Mechanical Integrity of Conductive Carbon-Black-Filled Aqueous Polymer Binder in Composite Electrode for Lithium-Ion Battery
Abstract
:1. Introduction
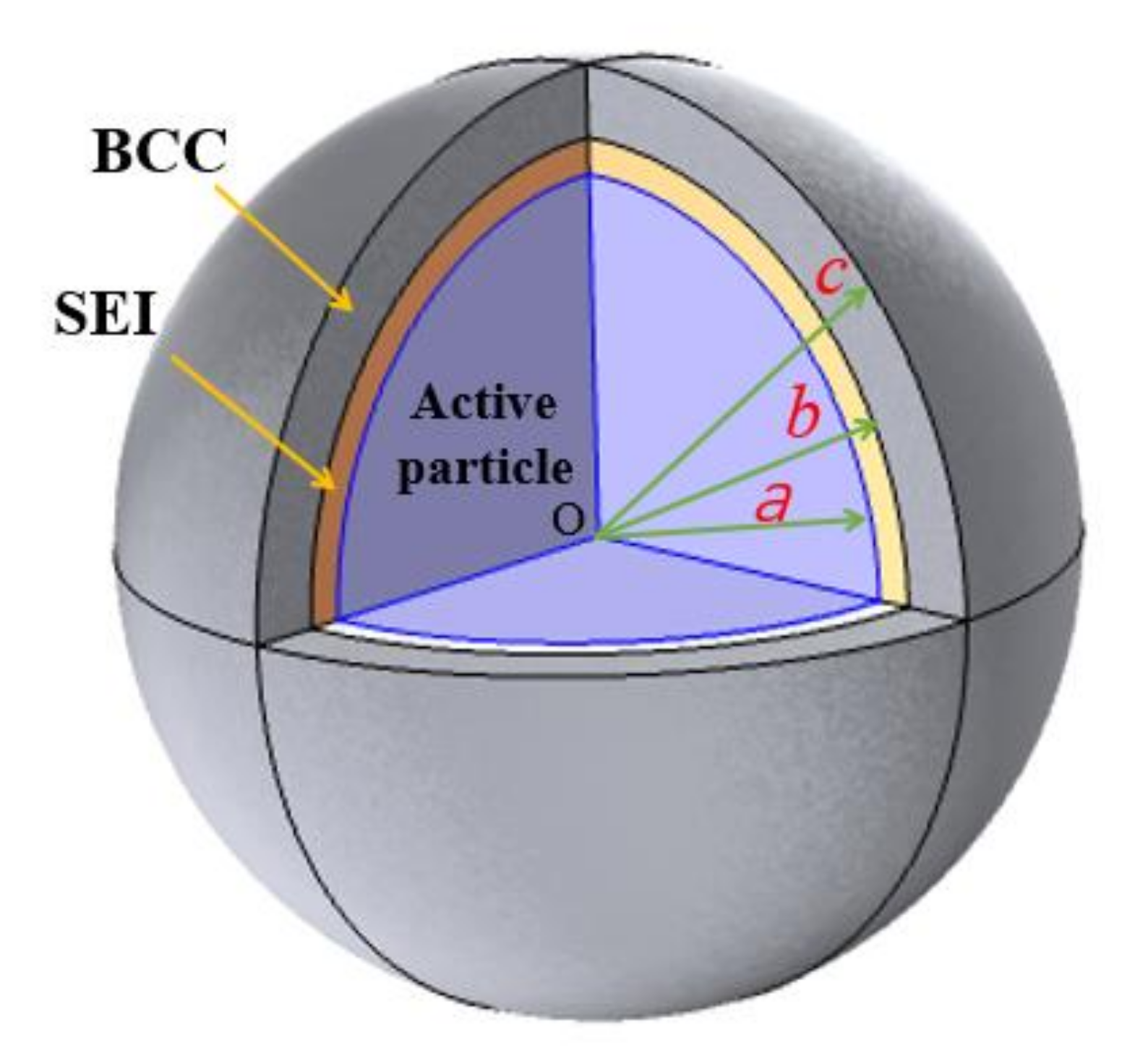
2. Model Description
3. Results and Discussion
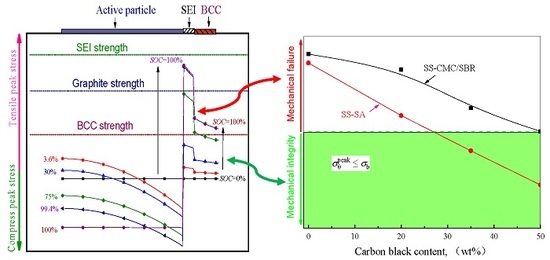
3.1. Distribution of Lithium Concentration and Stress in Electrode Particle System
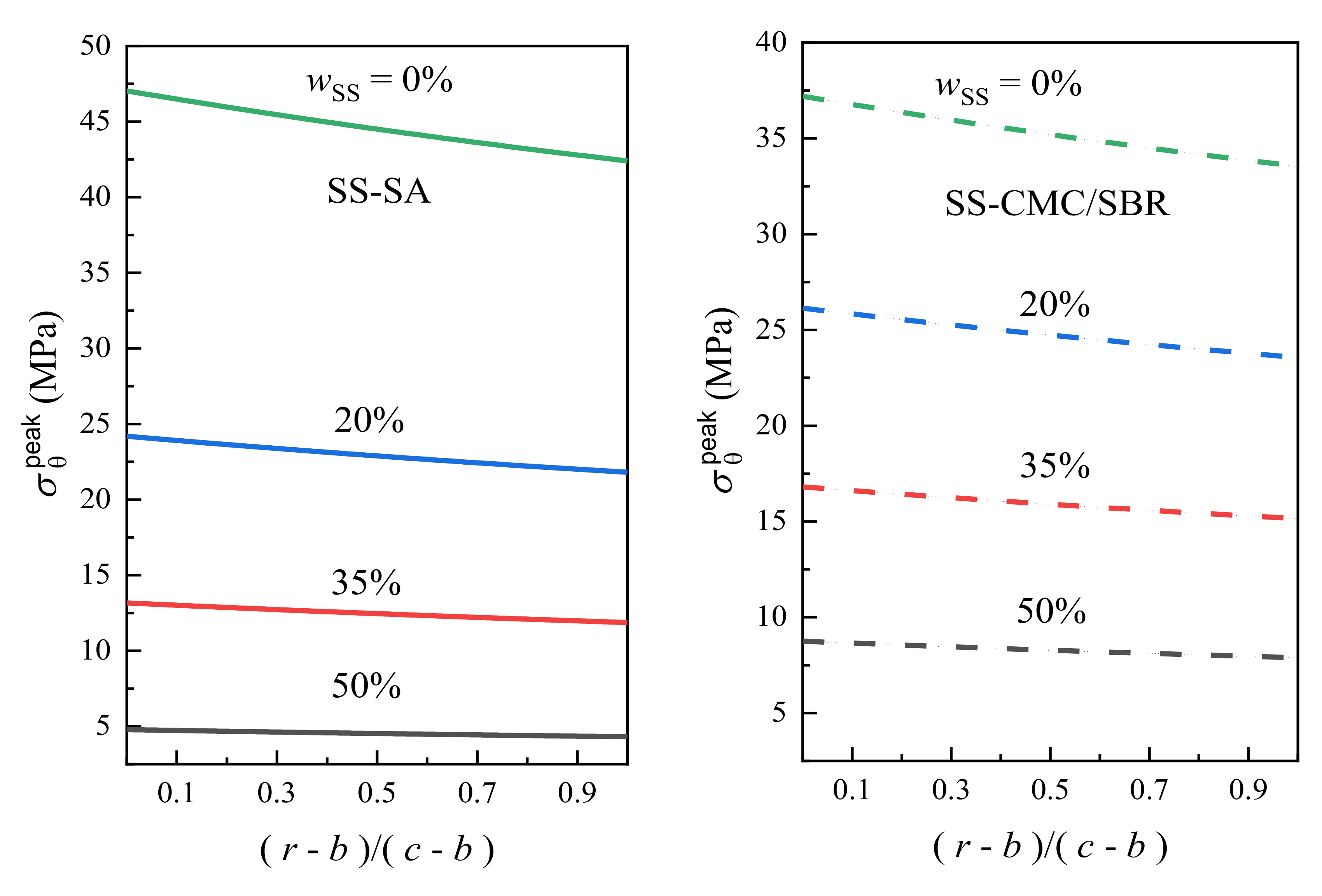
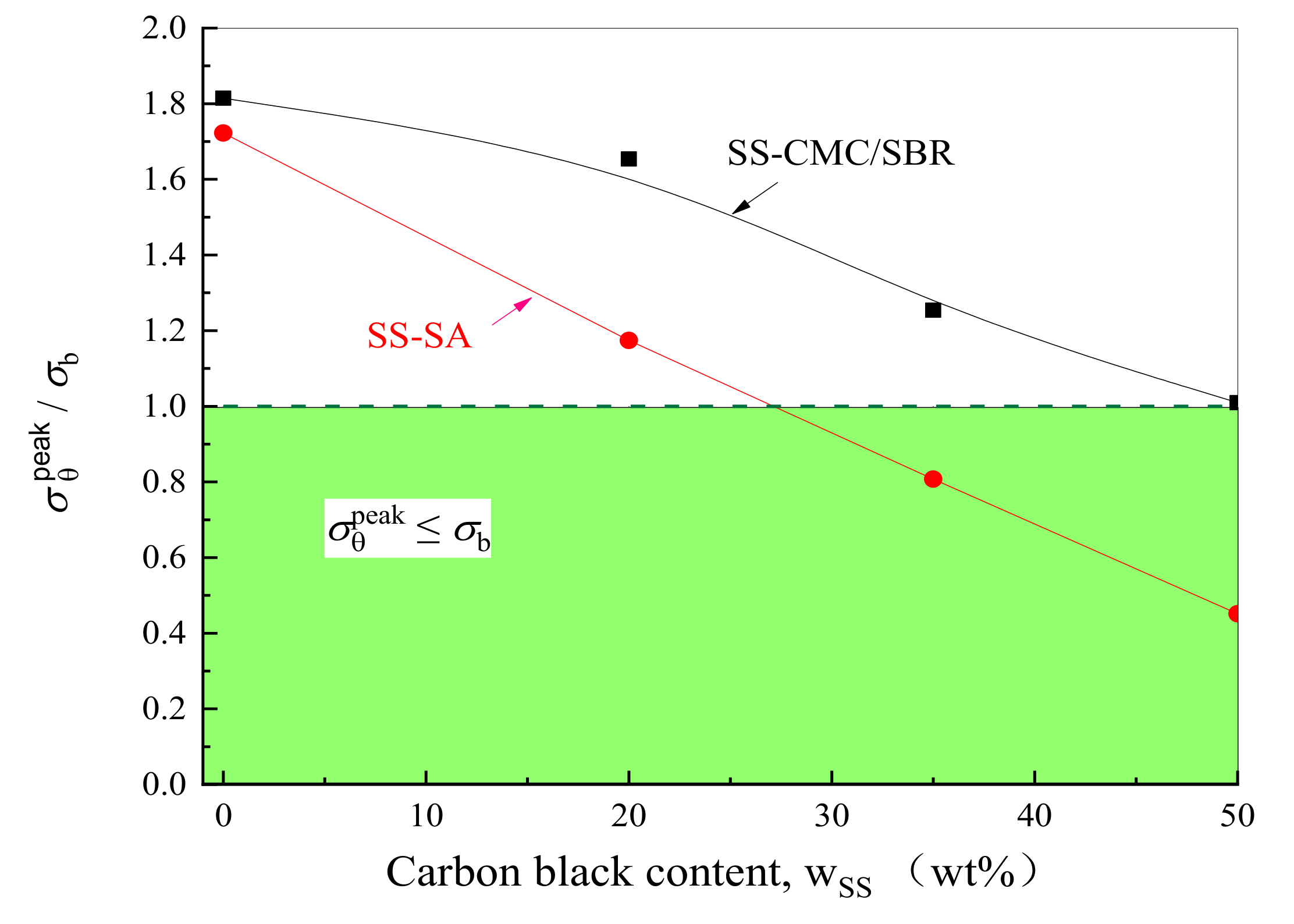
3.2. Effects of Carbon Black Contents and Polymer Type on Peak Stress in BCC
