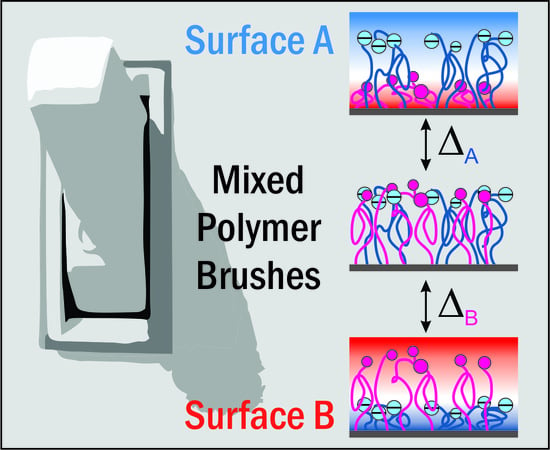
Mixed Polymer Brushes for “Smart” Surfaces
Abstract
1. Introduction
2. Synthetic Routes
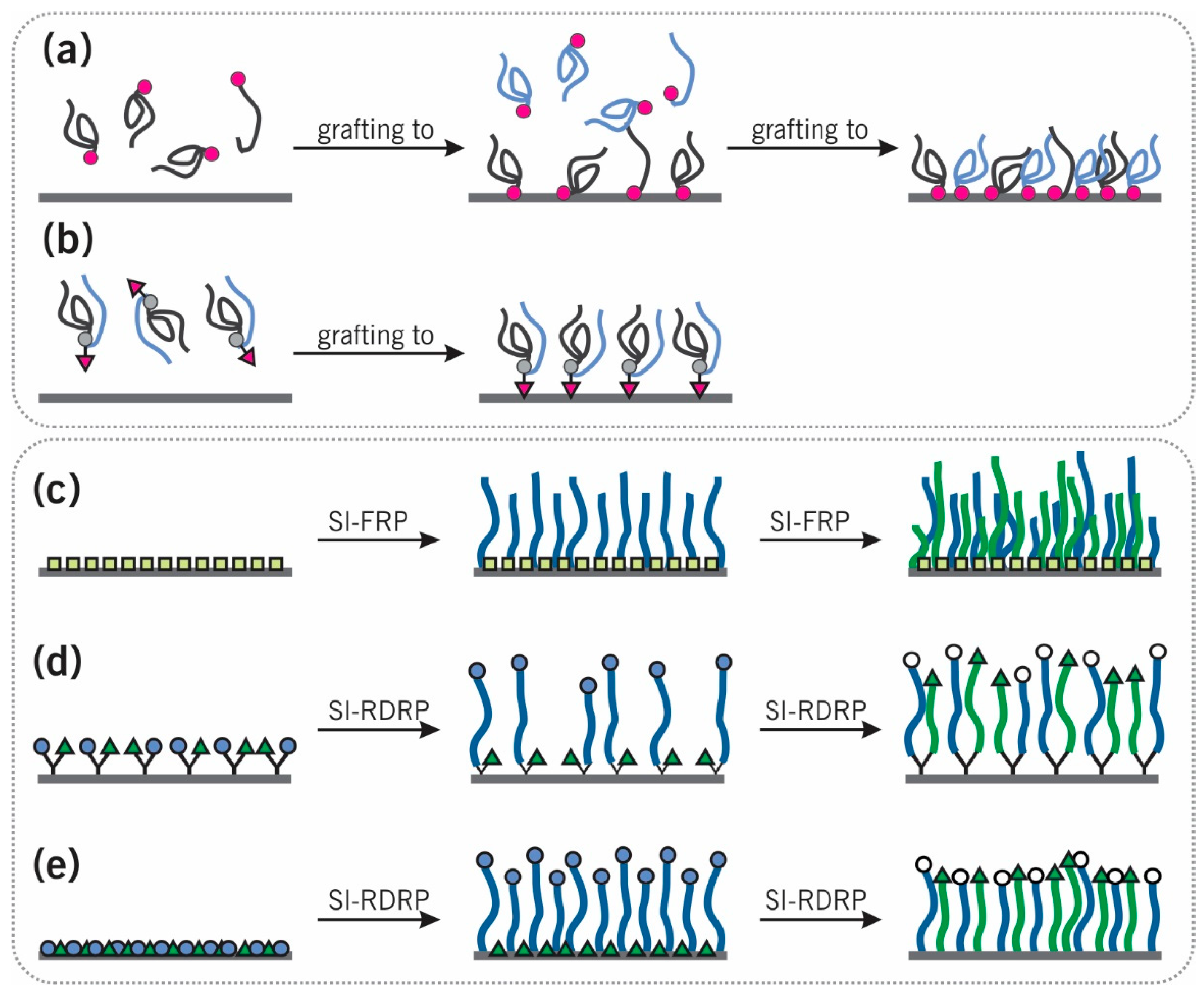
2.1. “Grafting to”
2.2. “Grafting from”
2.2.1. Sequential Surface-Initiated Free-Radical Polymerization (SI-FRP)
2.2.2. Sequential Surface-Initiated Reversible-Deactivation Radical Polymerization (SI-RDRP)
2.2.3. Orthogonal SI-RDRP and Surface-Initiated Ring-Opening Polymerization (SI-ROP)
3. Phase Behavior of Binary Mixed Brushes
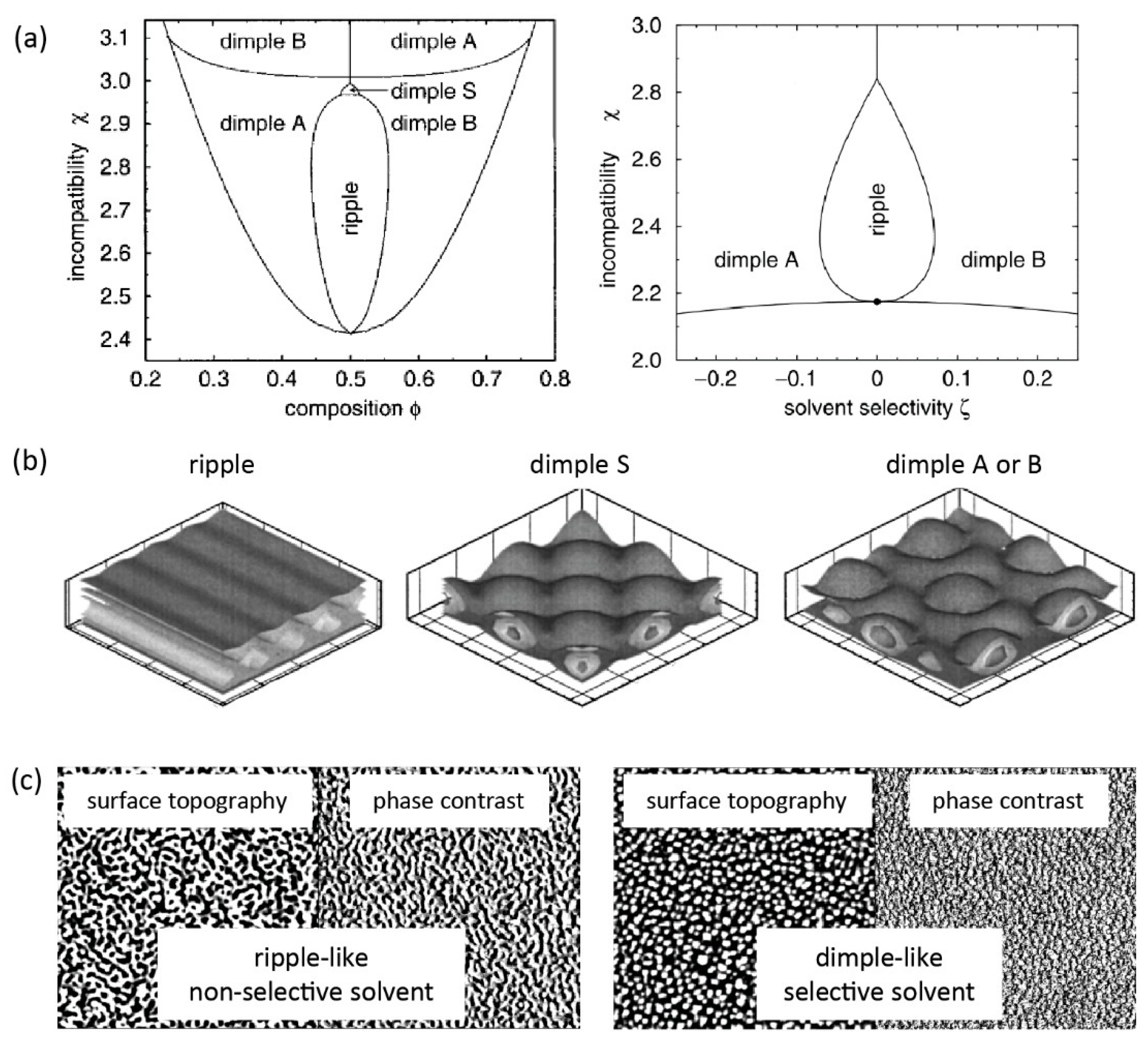
3.1. Theoretical Discussion
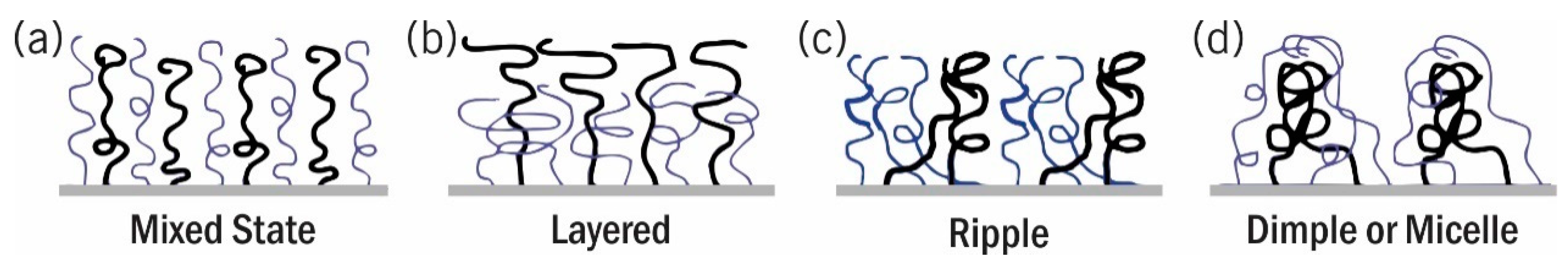
3.2. Mixed Polymer Brushes Nanostructures
3.2.1. Phase Segregation in Melt Condition
3.2.2. Phase Segregation in Non-Selective Solvents
3.2.3. Phase Segregation in Selective Solvents
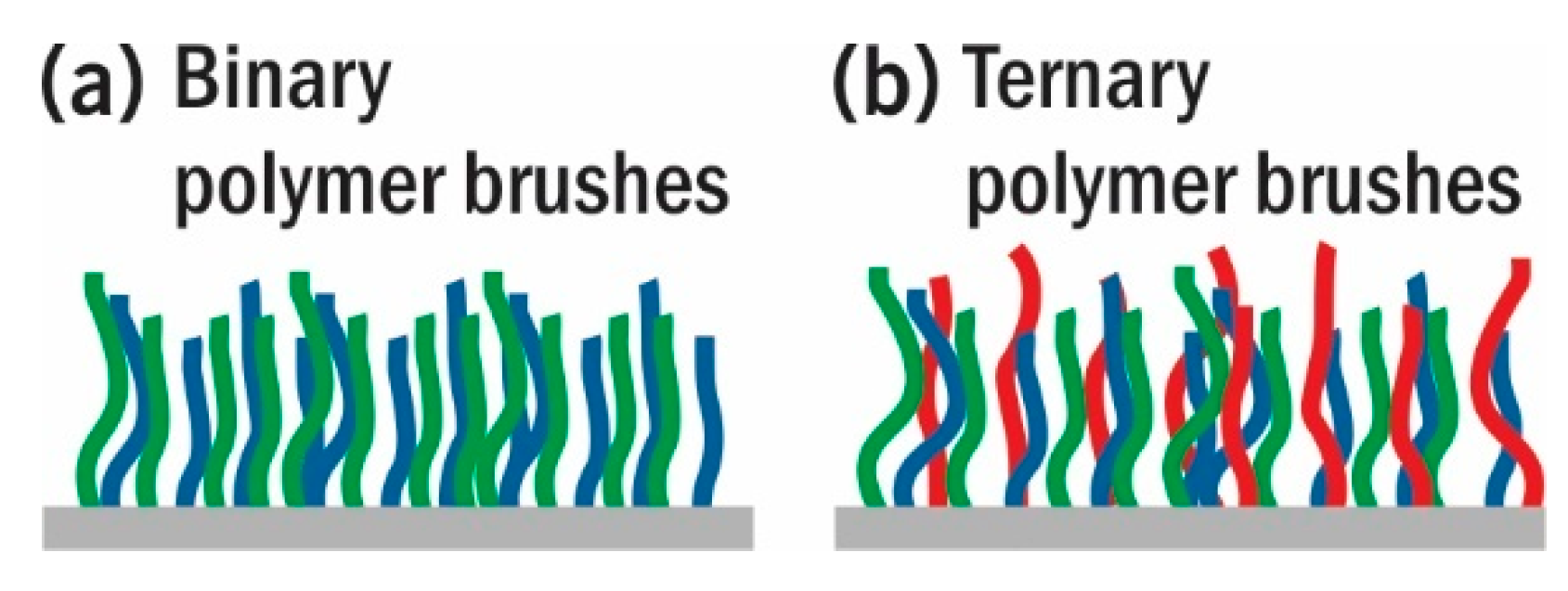
4. Phase Behavior of Ternary Mixed Brushes
5. External Stimuli
5.1. Solvents
5.2. pH and Ion Strength
5.3. Dry/Wet Condition
5.4. Temperature
6. Applications
7. Challenges and Future Directions
8. Conclusion and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Barbey, R.; Lavanant, L.; Paripovic, D.; Schüwer, N.; Sugnaux, C.; Tugulu, S.; Klok, H.A. Polymer Brushes via Surface-Initiated Controlled Radical Polymerization: Synthesis, Characterization, Properties, and Applications. Chem. Rev. 2009, 109, 5437–5527. [Google Scholar] [CrossRef] [PubMed]
- Zoppe, J.O.; Ataman, N.C.; Mocny, P.; Wang, J.; Moraes, J.; Klok, H.A. Surface-Initiated Controlled Radical Polymerization: State-of-the-Art, Opportunities, and Challenges in Surface and Interface Engineering with Polymer Brushes. Chem. Rev. 2017, 117, 1105–1318. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Orski, S.V.; Fries, K.H.; Sontag, S.K.; Locklin, J. Fabrication of nanostructures using polymer brushes. J. Mater. Chem. 2011, 21, 14135–14149. [Google Scholar] [CrossRef]
- Ogieglo, W.; Wormeester, H.; Eichhorn, K.J.; Wessling, M.; Benes, N.E. In situ ellipsometry studies on swelling of thin polymer films: A review. Prog. Polym. Sci. 2015, 42, 42–78. [Google Scholar] [CrossRef]
- Brittain, W.J.; Boyes, S.G.; Granville, A.M.; Baum, M.; Mirous, B.K.; Akgun, B.; Zhao, B.; Blickle, C.; Foster, M.D. Surface Rearrangement of Diblock Copolymer Brushes—Stimuli Responsive Films. In Surface-Initiated Polymerization II; Springer: Berlin/Heidelberg, Germany, 2006; pp. 125–147. ISBN 978-3-540-30251-3. [Google Scholar]
- Zhou, X.; Liu, X.; Xie, Z.; Zheng, Z. 3D-patterned polymer brush surfaces. Nanoscale 2011, 3, 4929–4939. [Google Scholar] [CrossRef]
- Olivier, A.; Meyer, F.; Raquez, J.M.; Damman, P.; Dubois, P. Surface-initiated controlled polymerization as a convenient method for designing functional polymer brushes: From self-assembled monolayers to patterned surfaces. Prog. Polym. Sci. 2012, 37, 157–181. [Google Scholar] [CrossRef]
- Chen, T.; Amin, I.; Jordan, R. Patterned polymer brushes. Chem. Soc. Rev. 2012, 41, 3280–3296. [Google Scholar] [CrossRef]
- Wu, L.; Glebe, U.; Böker, A. Surface-initiated controlled radical polymerizations from silica nanoparticles, gold nanocrystals, and bionanoparticles. Polym. Chem. 2015, 6, 5143–5184. [Google Scholar] [CrossRef]
- Li, B.; Yu, B.; Ye, Q.; Zhou, F. Tapping the potential of polymer brushes through synthesis. Acc. Chem. Res. 2015, 48, 229–237. [Google Scholar] [CrossRef]
- Chen, W.L.; Cordero, R.; Tran, H.; Ober, C.K. 50th Anniversary Perspective: Polymer Brushes: Novel Surfaces for Future Materials. Macromolecules 2017, 50, 4089–4113. [Google Scholar] [CrossRef]
- Kim, M.; Schmitt, S.; Choi, J.; Krutty, J.; Gopalan, P. From Self-Assembled Monolayers to Coatings: Advances in the Synthesis and Nanobio Applications of Polymer Brushes. Polymers 2015, 7, 1346–1378. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, X.; Yu, B.; Zhou, F. Brushing up functional materials. NPG Asia Mater. 2019, 11, 24. [Google Scholar] [CrossRef]
- Xu, F.J.; Yuan, Z.L.; Kang, E.T.; Neoh, K.G. Branched fluoropolymer-Si hybrids via surface-initiated ATRP of pentafluorostyrene on hydrogen-terminated Si(100) surfaces. Langmuir 2004, 20, 8200–8208. [Google Scholar] [CrossRef]
- Prucker, O.; Rühe, J. Synthesis of poly(styrene) monolayers attached to high surface area silica gels through self-assembled monolayers of azo initiators. Macromolecules 1998, 31, 592–601. [Google Scholar] [CrossRef]
- Jordan, R.; Ulman, A.; Kang, J.F.; Rafailovich, M.H.; Sokolov, J. Surface-initiated anionic polymerization of styrene by means of self assembled monolayers. J. Am. Chem. Soc. 1999, 121, 1016–1022. [Google Scholar] [CrossRef]
- Tam, T.K.; Pita, M.; Trotsenko, O.; Motornov, M.; Tokarev, I.; Halámek, J.; Minko, S.; Katz, E. Reversible “closing” of an electrode interface functionalized with a polymer brush by an electrochemical signal. Langmuir 2010, 26, 4506–4513. [Google Scholar] [CrossRef]
- Sheng, W.; Li, B.; Wang, X.; Dai, B.; Yu, B.; Jia, X.; Zhou, F. Brushing up from “anywhere” under sunlight: A universal surface-initiated polymerization from polydopamine-coated surfaces. Chem. Sci. 2015, 6, 2068–2073. [Google Scholar] [CrossRef]
- Prucker, O.; Schimmel, M.; Tovar, G.; Knoll, W.; Rühe, J. Microstructuring of Molecularly Thin Polymer Layers by Photolithography. Adv. Mater. 1998, 10, 1073–1077. [Google Scholar] [CrossRef]
- Amin, I.; Steenackers, M.; Zhang, N.; Beyer, A.; Zhang, X.; Pirzer, T.; Hügel, T.; Jordan, R.; Gölzhäuser, A. Polymer carpets. Small 2010, 6, 1623–1630. [Google Scholar] [CrossRef]
