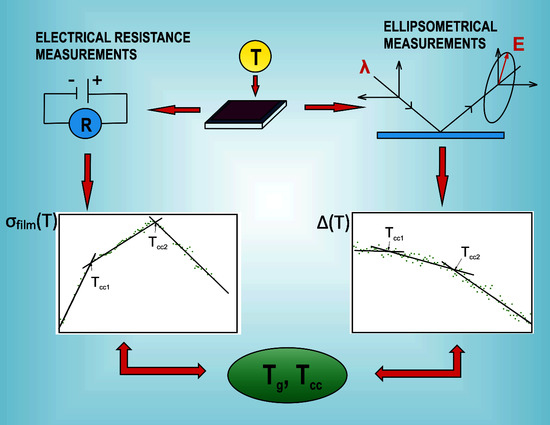
Thermal Transitions in P3HT:PC60BM Films Based on Electrical Resistance Measurements
Abstract
1. Introduction
2. Materials and Methods
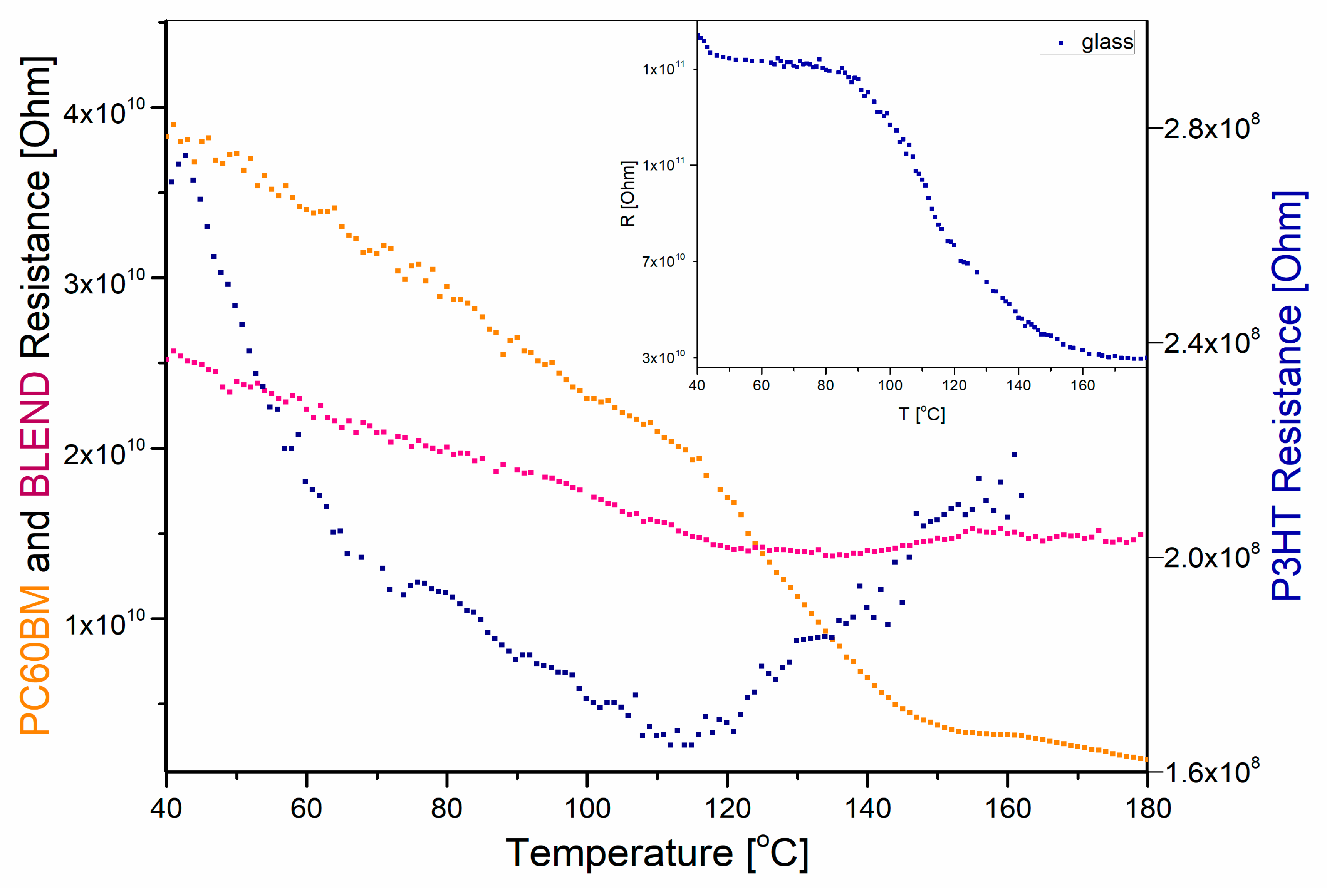
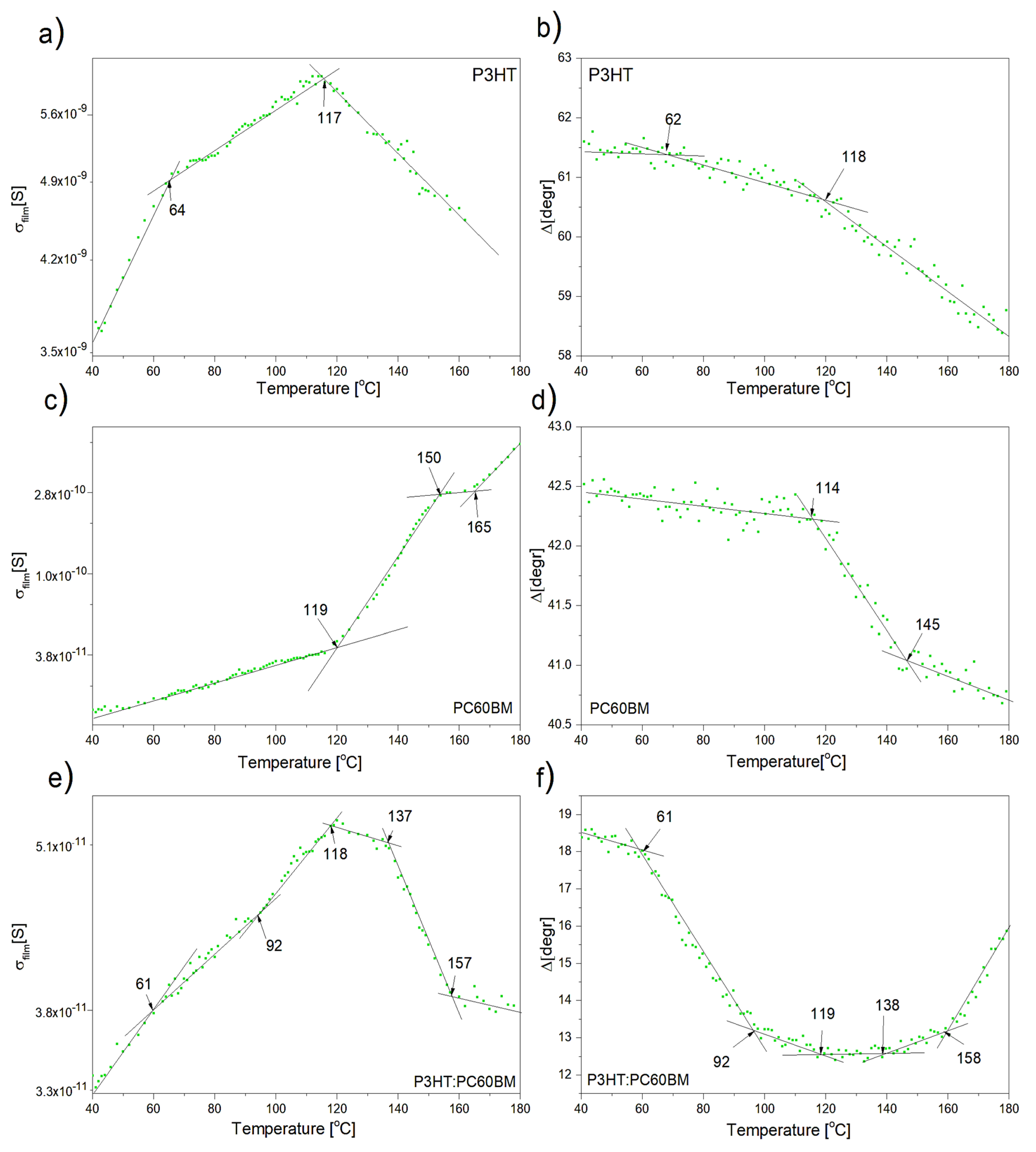
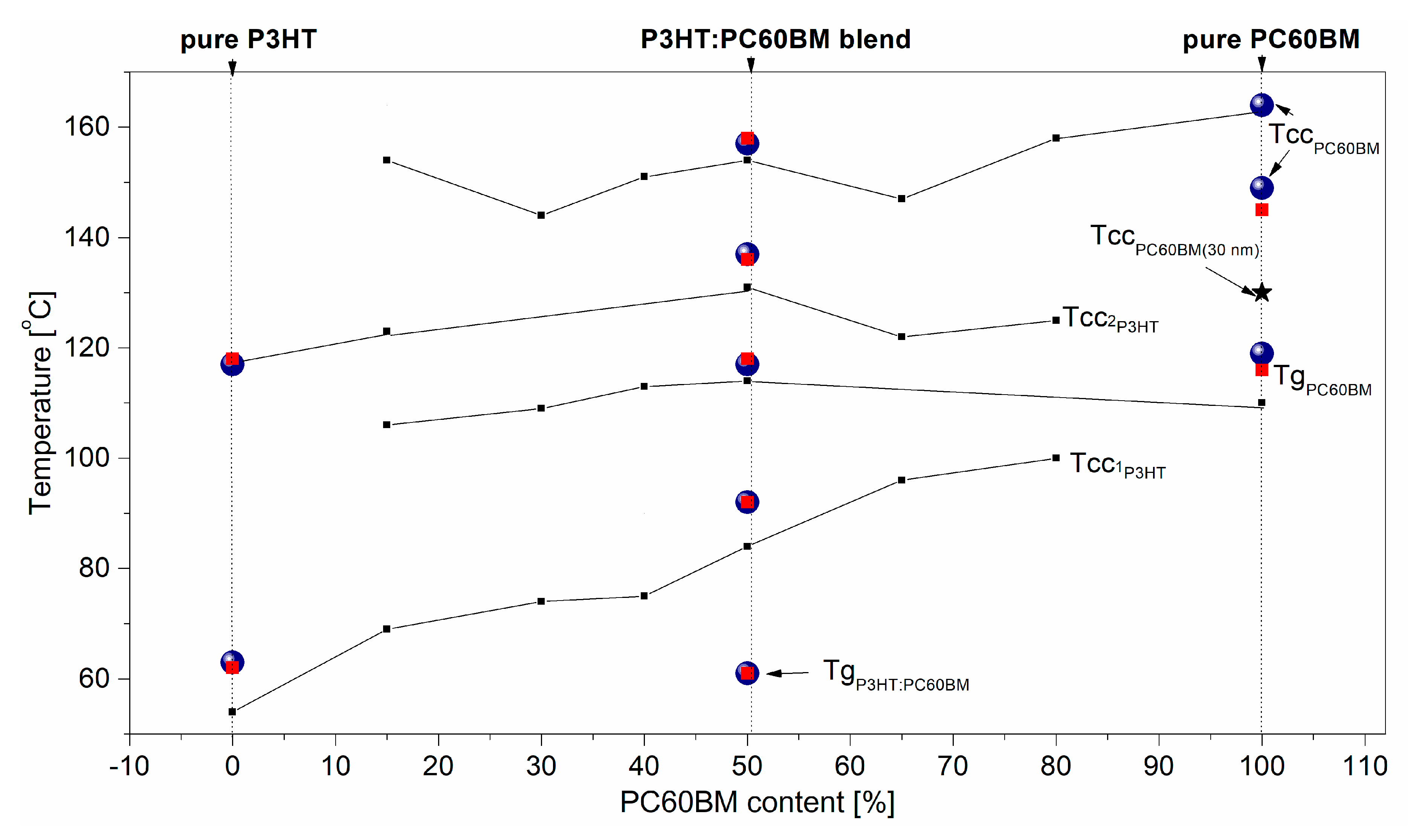
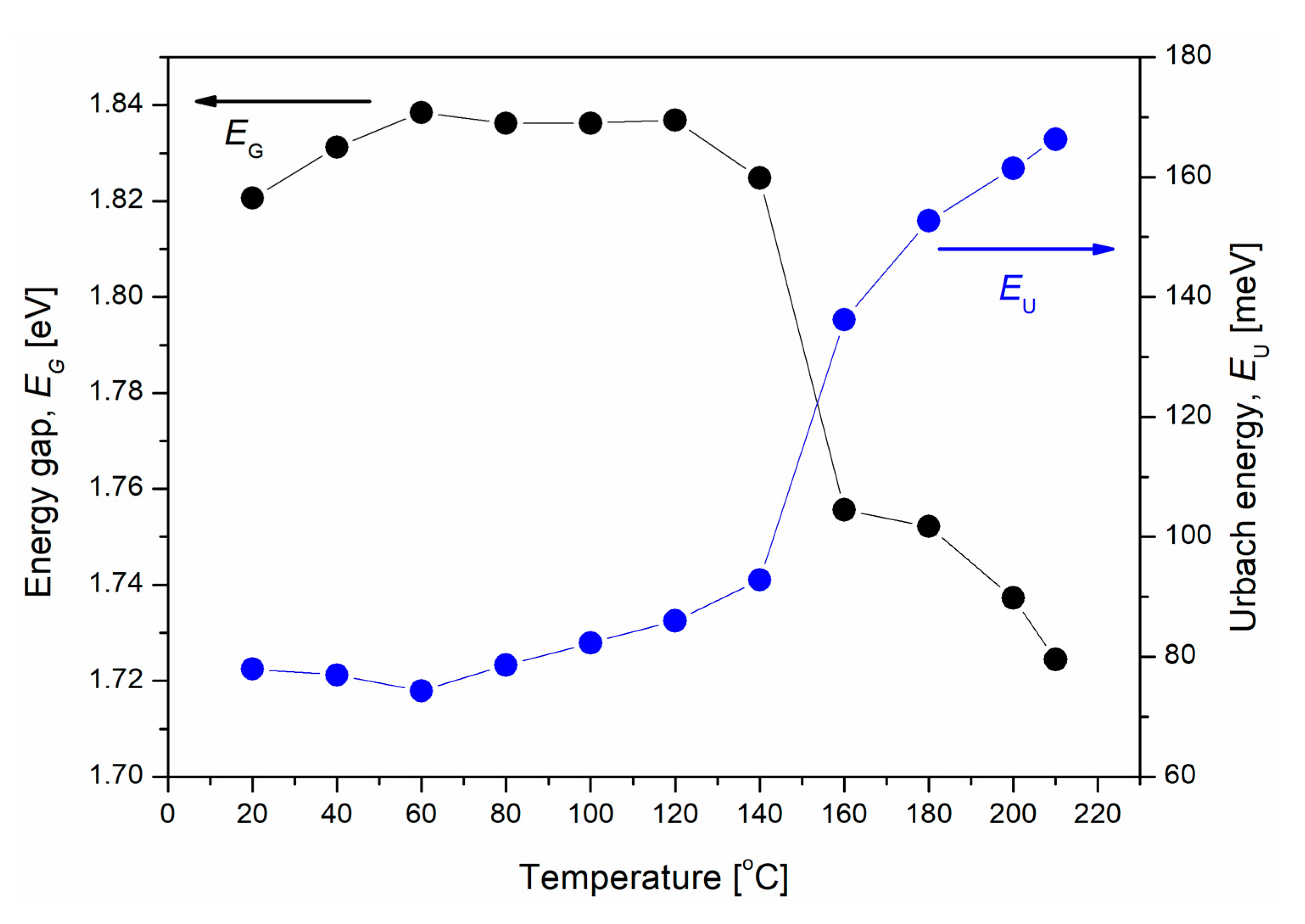
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ostroverkhova, O. Organic optoelectronic materials: Mechanisms and applications. Chem. Rev. 2016, 116, 13279–13412. [Google Scholar] [CrossRef] [PubMed]
- Abdulrazzaq, O.A.; Saini, V.; Bourdo, S.; Dervishi, E.; Biris, A.S. Organic solar cells: A review of materials, limitations and possibilities for improvement. Part. Sci. Technol. Int. J. 2013, 31, 427–442. [Google Scholar] [CrossRef]
- Kim, S.; Jahandar, M.; Jeong, J.H.; Lim, D.C. Recent progress in solar cell technology for low-light indoor applications. Curr. Altern. Energy 2018, 2, 1–15. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Bikiaris, D.N. Crystallization and melting behavior of three biodegradable poly(alkylene succinates). A comparative study. Polymer 2005, 46, 12081–12092. [Google Scholar] [CrossRef]
- Bikiaris, D.; Prinos, J.; Botev, M.; Betchev, C.; Panayiotou, C. Blends of polymers with similar glass transition temperatures: A DMTA and DSC study. J. Appl. Polym. Sci. 2004, 93, 726–735. [Google Scholar] [CrossRef]
- Giulianini, M.; Waclawik, E.R.; Bell, J.M.; Scarselli, M.; Castrucci, P.; De Crescenzi, M.; Motta, N. Microscopic and spectroscopic investigation of poly(3-hexylthiophene) interaction with carbon nanotubes. Polymers 2011, 3, 1433–1446. [Google Scholar] [CrossRef]
- Molefe, F.V.; Khenfouch, M.; Dhlamini, M.S.; Mothudi, B.M. Spectroscopic investigation of charge and energy transfer in P3HT/GO nanocomposite for solar cell applications. Adv. Mater. Lett. 2017, 8/3, 246–250. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, K.; Li, H.; Zhang, T.; Liu, D.; Han, Y. Liquid crystal ordering on conjugated polymers film morphology for high performance. J. Polym. Sci. Part B 2019. [Google Scholar] [CrossRef]
- Miller, E.D.; Jones, M.L.; Jankowski, E. Tying together multiscale calculations for charge transport in P3HT: Structural descriptors, morphology, and tie-chains. Polymers 2018, 10, 1358. [Google Scholar] [CrossRef]