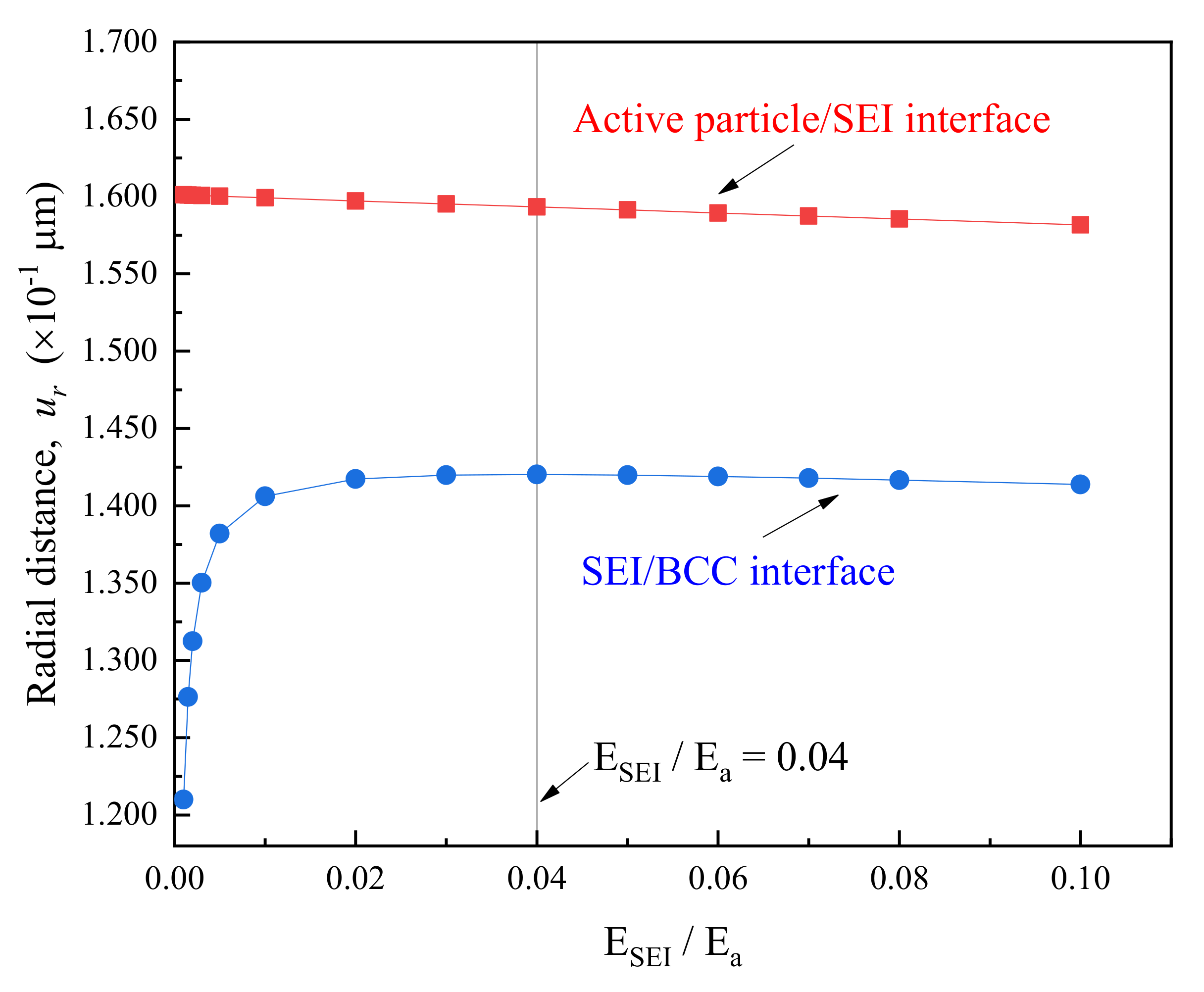
3.3. Effect of SEI Modulus and Thickness on Peak Stress in BCC
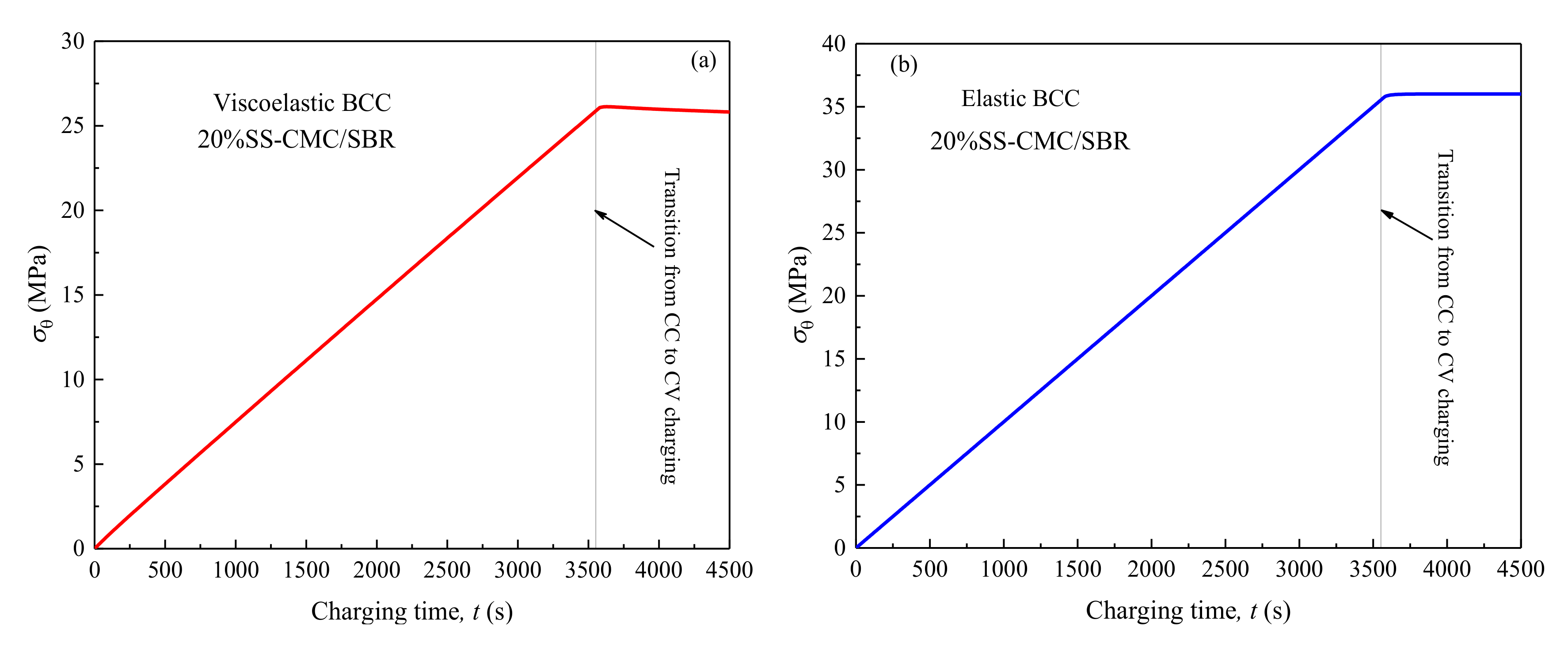
3.4. Effects of Lithiation Rate and Thickness of BCC on its Peak Stress
4. Conclusions
- Higher tensile hoop stress may occur for polymer binder and conductive composite (BCC) in the electrode during charging process. As a result, the circumferential cracks primarily initiated in these inactive materials rather than other electrode components. Further, mechanical damage did not take place at complete lithiation on account of the polymer viscoelasticity and would be greatly misled if neglecting the effect of SEI.
- With the increase of conductive agent content, the stress level of the bonding system decreased significantly under the electrochemical operation. To ensure the mechanical integrity of the graphite anode with aqueous binders, the minimum mass concentration of carbon black added in SA and CMC/SBR should be 27% and 50%, respectively. Moreover, SA-based composite exhibited much better rupture resistance compared to the counterparts at the same content of conductive agent.
- On the basis of the evolution of peak stress in multilayer spherical structures, a robust composite electrode may be obtained by: (1) reducing the elastic modulus of the SEI at least to less than that of the BCC; (2) lithiation at lower rate; (3) increasing the both BCC and SEI as much as possible in the context of meeting cell capacity requirements; (4) improving the tensile strength of BCC up to larger than the peak hoop stress in BCC at critical relative stiffness of SEI to active particle.
