A Novel Polymer Electrolyte Matrix Incorporating Ionic Liquid into Waterborne Polyurethane for Lithium-Ion Battery
Abstract
1. Introduction
2. Experimental
2.1. Materials and Reagents
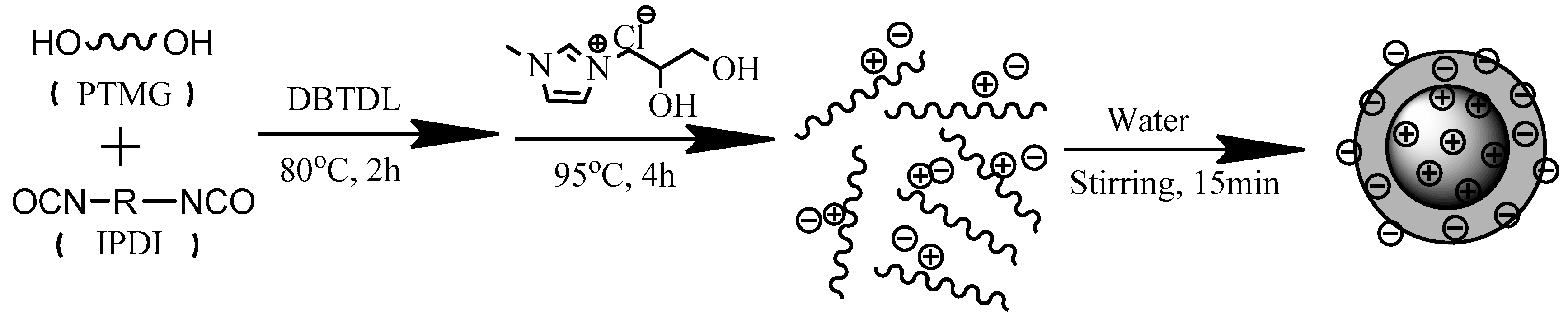
2.2. Preparation of WPU Films
2.3. Characterization
3. Results and Discussion
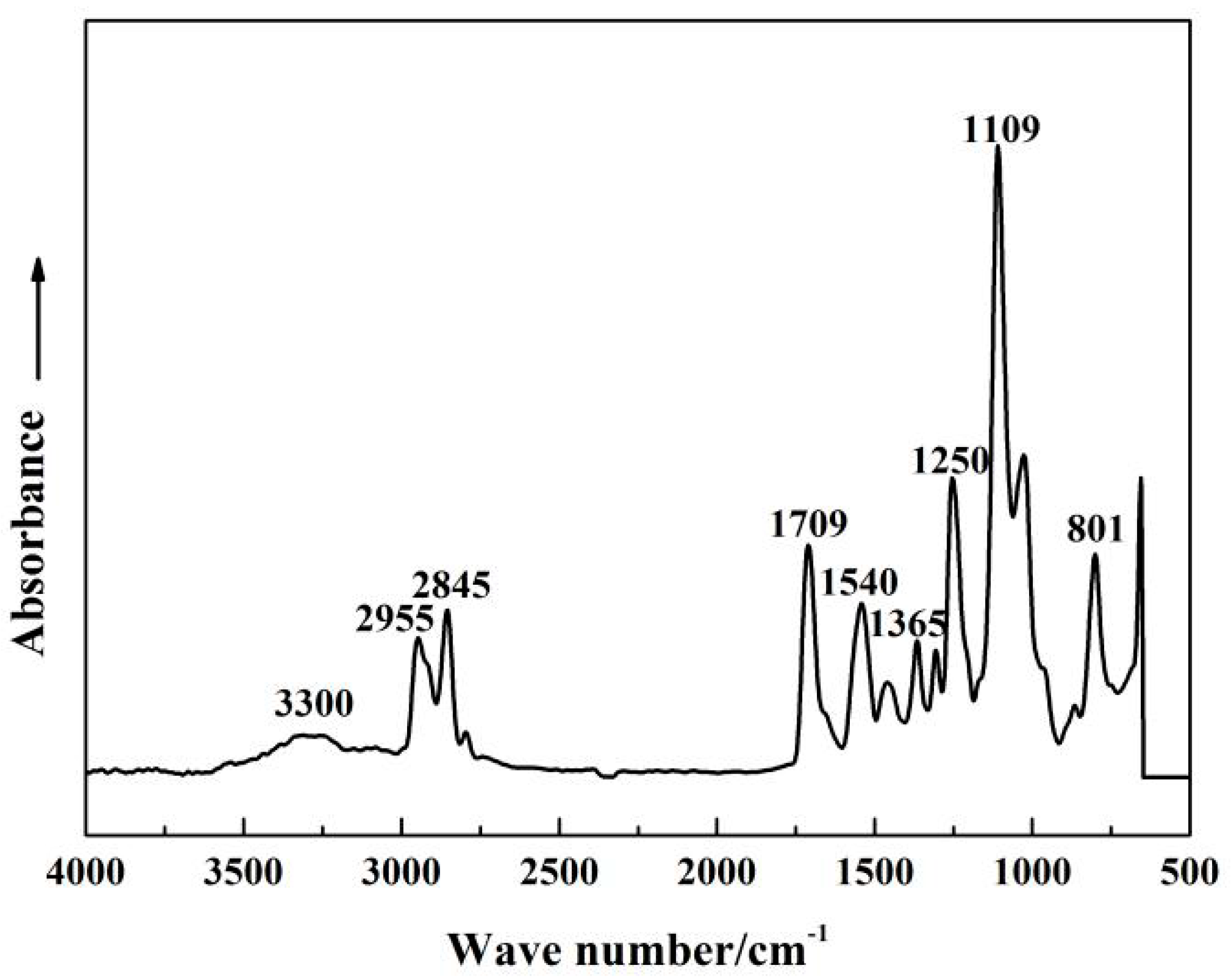
3.1. FTIR
3.2. Particle Size and Zeta Potential
3.3. GPC Analysis
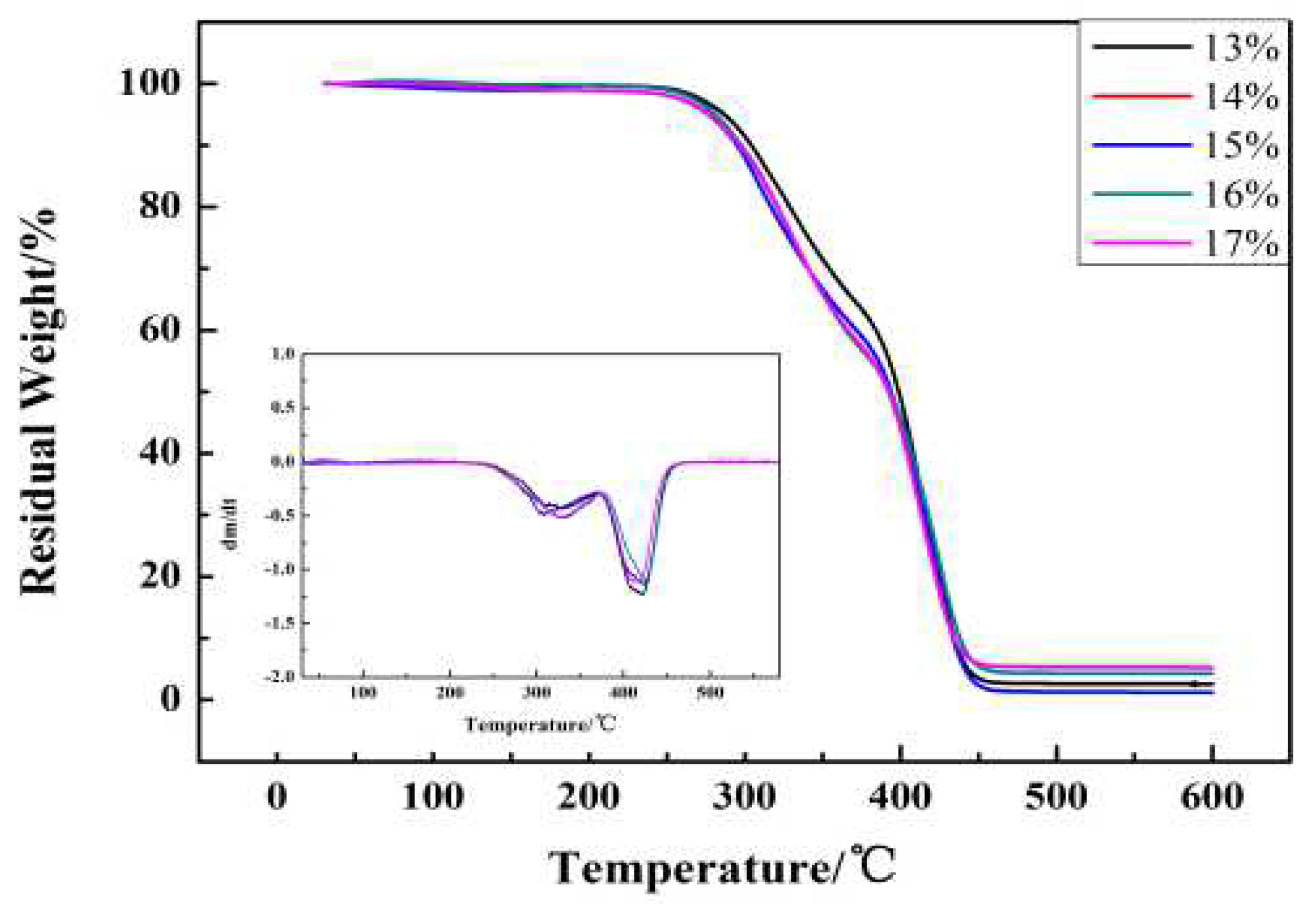
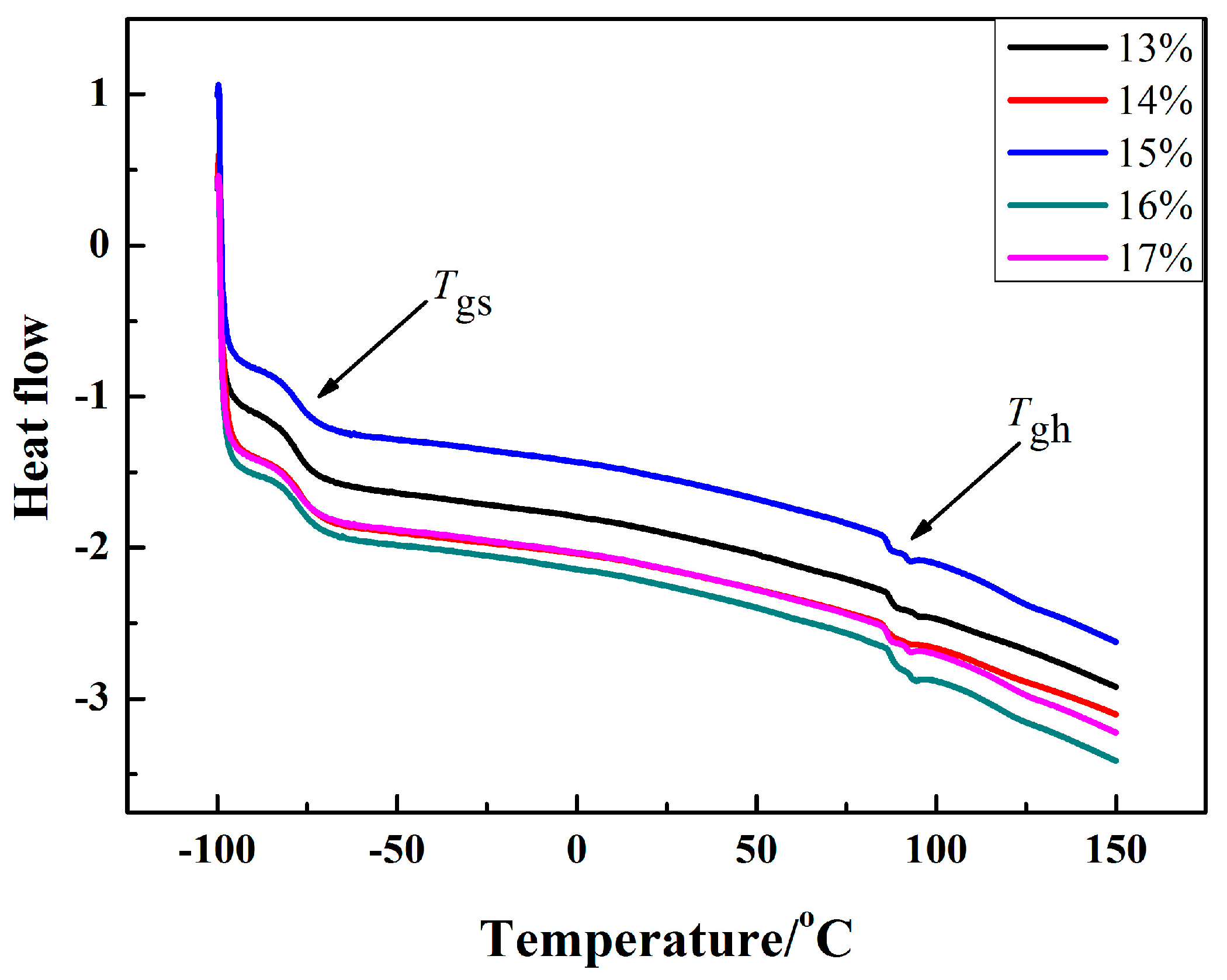
3.4. Thermal Analysis
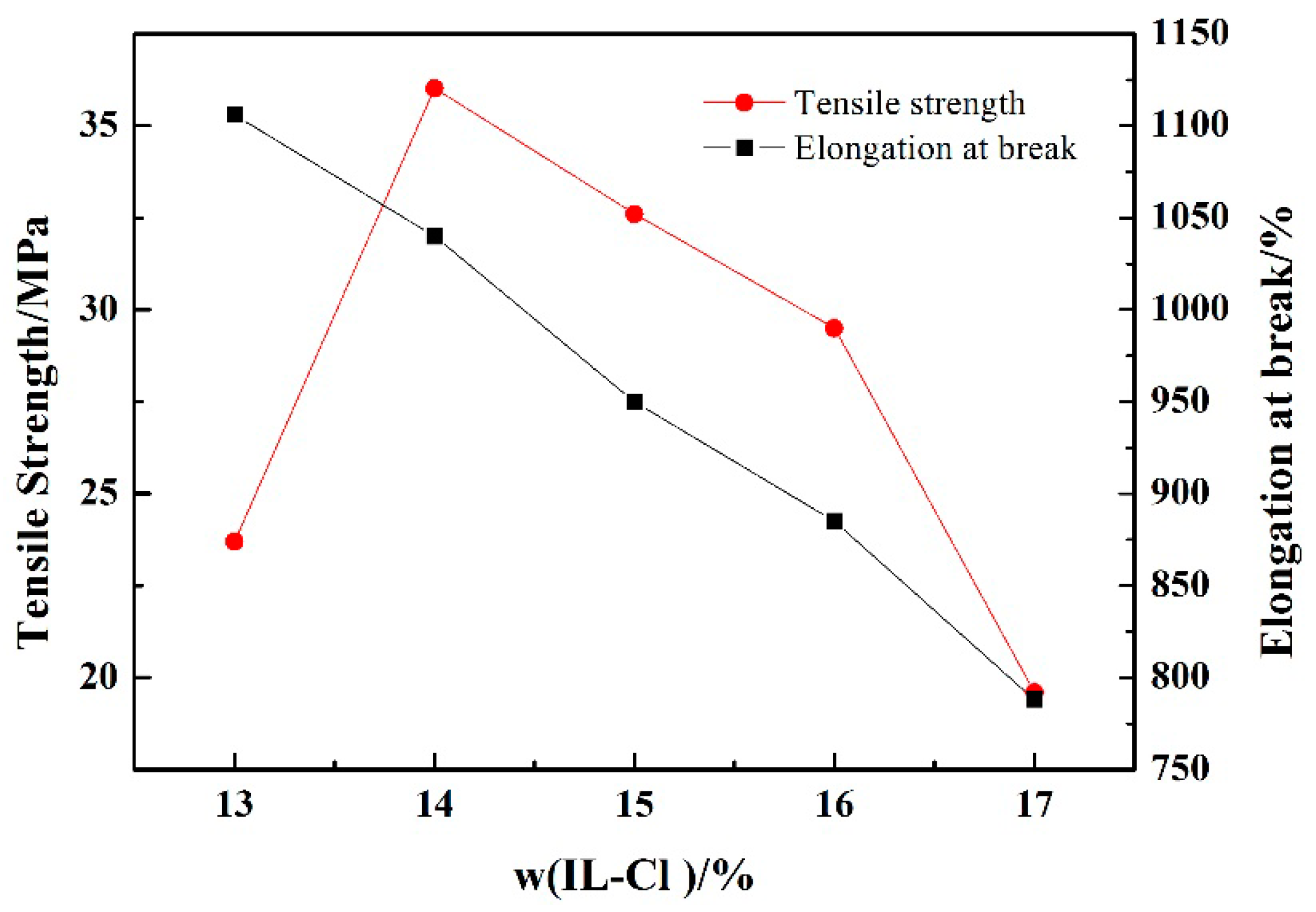
3.5. Mechanical Property
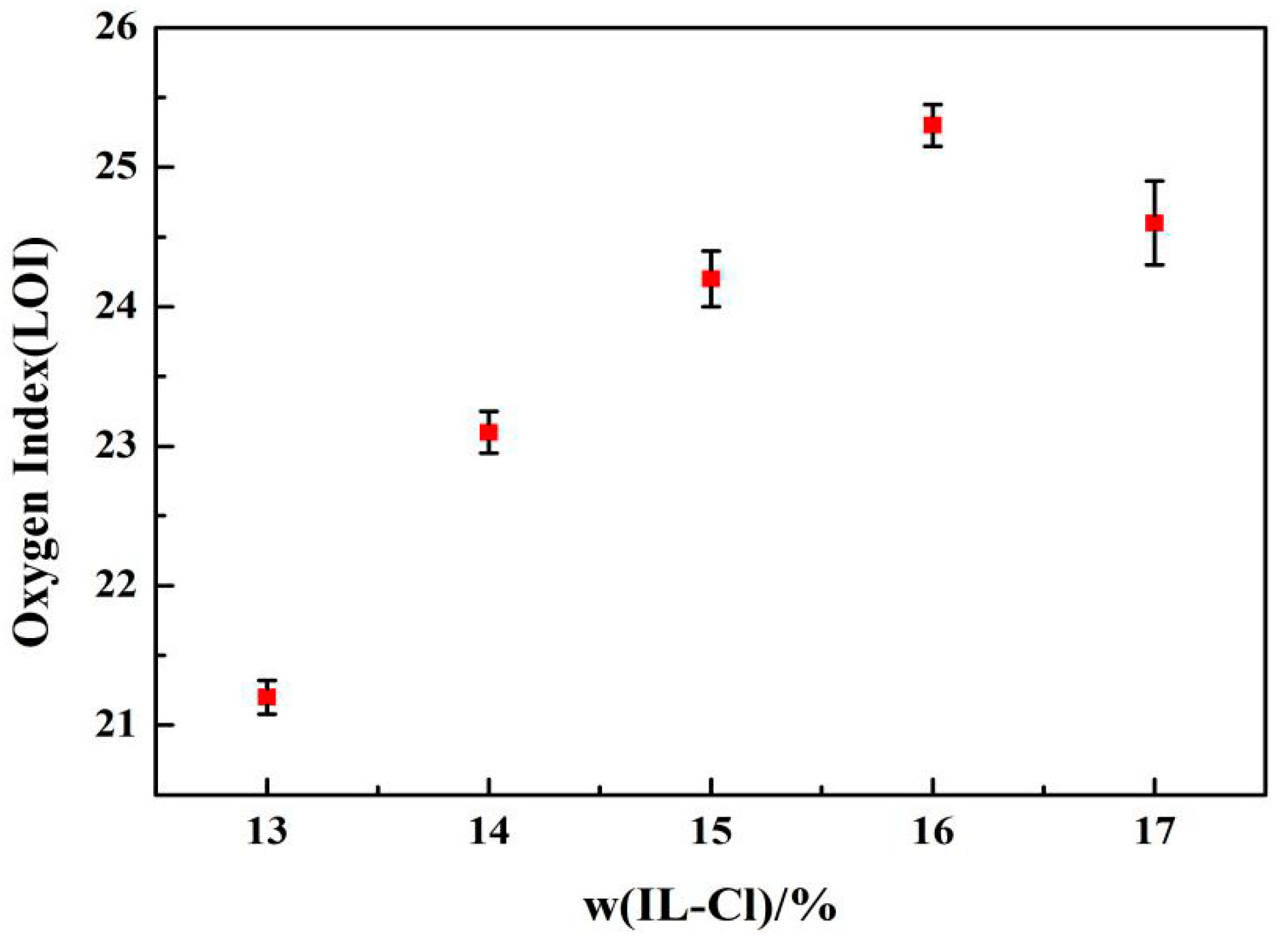
3.6. LOI Tests
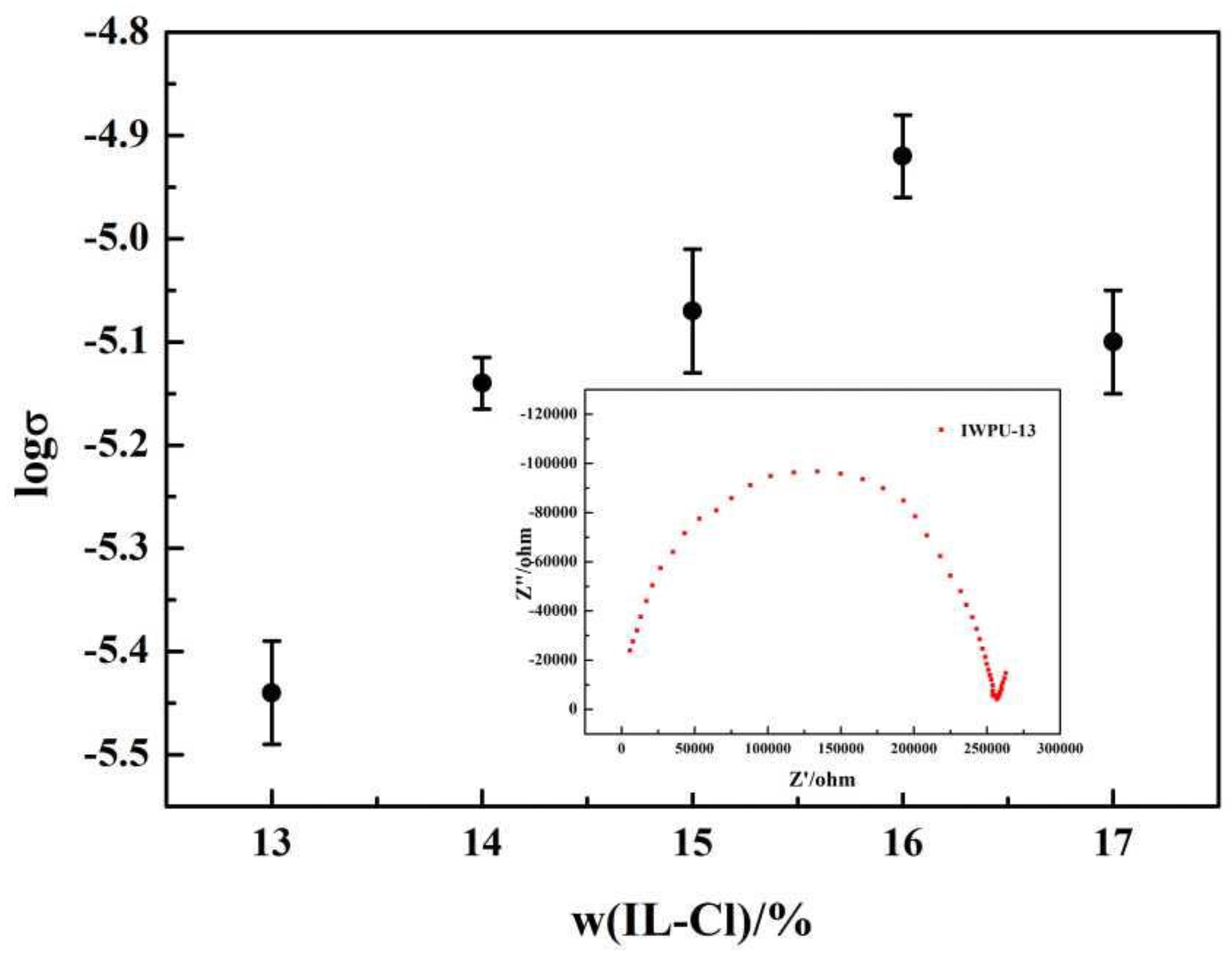
3.7. Ionic Conductivity Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, G.; Song, Y.; Wang, Q.; Zhang, L.; Deng, L. Review of ionic liquids containing, polymer/inorganic hybrid electrolytes for lithium metal batteries. Mater. Des. 2020, 190, 108563. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, R.; Zhang, J.; Qi, X.; He, Y.; Li, B.; Yang, Q.; He, Y.; Kang, F. In situ synthesis of hierarchical poly(ionic liquid)-based solid electrolytes for high-safety lithium-ion and sodium-ion batteries. Nano Energy 2017, 33, 45–54. [Google Scholar] [CrossRef]
