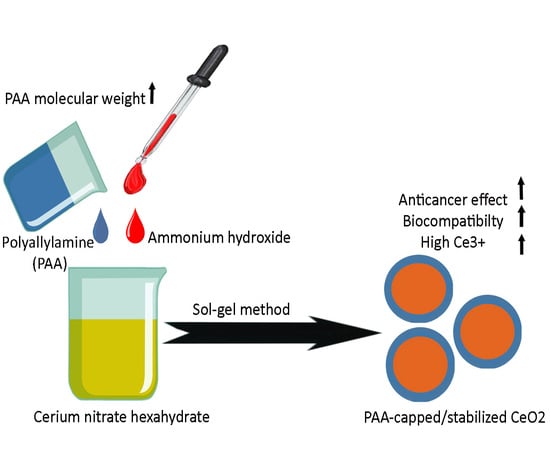
Sol–Gel Synthesis, Physico-Chemical and Biological Characterization of Cerium Oxide/Polyallylamine Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of CeO2-NPs
2.3. Characterization of CeO2-NPs
2.4. Evaluation of Cytotoxicity Effects
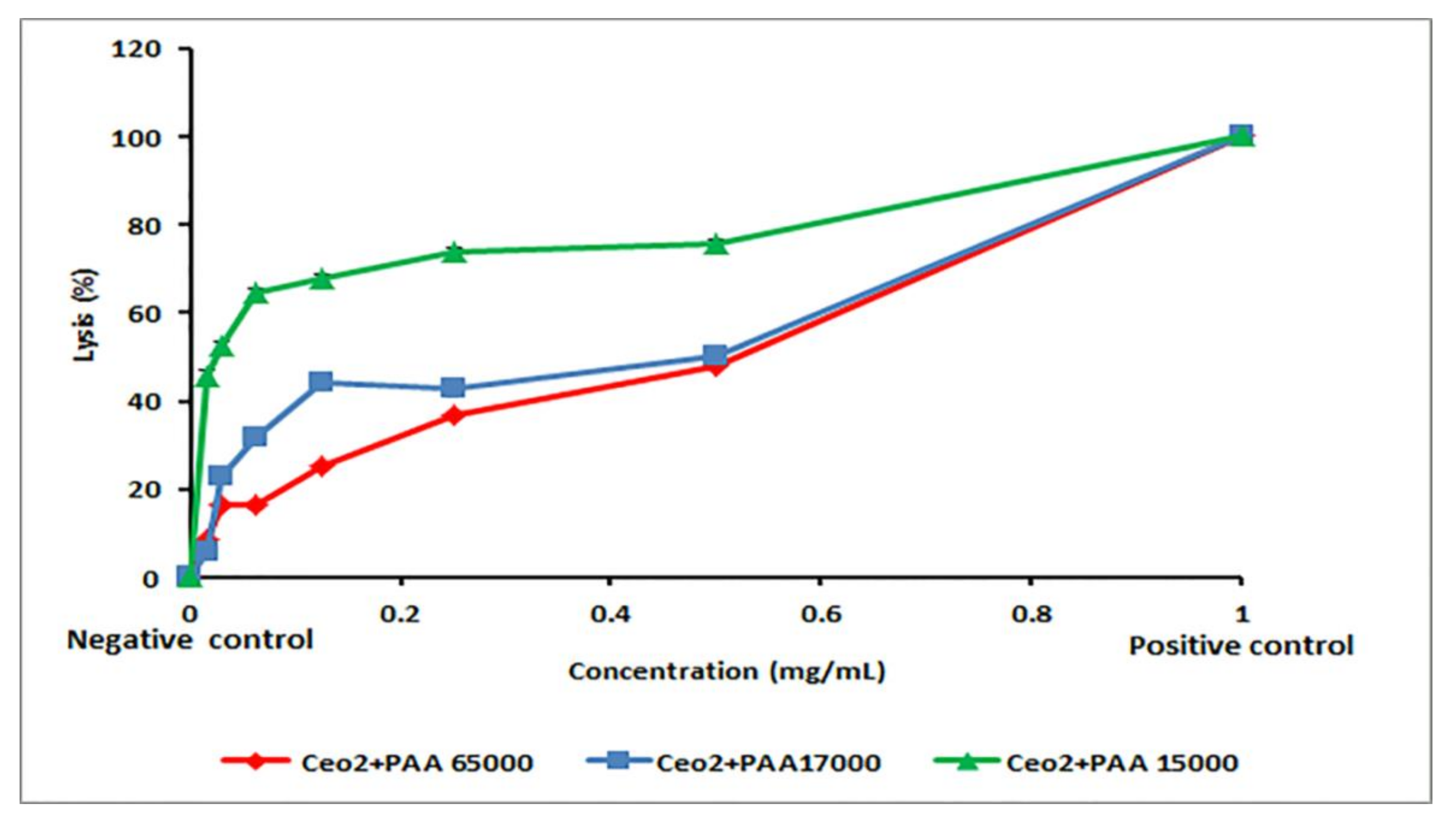
2.5. Hemolytic Activity of CeO2-NPs
2.6. Statistical Analysis
3. Results and Discussion
3.1. FTIR Spectroscopy
3.2. Thermal Analysis
3.3. PXRD Analysis
3.4. FESEM Analysis
3.5. Optical Properties of Calcined Nanoceria
3.6. Biocompatibility of the Synthesized Samples
3.7. Anticancer Activity of Nanoceria
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Amjadi, I.; Rabiee, M.; Hosseini, M.-S. Anticancer activity of nanoparticles based on PLGA and its co-polymer: In-vitro evaluation. Iran. J. Pharm. Res. IJPR 2013, 12, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Dadashi-Noshahr, K.; Islami, M.; Saburi, E.; Nikpoor, A.R.; Mellati, A.; Mossahebi-Mohammadi, M.; Soleimanifar, F.; Enderami, S.E. A novel silk/PES hybrid nanofibrous scaffold promotes the in vitro proliferation and differentiation of adipose-derived mesenchymal stem cells into insulin producing cells. Polym. Adv. Technol. 2020. [Google Scholar] [CrossRef]
- Hosseini, M.-S.; Tafazzoli-Shadpour, M.; Haghighipour, N.; Aghdami, N.; Goodarzi, A. The synergistic effects of shear stress and cyclic hydrostatic pressure modulate chondrogenic induction of human mesenchymal stem cells. Int. J. Artif. Organs 2015, 38, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.-S.; Amjadi, I.; Sheikhi, M.; Mozafari, M. Chapter 5–Supramolecular metallopolymers. In Advanced Functional Polymers for Biomedical Applications; Mozafari, M., Chauhan, N.P.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 83–110. [Google Scholar] [CrossRef]
- Charbgoo, F.; Ramezani, M.; Darroudi, M. Bio-sensing applications of cerium oxide nanoparticles: Advantages and disadvantages. Biosens. Bioelectron. 2017, 96, 33–43. [Google Scholar] [CrossRef]