Author Contributions
Funding
Conflicts of Interest
References
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Sarma, D.D.; Shukla, A.K. Building Better Batteries: A Travel Back in Time. ACS Energy Lett. 2018, 3, 2841–2845. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.T.; Verbrugge, M.W. Diffusion-Induced Stress, Interfacial Charge Transfer, and Criteria for Avoiding Crack Initiation of Electrode Particles. J. Electrochem. Soc. 2010, 157, A508–A516. [Google Scholar] [CrossRef]
- Ma, Z.S.; Xie, Z.C.; Wang, Y.; Zhang, P.P.; Pan, Y.; Zhou, Y.C.; Lu, C. Failure modes of hollow core-shell structural active materials during the lithiation-delithiation process. J. Power Sources 2015, 290, 114–122. [Google Scholar] [CrossRef]
- Xiao, X.R.; Wu, W.; Huang, X.S. A multi-scale approach for the stress analysis of polymeric separators in a lithium-ion battery. J. Power Sources 2010, 195, 7649–7660. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, K.; Zheng, B.L.; Yang, F.Q. Effect of local deformation on the coupling between diffusion and stress in lithium-ion battery. Int. J. Solids Struct. 2016, 87, 81–89. [Google Scholar] [CrossRef]
- Haftbaradaran, H.; Xiao, X.; Verbrugge, M.W.; Gao, H. Method to deduce the critical size for interfacial delamination of patterned electrode structures and application to lithiation of thin-film silicon islands. J. Power Sources 2012, 206, 357–366. [Google Scholar] [CrossRef]
- Chen, J.C.; Liu, J.Y.; Qi, Y.; Sun, T.; Li, X.D. Unveiling the Roles of Binder in the Mechanical Integrity of Electrodes for Lithium-Ion Batteries. J. Electrochem. Soc. 2013, 160, A1502–A1509. [Google Scholar] [CrossRef] [Green Version]
- Grillet, A.M.; Humplik, T.; Stirrup, E.K.; Roberts, S.A.; Barringer, D.A.; Snyder, C.M.; Janvrin, M.R.; Apblett, C.A. Conductivity Degradation of Polyvinylidene Fluoride Composite Binder during Cycling: Measurements and Simulations for Lithium-Ion Batteries. J. Electrochem. Soc. 2016, 163, A1859–A1871. [Google Scholar] [CrossRef] [Green Version]
- Zheng, T.Y.; Jia, Z.; Lin, N.; Langer, T.; Lux, S.; Lund, I.; Gentschev, A.C.; Qiao, J.; Liu, G. Molecular Spring Enabled High-Performance Anode for Lithium Ion Batteries. Polymers 2017, 9, 657. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.L.; Nadimpalli, S.P.V.; Shenoy, V.B. Inelastic shape changes of silicon particles and stress evolution at binder/particle interface in a composite electrode during lithiation/delithiation cycling. Extrem. Mech. Lett. 2016, 9, 430–438. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.S. A review on the separators of liquid electrolyte Li-ion batteries. J. Power Sources 2007, 164, 351–364. [Google Scholar] [CrossRef]
- Hao, W.; Kong, D.; Xie, J.; Chen, Y.; Ding, J.; Wang, F.; Xu, T. Self-Polymerized Dopamine Nanoparticles Modified Separators for Improving Electrochemical Performance and Enhancing Mechanical Strength of Lithium-Ion Batteries. Polymers 2020, 12, 648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falco, M.; Simari, C.; Ferrara, C.; Nair, J.R.; Meligrana, G.; Bella, F.; Nicotera, I.; Mustarelli, P.; Winter, M.; Gerbaldi, C. Understanding the Effect of UV-Induced Cross-Linking on the Physicochemical Properties of Highly Performing PEO/LiTFSI-Based Polymer Electrolytes. Langmuir 2019, 35, 8210–8219. [Google Scholar] [CrossRef]
- Guo, R.; Wang, J.; Zhang, S.; Han, W.-Q. Multifunctional Cross-Linked Polymer-Laponite Nanocomposite Binder for Lithium-Sulfur Batteries. Chem. Eng. J. 2020, 388, 124316. [Google Scholar] [CrossRef]