- Zhou, B.; Jo, Y.H.; Wang, R.; He, D.; Zhou, X.; Xie, X.; Xue, Z. Self-healing composite polymer electrolyte formed via supramolecular networks for high-performance lithium-ion batteries. J. Mater. Chem. A 2019, 7, 10354–10362. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, Z.; Yan, W.; Ma, X.; Sun, D.; Jin, Y. Solid Polymer Electrolyte Based on Polymerized Ionic Liquid for High Performance All-Solid-State Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2019, 7, 4675–4683. [Google Scholar] [CrossRef]
- YKhoon, L.T.; Fui, M.W.; Hassan, N.H.; Su’ait, M.S.; Vedarajan, R.; Matsumi, N.; Kassim, B.M.; Shyuan, L.K.; Ahmad, A. Development of electrolytes towards achieving safe and high-performance energy-storage devices: A review. Chemelectrochem 2015, 2, 22–36. [Google Scholar]
- Ma, Q.; Qi, X.; Tong, B.; Zheng, Y.; Feng, W.; Nie, J.; Hu, Y.S.; Li, H.; Huang, X.; Chen, L.; et al. Novel Li[(CF3SO2)(n-C4F9SO2)N]-based polymer electrolytes for solid-State lithium batteries with superior electrochemical performance. ACS Appl. Mater. Inter. 2016, 8, 29705–29712. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Cong, B.; Song, Y.; Ren, N. Polyethylene glycol-based waterborne polyurethane as solid polymer electrolyte for all-solid-state lithium ion batteries. Mater. Des. 2018, 142, 221–228. [Google Scholar] [CrossRef]