- Pester, C.W.; Poelma, J.E.; Narupai, B.; Patel, S.N.; Su, G.M.; Mates, T.E.; Luo, Y.; Ober, C.K.; Hawker, C.J.; Kramer, E.J. Ambiguous anti-fouling surfaces: Facile synthesis by light-mediated radical polymerization. J. Polym. Sci. Part. A Polym. Chem. 2016, 54, 253–262. [Google Scholar] [CrossRef]
- Krishnamoorthy, M.; Hakobyan, S.; Ramstedt, M.; Gautrot, J.E. Surface-Initiated Polymer Brushes in the Biomedical Field: Applications in Membrane Science, Biosensing, Cell Culture, Regenerative Medicine and Antibacterial Coatings. Chem. Rev. 2014, 114, 10976–11026. [Google Scholar] [CrossRef] [PubMed]
- Fromel, M.; Li, M.; Pester, C.W. Surface Engineering with Polymer Brush Photolithography. Macromol. Rapid Commun. 2020. [Google Scholar] [CrossRef]
- Lin, X.; He, Q.; Li, J. Complex polymer brush gradients based on nanolithography and surface-initiated polymerization. Chem. Soc. Rev. 2012, 41, 3584–3593. [Google Scholar] [CrossRef] [PubMed]
- Schmelmer, U.; Paul, A.; Küller, A.; Steenackers, M.; Ulman, A.; Grunze, M.; Gölzhäuser, A.; Jordan, R. Nanostructured Polymer Brushes. Small 2007, 3, 459–465. [Google Scholar] [CrossRef]
- Welch, M.; Rastogi, A.; Ober, C. Polymer brushes for electrochemical biosensors. Soft Matter 2011, 7, 297–302. [Google Scholar] [CrossRef]
- Tomlinson, M.R.; Bhat, R.R.; Genzer, J. Orthogonal polymer brush gradients: Formation and applications. Polym. Prepr. 2005, 46, 44–45. [Google Scholar]
- Günay, K.A.; Schüwer, N.; Klok, H.A. Synthesis and post-polymerization modification of poly(pentafluorophenyl methacrylate) brushes. Polym. Chem. 2012, 3, 2186–2192. [Google Scholar] [CrossRef]
- Page, Z.A.; Narupai, B.; Pester, C.W.; Bou Zerdan, R.; Sokolov, A.; Laitar, D.S.; Mukhopadhyay, S.; Sprague, S.; McGrath, A.J.; Kramer, J.W.; et al. Novel Strategy for Photopatterning Emissive Polymer Brushes for Organic Light Emitting Diode Applications. ACS Cent. Sci. 2017, 3, 654–661. [Google Scholar] [CrossRef]
- Keating, J.J.; Imbrogno, J.; Belfort, G. Polymer Brushes for Membrane Separations: A Review. ACS Appl. Mater. Interfaces 2016, 8, 28383–28399. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, B.; Luo, S.C.; Lin, H.A.; Nakao, A.; Yamashita, Y.; Yu, H.H. Controlled protein absorption and cell adhesion on polymer-brush-grafted poly(3,4-ethylenedioxythiophene) films. ACS Appl. Mater. Interfaces 2013, 5, 4536–4543. [Google Scholar] [CrossRef]
- Gras, S.L.; Mahmud, T.; Rosengarten, G.; Mitchell, A.; Kalantar-Zadeh, K. Intelligent control of surface hydrophobicity. ChemPhysChem 2007, 8, 2036–2050. [Google Scholar] [CrossRef] [PubMed]
- Costantini, F.; Bula, W.P.; Salvio, R.; Huskens, J.; Gardeniers, H.J.G.E.; Reinhoudt, D.N.; Verboom, W. Nanostructure based on polymer brushes for efficient heterogeneous catalysis in microreactors. J. Am. Chem. Soc. 2009, 131, 1650–1651. [Google Scholar] [CrossRef] [PubMed]
- Lego, B.; Skene, W.G.; Giasson, S. Swelling study of responsive polyelectrolyte brushes grafted from mica substrates: Effect of pH, salt, and grafting density. Macromolecules 2010, 43, 4384–4393. [Google Scholar] [CrossRef]
- Kizhakkedathu, J.N.; Norris-Jones, R.; Brooks, D.E. Synthesis of well-defined environmentally-responsive polymer brushes by aqueous ATRP. Macromolecules 2004, 37, 734–743. [Google Scholar] [CrossRef]
- Ito, Y.; Nishi, S.; Park, Y.S.; Imanishi, Y. Oxidoreduction-sensitive control of water permeation through a polymer brushes-grafted porous membrane. Macromolecules 1997, 30, 5856–5859. [Google Scholar] [CrossRef]
- Gupta, S.; Agrawal, M.; Uhlmann, P.; Simon, F.; Oertel, U.; Stamm, M. Gold nanoparticles immobilized on stimuli responsive polymer brushes as nanosensors. Macromolecules 2008, 41, 8152–8158. [Google Scholar] [CrossRef]
- Kong, B.; Choi, J.S.; Jeon, S.; Choi, I.S. The control of cell adhesion and detachment on thin films of thermoresponsive poly[(N-isopropylacrylamide)-r-((3-(methacryloylamino)propyl)-dimethyl(3-sulfopropyl)ammonium hydroxide)]. Biomaterials 2009, 30, 5514–5522. [Google Scholar] [CrossRef]
- Wang, D.; Ye, G.; Weng, X.; Wang, X. Graphene functionalized with azo polymer brushes: Surface-initiated polymerization and photoresponsive properties. Adv. Mater. 2011, 23, 1122–1125. [Google Scholar] [CrossRef]
- Zhou, F.; Biesheuvel, P.M.; Choi, E.Y.; Shu, W.; Poetes, R.; Steiner, U.; Huck, W.T.S. Polyelectrolyte brush amplified electroactuation of microcantilevers. Nano Lett. 2008, 8, 725–730. [Google Scholar] [CrossRef]
- Zakharchenko, A.; Guz, N.; Laradji, A.M.; Katz, E.; Minko, S. Magnetic field remotely controlled selective biocatalysis. Nat. Catal. 2018, 1, 73–81. [Google Scholar] [CrossRef]
- Simocko, C.K.; Frischknecht, A.L.; Huber, D.L. Phase Behavior of Ternary Polymer Brushes. ACS Macro Lett. 2016, 5, 149–153. [Google Scholar] [CrossRef]
- Okrugin, B.M.; Richter, R.P.; Leermakers, F.A.M.; Neelov, I.M.; Borisov, O.V.; Zhulina, E.B. Structure and properties of polydisperse polyelectrolyte brushes studied by self-consistent field theory. Soft Matter 2018, 14, 6230–6242. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hauptmann, N.; Appelhans, D.; Formanek, P.; Frank, S.; Kaskel, S.; Temme, A.; Voit, B. Synthesis of Hetero-Polymer Functionalized Nanocarriers by Combining Surface-Initiated ATRP and RAFT Polymerization. Small 2012, 8, 3579–3583. [Google Scholar] [CrossRef]
- Qian, X.; Lei, J.; Wickramasinghe, S.R. Novel polymeric solid acid catalysts for cellulose hydrolysis. RSC Adv. 2013, 3, 24280–24287. [Google Scholar] [CrossRef]