- Jeong, D.; Shim, J.; Shin, H.; Lee, J.C. Sustainable Lignin-Derived Cross-Linked Graft Polymers as Electrolyte and Binder Materials for Lithium Metal Batteries. ChemSusChem 2020, 13, 2642–2649. [Google Scholar] [CrossRef] [PubMed]
- Piana, G.; Bella, F.; Geobaldo, F.; Meligrana, G.; Gerbaldi, C. PEO/LAGP hybrid solid polymer electrolytes for ambient temperature lithium batteries by solvent-free, “one pot” preparation. J. Energy Storage 2019, 26, 100947. [Google Scholar] [CrossRef]
- Ramesh, S.; Liew, C.W. Development and investigation on PMMA–PVC blend-based solid polymer electrolytes with LiTFSI as dopant salt. Polym. Bull. 2013, 70, 1277–1288. [Google Scholar] [CrossRef]
- Ji, J.; Li, B.; Zhong, W.-H. Effects of a Block Copolymer as Multifunctional Fillers on Ionic Conductivity, Mechanical Properties, and Dimensional Stability of Solid Polymer Electrolytes. J. Phys. Chem. B 2010, 114, 13637–13643. [Google Scholar] [CrossRef]
- Rahani, E.K.; Shenoy, V.B. Role of Plastic Deformation of Binder on Stress Evolution during Charging and Discharging in Lithium-Ion Battery Negative Electrodes. J. Electrochem. Soc. 2013, 160, A1153–A1162. [Google Scholar] [CrossRef]
- Takahashi, K.; Higa, K.; Mair, S.; Chintapalli, M.; Balsara, N.; Srinivasan, V. Mechanical Degradation of Graphite/PVDF Composite Electrodes: A Model-Experimental Study. J. Electrochem. Soc. 2015, 163, A385–A395. [Google Scholar] [CrossRef] [Green Version]
- Singhz, G.; Bhandakkar, T.K. Analytical Investigation of Binder’s Role on the Diffusion Induced Stresses in Lithium Ion Battery through a Representative System of Spherical Isolated Electrode Particle Enclosed by Binder. J. Electrochem. Soc. 2017, 164, A608–A621. [Google Scholar] [CrossRef]
- Higa, K.; Srinivasan, V. Stress and Strain in Silicon Electrode Models. J. Electrochem. Soc. 2015, 162, A1111–A1122. [Google Scholar] [CrossRef]
- Lee, S.; Yang, J.; Lu, W. Debonding at the interface between active particles and PVDF binder in Li-ion batteries. Extrem. Mech. Lett. 2016, 6, 37–44. [Google Scholar] [CrossRef]
- Wu, W.; Xiao, X.R.; Wang, M.; Huang, X.S. A Microstructural Resolved Model for the Stress Analysis of Lithium-Ion Batteries. J. Electrochem. Soc. 2014, 161, A803–A813. [Google Scholar] [CrossRef]
- Liu, H.S.; Foster, J.M.; Gully, A.; Krachkovskiy, S.; Jiang, M.; Wu, Y.; Yang, X.Y.; Protas, B.; Goward, G.R.; Botton, G.A. Three-dimensional investigation of cycling-induced microstructural changes in lithium-ion battery cathodes using focused ion beam/scanning electron microscopy. J. Power Sources 2016, 306, 300–308. [Google Scholar] [CrossRef] [Green Version]
- He, Y.L.; Hu, H.J.; Zhang, K.F.; Li, S.; Chen, J.H. Mechanical insights into the stability of heterogeneous solid electrolyte interphase on an electrode particle. J. Mater. Sci. 2017, 52, 2836–2848. [Google Scholar] [CrossRef]
- Liu, G.; Zheng, H.; Simens, A.S.; Minor, A.M.; Song, X.; Battaglia, V.S. Optimization of acetylene black conductive additive and PVDF composition for high-power rechargeable lithium-ion cells. J. Electrochem. Soc. 2007, 154, A1129–A1134. [Google Scholar] [CrossRef]
- Liu, G.; Zheng, H.; Kim, S.; Deng, Y.; Minor, A.M.; Song, X.; Battaglia, V.S. Effects of Various Conductive Additive and Polymeric Binder Contents on the Performance of a Lithium-Ion Composite Cathode. J. Electrochem. Soc. 2008, 155, A887–A892. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.H.; Yang, R.Z.; Liu, G.; Song, X.Y.; Battaglia, V.S. Cooperation between Active Material, Polymeric Binder and Conductive Carbon Additive in Lithium Ion Battery Cathode. J. Phys. Chem. C 2012, 116, 4875–4882. [Google Scholar] [CrossRef]