- Deng, Y.; He, Z.; Cao, Q.; Jing, B.; Wang, X.; Peng, X. A novel high-performance electrospun thermoplastic polyurethane/poly(vinylidene fluoride)/polystyrene gel polymer electrolyte for lithium Batteries. Acta Chim. Slov. 2017, 64, 95–101. [Google Scholar] [CrossRef]
- Bao, J.; Qu, X.; Qi, G.; Huang, Q.; Wu, S.; Tao, C.; Gao, M.; Chen, C. Solid electrolyte based on waterborne polyurethane and poly(ethylene oxide) blend polymer for all-solid-state lithium ion batteries. Solid State Ion. 2018, 320, 55–63. [Google Scholar] [CrossRef]
- Bao, J.; Tao, C.; Yu, R.; Gao, M.; Huang, Y.; Chen, C. Solid polymer electrolyte based on waterborne polyurethane for all-solid-state lithium ion batterie. J. Appl. Polym. Sci. 2017, 134, 45554. [Google Scholar] [CrossRef]
- Ren, N.; Song, Y.; Tao, C.; Cong, B.; Cheng, Q.; Huang, Y.; Xu, G.; Bao, J. Effect of the soft and hard segment composition on the properties of waterborne polyurethane-based solid polymer electrolyte for lithium ion batteries. J. Solid State Electrochem. 2018, 22, 1109–1121. [Google Scholar] [CrossRef]
- Liu, L.; Wu, X.; Li, T. Novel polymer electrolytes based on cationic polyurethane with different alkyl chain length. J. Power Sources 2014, 249, 397–404. [Google Scholar] [CrossRef]
- Kuo, P.L.; Tsao, C.H.; Hsu, C.H.; Chen, S.T.; Hsu, H.M. A new strategy for preparing oligomeric ionic liquid gel polymer electrolytes for high-performance and nonflammable lithium ion batteries. J. Membr. Sci. 2016, 499, 462–469. [Google Scholar] [CrossRef]