- Santer, S.; Kopyshev, A.; Donges, J.; Yang, H.K.; Rühe, J. Dynamically Reconfigurable Polymer Films: Impact on Nanomotion. Adv. Mater. 2006, 18, 2359–2362. [Google Scholar] [CrossRef]
- Motornov, M.; Sheparovych, R.; Katz, E.; Minko, S. Chemical gating with nanostructured responsive polymer brushes: Mixed brush versus homopolymer brush. ACS Nano 2008, 2, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Chervanyov, A.; Heinrich, G. Polymer Adsorption onto Selective Mixed Brushes. MRS Proc. 2006, 947, 0947-A10-02. [Google Scholar] [CrossRef]
- Bratek-Skicki, A.; Eloy, P.; Morga, M.; Dupont-Gillain, C. Reversible Protein Adsorption on Mixed PEO/PAA Polymer Brushes: Role of Ionic Strength and PEO Content. Langmuir 2018, 34, 3037–3048. [Google Scholar] [CrossRef] [PubMed]
- Bittrich, E.; Burkert, S.; Eichhorn, K.J.; Stamm, M.; Uhlmann, P. Control of Protein Adsorption and Cell Adhesion by Mixed Polymer Brushes Made by the “Grafting-To” Approach. In Proteins at Interfaces III State of the Art; American Chemical Society: Washington, DC, USA, 2012; pp. 179–193. ISBN 9780841227965. [Google Scholar]
- Bratek-Skicki, A.; Cristaudo, V.; Savocco, J.; Nootens, S.; Morsomme, P.; Delcorte, A.; Dupont-Gillain, C. Mixed Polymer Brushes for the Selective Capture and Release of Proteins. Biomacromolecules 2019, 20, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Delcroix, M.F.; Huet, G.L.; Conard, T.; Demoustier-Champagne, S.; Du Prez, F.E.; Landoulsi, J.; Dupont-Gillain, C.C. Design of mixed PEO/PAA brushes with switchable properties toward protein adsorption. Biomacromolecules 2013, 14, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Psarra, E.; König, U.; Ueda, Y.; Bellmann, C.; Janke, A.; Bittrich, E.; Eichhorn, K.J.; Uhlmann, P. Nanostructured Biointerfaces: Nanoarchitectonics of Thermoresponsive Polymer Brushes Impact Protein Adsorption and Cell Adhesion. ACS Appl. Mater. Interfaces 2015, 7, 12516–12529. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhu, L. Mixed Polymer Brush-Grafted Particles: A New Class of Environmentally Responsive Nanostructured Materials. Macromolecules 2009, 42, 9369–9383. [Google Scholar] [CrossRef]
- Madsen, J.; Ducker, R.E.; Al Jaf, O.; Cartron, M.L.; Alswieleh, A.M.; Smith, C.H.; Hunter, C.N.; Armes, S.P.; Leggett, G.J. Fabrication of microstructured binary polymer brush “corrals” with integral pH sensing for studies of proton transport in model membrane systems. Chem. Sci. 2018, 9, 2238–2251. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Madsen, J.; Chapman, P.; Alswieleh, A.; Al-Jaf, O.; Bao, P.; Hurley, C.R.; Cartron, M.L.; Evans, S.D.; Hobbs, J.K.; et al. Micrometre and nanometre scale patterning of binary polymer brushes, supported lipid bilayers and proteins. Chem. Sci. 2017, 8, 4517–4526. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Cui, L.; Chen, R.; Xu, X.; Chen, J.; Yin, L.; Liu, J.; Shi, Q.; Yin, J. Facile Fabrication of Hierarchically Thermoresponsive Binary Polymer Pattern for Controlled Cell Adhesion. Macromol. Rapid Commun. 2018, 39, 1700572. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Nakaji-Hirabayashi, T.; Kitano, H.; Ohno, K.; Saruwatari, Y.; Matsuoka, K. A novel approach for UV-patterning with binary polymer brushes. Colloids Surf. B Biointerfaces 2018, 161, 42–50. [Google Scholar] [CrossRef]
- Narupai, B.; Page, Z.A.; Treat, N.J.; McGrath, A.J.; Pester, C.W.; Discekici, E.H.; Dolinski, N.D.; Meyers, G.F.; Read de Alaniz, J.; Hawker, C.J. Simultaneous Preparation of Multiple Polymer Brushes under Ambient Conditions using Microliter Volumes. Angew. Chem. 2018, 57, 13433–13438. [Google Scholar] [CrossRef]
- Pester, C.W.; Narupai, B.; Mattson, K.M.; Bothman, D.P.; Klinger, D.; Lee, K.W.; Discekici, E.H.; Hawker, C.J. Engineering Surfaces through Sequential Stop-Flow Photopatterning. Adv. Mater. 2016, 28, 9292–9300. [Google Scholar] [CrossRef] [PubMed]
- Arnold, R.M.; Locklin, J. Self-Sorting Click Reactions That Generate Spatially Controlled Chemical Functionality on Surfaces. Langmuir 2013, 29, 5920–5926. [Google Scholar] [CrossRef]
- del Campo, A.; Boos, D.; Spiess, H.W.; Jonas, U. Surface Modification with Orthogonal Photosensitive Silanes for Sequential Chemical Lithography and Site-Selective Particle Deposition. Angew. Chem. 2005, 44, 4707–4712. [Google Scholar] [CrossRef] [PubMed]
- Arnold, R.M.; Patton, D.L.; Popik, V.V.; Locklin, J. A Dynamic Duo: Pairing Click Chemistry and Postpolymerization Modification To Design Complex Surfaces. Acc. Chem. Res. 2014, 47, 2999–3008. [Google Scholar] [CrossRef] [PubMed]
- Uhlmann, P.; Ionov, L.; Houbenov, N.; Nitschke, M.; Grundke, K.; Motornov, M.; Minko, S.; Stamm, M. Surface functionalization by smart coatings: Stimuli-responsive binary polymer brushes. Prog. Org. Coat. 2006, 55, 168–174. [Google Scholar] [CrossRef]
- Tsujii, Y.; Ohno, K.; Yamamoto, S.; Goto, A.; Fukuda, T. Structure and properties of high-density polymer brushes prepared by surface-initiated living radical polymerization. Adv. Polym. Sci. 2006, 197, 1–45. [Google Scholar] [CrossRef]
- Zhao, B.; Brittain, W.J. Polymer brushes: Surface-immobilized macromolecules. Prog. Polym. Sci. 2000, 25, 677–710. [Google Scholar] [CrossRef]
- Léonforte, F.; Müller, M. Poly(N -isopropylacrylamide)-Based Mixed Brushes: A Computer Simulation Study. ACS Appl. Mater. Interfaces 2015, 7, 12450–12462. [Google Scholar] [CrossRef]
- Minko, S.; Patil, S.; Datsyuk, V.; Simon, F.; Eichhorn, K.J.; Motornov, M.; Usov, D.; Tokarev, I.; Stamm, M. Synthesis of adaptive polymer brushes via “grafting to” approach from melt. Langmuir 2002, 18, 289–296. [Google Scholar] [CrossRef]
