Structural, Impedance and Electrochemical Characteristics of Electrical Double Layer Capacitor Devices Based on Chitosan: Dextran Biopolymer Blend Electrolytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Structural and Impedance Characterizations
2.3. Transference Number Measurement (TNM)
2.4. Linear Sweep Voltammetry (LSV) Study
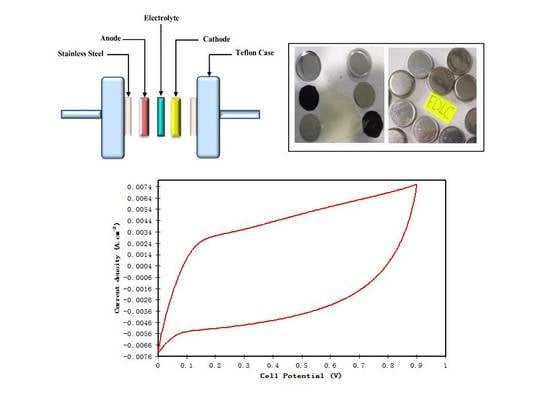
2.5. EDLC Fabrication
3. Results
3.1. XRD Study
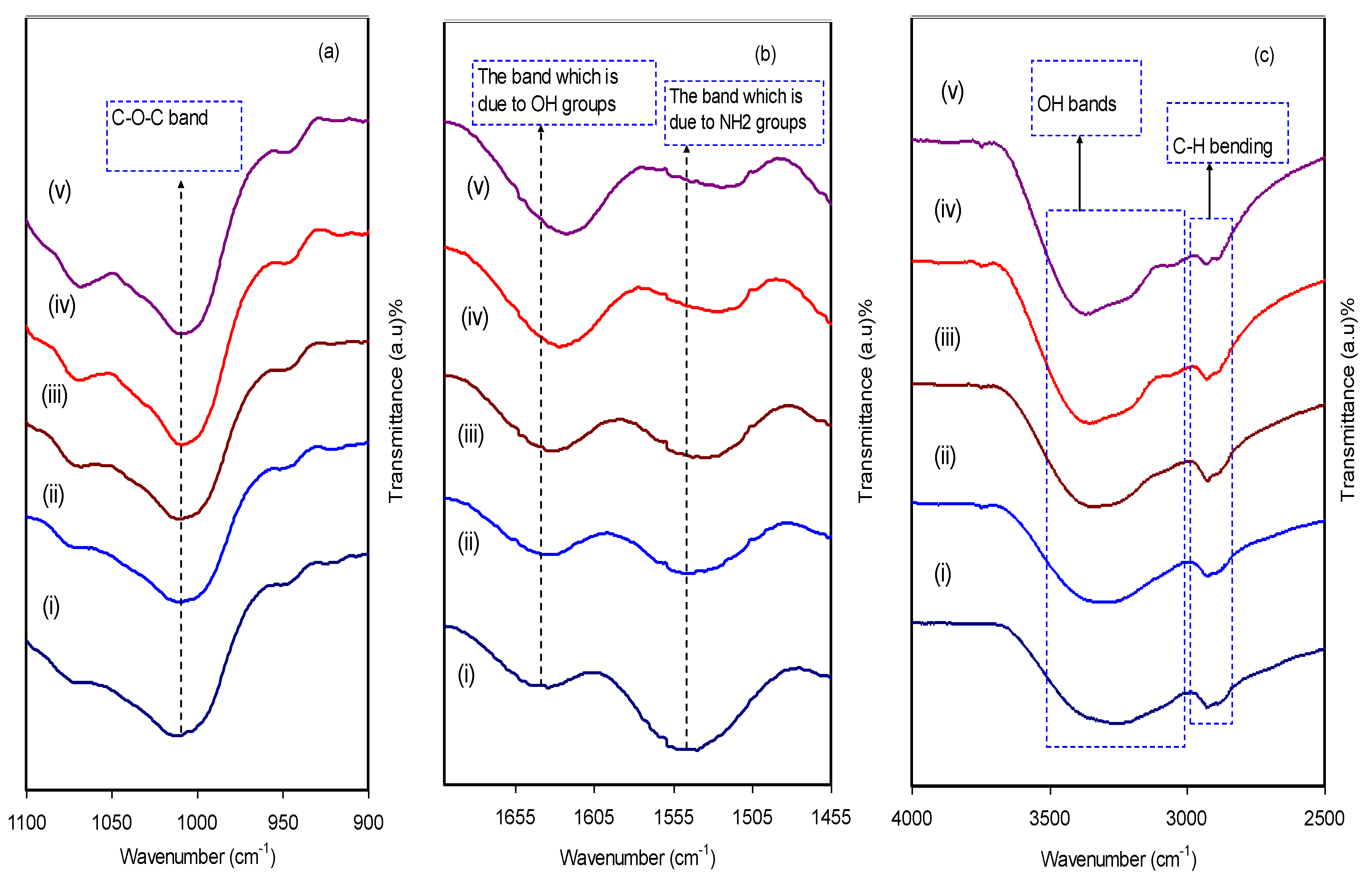
3.2. FTIR Analysis
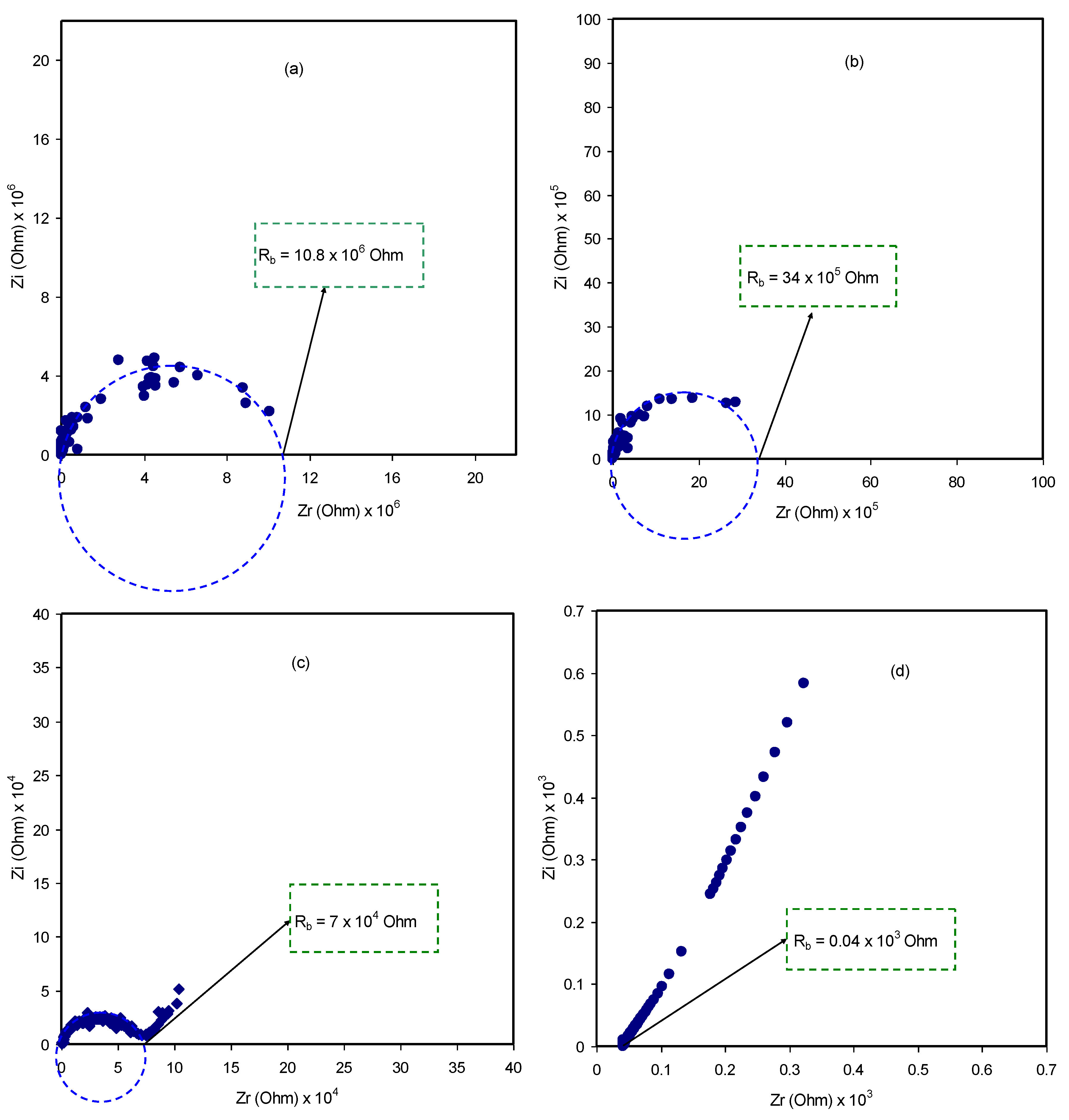
3.3. Impedance Study
3.4. EDLC Study
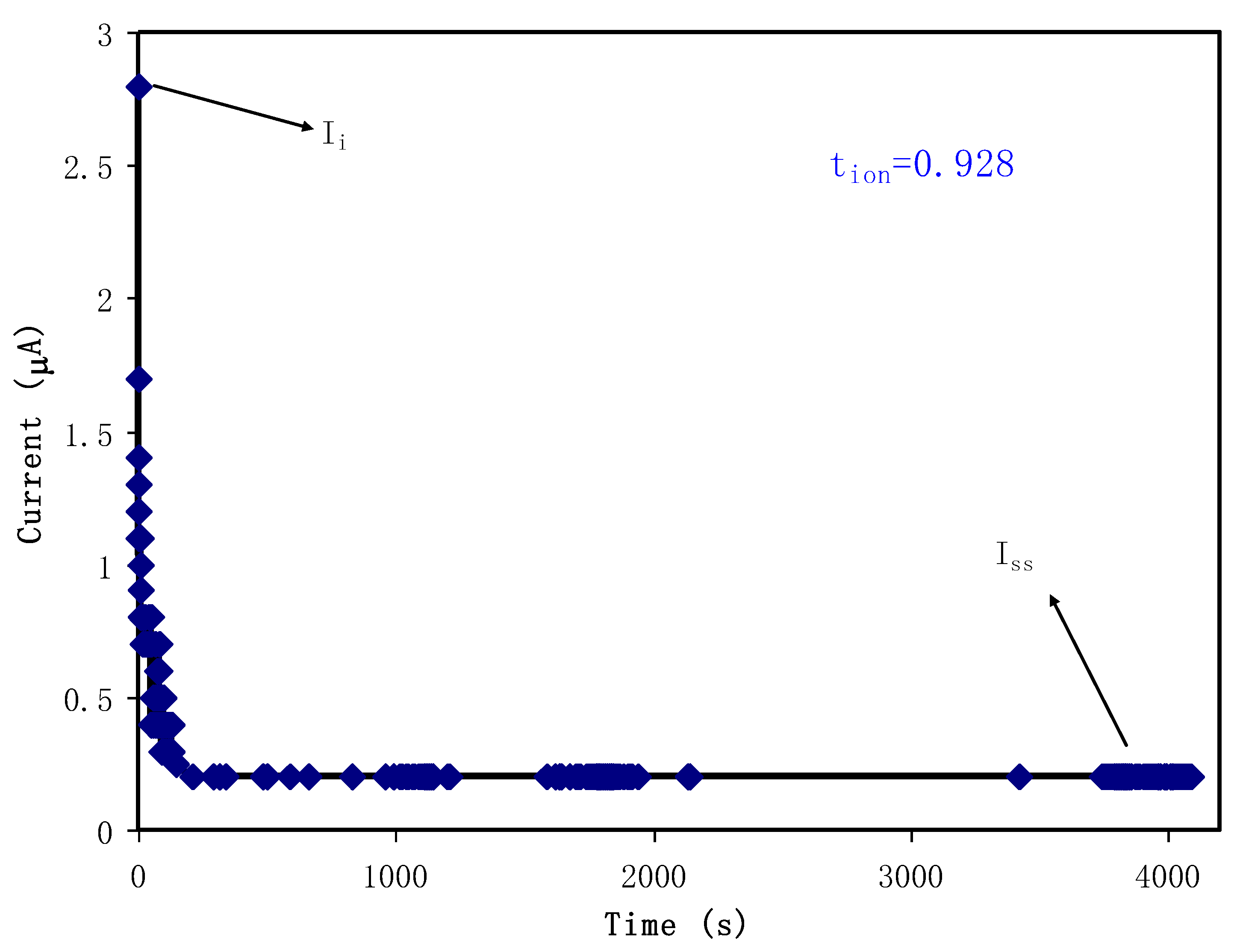