- Liu, C.; Qiu, S.; Du, P.; Zhao, H.; Wang, L. An ionic liquid–graphene oxide hybrid nanomaterial: Synthesis and anticorrosive applications. Nanoscale 2018, 10, 8115–8124. [Google Scholar] [CrossRef]
- Li, L.; Yang, X.; Li, J.; Xu, Y. A novel and shortcut method to prepare ionic liquid gel polymer electrolyte membranes for lithium-ion battery. Ionics 2017, 24, 1–7. [Google Scholar] [CrossRef]
- Fasciani, C.; Panero, S.; Hassoun, J.; Scrosati, B. Novel configuration of poly(vinylidenedifluoride)-based gel polymer electrolyte for application in lithium-ion batteries. J. Power Sources 2015, 294, 180–186. [Google Scholar] [CrossRef]
- Khanmirzaei, M.H.; Ramesh, S.; Ramesh, K. Polymer electrolyte based dye-sensitized solar cell with rice starch and 1-methyl-3-propylimidazolium iodide ionic liquid. Mater. Des. 2015, 85, 833–837. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, Y.; Zhou, M.; Mao, C.; Shen, J. Preparation of anionic polyurethane nanoparticles and blood compatible behaviors. J. Nanosci. Nanotechnol. 2012, 12, 4051–4056. [Google Scholar] [CrossRef]
- Behara, P.K.; Usha, K.M.; Guchhait, P.K.; Jehnichen, D.; Das, A.; Voit, B.; Singha, N. A novel ionomeric polyurethane elastomer based on ionic liquid as crosslinker. RSC Adv. 2016, 6, 99404–99413. [Google Scholar] [CrossRef]