- Houbenov, N.; Minko, S.; Stamm, M. Mixed polyelectrolyte brush from oppositely charged polymers for switching of surface charge and composition in aqueous environment. Macromolecules 2003, 36, 5897–5901. [Google Scholar] [CrossRef]
- Xu, G.; Liu, P.; Pranantyo, D.; Xu, L.; Neoh, K.G.; Kang, E.T. Antifouling and Antimicrobial Coatings from Zwitterionic and Cationic Binary Polymer Brushes Assembled via “click” Reactions. Ind. Eng. Chem. Res. 2017, 56, 14479–14488. [Google Scholar] [CrossRef]
- Motornov, M.; Sheparovych, R.; Tokarev, I.; Roiter, Y.; Minko, S. Nonwettable thin films from hybrid polymer brushes can be hydrophilic. Langmuir 2007, 23, 13–19. [Google Scholar] [CrossRef]
- Arnold, R.M.; McNitt, C.D.; Popik, V.V.; Locklin, J. Direct grafting of poly(pentafluorophenyl acrylate) onto oxides: Versatile substrates for reactive microcapillary printing and self-sorting modification. Chem. Commun. 2014, 50, 5307–5309. [Google Scholar] [CrossRef] [PubMed]
- Escorihuela, J.; Marcelis, A.T.M.; Zuilhof, H. Metal-Free Click Chemistry Reactions on Surfaces. Adv. Mater. Interfaces 2015, 2, 1500135. [Google Scholar] [CrossRef]
- Julthongpiput, D.; Lin, Y.H.; Teng, J.; Zubarev, E.R.; Tsukruk, V.V. Y-shaped polymer brushes: Nanoscale switchable surfaces. Langmuir 2003, 19, 7832–7836. [Google Scholar] [CrossRef]
- Julthongpiput, D.; Lin, Y.H.; Teng, J.; Zubarev, E.R.; Tsukruk, V.V. Y-Shaped Amphiphilic Brushes with Switchable Micellar Surface Structures. J. Am. Chem. Soc. 2003, 125, 15912–15921. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhou, S.; You, B.; Wu, L. Polymer brush-functionalized surfaces with unique reversible double-stimulus responsive wettability. J. Mater. Chem. A 2013, 1, 10646–10654. [Google Scholar] [CrossRef]
- Wenning, L.; Müller, M.; Binder, K. How does the pattern of grafting points influence the structure of one-component and mixed polymer brushes? Europhys. Lett. 2005, 71, 639–645. [Google Scholar] [CrossRef]
- Santer, S.; Kopyshev, A.; Donges, J.; Rühe, J.; Jiang, X.; Zhao, B.; Müller, M. Memory of Surface Patterns in Mixed Polymer Brushes: Simulation and Experiment. Langmuir 2007, 23, 279–285. [Google Scholar] [CrossRef]
- Boven, G.; Oosterling, M.L.C.M.; Challa, G.; Jan Schouten, A. Grafting kinetics of poly(methyl methacrylate) on microparticulate silica. Polymer 1990, 31, 2377–2383. [Google Scholar] [CrossRef]
- Li, M.; Fromel, M.; Ranaweera, D.; Rocha, S.; Boyer, C.; Pester, C.W. SI-PET-RAFT: Surface-Initiated Photoinduced Electron Transfer-Reversible Addition–Fragmentation Chain Transfer Polymerization. ACS Macro Lett. 2019, 8, 374–380. [Google Scholar] [CrossRef]
- Discekici, E.H.; Pester, C.W.; Treat, N.J.; Lawrence, J.; Mattson, K.M.; Narupai, B.; Toumayan, E.P.; Luo, Y.; McGrath, A.J.; Clark, P.G.; et al. Simple Benchtop Approach to Polymer Brush Nanostructures Using Visible-Light-Mediated Metal-Free Atom Transfer Radical Polymerization. ACS Macro Lett. 2016, 5, 258–262. [Google Scholar] [CrossRef]
- Poelma, J.E.; Fors, B.P.; Meyers, G.F.; Kramer, J.W.; Hawker, C.J. Fabrication of complex three-dimensional polymer brush nanostructures through light-mediated living radical polymerization. Angew. Chem. 2013, 52, 6844–6848. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yu, B.; Huck, W.T.S.; Zhou, F.; Liu, W. Electrochemically induced surface-initiated atom-transfer radical polymerization. Angew. Chem. 2012, 51, 5092–5095. [Google Scholar] [CrossRef] [PubMed]
- Sidorenko, A.; Minko, S.; Schenk-Meuser, K.; Duschner, H.; Stamm, M. Switching of polymer brushes. Langmuir 1999, 15, 8349–8355. [Google Scholar] [CrossRef]
- Hiemenz, P.C.; Lodge, T.P. Polymer Chemistry, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-1574447798. [Google Scholar]
- Minko, S.; Usov, D.; Goreshnik, E.; Stamm, M. Environment-Adopting Surfaces with Reversibly Switchable Morphology. Macromol. Rapid Commun. 2001, 22, 206–211. [Google Scholar] [CrossRef]
- Minko, S.; Müller, M.; Usov, D.; Scholl, A.; Froeck, C.; Stamm, M. Lateral versus Perpendicular Segregation in Mixed Polymer Brushes. Phys. Rev. Lett. 2002, 88, 035502. [Google Scholar] [CrossRef]
- Motornov, M.; Minko, S.; Eichhorn, K.J.; Nitschke, M.; Simon, F.; Stamm, M. Reversible tuning of wetting behavior of polymer surface with responsive polymer brushes. Langmuir 2003, 19, 8077–8085. [Google Scholar] [CrossRef]
- Draper, J.; Luzinov, I.; Minko, S.; Tokarev, I.; Stamm, M. Mixed polymer brushes by sequential polymer addition: Anchoring layer effect. Langmuir 2004, 20, 4064–4075. [Google Scholar] [CrossRef]
- Kumar Vyas, M.; Schneider, K.; Nandan, B.; Stamm, M. Switching of friction by binary polymer brushes. Soft Matter 2008, 4, 1024–1032. [Google Scholar] [CrossRef]
- Minko, S.; Ionov, L.; Sydorenko, A.; Houbenov, N.; Stamm, M.; Zdyrko, B.; Klep, V.; Luzinov, I. Gradient Stimuli-Responsive Polymer Grafted Layers. In Stimuli-Responsive Polymeric Films and Coatings; American Chemical Society: Washington, DC, USA, 2005; pp. 68–83. ISBN 9780841239326. [Google Scholar]
- Lemieux, M.; Usov, D.; Minko, S.; Stamm, M.; Shulha, H.; Tsukruk, V.V. Reorganization of binary polymer brushes: Reversible switching of surface microstructures and nanomechanical properties. Macromolecules 2003, 36, 7244–7255. [Google Scholar] [CrossRef]
- Zhao, B. Synthesis of binary mixed homopolymer brushes by combining atom transfer radical polymerization and nitroxide-mediated radical polymerization. Polymer 2003, 44, 4079–4083. [Google Scholar] [CrossRef]
