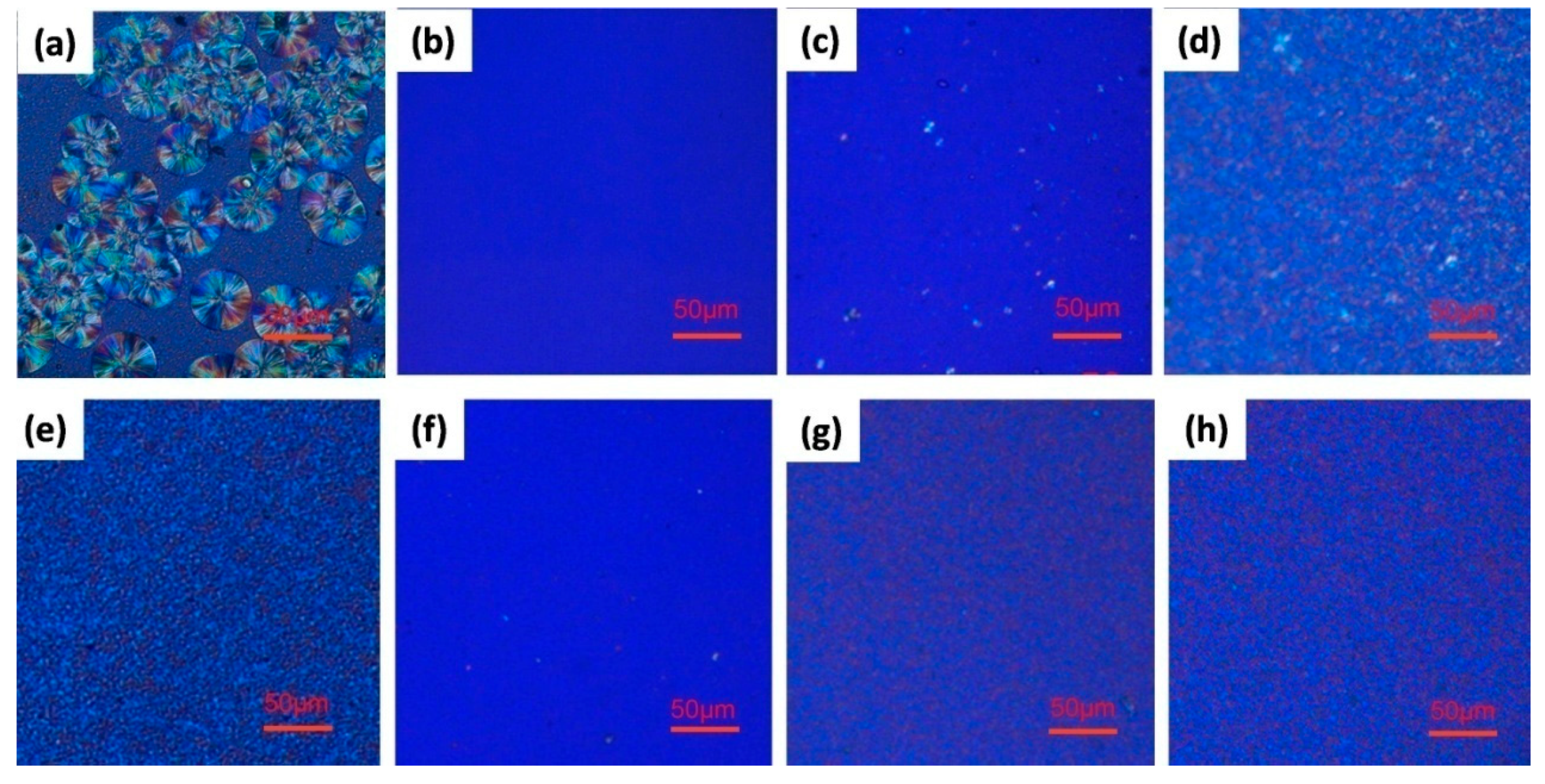
3.4. Polarized Optical Microscopy (POM)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhasney, S.M.; Bhagabati, P.; Kumar, A.; Katiyar, V. Morphology and crystalline characteristics of polylactic acid [PLA]/linear low density polyethylene [LLDPE]/microcrystalline cellulose [MCC] fiber composite. Compos. Sci. Technol. 2019, 171, 54–61. [Google Scholar] [CrossRef]
- Wang, F.; Sun, Z.; Yin, J.; Xu, L. Preparation, characterization and properties of porous PLA/PEG/Curcumin composite nanofibers for antibacterial application. Nanomaterials (Basel) 2019, 9, 508. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Muiruri, J.K.; Thitsartarn, W.; Zhang, X.; Tan, B.H.; He, C. Biodegradable silica rubber core-shell nanoparticles and their stereocomplex for efficient PLA toughening. Compos. Sci. Technol. 2018, 159, 11–17. [Google Scholar] [CrossRef]
- Yeo, J.C.C.; Muiruri, J.K.; Tan, B.H.; Thitsartarn, W.; Kong, J.; Zhang, X.; Li, Z.; He, C. Biodegradable PHB-rubber copolymer toughened PLA green composites with ultrahigh extensibility. ACS Sustain. Chem. Eng. 2018, 6, 15517–15527. [Google Scholar] [CrossRef]
- Li, C.; Guo, J.; Jiang, T.; Zhang, X.; Xia, L.; Wu, H.; Guo, S.; Zhang, X. Extensional flow-induced hybrid crystalline fibrils (shish) in CNT/PLA nanocomposite. Carbon 2018, 129, 720–729. [Google Scholar] [CrossRef]
- Zhang, L.; Lv, S.; Sun, C.; Wan, L.; Tan, H.; Zhang, Y. Effect of MAH-g-PLA on the properties of wood fiber/polylactic acid composites. Polymers-Basel 2017, 9, 591. [Google Scholar] [CrossRef]
- Del Rosario Salazar-Sánchez, M.; Campo-Erazo, S.D.; Villada-Castillo, H.S.; Solanilla-Duque, J.F. Structural changes of cassava starch and polylactic acid films submitted to biodegradation process. Int. J. Biol. Macromol. 2019, 129, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Tabi, T.; Sajo, I.E.; Szabo, F.; Luyt, A.S.; Kovacs, J.G. Crystalline structure of annealed polylactic acid and its relation to processing. Express Polym. Lett. 2010, 4, 659–668. [Google Scholar] [CrossRef]