- Chen, G.; Xu, Y. Biosynthesis of cerium oxide nanoparticles and their effect on lipopolysaccharide (LPS) induced sepsis mortality and associated hepatic dysfunction in male Sprague Dawley rats. Mater. Sci. Eng. C 2018, 83, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Khorrami, M.B.; Sadeghnia, H.R.; Pasdar, A.; Ghayour-Mobarhan, M.; Riahi-Zanjani, B.; Darroudi, M. Role of Pullulan in preparation of ceria nanoparticles and investigation of their biological activities. J. Mol. Struct. 2018, 1157, 127–131. [Google Scholar] [CrossRef]
- Milani, Z.M.; Charbgoo, F.; Darroudi, M. Impact of physicochemical properties of cerium oxide nanoparticles on their toxicity effects. Ceram. Int. 2017, 43, 14572–14581. [Google Scholar] [CrossRef]
- Cassee, F.R.; van Balen, E.C.; Singh, C.; Green, D.; Muijser, H.; Weinstein, J.; Dreher, K. Exposure, health and ecological effects review of engineered nanoscale cerium and cerium oxide associated with its use as a fuel additive. Crit. Rev. Toxicol. 2011, 41, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Arzaluz, M.; Noreña-Franco, L.; Ángel-Cuevas, S.; Mugica-Álvarez, V.; Torres-Rodríguez, M. Catalysts with cerium in a membrane reactor for the removal of formaldehyde pollutant from water effluents. Molecules 2016, 21, 668. [Google Scholar] [CrossRef]
- Chan, S.H.S.; Wu, T.Y.; Juan, J.C.; Teh, C.Y. Recent developments of metal oxide semiconductors as photocatalysts in advanced oxidation processes (AOPs) for treatment of dye waste-water. J. Chem. Technol. Biotechnol. 2011, 86, 1130–1158. [Google Scholar] [CrossRef]
- Ghafoori, M.; Ghobadian, B.; Najafi, G.; Layeghi, M.; Rashidi, A.; Mamat, R. Effect of nano-particles on the performance and emission of a diesel engine using biodiesel-diesel blend. Int. J. Automot. Mech. Eng. 2015, 12, 3097–3108. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar] [CrossRef]
- Liying, H.; Yumin, S.; Lanhong, J.; Shikao, S. Recent advances of cerium oxide nanoparticles in synthesis, luminescence and biomedical studies: A review. J. Rare Earths 2015, 33, 791–799. [Google Scholar] [CrossRef]
- Li, H.; Liu, C.; Zeng, Y.-P.; Hao, Y.-H.; Huang, J.-W.; Yang, Z.-Y.; Li, R. Nanoceria-mediated drug delivery for targeted photodynamic therapy on drug-resistant breast cancer. ACS Appl. Mater. Interfaces 2016, 8, 31510–31523. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Naik, P. Synthesis and biomedical applications of Cerium oxide nanoparticles–A Review. Biotechnol. Rep. 2018, 17, 1–5. [Google Scholar] [CrossRef]