- Zhao, B.; Haasch, R.T.; MacLaren, S. Solvent-Induced Self-Assembly of Mixed Poly(methyl methacrylate)/Polystyrene Brushes on Planar Silica Substrates: Molecular Weight Effect. J. Am. Chem. Soc. 2004, 126, 6124–6134. [Google Scholar] [CrossRef]
- Zhao, B.; Haasch, R.T.; MacLaren, S. Self-reorganization of mixed poly(methyl methacrylate)/polystyrene brushes on planar silica substrates in response to combined selective solvent treatments and thermal annealing. Polymer 2004, 45, 7979–7988. [Google Scholar] [CrossRef]
- Santer, S.; Kopyshev, A.; Yang, H.K.; Rühe, J. Local Composition of Nanophase-Separated Mixed Polymer Brushes. Macromolecules 2006, 39, 3056–3064. [Google Scholar] [CrossRef]
- Filimon, M.; Kopf, I.; Ballout, F.; Schmidt, D.A.; Bründermann, E.; Rühe, J.; Santer, S.; Havenith, M. Smart polymer surfaces: Mapping chemical landscapes on the nanometre scale. Soft Matter 2010, 6, 3764–3768. [Google Scholar] [CrossRef]
- Price, A.D.; Hur, S.M.; Fredrickson, G.H.; Frischknecht, A.L.; Huber, D.L. Exploring lateral microphase separation in mixed polymer brushes by experiment and self-consistent field theory simulations. Macromolecules 2012, 45, 510–524. [Google Scholar] [CrossRef]
- Li, D.; Sheng, X.; Zhao, B. Environmentally responsive “hairy” nanoparticles: Mixed homopolymer brushes on silica nanoparticles synthesized by living radical polymerization techniques. J. Am. Chem. Soc. 2005, 127, 6248–6256. [Google Scholar] [CrossRef] [PubMed]
- Fox, T.L.; Tang, S.; Horton, J.M.; Holdaway, H.A.; Zhao, B.; Zhu, L.; Stewart, P.L. In Situ Characterization of Binary Mixed Polymer Brush-Grafted Silica Nanoparticles in Aqueous and Organic Solvents by Cryo-Electron Tomography. Langmuir 2015, 31, 8680–8688. [Google Scholar] [CrossRef] [PubMed]
- Thiessen, W.; Wolff, T. NMRP and ATRP double initiators for the formation of binary polymer brushes via grafting-from methods. Des. Monomers Polym. 2011, 14, 287–302. [Google Scholar] [CrossRef]
- Jiang, X.; Zhong, G.; Horton, J.M.; Jin, N.; Zhu, L.; Zhao, B. Evolution of phase morphology of mixed poly(tert -butyl acrylate)/Polystyrene brushes grafted on silica particles with the change of chain length disparity. Macromolecules 2010, 43, 5387–5395. [Google Scholar] [CrossRef]
- Jiang, X.; Zhao, B.; Zhong, G.; Jin, N.; Horton, J.M.; Zhu, L.; Hafner, R.S.; Lodge, T.P. Microphase separation of high grafting density asymmetric mixed homopolymer brushes on silica particles. Macromolecules 2010, 43, 8209–8217. [Google Scholar] [CrossRef]
- Horton, J.M.; Tang, S.; Bao, C.; Tang, P.; Qiu, F.; Zhu, L.; Zhao, B. Truncated wedge-shaped nanostructures formed from lateral microphase separation of mixed homopolymer brushes grafted on 67 nm silica nanoparticles: Evidence of the effect of substrate curvature. ACS Macro Lett. 2012, 1, 1061–1065. [Google Scholar] [CrossRef]
- Bao, C.; Tang, S.; Horton, J.M.; Jiang, X.; Tang, P.; Qiu, F.; Zhu, L.; Zhao, B. Effect of overall grafting density on microphase separation of mixed homopolymer brushes synthesized from Y-initiator-functionalized silica particles. Macromolecules 2012, 45, 8027–8036. [Google Scholar] [CrossRef]
- Tang, S.; Lo, T.Y.; Horton, J.M.; Bao, C.; Tang, P.; Qiu, F.; Ho, R.M.; Zhao, B.; Zhu, L. Direct visualization of three-dimensional morphology in hierarchically self-assembled mixed poly(tert-butyl acrylate)/polystyrene brush-grafted silica nanoparticles. Macromolecules 2013, 46, 6575–6584. [Google Scholar] [CrossRef]
- Bao, C.; Tang, S.; Wright, R.A.E.; Tang, P.; Qiu, F.; Zhu, L.; Zhao, B. Effect of molecular weight on lateral microphase separation of mixed homopolymer brushes grafted on silica particles. Macromolecules 2014, 47, 6824–6835. [Google Scholar] [CrossRef]
- Tang, S.; Fox, T.L.; Lo, T.Y.; Horton, J.M.; Ho, R.M.; Zhao, B.; Stewart, P.L.; Zhu, L. Environmentally responsive self-assembly of mixed poly(tert-butyl acrylate)–polystyrene brush-grafted silica nanoparticles in selective polymer matrices. Soft Matter 2015, 11, 5501–5512. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, Y.; Xiao, S.; Zhang, L.; Huang, L.; Chen, F.; Fan, P.; Zhong, M.; Tan, J.; Yang, J. Mixed polymer brushes with integrated antibacterial and antifouling properties. Prog. Org. Coat. 2019, 130, 75–82. [Google Scholar] [CrossRef]
- Estillore, N.C.; Advincula, R.C. Stimuli-responsive binary mixed polymer brushes and free-standing films by LbL-SIP. Langmuir 2011, 27, 5997–6008. [Google Scholar] [CrossRef]
- Priftis, D.; Sakellariou, G.; Baskaran, D.; Mays, J.W.; Hadjichristidis, N. Polymer grafted Janus multi-walled carbon nanotubes. Soft Matter 2009, 5, 4272–4278. [Google Scholar] [CrossRef]
- Li, W.; Bao, C.; Wright, R.A.E.; Zhao, B. Synthesis of mixed poly(ε-caprolactone)/polystyrene brushes from Y-initiator-functionalized silica particles by surface-initiated ring-opening polymerization and nitroxide-mediated radical polymerization. RSC Adv. 2014, 4, 18772–18781. [Google Scholar] [CrossRef]
- Wang, Y.; Brittain, W.J. Simultaneous Binary Mixed Homopolymer Brush Formation by Combining Nitroxide-Mediated Radical Polymerization and Living Cationic Ring-Opening Polymerization. Macromol. Rapid Commun. 2007, 28, 811–815. [Google Scholar] [CrossRef]