- Girdthep, S.; Sankong, W.; Pongmalee, A.; Saelee, T.; Punyodom, W.; Meepowpan, P.; Worajittiphon, P. Enhanced crystallization, thermal properties, and hydrolysis resistance of poly(l-lactic acid) and its stereocomplex by incorporation of graphene nanoplatelets. Polym. Test. 2017, 61, 229–239. [Google Scholar] [CrossRef]
- Peelman, N.; Ragaert, P.; Ragaert, K.; Erkoç, M.; Van Brempt, W.; Faelens, F.; Devlieghere, F.; De Meulenaer, B.; Cardon, L. Heat resistance of biobased materials, evaluation and effect of processing techniques and additives. Polym. Eng. Sci. 2018, 58, 513–520. [Google Scholar] [CrossRef]
- Zhang, H.; Ming, R.; Yang, G.; Li, Y.; Li, Q.; Shao, H. Influence of alkali treatment on flax fiber for use as reinforcements in polylactide stereocomplex composites. Polym. Eng. Sci. 2015, 55, 2553–2558. [Google Scholar] [CrossRef]
- Dhar, P.; Katiyar, V. Thermal degradation kinetics of polylactic acid/acid fabricated cellulose nanocrystal based bionanocomposites. Int. J. Biol. Macromol. 2017, 104, 827–836. [Google Scholar]
- Wang, L.; Wang, Y.; Huang, Z.; Weng, Y. Heat resistance, crystallization behavior, and mechanical properties of polylactide/nucleating agent composites. Mater. Design (1980–2015) 2015, 66, 7–15. [Google Scholar] [CrossRef]
- Liu, Y.; He, M.; Yan, W.; Zhang, D.; Zhao, Q.; Qin, S.; Yu, J. P(N-phenylmaleimide-alt-styrene) as a heat-resistant agent in the application of nylon 6. J. Appl. Polym. Sci. 2019, 136, 47689. [Google Scholar] [CrossRef]
- Liu, Y.; He, M.; Zhang, D.; Zhao, Q.; Li, Y.; Qin, S.; Yu, J. P(N-Phenylmaleimide-Alt-Styrene) introduced with 4-carboxyl and its effect on the heat deflection temperature of nylon 6. Materials 2018, 11, 2330. [Google Scholar] [CrossRef]
- Rigolin, T.R.; Takahashi, M.C.; Kondo, D.L.; Bettini, S.H.P. Compatibilizer acidity in Coir-Reinforced PLA composites: Matrix degradation and composite properties. J. Polym. Environ. 2019, 27, 1096–1104. [Google Scholar] [CrossRef]