- Jaglarz, J.; Nosidlak, N.; Wolska, N. Thermo-optical properties of conducted polythiophene polymer films used in electroluminescent devices. Opt. Quantum Electron. 2016, 48, 392. [Google Scholar] [CrossRef]
- Sheng, C.-Q.; Wang, P.; Shen, Y.; Li, Y.-J.; Zhang, W.-H.; Xu, F.-Q.; Zhu, J.-F.; Lai, G.-Q.; Li, H.-N. Electronic structure of PC60BM. Chin. Phys. B 2012, 21/1, 017102. [Google Scholar] [CrossRef]
- Santo, Y.; Jeon, I.; Sheng Yeo, K.; Nakagawa, T.; Matsuo, Y. Mixture of [60] and [70] PC60BM giving morphological stability in organic solar cells. Appl. Phys. Lett. 2013, 103, 073306. [Google Scholar] [CrossRef]
- Yu, Y.; Jin, B.; Peng, R.; Fan, L.; Cai, L.; Fan, B.; Chu, S. Photovoltaic performance of PCBM analogs with different ester groups as acceptor in the polymer solar cells. Synth. Met. 2016, 212, 44–50. [Google Scholar] [CrossRef]
- Ismail, Y.A.M.; Soga, T.; Jimbo, T. Investigation of PC60BM concentration on the performance of small organic solar cell. ISRN Renew. Energy 2012, 2012, 385415. [Google Scholar]
- Zhang, F.; Zhuo, Z.; Zhang, J.; Wang, X.; Xu, X.; Wang, Z.; Xin, Y.; Wang, J.; Wang, J.; Tang, W.; et al. Influence of PC60BM or PC70BM as electron acceptor on the performance of polymer solar cells. Sol. Energy Mater. Sol. Cells 2012, 97, 71–77. [Google Scholar] [CrossRef]
- Schmerl, N.; Andersson, G. A layered structure at the surface of P3HT/PCBM blends. Phys. Chem. Chem. Phys. 2011, 13, 14993–15002. [Google Scholar] [CrossRef] [PubMed]
- Ourida, O.; Said, B.M. Influence of the blend concentration of P3HT: PCBM in the performances of BHJ solar cells. Sci. Acad. Trans. Renew. Energy Syst. Eng. Technol. 2011, 1/3, 90–92. [Google Scholar]
- Chen, D.; Nakahara, A.; Wei, D.; Nordlund, D.; Russell, T.P. P3HT/PC60BM bulk heterojunction organic photovoltaics: Correlating efficiency and morphology. Nano Lett. 2011, 11, 561–567. [Google Scholar] [CrossRef]
- Munshi, J.; Balasubramanian, G. Investigating blend morphology of P3HT:PCBM bulk heterojunction solar cells by classical atomistic simulations—Progress and prospects. Soft Mater. 2020. [Google Scholar] [CrossRef]