- Jenkins, A.D.; Jones, R.G.; Moad, G. Terminology for reversible-deactivation radical polymerization previously called “controlled” radical or “living” radical polymerization (IUPAC Recommendations 2010). Pure Appl. Chem. 2009, 82, 483–491. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Atom Transfer Radical Polymerization (ATRP): Current status and future perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Pearson, S.; St Thomas, C.; Guerrero-Santos, R.; D’Agosto, F. Opportunities for dual RDRP agents in synthesizing novel polymeric materials. Polym. Chem. 2017, 8, 4916–4946. [Google Scholar] [CrossRef]
- Nicolaÿ, R.; Kwak, Y.; Matyjaszewski, K. Synthesis of poly(vinyl acetate) block copolymers by successive RAFT and ATRP with a bromoxanthate iniferter. Chem. Commun. 2008, 5336–5338. [Google Scholar] [CrossRef]
- Huang, C.F.; Nicolaÿ, R.; Kwak, Y.; Chang, F.C.; Matyjaszewski, K. Homopolymerization and Block Copolymerization of N-Vinylpyrrolidone by ATRP and RAFT with Haloxanthate Inifers. Macromolecules 2009, 42, 8198–8210. [Google Scholar] [CrossRef]
- Kwak, Y.; Matyjaszewski, K. Effect of Initiator and Ligand Structures on ATRP of Styrene and Methyl Methacrylate Initiated by Alkyl Dithiocarbamate. Macromolecules 2008, 41, 6627–6635. [Google Scholar] [CrossRef]
- Xue, Y.; Li, X.; Li, H.; Zhang, W. Quantifying thiol–gold interactions towards the efficient strength control. Nat. Commun. 2014, 5, 4348. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Miller, P.J.; Shukla, N.; Immaraporn, B.; Gelman, A.; Luokala, B.B.; Siclovan, T.M.; Kickelbick, G.; Vallant, T.; Hoffmann, H.; et al. Polymers at Interfaces: Using Atom Transfer Radical Polymerization in the Controlled Growth of Homopolymers and Block Copolymers from Silicon Surfaces in the Absence of Untethered Sacrificial Initiator. Macromolecules 1999, 32, 8716–8724. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Dong, H.; Jakubowski, W.; Pietrasik, J.; Kusumo, A. Grafting from Surfaces for “Everyone”: ARGET ATRP in the Presence of Air. Langmuir 2007, 23, 4528–4531. [Google Scholar] [CrossRef]
- Husseman, M.; Malmström, E.E.; McNamara, M.; Mate, M.; Mecerreyes, D.; Benoit, D.G.; Hedrick, J.L.; Mansky, P.; Huang, E.; Russell, T.P.; et al. Controlled Synthesis of Polymer Brushes by “Living” Free Radical Polymerization Techniques. Macromolecules 1999, 32, 1424–1431. [Google Scholar] [CrossRef]
- Genzer, J. In silico polymerization: Computer simulation of controlled radical polymerization in bulk and on flat surfaces. Macromolecules 2006, 39, 7157–7169. [Google Scholar] [CrossRef]
- Baum, M.; Brittain, W.J. Synthesis of polymer brushes on silicate substrates via reversible addition fragmentation chain transfer technique. Macromolecules 2002, 35, 610–615. [Google Scholar] [CrossRef]
- Mattson, K.M.; Pester, C.W.; Gutekunst, W.R.; Hsueh, A.T.; Discekici, E.H.; Luo, Y.; Schmidt, B.V.K.J.; McGrath, A.J.; Clark, P.G.; Hawker, C.J. Metal-Free Removal of Polymer Chain Ends Using Light. Macromolecules 2016, 49, 8162–8166. [Google Scholar] [CrossRef]
- Discekici, E.H.; Shankel, S.L.; Anastasaki, A.; Oschmann, B.; Lee, I.H.; Niu, J.; McGrath, A.J.; Clark, P.G.; Laitar, D.S.; De Alaniz, J.R.; et al. Dual-pathway chain-end modification of RAFT polymers using visible light and metal-free conditions. Chem. Commun. 2017, 53, 1888–1891. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, J.; Häkkinen, S.; Boeck, P.T.; Cong, Y.; Perrier, S.; Sheiko, S.S.; You, W. Orthogonal Cationic and Radical RAFT Polymerizations to Prepare Bottlebrush Polymers. Angew. Chem. 2020, 59, 7203–7208. [Google Scholar] [CrossRef]
- Aoshima, H.; Uchiyama, M.; Satoh, K.; Kamigaito, M. Interconvertible Living Radical and Cationic Polymerization through Reversible Activation of Dormant Species with Dual Activity. Angew. Chem. 2014, 53, 10932–10936. [Google Scholar] [CrossRef]
- Uchiyama, M.; Satoh, K.; Kamigaito, M. Cationic RAFT Polymerization Using ppm Concentrations of Organic Acid. Angew. Chem. 2015, 54, 1924–1928. [Google Scholar] [CrossRef]
- Guerre, M.; Uchiyama, M.; Folgado, E.; Semsarilar, M.; Améduri, B.; Satoh, K.; Kamigaito, M.; Ladmiral, V. Combination of Cationic and Radical RAFT Polymerizations: A Versatile Route to Well-Defined Poly(ethyl vinyl ether)- block -poly(vinylidene fluoride) Block Copolymers. ACS Macro Lett. 2017, 6, 393–398. [Google Scholar] [CrossRef]
- Satoh, K.; Hashimoto, H.; Kumagai, S.; Aoshima, H.; Uchiyama, M.; Ishibashi, R.; Fujiki, Y.; Kamigaito, M. One-shot controlled/living copolymerization for various comonomer sequence distributions via dual radical and cationic active species from RAFT terminals. Polym. Chem. 2017, 8, 5002–5011. [Google Scholar] [CrossRef]
- Guerre, M.; Uchiyama, M.; Lopez, G.; Améduri, B.; Satoh, K.; Kamigaito, M.; Ladmiral, V. Synthesis of PEVE- b -P(CTFE- alt -EVE) block copolymers by sequential cationic and radical RAFT polymerization. Polym. Chem. 2018, 9, 352–361. [Google Scholar] [CrossRef]
- Wong, C.H.; Zimmerman, S.C. Orthogonality in organic, polymer, and supramolecular chemistry: From Merrifield to click chemistry. Chem. Commun. 2013, 49, 1679–1695. [Google Scholar] [CrossRef]
- Runge, M.B.; Dutta, S.; Bowden, N.B. Synthesis of Comb Block Copolymers by ROMP, ATRP, and ROP and Their Assembly in the Solid State. Macromolecules 2006, 39, 498–508. [Google Scholar] [CrossRef]
- Cheng, C.; Khoshdel, E.; Wooley, K.L. Facile One-Pot Synthesis of Brush Polymers through Tandem Catalysis Using Grubbs’ Catalyst for Both Ring-Opening Metathesis and Atom Transfer Radical Polymerizations. Nano Lett. 2006, 6, 1741–1746. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Xu, J.; Kokotovic, M.; Boyer, C. One-Pot Synthesis of Block Copolymers by Orthogonal Ring-Opening Polymerization and PET-RAFT Polymerization at Ambient Temperature. ACS Macro Lett. 2016, 5, 444–449. [Google Scholar] [CrossRef]