- Deshmukh, K.; Ahmad, J.; Hägg, M.B. Fabrication and characterization of polymer blends consisting of cationic polyallylamine and anionic polyvinyl alcohol. Ionics 2014, 20, 957–967. [Google Scholar] [CrossRef]
- Jian, W.; Xu, S.; Wang, J.; Feng, S. Layer-by-layer assembly of poly (allylamine hydrochloride)/polyurethane and its loading and release behavior for methylene orange. J. Appl. Polym. Sci. 2013, 129, 2070–2075. [Google Scholar] [CrossRef]
- Luo, R.; Neu, B.; Venkatraman, S.S. Surface functionalization of nanoparticles to control cell interactions and drug release. Small 2012, 8, 2585–2594. [Google Scholar] [CrossRef]
- Sarwar, M.S.; Huang, Q.; Ghaffar, A.; Abid, M.A.; Zafar, M.S.; Khurshid, Z.; Latif, M. A Smart Drug Delivery System Based on Biodegradable Chitosan/Poly (allylamine hydrochloride) Blend Films. Pharmaceutics 2020, 12, 131. [Google Scholar] [CrossRef]
- Shanavas, A.; Bahadur, D.; Srivastava, R. Core/surface modified nanomedicines for controlled release of drug. In Proceedings of the 12th IEEE International Conference on Nanotechnology (IEEE-NANO), Birmingham, UK, 20–23 August 2012; pp. 1–4. [Google Scholar] [CrossRef]
- Darroudi, M.; Sarani, M.; Oskuee, R.K.; Zak, A.K.; Amiri, M.S. Nanoceria: Gum mediated synthesis and in vitro viability assay. Ceram. Int. 2014, 40, 2863–2868. [Google Scholar] [CrossRef]
- Bagheri, B.; Zarrintaj, P.; Samadi, A.; Zarrintaj, R.; Ganjali, M.R.; Saeb, M.R.; Mozafari, M.; Park, O.O.; Kim, Y.C. Tissue engineering with electrospun electro-responsive chitosan-aniline oligomer/polyvinyl alcohol. Int. J. Biol. Macromol. 2020, 147, 160–169. [Google Scholar] [CrossRef]
- Bagheri, B.; Zarrintaj, P.; Surwase, S.S.; Baheiraei, N.; Saeb, M.R.; Mozafari, M.; Kim, Y.C.; Park, O.O. Self-gelling electroactive hydrogels based on chitosan–aniline oligomers/agarose for neural tissue engineering with on-demand drug release. Colloids Surf. B Biointerfaces 2019, 184, 110549. [Google Scholar] [CrossRef]
- Auffan, M.; Achouak, W.; Rose, J.; Roncato, M.-A.; Chaneac, C.; Waite, D.T.; Masion, A.; Woicik, J.C.; Wiesner, M.R.; Bottero, J.-Y. Relation between the redox state of iron-based nanoparticles and their cytotoxicity toward Escherichia coli. Environ. Sci. Technol. 2008, 42, 6730–6735. [Google Scholar] [CrossRef]