- Liu, G.; Kong, Z.; Wu, G.; Chen, J.; Huo, S. Preparation and properties of waterborne polyurethane/epoxy resin composite coating from anionic terpene-based polyol dispersion. Prog. Org. Coat. 2014, 77, 315–321. [Google Scholar]
- Mondal, T.; Basak, S.; Bhowmick, A.K. Ionic liquid modification of graphene oxide and its role towards controlling the porosity, and mechanical robustness of polyurethane foam. Polymer 2017, 127, 106–118. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, J.; Chao, Z. Synthesis and properties of waterborne polyurethane-based PTMG and PDMS as soft segment. Polym. Bull. 2016, 73, 293–308. [Google Scholar]
- Kumagai, S.; Motokucho, S.; Yabuki, R.; Anzai, A.; Kameda, T.; Watanabe, A. Effects of hard- and soft-segment composition on pyrolysis characteristics of MDI, BD, and PTMG-based polyurethane elastomers. J. Anal. Appl. Pyrol. 2016, 126, 337–345. [Google Scholar] [CrossRef]
- Luo, F.; Wu, K.; Li, Y.; Zheng, J.; Guo, H.; Lu, M. Reactive flame retardant with core-shell structure and its flame retardancy in rigid polyurethane foam. J. Appl. Polym. Sci. 2015, 132, 42800. [Google Scholar] [CrossRef]
- Wei, H.; Zhu, Z.; Sun, H.; Mu, P.; Li, A. Graphene and poly(ionic liquid) modified polyurethane sponges with enhanced flame-retardant properties. J. Appl. Polym. Sci. 2017, 34, 45477. [Google Scholar] [CrossRef]