- Fu, C.; Xu, J.; Boyer, C. Photoacid-mediated ring opening polymerization driven by visible light. Chem. Commun. 2016, 52, 7126–7129. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, N.; Boyer, C. 100th Anniversary of Macromolecular Science Viewpoint: Photochemical Reaction Orthogonality in Modern Macromolecular Science. ACS Macro Lett. 2019, 8, 812–818. [Google Scholar] [CrossRef]
- Peterson, B.M.; Kottisch, V.; Supej, M.J.; Fors, B.P. On Demand Switching of Polymerization Mechanism and Monomer Selectivity with Orthogonal Stimuli. ACS Cent. Sci. 2018, 4, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Supej, M.J.; Peterson, B.M.; Fors, B.P. Dual Stimuli Switching: Interconverting Cationic and Radical Polymerizations with Electricity and Light. Chem 2020. [Google Scholar] [CrossRef]
- Satoh, K.; Sun, Z.; Uchiyama, M.; Kamigaito, M.; Xu, J.; Boyer, C. Interconvertible and switchable cationic/PET-RAFT copolymerization triggered by visible light. Polym. J. 2020, 52, 65–73. [Google Scholar] [CrossRef]
- Qi, M.; Dong, Q.; Wang, D.; Byers, J.A. Electrochemically Switchable Ring-Opening Polymerization of Lactide and Cyclohexene Oxide. J. Am. Chem. Soc. 2018, 140, 5686–5690. [Google Scholar] [CrossRef]
- Teator, A.J.; Lastovickova, D.N.; Bielawski, C.W. Switchable polymerization catalysts. Chem. Rev. 2016, 116, 1969–1992. [Google Scholar] [CrossRef] [PubMed]
- Bates, C.M.; Bates, F.S. 50th Anniversary Perspective: Block Polymers—Pure Potential. Macromolecules 2017, 50, 3–22. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, X.; Yang, P.; Peng, J.; Luo, C.; Huang, W.; Han, Y. Microphase Separation of Block Copolymer Thin Films. Macromol. Rapid Commun. 2010, 31, 591–608. [Google Scholar] [CrossRef]
- Leibler, L. Theory of Microphase Separation in Block Copolymers. Macromolecules 1980, 13, 1602–1617. [Google Scholar] [CrossRef]
- Zhulina, E.; Balazs, A.C. Designing Patterned Surfaces by Grafting Y-Shaped Copolymers. Macromolecules 1996, 29, 2667–2673. [Google Scholar] [CrossRef]
- Wang, J.; Müller, M. Microphase Separation of Mixed Polymer Brushes: Dependence of the Morphology on Grafting Density, Composition, Chain-Length Asymmetry, Solvent Quality, and Selectivity. J. Phys. Chem. B 2009, 113, 11384–11402. [Google Scholar] [CrossRef]
- Witten, T.A.; Milner, S.T. Two-Component Grafted Polymer Layers. MRS Proc. 1989, 177, 37. [Google Scholar] [CrossRef]
- Marko, J.F.; Witten, T.A. Phase separation in a grafted polymer layer. Phys. Rev. Lett. 1991, 66, 1541–1544. [Google Scholar] [CrossRef]
- Müller, M. Phase diagram of a mixed polymer brush. Phys. Rev. E 2002, 65, 030802. [Google Scholar] [CrossRef]
- Brittain, W.J.; Minko, S. A structural definition of polymer brushes. J. Polym. Sci. Part. A Polym. Chem. 2007, 45, 3505–3512. [Google Scholar] [CrossRef]
- Matsen, M.W.; Bates, F.S. Unifying weak- and strong-segregation block copolymer theories. Macromolecules 1996, 29, 1091–1098. [Google Scholar] [CrossRef]
- Akgun, B.; Uğur, G.; Brittain, W.J.; Majkrzak, C.F.; Li, X.; Wang, J.; Li, H.; Wu, D.T.; Wang, Q.; Foster, M.D. Internal Structure of Ultrathin Diblock Copolymer Brushes. Macromolecules 2009, 42, 8411–8422. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, G.; Tang, P.; Qiu, F.; Yang, Y.; Zhu, L. Mixed homopolymer brushes grafted onto a nanosphere. J. Chem. Phys. 2011, 134, 134903. [Google Scholar] [CrossRef] [PubMed]
- Egorov, S.A. Microphase separation of mixed polymer brushes physisorbed on cylindrical surfaces. Soft Matter 2012, 8, 3971–3979. [Google Scholar] [CrossRef]
- Ma, X.; Yang, Y.; Zhu, L.; Zhao, B.; Tang, P.; Qiu, F. Binary mixed homopolymer brushes grafted on nanorod particles: A self-consistent field theory study. J. Chem. Phys. 2013, 139, 214902. [Google Scholar] [CrossRef]
- Ma, X.; Chen, C.Y.; Yang, Y.Z.; Qiu, F. Ripple structures of mixed homopolymer brushes grafted on cylindrical surfaces: Controlling the orientation of the pattern by attuning the substrate curvatures. Soft Matter 2014, 10, 6005–6013. [Google Scholar] [CrossRef]
- Dong, H. Phase separation of grafted polymers under strong demixing interaction. J. Phys. II 1993, 3, 999–1020. [Google Scholar] [CrossRef]
- Lai, P.Y. Binary mixture of grafted polymer chains: A Monte Carlo simulation. J. Chem. Phys. 1994, 100, 3351–3357. [Google Scholar] [CrossRef]
- Soga, K.G.; Zuckermann, M.J.; Guo, H. Binary polymer brush in a solvent. Macromolecules 1996, 29, 1998–2005. [Google Scholar] [CrossRef]
- Hur, S.M.; Frischknecht, A.L.; Huber, D.L.; Fredrickson, G.H. Self-assembly in a mixed polymer brush with inhomogeneous grafting density composition. Soft Matter 2013, 9, 5341–5354. [Google Scholar] [CrossRef]
- Yin, Y.; Jiang, R.; Wang, Z.; Li, B.; Shi, A.C. Influence of Grafting Point Distribution on the Surface Structures of Y-Shaped Polymer Brushes in Solution. Langmuir 2016, 32, 7467–7475. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Chapman, W.G. Solvent response of mixed polymer brushes. J. Chem. Phys. 2011, 135, 214901. [Google Scholar] [CrossRef] [PubMed]
- Romeis, D.; Sommer, J.U. Binary and Bidisperse Polymer Brushes: Coexisting Surface States. ACS Appl. Mater. Interfaces 2015, 7, 12496–12504. [Google Scholar] [CrossRef] [PubMed]
- Ionov, L.; Minko, S. Mixed polymer brushes with locking switching. ACS Appl. Mater. Interfaces 2012, 4, 483–489. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, X.; Han, X.; Xu, S.; Liu, H.; Hu, Y. Lock/Unlock Mechanism of Solvent-Responsive Binary Polymer Brushes: Density Functional Theory Approach. Langmuir 2013, 29, 4988–4997. [Google Scholar] [CrossRef]