- Laquai, F.; Andrienko, D.; Mauer, R.; Blom, P.W.M. Charge carrier transport and photogeneration in P3HT:PCBM photovoltaic blends. Macromol. Rapid Commun. 2015, 36/11, 1001–1025. [Google Scholar] [CrossRef]
- Bednarski, H.; Hajduk, B.; Domański, M.; Jarząbek, B.; Nitschke, P.; Łaba, K.; Wanic, A.; Łapkowski, M. Unveiling of polymer/fullerene blend films morphology by ellipsometrically determined optical order within polymer and fullerene phases. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 1094–1100. [Google Scholar] [CrossRef]
- Xie, Y.; Zabihi, F.; Eslamian, M. Fabrication of highly reproducible polymer solar cell using ultrasonic substrate vibration posttreatment. J. Photonics Energy 2016, 6/4, 045502. [Google Scholar] [CrossRef]
- Oyewole, O.K.; Yu, D.; Du, J.; Asare, J.; Anye, V.C.; Fashina, A.; Zebaze Kana, M.G.; Soboyejo, W.O. Lamination of organic solar cells and organic light emitting devices: Models and experiments. J. Appl. Phys. 2015, 118, 075302. [Google Scholar] [CrossRef]
- Zhang, Y.; Basel, T.P.; Gautam, B.R.; Yang, X.; Mascaro, D.J.; Liu, F.; Valy Vardeny, Z. Spin-enhanced organic bulk heterojunction photovoltaic solar cells. Nat. Commun. 2012, 3, 1043. [Google Scholar] [CrossRef] [PubMed]
- Tore, N.; Parlak, E.; Gunes, S.; Ozturk, U.; Utkan, G.; Denizci, A.; Basarir, F. Efficiency enhancement of P3HT: PCBM based organic photovoltaic devices via incorporation of bio-synthesized gold nanoparticles. Austin J. Nanomed. Nanotechnol. 2014, 2/6, 1036. [Google Scholar]
- Shin, W.S.; Hwang, Y.M.; So, W.-W.; Yoon, S.C.; Lee, C.J.; Moon, S.-J. Performance of P3HT/C70-PCBM polymer photovoltaic devices according to manufacturing conditions. Mol. Cryst. Liq. Cryst. 2008, 491, 331–338. [Google Scholar] [CrossRef]
- Li, G.; Zhu, R.; Yang, Y. Polymer solar cells. Nat. Photonics 2012, 6, 153–161. [Google Scholar] [CrossRef]
- Kroon, R.; Lenes, M.; Hummelen, J.C.; Blom, P.W.M.; De Boer, B. Small bandgap polymers for organic solar cells (polymer material development in the last 5 years). Polym. Rev. 2008, 48/3, 531–582. [Google Scholar] [CrossRef]
- Ciammaruchi, L.; Oliveira, R.; Charas, A.; Tulus; Von Hauff, E.; Polino, G.; Brunetti, F.; Hansson, R.; Moons, E.; Krassas, M.; et al. Stability of organic solar cells with PCDTBT donor polymer: An interlaboratory study. J. Mater. Res. 2018, 33/13, 1909–1924. [Google Scholar] [CrossRef]