- Li, Y. The study of melamine modified by imidazolium based ionic liquid [BMIM]PF6 on the flame retardancy of rigid polyurethane foam. Adv. Mater. Res. 2014, 1030, 241–245. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, Z.; Miao, J.; Yu, Y.; Zhang, L. Properties of flame-retardant cellulose fibers with ionic liquid. Fiber. Polym. 2017, 18, 915–921. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, L.; Yang, K.; Wang, H.; Yu, C.; Xu, D. Superior blends solid polymer electrolyte with integrated hierarchical architectures for all-solid-state lithium ion batteries. ACS Appl. Mater. Inter. 2017, 9, 36886–36896. [Google Scholar] [CrossRef]
- Bao, J.; Shi, G.; Tao, C.; Wang, C.; Zhu, C.; Cheng, L. Polycarbonate-based polyurethane as a polymer electrolyte matrix for all-solid-state lithium batteries. J. Power Sources 2018, 389, 84–92. [Google Scholar] [CrossRef]
- Kim, B.G.; Kim, J.S.; Min, J.; Lee, Y.H.; Choi, J.H.; Jang, M.C. A moisture-and oxygen-impermeable separator for aprotic Li-O-2 batteries. Adv. Funct. Mater. 2016, 26, 1747–1756. [Google Scholar] [CrossRef]
| Sample Name | m (PTMG)/g | m (IPDI)/g | m (IL-Cl)/g | m (H2O)/g |
|---|---|---|---|---|
| IWPU-13 | 20.00 | 8.57 | 4.27 | 131.36 |
| IWPU-14 | 20.00 | 9.25 | 4.76 | 136.04 |
| IWPU-15 | 20.00 | 9.98 | 5.20 | 140.72 |
| IWPU-16 | 20.00 | 10.77 | 5.86 | 146.52 |