- Koenig, M.; Magerl, D.; Philipp, M.; Eichhorn, K.J.; Müller, M.; Müller-Buschbaum, P.; Stamm, M.; Uhlmann, P. Nanocomposite coatings with stimuli-responsive catalytic activity. RSC Adv. 2014, 4, 17579–17586. [Google Scholar] [CrossRef]
- Szleifer, I. Protein Adsorption on Surfaces with Grafted Polymers. Biophys. J. 1997, 72, 595–612. [Google Scholar] [CrossRef]
- Satulovsky, J.; Carignano, M.A.; Szleifer, I. Kinetic and thermodynamic control of protein adsorption. Proc. Natl. Acad. Sci. USA 2000, 97, 9037–9041. [Google Scholar] [CrossRef]
- Halperin, A. Polymer Brushes that Resist Adsorption of Model Proteins: Design Parameters. Langmuir 1999, 15, 2525–2533. [Google Scholar] [CrossRef]
- Zhou, Y.N.; Li, J.J.; Wu, Y.Y.; Luo, Z.H. Role of External Field in Polymerization: Mechanism and Kinetics. Chem. Rev. 2020, 120, 2950–3048. [Google Scholar] [CrossRef]
- Pan, X.; Fantin, M.; Yuan, F.; Matyjaszewski, K. Externally controlled atom transfer radical polymerization. Chem. Soc. Rev. 2018, 47, 5457–5490. [Google Scholar] [CrossRef] [PubMed]
- Reyhani, A.; Mazaheri, O.; Alivand, M.S.; Mumford, K.A.; Qiao, G. Temporal control of RAFT polymerization via magnetic catalysis. Polym. Chem. 2020, 11, 2838–2846. [Google Scholar] [CrossRef]
- Hu, L.; Wang, Q.; Zhang, X.; Zhao, H.; Cui, Z.; Fu, P.; Liu, M.; Liu, N.; He, S.; Pang, X.; et al. Light and magnetism dual-gated photoinduced electron transfer-reversible addition–fragmentation chain transfer (PET-RAFT) polymerization. RSC Adv. 2020, 10, 6850–6857. [Google Scholar] [CrossRef]
- Zaborniak, I.; Chmielarz, P. Ultrasound-Mediated Atom Transfer Radical Polymerization (ATRP). Materials 2019, 12, 3600. [Google Scholar] [CrossRef]
- Leibfarth, F.A.; Mattson, K.M.; Fors, B.P.; Collins, H.A.; Hawker, C.J. External Regulation of Controlled Polymerizations. Angew. Chem. 2013, 52, 199–210. [Google Scholar] [CrossRef]
- Kikuchi, S.; Saito, K.; Akita, M.; Inagaki, A. Nonradical Light-Controlled Polymerization of Styrene and Vinyl Ethers Catalyzed by an Iridium–Palladium Photocatalyst. Organometallics 2018, 37, 359–366. [Google Scholar] [CrossRef]
- Turgman-Cohen, S.; Genzer, J. Simultaneous Bulk- and Surface-Initiated Controlled Radical Polymerization from Planar Substrates. J. Am. Chem. Soc. 2011, 133, 17567–17569. [Google Scholar] [CrossRef]
- Wang, C.; Araki, T.; Watts, B.; Harton, S.; Koga, T.; Basu, S.; Ade, H. Resonant soft x-ray reflectivity of organic thin films. J. Vac. Sci. Technol. A Vac. Surf. Film. 2007, 25, 575–586. [Google Scholar] [CrossRef]
- Welch, C.F.; Hjelm, R.P.; Mang, J.T.; Hawley, M.E.; Wrobleski, D.A.; Bruce Orler, E.; Kortright, J.B. Resonant soft x-ray scattering and reflectivity study of the phase-separated structure of thin poly(styrene-b-methyl methacrylate) films. J. Polym. Sci. Part. B Polym. Phys. 2013, 51, 149–157. [Google Scholar] [CrossRef]




| Polymer Combination | Synthetic Approach | Surface Initiator | Substrate | Stimuli | Reference |
|---|---|---|---|---|---|
| PEO/PAA | “grafting to” | N/A | gold | Ion strength | [50] |
| PDMS/EPEI | “grafting to” | N/A | Si | dry/wet | [72] |
| PDMS/P2VP | “grafting to” | N/A | Si/ITO | pH | [48] |
| PAA/P2VP | “grafting to” | N/A | Si | pH | [70] |
| PS/P2VP | sequential SI-FRP | non-selective initiator | Si, polyamides film | solvent | [85,87,88,89] |
| “grafting to” | N/A | Si, silica NP | [69,90,91,92] | ||
| PSF/PMMA | sequential SI-FRP | non-selective | Si | solvent | [88,93] |
| PMMA/PS | sequential SI-ATRP/SI-NMP | co-deposited | Si, silica | solvent | [79,94] |
| Y-shaped | [79,95,96] | ||||
| sequential SI-FRP | non-selective | [47,97,98,99] | |||
| PS/PMMA/P4VP | sequential SI-FRP | non-selective | Si | N/A | [43] |
| PAA/PS | sequential SI-FRP | non-selective | Si | solvent | [88] |
| “grafting to” (Y-shaped diblock) | N/A | [75,76] | |||
| sequential SI-ATRP/SI-NMP | Y-shaped | silica NP | [100,101] | ||
| PS/P4VP | sequential SI-ATRP/SI-NMP | co-deposited | silica | solvent | [102] |
| Y-shaped | |||||
| PtBA/PS | sequential SI-ATRP/SI-NMP | Y-shaped | silica NP | N/A | [103,104,105,106,107,108,109] |
| PHPMA/PDEAEMA | sequential SI-ATRP/SI-RAFT | Y-shaped | MSN | pH | [45] |
| PHEAA/PMETA | sequential SI-ATRP/SI-PIMP | co-deposited | Si | N/A | [110] |
| PSSA/PIL | sequential SI-ATRP/SI-FRP | co-deposited | glass, ceramic membrane | N/A | [46] |
| PNIPAm/PS | sequential SI-ATRP/SI-FRP | Layer-by-Layer deposited | Si, free standing polymer film | solvent, temperature | [111] |
| PS/PεCL | one-pot SI-ATRP/SI-ROP | co-deposited | CNT | solvent | [112] |
| sequential SI-ROP/SI-NMP | Y-shaped | silica NP | [113] | ||
| PMMA/PLLA | one-pot SI-ATRP/SI-ROP | co-deposited | CNT | solvent | [112] |
| PS/PPhOXA | one-pot SI-NMP/SI-ROP | Y-shaped | Si | solvent | [114] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Pester, C.W. Mixed Polymer Brushes for “Smart” Surfaces. Polymers 2020, 12, 1553. https://doi.org/10.3390/polym12071553
Li M, Pester CW. Mixed Polymer Brushes for “Smart” Surfaces. Polymers. 2020; 12(7):1553. https://doi.org/10.3390/polym12071553
Chicago/Turabian StyleLi, Mingxiao, and Christian W. Pester. 2020. "Mixed Polymer Brushes for “Smart” Surfaces" Polymers 12, no. 7: 1553. https://doi.org/10.3390/polym12071553
APA StyleLi, M., & Pester, C. W. (2020). Mixed Polymer Brushes for “Smart” Surfaces. Polymers, 12(7), 1553. https://doi.org/10.3390/polym12071553