- Kumar, A.; Tumu, V.R.; Ray Chowdhury, S.; Ramana Reddy, S.V.S. A green physical approach to compatibilize a bio-based poly (lactic acid)/lignin blend for better mechanical, thermal and degradation properties. Int. J. Biol. Macromol. 2019, 121, 588–600. [Google Scholar] [CrossRef]
- Spinella, S.; Lo Re, G.; Liu, B.; Dorgan, J.; Habibi, Y.; Leclère, P.; Raquez, J.; Dubois, P.; Gross, R.A. Polylactide/cellulose nanocrystal nanocomposites: Efficient routes for nanofiber modification and effects of nanofiber chemistry on PLA reinforcement. Polymer 2015, 65, 9–17. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Franciszczak, P.; Meljon, A. High performance hybrid PP and PLA biocomposites reinforced with short man-made cellulose fibres and softwood flour. Compos. Part A Appl. Sci. Manuf. 2015, 74, 132–139. [Google Scholar] [CrossRef]
- Luo, H.; Zhang, C.; Xiong, G.; Wan, Y. Effects of alkali and alkali/silane treatments of corn fibers on mechanical and thermal properties of its composites with polylactic acid. Polym. Compos. 2016, 37, 3499–3507. [Google Scholar] [CrossRef]
- Tábi, T.; Wacha, A.F.; Hajba, S. Effect of D-lactide content of annealed poly(lactic acid) on its thermal, mechanical, heat deflection temperature, and creep properties. J. Appl. Polym. Sci. 2018, 136, 47103. [Google Scholar] [CrossRef]
- Chen, P.; Lian, H.; Shih, Y.; Chen-Wei, S.; Jeng, R. Preparation, characterization and crystallization kinetics of Kenaf fiber/multi-walled carbon nanotube/polylactic acid (PLA) green composites. Mater. Chem. Phys. 2017, 196, 249–255. [Google Scholar] [CrossRef]
- Spinella, S.; Samuel, C.; Raquez, J.; McCallum, S.A.; Gross, R.; Dubois, P. Green and efficient synthesis of dispersible cellulose nanocrystals in biobased polyesters for engineering applications. ACS Sustain. Chem. Eng. 2016, 4, 2517–2527. [Google Scholar] [CrossRef]
- Burzic, I.; Pretschuh, C.; Kaineder, D.; Eder, G.; Smilek, J.; Másilko, J.; Kateryna, W. Impact modification of PLA using biobased biodegradable PHA biopolymers. Eur. Polym. J. 2019, 114, 32–38. [Google Scholar] [CrossRef]