- Pierini, F.; Lanzi, M.; Nakielski, P.; Pawłowska, S.; Urbanek, O.; Zembrzycki, K.; Kowalewski, T.A. Single-material organic solar cells based on electrospun fullerenegrafted polythiophene nanofibers. Macromolecules 2017, 50, 4972–4981. [Google Scholar] [CrossRef]
- Cui, Y.; Yao, H.; Zhang, J.; Zhang, T.; Wang, Y.; Hong, L.; Xian, K.; Xu, B.; Zhang, S.; Peng, J.; et al. Over 16% efficiency organic photovoltaic cells enabled by a chlorinated acceptor with increased open-circuit voltages. Nat. Commun. 2019, 10, 2515. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jiang, Y.; Jin, K.; Qin, J.; Xu, J.; Li, W.; Xiong, J.; Liu, J.; Xiao, Z.; Sun, K.; et al. 18% efficiency organic solar cells. Sci. Bull. 2020, 65/4, 272–275. [Google Scholar] [CrossRef]
- Lin, Y.; Adilbekova, B.; Firdaus, Y.; Yengel, E.; Faber, H.; Sajjad, M.; Zheng, X.; Yarali, E.; Seitkhan, A.; Bakr, O.M.; et al. 17% efficient organic solar cells based on liquid exfoliated WS2 as a replacement for PEDOT:PSS. Adv. Mater. 2019, 31, 1902965. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.M.; Chen, C.S.; Yang, P.C.; Ma, S.Y.; Lin, C.F. Improvement of surface morphology of thin films and performance by applying electric field on P3HT:PCBM based solar cells. Sol. Energy Mater. Sol. Cells 2012, 99, 263–267. [Google Scholar] [CrossRef]
- Ma, S.Y.; Shen, Y.M.; Yang, P.C.; Chen, C.S.; Lin, C.F. Morphological modification induced by external electric field during solution process of organic solar cells. Org. Electron. 2012, 13, 297–301. [Google Scholar] [CrossRef]
- Dang, M.T.; Hirsch, L.; Wantz, G. P3HT:PCBM, best seller in polymer photovoltaic research. Adv. Mater. 2011, 23/31, 3597–3602. [Google Scholar] [CrossRef]
- Pearson, A.J.; Wang, T.; Jones, R.A.L.; Lidzey, D.G.; Staniec, P.A.; Hopkinson, P.E.; Donald, A.M. Rationalizing phase transitions with thermal annealing temperatures for P3HT:PCBM organic photovoltaic devices. Macromolecules 2012, 45, 1499–1508. [Google Scholar] [CrossRef]
- Baek, W.H.; Yang, H.; Yoon, T.S.; Kang, C.J.; Lee, H.H.; Kim, Y.S. Effect of P3HT:PCBM concentration in solvent on performances of organic solar cells. Sol. Energy Mater. Sol. Cells 2009, 93, 1263–1267. [Google Scholar] [CrossRef]