- Palermo, E.F.; Sovadinova, I.; Kuroda, K. Structural determinants of antimicrobial activity and biocompatibility in membrane-disrupting methacrylamide random copolymers. Biomacromolecules 2009, 10, 3098–3107. [Google Scholar] [CrossRef] [PubMed]
- Kargar, H.; Ghasemi, F.; Darroudi, M. Bioorganic polymer-based synthesis of cerium oxide nanoparticles and their cell viability assays. Ceram. Int. 2015, 41, 1589–1594. [Google Scholar] [CrossRef]
- Kargar, H.; Ghazavi, H.; Darroudi, M. Size-controlled and bio-directed synthesis of ceria nanopowders and their in vitro cytotoxicity effects. Ceram. Int. 2015, 41, 4123–4128. [Google Scholar] [CrossRef]
- Alpaslan, E.; Yazici, H.; Golshan, N.H.; Ziemer, K.S.; Webster, T.J. pH-Dependent Activity of Dextran-Coated Cerium Oxide Nanoparticles on Prohibiting Osteosarcoma Cell Proliferation. ACS Biomater. Sci. Eng. 2015, 1, 1096–1103. [Google Scholar] [CrossRef]
- Eriksson, P.; Tal, A.A.; Skallberg, A.; Brommesson, C.; Hu, Z.; Boyd, R.D.; Olovsson, W.; Fairley, N.; Abrikosov, I.A.; Zhang, X.; et al. Cerium oxide nanoparticles with antioxidant capabilities and gadolinium integration for MRI contrast enhancement. Sci. Rep. 2018, 8, 6999. [Google Scholar] [CrossRef]
- Giri, S.; Karakoti, A.; Graham, R.P.; Maguire, J.L.; Reilly, C.M.; Seal, S.; Rattan, R.; Shridhar, V. Nanoceria: A rare-earth nanoparticle as a novel anti-angiogenic therapeutic agent in ovarian cancer. PLoS ONE 2013, 8, e54578. [Google Scholar] [CrossRef]
- Elahi, B.; Mirzaee, M.; Darroudi, M.; Oskuee, R.K.; Sadri, K.; Amiri, M.S. Preparation of cerium oxide nanoparticles in Salvia Macrosiphon Boiss seeds extract and investigation of their photo-catalytic activities. Ceram. Int. 2019, 45, 4790–4797. [Google Scholar] [CrossRef]
- Gu, H.; Soucek, M.D. Preparation and Characterization of Monodisperse Cerium Oxide Nanoparticles in Hydrocarbon Solvents. Chem. Mater. 2007, 19, 1103–1110. [Google Scholar] [CrossRef]
- Nourmohammadi, E.; Khoshdel-Sarkarizi, H.; Nedaeinia, R.; Sadeghnia, H.R.; Hasanzadeh, L.; Darroudi, M.; Oskuee, R.K. Evaluation of anticancer effects of cerium oxide nanoparticles on mouse fibrosarcoma cell line. J. Cell. Physiol. 2019, 234, 4987–4996. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-I.; Chang, H.-Y. Synthesis of nanocrystalline cerium oxide particles by the precipitation method. Ceram. Int. 2005, 31, 795–802. [Google Scholar] [CrossRef]