- Garcia-Campo, M.J.; Quiles-Carrillo, L.; Sanchez-Nacher, L.; Balart, R.; Montanes, N. High toughness poly(lactic acid) (PLA) formulations obtained by ternary blends with poly(3-hydroxybutyrate) (PHB) and flexible polyesters from succinic acid. Polym. Bull. 2019, 76, 1839–1859. [Google Scholar] [CrossRef]
- Bai, Z.; Dou, Q. Rheology, morphology, crystallization behaviors, mechanical and thermal properties of poly(lactic acid)/polypropylene/maleic anhydride-graftedpolypropylene blends. J. Polym. Environ. 2018, 26, 959–969. [Google Scholar] [CrossRef]
- Gonzalez-Garzon, M.; Shahbikian, S.; Huneault, M.A. Properties and phase structure of melt-processed PLA/PMMA blends. J. Polym. Res. 2018, 25, 58. [Google Scholar] [CrossRef]
- Yuryev, Y.; Mohanty, A.K.; Misra, M. Novel biocomposites from biobased PC/PLA blend matrix system for durable applications. Compos. Part B Eng. 2017, 130, 158–166. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, J.; Huang, J. Poly(lactic acid)/polyoxymethylene blends: Morphology, crystallization, rheology, and thermal mechanical properties. Polymer 2015, 69, 103–109. [Google Scholar] [CrossRef]
- Tábi, T.; Hajba, S.; Kovács, J.G. Effect of crystalline forms (α′ and α) of poly(lactic acid) on its mechanical, thermo-mechanical, heat deflection temperature and creep properties. Eur. Polym. J. 2016, 82, 232–243. [Google Scholar] [CrossRef]
- Yu, T.; Jiang, N.; Li, Y. Study on short ramie fiber/poly(lactic acid) composites compatibilized by maleic anhydride. Compos. Part A Appl. Sci. 2014, 64, 139–146. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, D.; Li, B.; Wan, G.; Zhao, G.; Zhang, A. Strong and thermal-resistance glass fiber-reinforced polylactic acid (PLA) composites enabled by heat treatment. Int. J. Biol. Macromol. 2019, 129, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Kong, J.; Chen, Y.; Zhang, H.; Dong, L. Improved heat resistance properties of poly(l-lactide)/basalt fiber biocomposites with high crystallinity under forming hybrid-crystalline morphology. Int. J. Biol. Macromol. 