- Yu, J.; Zheng, Y.; Huang, J. Towards high performance organic photovoltaic cells: A review of recent development in organic photovoltaics. Polymers 2014, 6, 2473–2509. [Google Scholar] [CrossRef]
- Park, S.; Seo, Y.S.; Shin, W.S.; Moon, S.J.; Hwang, J. Rapid and checkable electrical post-treatment method for organic photovoltaic devices. Sci. Rep. 2016, 6, 22604. [Google Scholar] [CrossRef]
- Jung, J.; Bork, J.; Holmgaard, T.; Kortbek, N.A.; Pedersen, K. “Ellipsometry”, Scientific Project 2004, 1-132; Institute of Physics and Nanotechnology, Aalborg University: Aalborg, Denmark, 2004. [Google Scholar]
- Nestler, P.; Helm, C.A. Determination of refractive index and layer thickness of nm-thin films via ellipsometry. Opt. Express 2017, 25/22, 27077–27085. [Google Scholar] [CrossRef] [PubMed]
- Hilfiker, J.N.; Bungay, C.L.; Synowicki, R.A.; Tiwald, T.E.; Herzinger, C.M.; Johs, B.; Pribil, G.K.; Woollam, J.A. Progress in spectroscopic ellipsometry: Applications from vacuum ultraviolet to infrared. J. Vac. Sci. Technol. A Vac. Surf. Film. 2013, 21/4, 1103–1108. [Google Scholar] [CrossRef]
- Singh, L.; Ludovice, P.J.; Henderson, C.L. Influence of molecular weight and film thickness on the glass transition temperature and coefficient of thermal expansion of supported ultrathin polymer films. Thin Solid Film. 2004, 449, 231–241. [Google Scholar] [CrossRef]
- Wang, T.; Pearson, A.J.; Dunbar, A.D.F.; Staniec, P.A.; Watter, D.C.; Coles, D.; Yi, H.; Iraqi, A.; Lidzey, D.G.; Jones, R.A.L. Competition between substrate-mediated π-π stacking and surface-mediated Tg depression in ultrathin conjugated polymer films. Eur. Phys. J. E 2012, 35, 129. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jang, J.; Zin, W.-C. Estimation of the Thickness dependence of the glass transition temperature in various thin polymer films. Langmuir 2000, 16, 4064–4067. [Google Scholar] [CrossRef]
- Keddie, J.L.; Jones, R.A.L.; Cory, R.A. Size-dependent depression of the glass transition temperature in polymer films. Europhys. Lett. 1994, 27, 59–64. [Google Scholar] [CrossRef]
- Hajduk, B.; Bednarski, H.; Trzebicka, B. Temperature-dependent spectroscopic ellipsometry of thin polymer films. J. Phys. Chem. B 2020, 124, 3229–3251. [Google Scholar] [CrossRef]