- Brus, L. Electronic wave functions in semiconductor clusters: Experiment and theory. J. Phys. Chem. 1986, 90, 2555–2560. [Google Scholar] [CrossRef]
- Afsar, T.; Razak, S.; Khan, M.R.; Mawash, S.; Almajwal, A.; Shabir, M.; Haq, I.U. Evaluation of antioxidant, anti-hemolytic and anticancer activity of various solvent extracts of Acacia hydaspica R. Parker aerial parts. BMC Complement. Altern. Med. 2016, 16, 258. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Ansari, A.A.; Khan, A.A.; Al-Kattan, W.; Al-Obeed, O.; Ahmad, R. Design, synthesis and in vitro evaluation of anticancer and antibacterial potential of surface modified Tb(OH)3@SiO2 core–shell nanoparticles. RSC Adv. 2016, 6, 18667–18677. [Google Scholar] [CrossRef]
- Sathishkumar, G.; Bharti, R.; Jha, P.K.; Selvakumar, M.; Dey, G.; Jha, R.; Jeyaraj, M.; Mandal, M.; Sivaramakrishnan, S. Dietary flavone chrysin (5,7-dihydroxyflavone ChR) functionalized highly-stable metal nanoformulations for improved anticancer applications. RSC Adv. 2015, 5, 89869–89878. [Google Scholar] [CrossRef]
- Benameur, L.; Auffan, M.; Cassien, M.; Liu, W.; Culcasi, M.; Rahmouni, H.; Stocker, P.; Tassistro, V.; Bottero, J.Y.; Rose, J.; et al. DNA damage and oxidative stress induced by CeO2 nanoparticles in human dermal fibroblasts: Evidence of a clastogenic effect as a mechanism of genotoxicity. Nanotoxicology 2015, 9, 696–705. [Google Scholar] [CrossRef]
- Hussain, S.; Kodavanti, P.P.; Marshburg, J.D.; Janoshazi, A.; Marinakos, S.M.; George, M.; Rice, A.; Wiesner, M.R.; Garantziotis, S. Decreased Uptake and Enhanced Mitochondrial Protection Underlie Reduced Toxicity of Nanoceria in Human Monocyte-Derived Macrophages. J. Biomed. Nanotechnol. 2016, 12, 2139–2150. [Google Scholar] [CrossRef]
- Asati, A.; Santra, S.; Kaittanis, C.; Nath, S.; Perez, J.M. Oxidase-like activity of polymer-coated cerium oxide nanoparticles. Angew. Chem. Int. Ed. Engl. 2009, 48, 2308–2312. [Google Scholar] [CrossRef]
- Gharbi, N.; Pressac, M.; Hadchouel, M.; Szwarc, H.; Wilson, S.R.; Moussa, F. Fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano Lett. 2005, 5, 2578–2585. [Google Scholar] [CrossRef] [PubMed]