2019, 122, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Deng, C.; Lin, G.; Wang, Y. Super toughened and high heat-resistant poly(lactic acid) (PLA)-based blends by enhancing interfacial bonding and PLA phase crystallization. Ind. Eng. Chem. Res. 2015, 54, 5643–5655. [Google Scholar] [CrossRef]
- Xu, T.; Wang, Y.; Han, Q.; He, D.; Li, Q.; Shen, C. Nonisothermal crystallization kinetics of poly(lactic acid) nucleated with a multiamide nucleating agent. J. Macromol. Sci. Part B 2014, 53, 1680–1694. [Google Scholar] [CrossRef]
- Shen, X.; Hu, W.; Russell, T.P. Measuring the degree of crystallinity in semicrystalline regioregular poly(3-hexylthiophene). Macromolecules 2016, 49, 4501–4509. [Google Scholar] [CrossRef]
- Li, X.; Dong, F.; Zhang, L.; Xu, Q.; Zhu, X.; Liang, S.; Hu, L.; Xie, H. Cellulosic protic ionic liquids hydrogel: A green and efficient catalyst carrier for Pd nanoparticles in reduction of 4-nitrophenol in water. Chem. Eng. J. 2019, 372, 516–525. [Google Scholar] [CrossRef]
- Xue, B.; Guo, D.; Bao, J. Effect of sebacate benzohydydrazide on heat-resistance property of poly(lactic acid). Polym. Mate. Sci. Eng. 2014, 30, 59–63. [Google Scholar]
- Tsuji, H.; Ikada, Y. Properties and morphologies of L-lactide): 1. Annealing condition effects on properties and morphologie poly(L-lactide). Polymer 1995, 36, 2709–2716. [Google Scholar] [CrossRef]
- Han, Q.; Wang, Y.; Shao, C.; Zheng, G.; Li, Q.; Shen, C. Nonisothermal crystallization kinetics of biodegradable poly (lactic acid)/zinc phenylphosphonate composites. J. Compos. Mater. 2014, 48, 2737–2746. [Google Scholar] [CrossRef]
- Wu, N.; Wang, H. Effect of Zinc Phenylphosphonate on the Crystallization Behavior of Poly(L-lactide). J. Appl. Polym. Sci. 2013, 130, 2744–2752. [Google Scholar] [CrossRef]
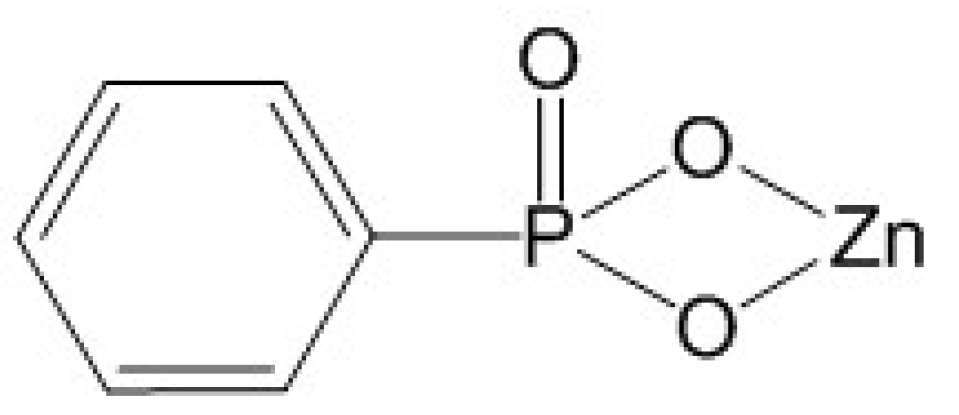
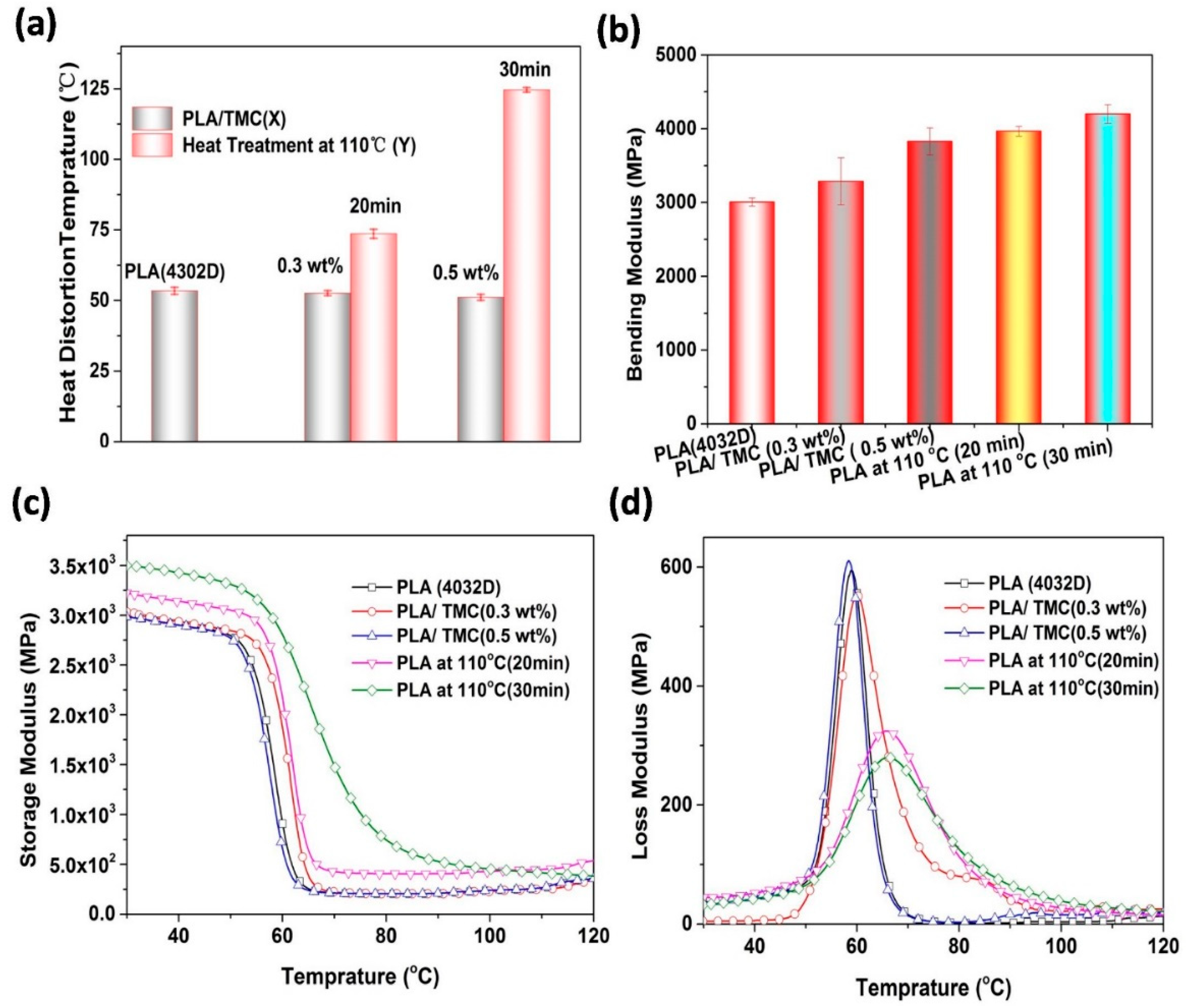

| PLA and Its Composites | HDT(°C) | Test Method | Heat Treatment | References |