- El Ouakili, A.; Vignaud, G.; Balnois, E.; Bardeau, J.-F.; Grohens, Y. Glass transition temperatures of isotactic poly(methymethacrylate) thin films and individual chains probed by multi wavelength ellipsometry. Eur. Phys. J. Appl. Phys. 2011, 56, 13703. [Google Scholar] [CrossRef]
- Geng, K.; Tsui, O.K.C. Effects of Polymer tacticity and molecular weight on the glass transition temperature of poly(methyl methacrylate) films on silica. Macromolecules 2016, 49, 2671–2678. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Z.; Lan, Y.; Zuo, B.; Wang, X.; Yang, J.; Zhang, W.; Hu, W. Mobility gradient of poly(ethylene terephthalate) chains near a substrate scaled by the thickness of the adsorbed layer. Macromolecules 2017, 50, 6804–6812. [Google Scholar] [CrossRef]
- Efremov, M.Y.; Kiyanova, A.V.; Nealey, P.F. Temperature-modulated ellipsometry: A new probe for glass transition in thin supported polymer films. Macromolecules 2008, 41, 5978–5980. [Google Scholar] [CrossRef]
- Yamamoto, S.; Tsujii, Y.; Fukuda, T. Glass transition temperatures of high-density poly(methyl methacrylate) brushes. Macromolecules 2002, 35, 6077–6079. [Google Scholar] [CrossRef]
- Fryer, D.S.; Peters, R.D.; Kim, E.J.; Tomaszewski, J.E.; De Pablo, J.J.; Nealey, P.F.; White, C.C.; Wu, W.-L. Dependence of the glass transition temperature of polymer films on interfacial energy and thickness. Macromolecules 2001, 34, 5627–5634. [Google Scholar] [CrossRef]
- Christian, P.; Coclite, A.M. Vapor-phase-synthesized fluoroacrylate polymer thin films: Thermal stability and structural properties. Beilstein J. Nanotechnol. 2017, 8, 933–942. [Google Scholar] [CrossRef]
- Askar, S.; Evans, C.M.; Torkelson, J.M. Residual stress relaxation and stiffness in spin-coated polymer films: Characterization by ellipsometry and fluorescence. Polymer 2015, 76, 113–122. [Google Scholar] [CrossRef]
- Sharp, J.S.; Forrest, J.A. Dielectric and ellipsometric studies of the dynamics in thin films of isotactic poly(methylmethacrylate) with one free surface. Phys. Rev. E 2003, 67, 031805. [Google Scholar] [CrossRef]
- Beaucage, C.; Composto, R.; Stein, R.S. Ellipsometric study of the glass transition and thermal expansion coefficients of thin polymer films. J. Polym. Sci. Polym. Phys. Part B 1993, 31, 319–326. [Google Scholar] [CrossRef]