- Lucente-Schultz, R.M.; Moore, V.C.; Leonard, A.D.; Price, B.K.; Kosynkin, D.V.; Lu, M.; Partha, R.; Conyers, J.L.; Tour, J.M. Antioxidant single-walled carbon nanotubes. J. Am. Chem. Soc. 2009, 131, 3934–3941. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.X.; Tang, J.J.; Luo, H.; Jin, X.L.; Dai, F.; Yang, J.; Qian, Y.P.; Li, X.Z.; Zhou, B. Antioxidant and antiproliferative activities of hydroxyl-substituted Schiff bases. Bioorganic Med. Chem. Lett. 2010, 20, 2417–2420. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Mostafapoor, F.; Milan, P.B.; Saeb, M.R. Theranostic platforms proposed for cancerous stem cells: A review. Curr. Stem Cell Res. Ther. 2019, 14, 137–145. [Google Scholar] [CrossRef] [PubMed]



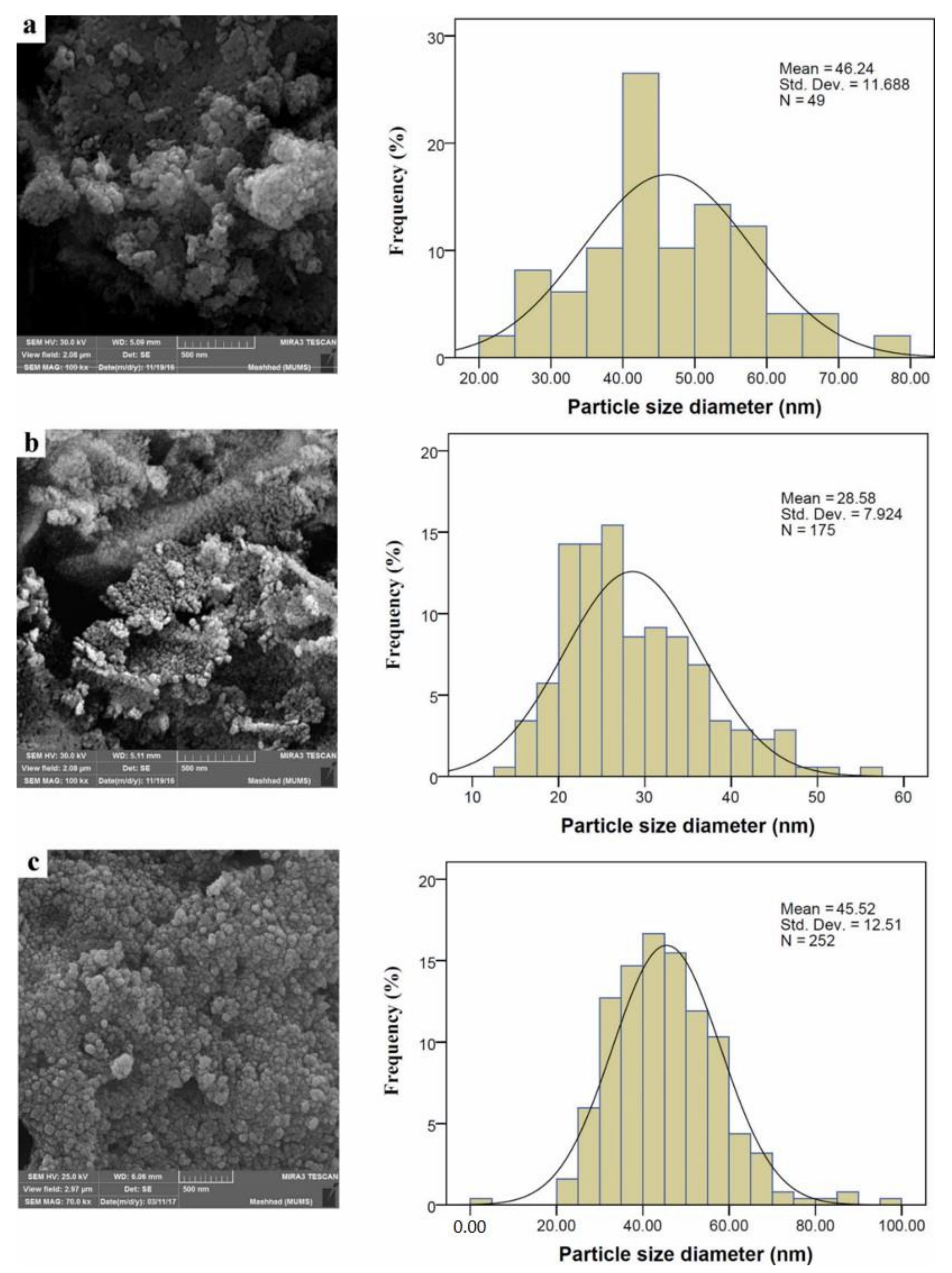


| PAA Molecular Weight in CeO2-NPs, g/mole | λmax, nm | Eg, eV | Crystallite Size (nm) | |
|---|---|---|---|---|
| Calculated 1 | Observed 2 | |||
| 15,000 | 348 | 2.47 | 12.26 | 46.24 |
| 17,000 | 354 | 2.52 | 13.66 | 28.58 |
| 65,000 | 298 | 3.51 | 15.04 | 45.52 |
| PAA Molecular Weight in CeO2-NPs, g/mole | HC50 (mg/mL) | IC50 (μg/mL) | |
|---|---|---|---|
| MCF-7 | HeLa | ||
| 15,000 | 0.022 ± 0.001 | 17.44 ± 7.32 | 8.09 ± 1.55 |
| 17,000 | 3.74 ± 0.58 | 6.17 ± 1.01 | 2.11 ± 0.33 |
| 65,000 | 7.35 ± 1.32 | 0.12 ± 0.03 | 0.20 ± 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosseini, M.; Amjadi, I.; Mohajeri, M.; Mozafari, M. Sol–Gel Synthesis, Physico-Chemical and Biological Characterization of Cerium Oxide/Polyallylamine Nanoparticles. Polymers 2020, 12, 1444. https://doi.org/10.3390/polym12071444
Hosseini M, Amjadi I, Mohajeri M, Mozafari M. Sol–Gel Synthesis, Physico-Chemical and Biological Characterization of Cerium Oxide/Polyallylamine Nanoparticles. Polymers. 2020; 12(7):1444. https://doi.org/10.3390/polym12071444
Chicago/Turabian StyleHosseini, Motaharesadat, Issa Amjadi, Mohammad Mohajeri, and Masoud Mozafari. 2020. "Sol–Gel Synthesis, Physico-Chemical and Biological Characterization of Cerium Oxide/Polyallylamine Nanoparticles" Polymers 12, no. 7: 1444. https://doi.org/10.3390/polym12071444
APA StyleHosseini, M., Amjadi, I., Mohajeri, M., & Mozafari, M. (2020). Sol–Gel Synthesis, Physico-Chemical and Biological Characterization of Cerium Oxide/Polyallylamine Nanoparticles. Polymers, 12(7), 1444. https://doi.org/10.3390/polym12071444