|---|---|---|---|---|
| PLA | 50 | 0.45 MPa | No | [16] |
| PLA/LG (20%) | 54 | 0.45 MPa | No | [17] |
| PLA/CNC (20%) | 78 | DMA | Yes, at 130 °C | [18] |
| PLA/GF (40%) | 55 | 1.82 MPa | No | [19] |
| PLA/GF (20%) | 150 | 0.45 MPa | Yes, at 100 °C | [32] |
| PLA/CF (10%) | 60 | 1.82 MPa | No | [20] |
| PLA/KF (40%) | 135 | 0.45 MPa | Yes, at 80 °C | [22] |
| PLA/D-lactide (0.5 wt%) | 150 | 0.45 MPa | Yes, at 140 °C | [21] |
| PLA/PHA (90/10)PLA/PHA | 62 | 1.82 MPa | Yes, at 100 °C | [24] |
| 50 | 1.82 MPa | No | [24] | |
| PLA/PP (60/40) | 60 | 0.45 MPa | No | [26] |
| PLA/POM (40/60) | 140 | 0.46 MPa | No | [29] |
| Samples | Xna (%) | T0b (°C) | Tpc (°C) | ΔH d (J/g) | Tme (°C) | HDT (°C) |
|---|---|---|---|---|---|---|
| PLA (4032D) | 11.7 | 113.7 | 94.5 | 10.9 | 168.0 | 53.4 |
| PLA/TMC (0.3 wt%) | 21.3 | 126.1 | 113.8 | 15.8 | 167.6 | 51.1 |
| PLA at 110 °C (20 min) | 25.3 | 126.7 | 113.3 | 47.9 | 169.7 | 73.6 |
| PLA/TMC (0.5 wt%) | 30.1 | 139.1 | 136.3 | 28.02 | 167.5 | 52.6 |
| PLA at 110 °C (30 min) | 30.84 | 139.0 | 135.9 | 43.3 | 167.7 | 124.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Jiang, S.; Yan, W.; He, M.; Qin, J.; Qin, S.; Yu, J. Crystallization Morphology Regulation on Enhancing Heat Resistance of Polylactic Acid. Polymers 2020, 12, 1563. https://doi.org/10.3390/polym12071563
Liu Y, Jiang S, Yan W, He M, Qin J, Qin S, Yu J. Crystallization Morphology Regulation on Enhancing Heat Resistance of Polylactic Acid. Polymers. 2020; 12(7):1563. https://doi.org/10.3390/polym12071563
Chicago/Turabian StyleLiu, Yufei, Siyuan Jiang, Wei Yan, Min He, Jun Qin, Shuhao Qin, and Jie Yu. 2020. "Crystallization Morphology Regulation on Enhancing Heat Resistance of Polylactic Acid" Polymers 12, no. 7: 1563. https://doi.org/10.3390/polym12071563
APA StyleLiu, Y., Jiang, S., Yan, W., He, M., Qin, J., Qin, S., & Yu, J. (2020). Crystallization Morphology Regulation on Enhancing Heat Resistance of Polylactic Acid. Polymers, 12(7), 1563. https://doi.org/10.3390/polym12071563