- Kawana, S.; Jones, R.A.L. Character of the glass transition in thin supported polymer films. Phys. Rev. E 2001, 63, 021501. [Google Scholar] [CrossRef]
- Dos Santos, W.N.; De Sousa, J.A.; Gregorio, R., Jr. Thermal conductivity behaviour of polymers around glass transition and crystalline melting temperatures. Polym. Test. 2013, 32, 987–994. [Google Scholar] [CrossRef]
- Hajduk, B.; Bednarski, H.; Jarząbek, B.; Janeczek, H.; Nitschke, P. P3HT:PCBM blend films phase diagram on the base of variable-temperature spectroscopic ellipsometry. Beilstein J. Nanotechnol. 2018, 9, 1108–1115. [Google Scholar] [CrossRef]
- Hajduk, B.; Bednarski, H.; Jarząbek, B.; Nitschke, P.; Janeczek, H. Phase diagram of P3HT:PC70BM thin films based on variable-temperature spectroscopic ellipsometry. Polym. Test. 2020, 84, 106383. [Google Scholar] [CrossRef]
- Farbod, M.; Tadavani, S.K. Electrical properties and glass transition temperature of multiwalled carbon nanotube/polyaniline composites. J. Non Cryst. Solids 2012, 358, 1339–1344. [Google Scholar] [CrossRef]
- Girtan, M. On the stability of the electrical and photoelectrical properties of P3HT and P3HT:PCBM blends thin films. Org. Electr. 2013, 14, 200–205. [Google Scholar] [CrossRef]
- Mei, Z.; Chung, D.D.L. Glass transition and melting behavior of carbon fiber reinforced thermoplastic composite, studied by electrical resistance measurement. Polym. Compos. 2000, 21, 711–715. [Google Scholar] [CrossRef]
- Kline, R.J.; McGehee, M.D.; Kadnikova, E.N.; Liu, J.; Fréchet, J.M.J.; Toney, M.F. Dependence of regioregular poly(3-hexylthiophene) film morphology, and field-effect mobility on molecular weight. Macromolecules 2005, 38, 3312–3319. [Google Scholar] [CrossRef]
- Liu, C.; Oshima, K.; Shimomura, M.; Miyauchi, S. Anisotropic conductivity–temperature characteristic of solution-cast poly(3-hexylthiophene) films. Synth. Met. 2006, 156, 1362–1367. [Google Scholar] [CrossRef]
- Ngo, T.T.; Nguyen, D.N.; Nguyen, V.T. Glass transition of PC60BM, P3HT and their blends in quenched state. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 045001. [Google Scholar] [CrossRef]
- Fernandes, L.; Gaspar, H.; Bernardo, G. Inhibition of thermal degradation of polystyrene by C60 and PC60BM: A comparative study. Polym. Test. 2014, 40, 63–69. [Google Scholar] [CrossRef]