- Chen, Z.H.; Christensen, L.; Dahn, J.R. A study of the mechanical and electrical properties of a polymer/carbon black binder system used in battery electrodes. J. Appl. Polym. Sci. 2003, 90, 1891–1899. [Google Scholar] [CrossRef]
- Hu, H.J.; Tao, B.; HE, Y.L.; Zhou, S.H. Effect of conductive carbon black on mechanical properties of aqueous polymer binders for secondary battery electrode. Polymers 2019, 11, 1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, L.M.; Bai, Y.; Wang, Z.H.; Ni, Q.; Chen, G.H.; Zhou, Z.M.; Wu, C. Remarkable Effect of Sodium Alginate Aqueous Binder on Anatase TiO2 as High-Performance Anode in Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 5560–5568. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.Y.; Wohlfahrt-Mehrens, M.; Dsoke, S. Influence of the binder nature on the performance and cycle life of activated carbon electrodes in electrolytes containing Li-salt. J. Power Sources 2017, 342, 301–312. [Google Scholar] [CrossRef]
- Kovalenko, I.; Zdyrko, B.; Magasinski, A.; Hertzberg, B.; Milicev, Z.; Burtovyy, R.; Luzinov, I.; Yushin, G. A major constituent of brown algae for use in high-capacity Li-ion batteries. Science 2011, 334, 75. [Google Scholar] [CrossRef]
- Lee, J.-H.; Paik, U.; Hackley, V.A.; Choi, Y.-M. Effect of poly(acrylic acid) on adhesion strength and electrochemical performance of natural graphite negative electrode for lithium-ion batteries. J. Power Sources 2006, 161, 612–616. [Google Scholar] [CrossRef]
- Shin, D.; Park, H.; Paik, U. Cross-linked poly(acrylic acid)-carboxymethyl cellulose and styrene-butadiene rubber as an efficient binder system and its physicochemical effects on a high energy density graphite anode for Li-ion batteries. Electrochem. Commun. 2017, 77, 103–106. [Google Scholar] [CrossRef]
- Nirmale, T.C.; Kale, B.B.; Varma, A.J. A review on cellulose and lignin based binders and electrodes: Small steps towards a sustainable lithium ion battery. Int. J. Biol. Macromol. 2017, 103, 1032–1043. [Google Scholar] [CrossRef]
- Chou, S.L.; Pan, Y.D.; Wang, J.Z.; Liu, H.K.; Dou, S.X. Small things make a big difference: Binder effects on the performance of Li and Na batteries. Phys. Chem. Chem. Phys. 2014, 16, 20347–20359. [Google Scholar] [CrossRef]
- Li, D.W.; Wang, Y.K.; Hu, J.Z.; Lu, B.; Dang, D.Y.; Zhang, J.G.; Cheng, Y.T. Role of polymeric binders on mechanical behavior and cracking resistance of silicon composite electrodes during electrochemical cycling. J. Power Sources 2018, 387, 9–15. [Google Scholar] [CrossRef]
- He, Y.L.; Hu, H.J.; Song, Y.C.; Guo, Z.S.; Liu, C.; Zhang, J.Q. Effects of concentration-dependent elastic modulus on the diffusion of lithium ions and diffusion induced stress in layered battery electrodes. J. Power Sources 2014, 248, 517–523. [Google Scholar] [CrossRef]
- Zhang, K.; Li, Y.; Zheng, B.L. Effects of concentration-dependent elastic modulus on Li-ions diffusion and diffusion-induced stresses in spherical composition-gradient electrodes. J. Appl. Phys. 2015, 118. [Google Scholar] [CrossRef]
- Deshpande, R.; Qi, Y.; Cheng, Y.T. Effects of Concentration-Dependent Elastic Modulus on Diffusion-Induced Stresses for Battery Applications. J. Electrochem. Soc. 2010, 157, A967–A971. [Google Scholar] [CrossRef]