| IWPU-17 | 20.00 | 11.62 | 6.48 | 152.40 |
| Wave Number/cm−1 | Assignments |
|---|---|
| 3300 | N-H stretching |
| 2945, 2855 | -CH3, -CH2- stretching |
| 1709 | -C=O stretching |
| 1540 | -C=C, -C=N deformation |
| 1365 | Imidazole skeletal stretching |
| 1250 | Imidazole symmetric stretching |
| 1109, 1027 | -C-O-C- asymmetric stretching |
| 801 | -C-O-C- symmetric stretching |
| Sample Name | Appearance | Particle Size/nm (PDI) | Zeta Potential/mV |
|---|---|---|---|
| IWPU-13 | Milky white, uniform | 106.8 (0.134) | 43.5 |
| IWPU-14 | Milky white, uniform | 123.4 (0.087) | 43.8 |
| IWPU-15 | Milky white, uniform | 185.7 (0.143) | 43.0 |
| IWPU-16 | Milky white, uniform | 179.3 (0.143) | 41.8 |
| IWPU-17 | Milky white, uniform | 178.9 (0.167) | 40.1 |
| Sample Name | Mn/(104g·mol−1) | Mw/(104g·mol−1) | Mw/Mn |
|---|---|---|---|
| IWPU-13 | 1.91 | 3.92 | 1.45 |
| IWPU-14 | 3.25 | 4.16 | 1.28 |
| IWPU-15 | 3.81 | 4.85 | 1.27 |
| IWPU-16 | 3.91 | 4.89 | 1.25 |
| IWPU-17 | 3.65 | 4.45 | 1.21 |
| Sample Name | Ti/°C | Tmax1/°C | Tmax2/°C | Char Yields at 500 °C/% |
|---|---|---|---|---|
| IWPU-13 | 276.17 | 309.67 | 421.83 | 1.52 |
| IWPU-14 | 286.67 | 319.33 | 422.33 | 2.68 |
| IWPU-15 | 284.67 | 326.00 | 423.67 | 3.87 |
| IWPU-16 | 281.00 | 329.33 | 424.17 | 4.47 |
| IWPU-17 | 276.67 | 327.67 | 419.67 | 5.15 |
| Sample Name | Tgs/°C | Tgh/°C |
|---|---|---|