- Rodrigues, A.; Cidália, M.; Castro, R.; Farinha, A.S.F.; Oliveira, M.; Tomé, J.P.C.; Machado, A.V.; Raposo, M.M.M.; Hilliou, L.; Bernardo, G. Thermal stability of P3HT and P3HT:PC60BM blends in the molten state. Polym. Test. 2013, 32, 1192–1201. [Google Scholar] [CrossRef]
- Adhikari, A.R.; Huang, M.; Bakhru, H.; Chipara, M.; Ryu, C.Y.; Ajayan, P.M. Thermal property of regioregular poly(3-hexylthiophene)/nanotube composites using modified single-walled carbon nanotubes via ion irradiation. Nanotechnology 2006, 17, 5947–5953. [Google Scholar] [CrossRef]
- Zhao, J.; Swinnen, A.; Van Assche, G.; Manca, J.; Vanderzande, D.; Van Mele, B. Phase diagram of P3HT/PCBM blends and its implication for the stability of morphology. J. Phys. Chem. B 2009, 113, 1587–1591. [Google Scholar] [CrossRef] [PubMed]
- Jarząbek, B.; Nitschke, P.; Hajduk, B.; Domański, M.; Bednarski, H. In situ thermo-optical studies of polymer:fullerene blend films. Polym. Test. 2020, 88, 106573. [Google Scholar] [CrossRef]

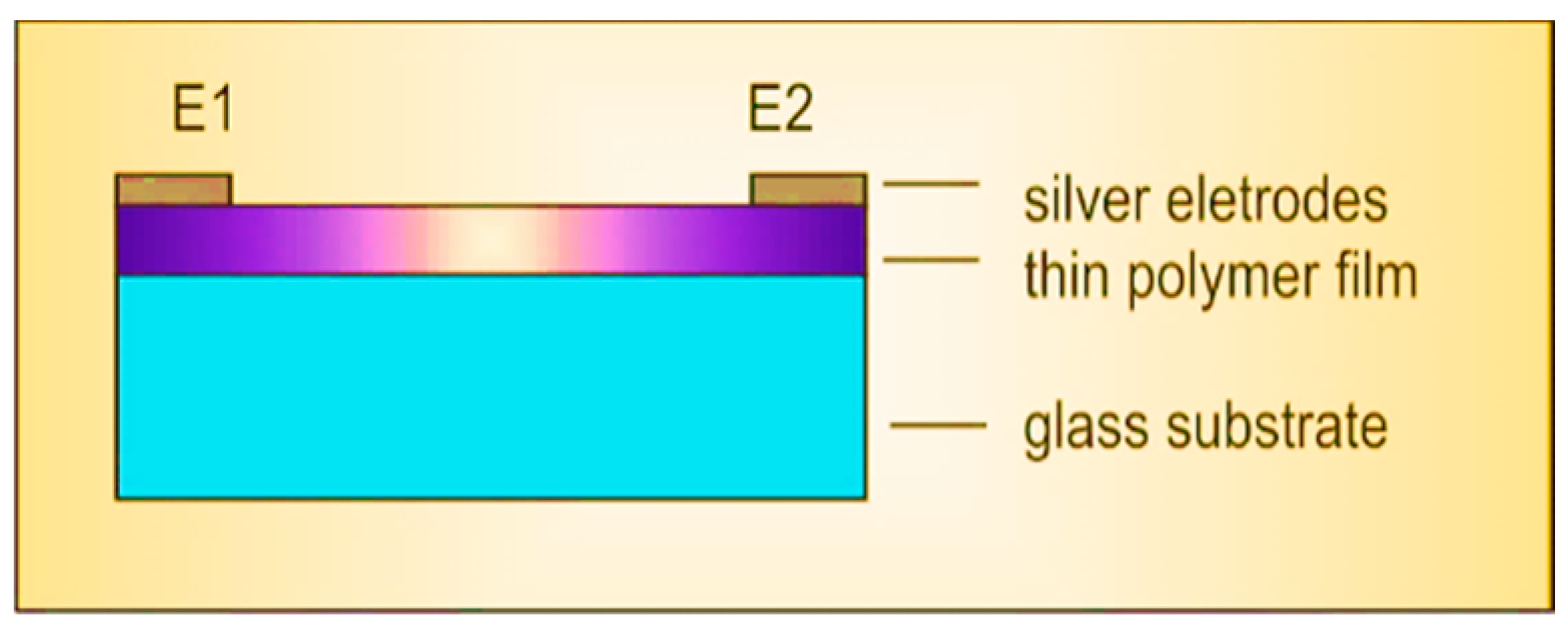
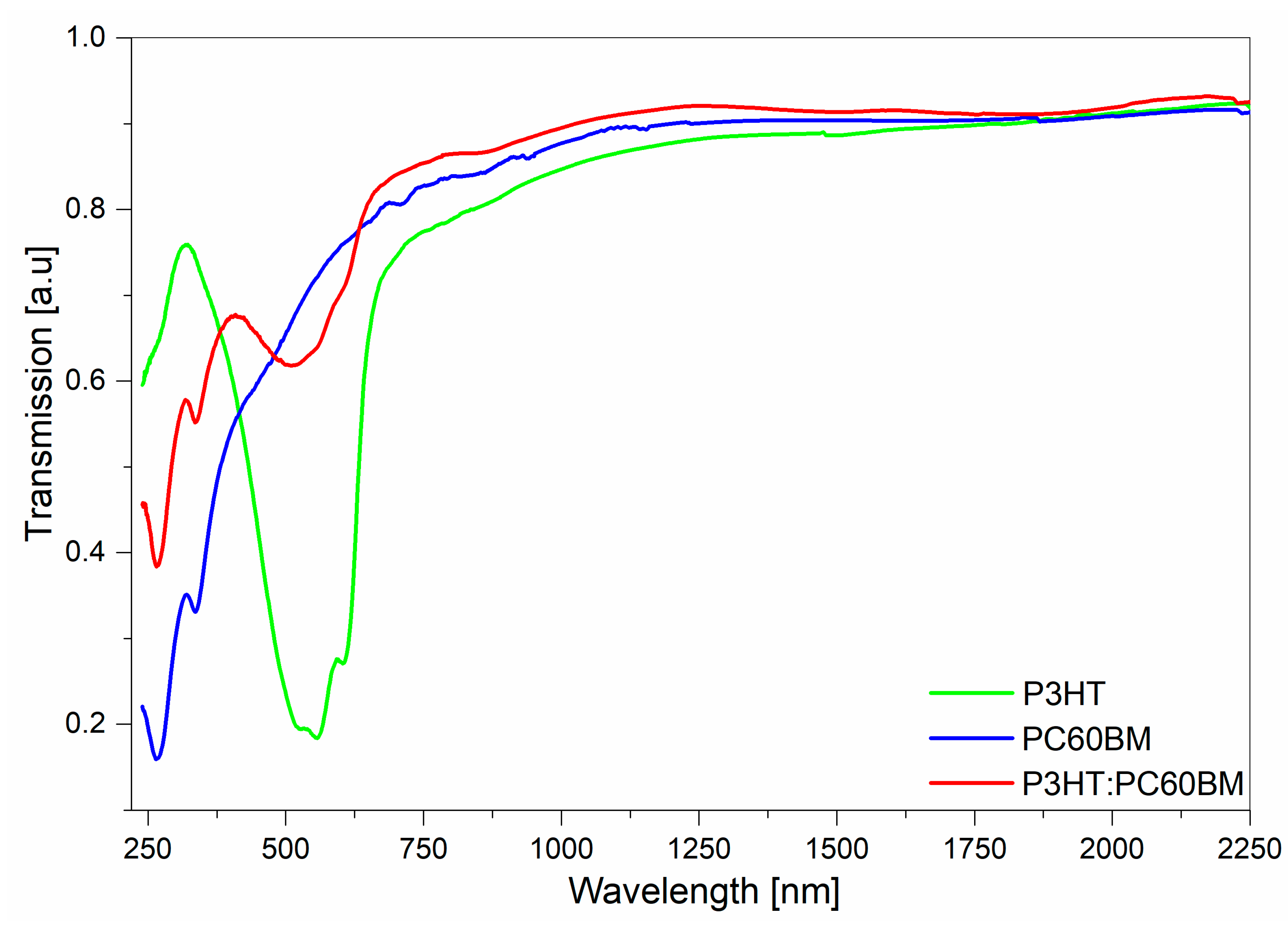
| Sample | P3HT | PC60BM | P3HT:PC60BM | |
|---|---|---|---|---|
| thickness [nm] | quartz | 150 | 80 | 120 |
| microscope glass | 250 | 400 | 250 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajduk, B.; Bednarski, H.; Domański, M.; Jarząbek, B.; Trzebicka, B. Thermal Transitions in P3HT:PC60BM Films Based on Electrical Resistance Measurements. Polymers 2020, 12, 1458. https://doi.org/10.3390/polym12071458
Hajduk B, Bednarski H, Domański M, Jarząbek B, Trzebicka B. Thermal Transitions in P3HT:PC60BM Films Based on Electrical Resistance Measurements. Polymers. 2020; 12(7):1458. https://doi.org/10.3390/polym12071458
Chicago/Turabian StyleHajduk, Barbara, Henryk Bednarski, Marian Domański, Bożena Jarząbek, and Barbara Trzebicka. 2020. "Thermal Transitions in P3HT:PC60BM Films Based on Electrical Resistance Measurements" Polymers 12, no. 7: 1458. https://doi.org/10.3390/polym12071458
APA StyleHajduk, B., Bednarski, H., Domański, M., Jarząbek, B., & Trzebicka, B. (2020). Thermal Transitions in P3HT:PC60BM Films Based on Electrical Resistance Measurements. Polymers, 12(7), 1458. https://doi.org/10.3390/polym12071458