- He, Y.L.; Hu, H.J.; Huang, D.W. Effects of stoichiometric maximum concentration on lithium diffusion and stress within an insertion electrode particle. Mater. Des. 2016, 92, 438–444. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Verbrugge, M.W. Evolution of stress within a spherical insertion electrode particle under potentiostatic and galvanostatic operation. J. Power Sources 2009, 190, 453–460. [Google Scholar] [CrossRef]
- Christensen, J.; Newman, J. Stress generation and fracture in lithium insertion materials. J. Solid State Electrochem. 2006, 10, 293–319. [Google Scholar] [CrossRef]
- Deshpande, R.D.; Bernardi, D.M. Modeling Solid-Electrolyte Interphase (SEI) Fracture: Coupled Mechanical/Chemical Degradation of the Lithium Ion Battery. J. Electrochem. Soc. 2017, 164, A461–A474. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, R.; Yang, X.C.; Lu, W.; Wu, X.D.; Wang, X.P.; Li, H.; Chen, L.W. Direct Observation of Inhomogeneous Solid Electrolyte Interphase on MnO Anode with Atomic Force Microscopy and Spectroscopy. Nano Lett. 2012, 12, 2153–2157. [Google Scholar] [CrossRef]
- Verma, P.; Maire, P.; Novak, P. A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries. Electrochim. Acta 2010, 55, 6332–6341. [Google Scholar] [CrossRef]
- Zhang, X.; Shyy, W.; Sastry, A.M. Numerical simulation of intercalation-induced stress in Li-ion battery electrode particles. J. Electrochem. Soc. 2007, 154, A910–A916. [Google Scholar] [CrossRef]
- He, Y.L.; Hu, H.J. Analysis of lithium ion concentration and stress in the solid electrolyte interphase on the graphite anode. Phys. Chem. Chem. Phys. 2015, 17, 23565–23572. [Google Scholar] [CrossRef] [PubMed]
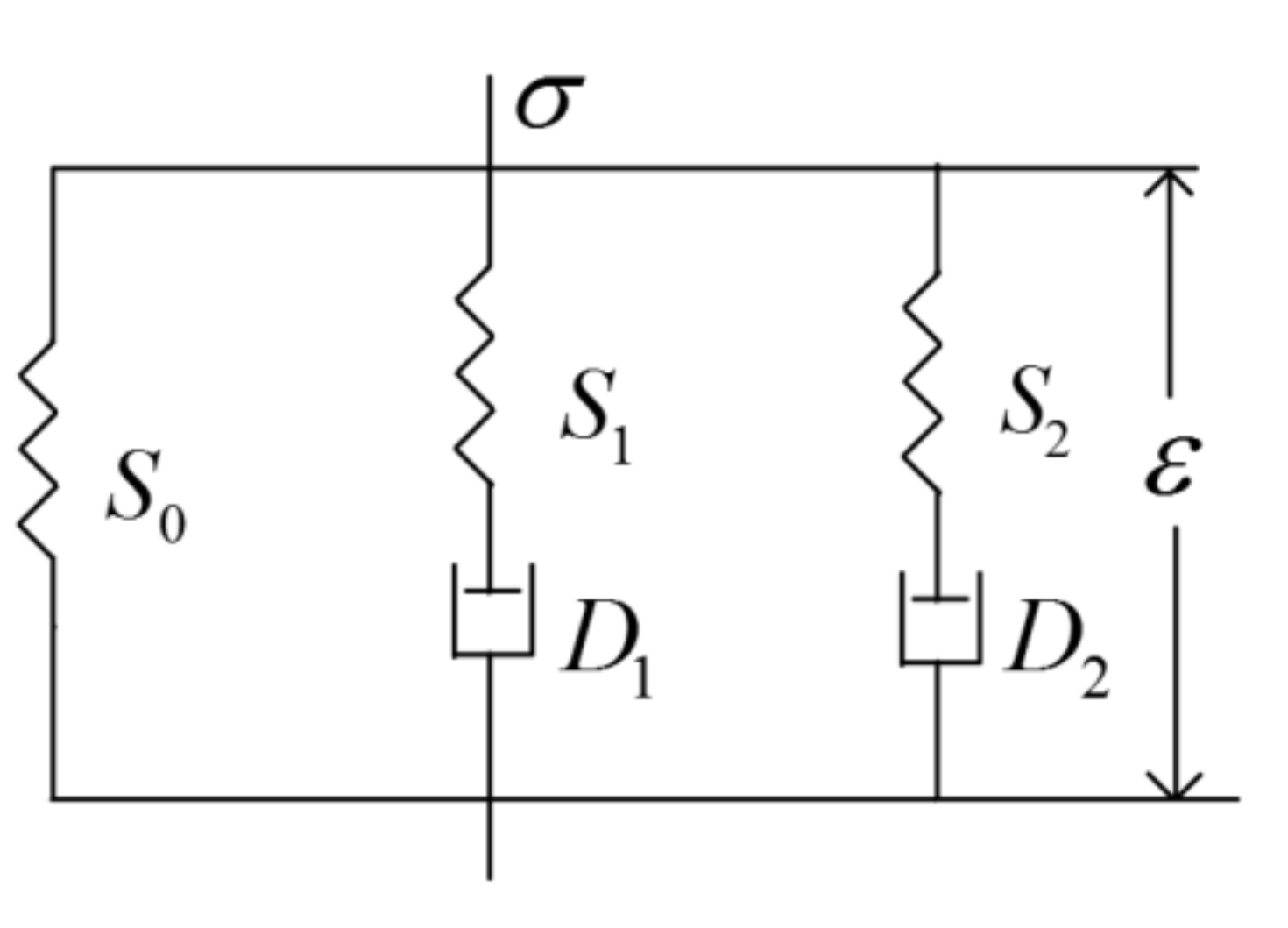



| SS Contents (wt%) | G0 (MPa) | G1 (MPa) | τ1 (min) | G2 (MPa) | τ2 (min) |
|---|---|---|---|---|---|
| 0 | 320.3 | 195.3 | 51.85 | 132.67 | 2.1 |
| 20 | 163.6 | 140.11 | 28.26 | 94.9 | 1.6 |
| 35 | 84.2 | 103.87 | 24.13 | 69.24 | 1.33 |
| 50 | 27.9 | 57.56 | 19.57 | 35.04 | 1.18 |
| SS Contents (wt%) | G0 (MPa) | G1 (MPa) | τ1 (min) | G2 (MPa) | τ2 (min) |
|---|---|---|---|---|---|
| 0 | 247.4 | 110.44 | 295.6 | 73.62 | 3.18 |
| 20 | 176.2 | 74.42 | 253.16 | 56.03 | 1.89 |
| 35 | 104.2 | 68.06 | 174.45 | 45.38 | 1.72 |
| 50 | 51.6 | 43.81 | 130.55 | 21.8 | 0.59 |
| Poisson’s ratio | 0.3 |
| Young’s modulus (GPa) | 10.0 |
| Partial molar volume of Li (m3/mol) | 3.17 × 10−6 |
| Li diffusion coefficient (m2/s) | 4.9 × 10−14 |
| Saturated Li concentration (mol/m3) | 3.05 × 104 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, K.; He, Y.; Hu, H.; Li, S.; Tao, B. Mechanical Integrity of Conductive Carbon-Black-Filled Aqueous Polymer Binder in Composite Electrode for Lithium-Ion Battery. Polymers 2020, 12, 1460. https://doi.org/10.3390/polym12071460
Peng K, He Y, Hu H, Li S, Tao B. Mechanical Integrity of Conductive Carbon-Black-Filled Aqueous Polymer Binder in Composite Electrode for Lithium-Ion Battery. Polymers. 2020; 12(7):1460. https://doi.org/10.3390/polym12071460
Chicago/Turabian StylePeng, Kehua, Yaolong He, Hongjiu Hu, Shufeng Li, and Bao Tao. 2020. "Mechanical Integrity of Conductive Carbon-Black-Filled Aqueous Polymer Binder in Composite Electrode for Lithium-Ion Battery" Polymers 12, no. 7: 1460. https://doi.org/10.3390/polym12071460
APA StylePeng, K., He, Y., Hu, H., Li, S., & Tao, B. (2020). Mechanical Integrity of Conductive Carbon-Black-Filled Aqueous Polymer Binder in Composite Electrode for Lithium-Ion Battery. Polymers, 12(7), 1460. https://doi.org/10.3390/polym12071460