| IWPU-13 | −77.64 | 87.36 |
| IWPU-14 | −76.86 | 85.84 |
| IWPU-15 | −78.01 | 87.89 |
| IWPU-16 | −78.38 | 88.36 |
| IWPU-17 | −77.64 | 86.58 |
| Sample Name | IWPU-13 | IWPU-14 | IWPU-15 | IWPU-16 | IWPU-17 |
|---|---|---|---|---|---|
| σ/(S·cm−1) | 3.6 × 10−6 | 7.3 × 10−6 | 8.4 × 10−6 | 1.2 × 10−5 | 7.9 × 10−6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhan, Y.; Zhao, C.; Su, Y.; Ge, Z.; Luo, Y. A Novel Polymer Electrolyte Matrix Incorporating Ionic Liquid into Waterborne Polyurethane for Lithium-Ion Battery. Polymers 2020, 12, 1513. https://doi.org/10.3390/polym12071513
Liu X, Zhan Y, Zhao C, Su Y, Ge Z, Luo Y. A Novel Polymer Electrolyte Matrix Incorporating Ionic Liquid into Waterborne Polyurethane for Lithium-Ion Battery. Polymers. 2020; 12(7):1513. https://doi.org/10.3390/polym12071513
Chicago/Turabian StyleLiu, Xiaoli, Yu Zhan, Chenying Zhao, Yuefeng Su, Zhen Ge, and Yunjun Luo. 2020. "A Novel Polymer Electrolyte Matrix Incorporating Ionic Liquid into Waterborne Polyurethane for Lithium-Ion Battery" Polymers 12, no. 7: 1513. https://doi.org/10.3390/polym12071513
APA StyleLiu, X., Zhan, Y., Zhao, C., Su, Y., Ge, Z., & Luo, Y. (2020). A Novel Polymer Electrolyte Matrix Incorporating Ionic Liquid into Waterborne Polyurethane for Lithium-Ion Battery. Polymers, 12(7), 1513. https://doi.org/10.3390/polym12071513