3.4.1. TNM Study
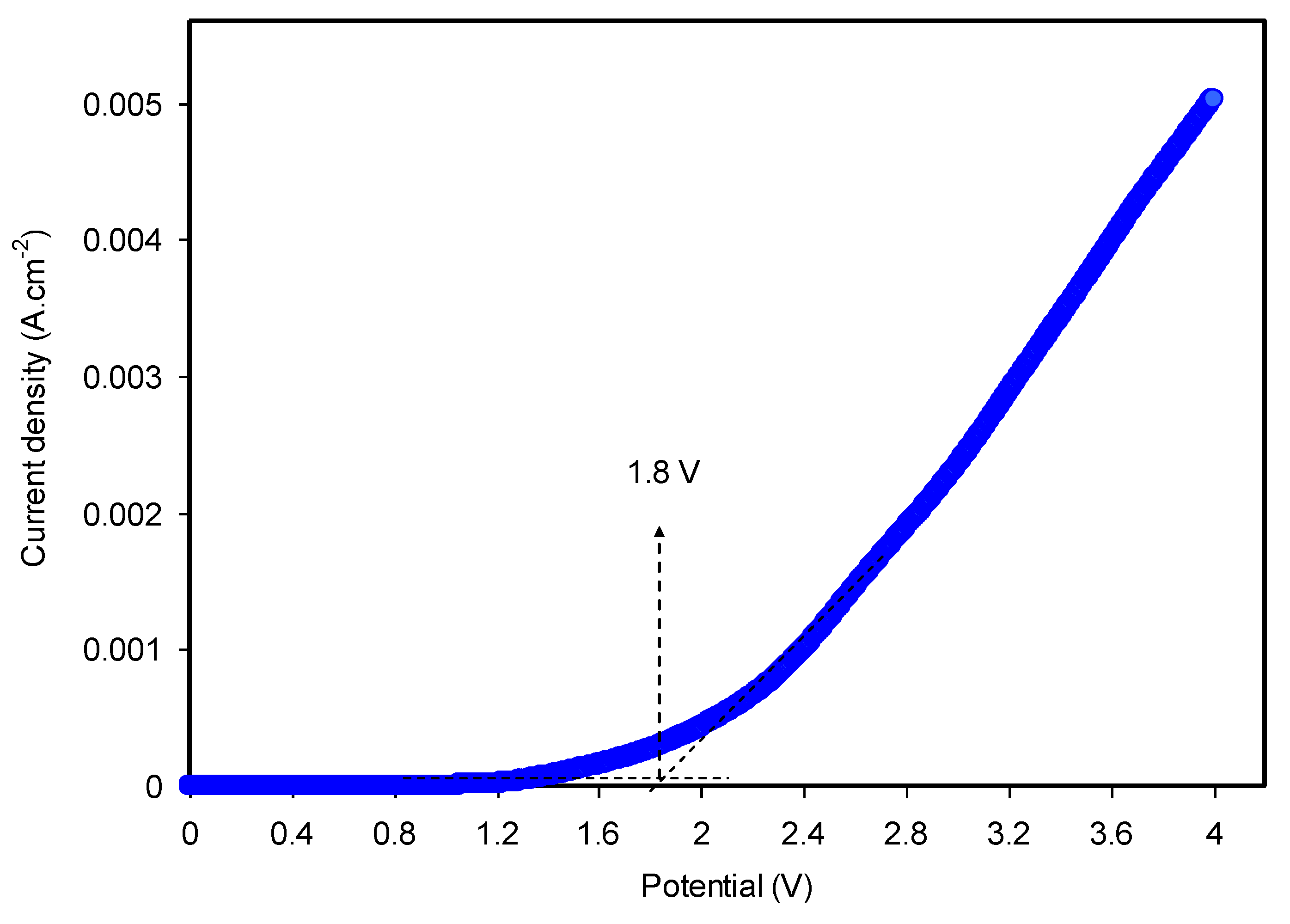
3.4.2. LSV Study
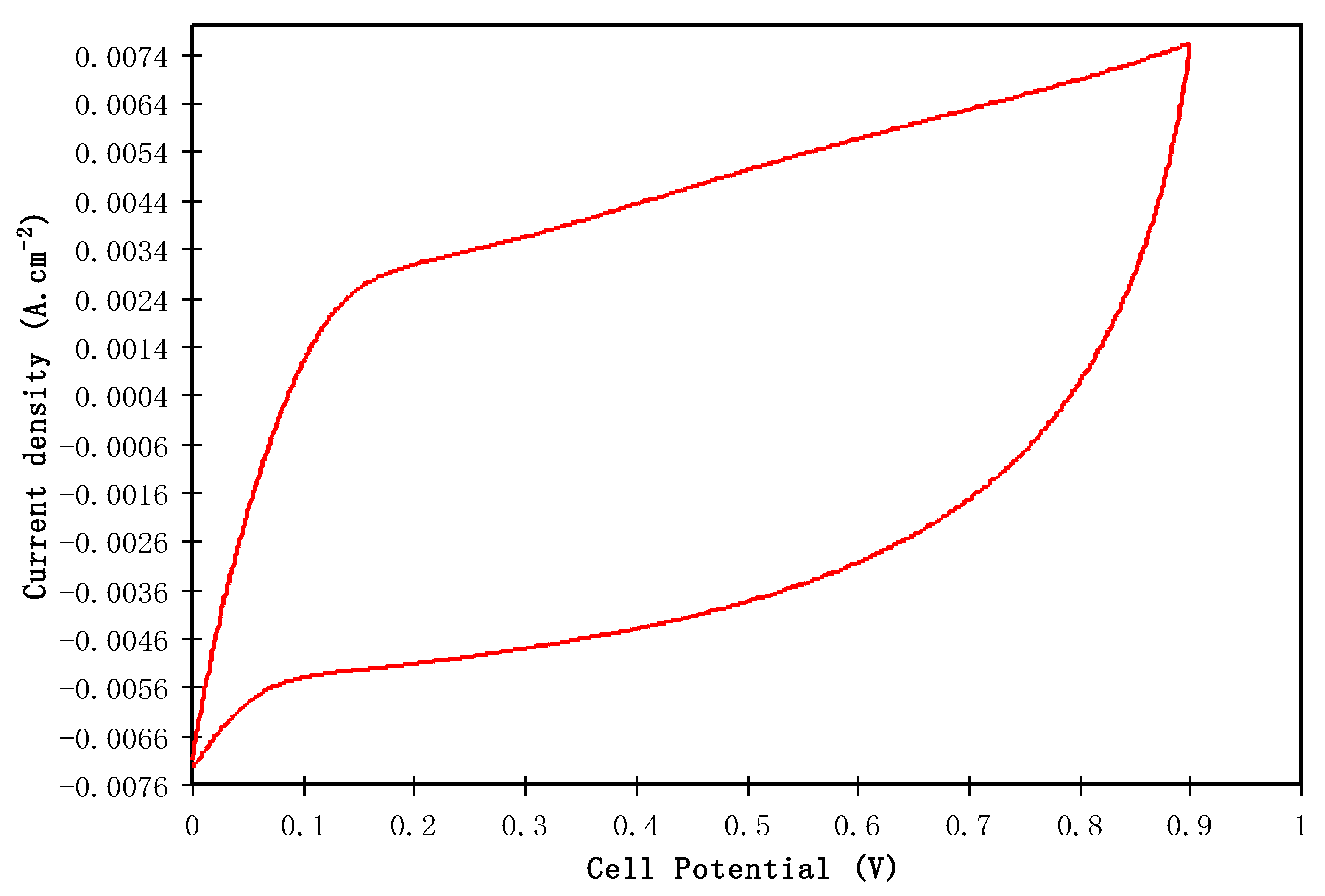
3.4.3. CV Study
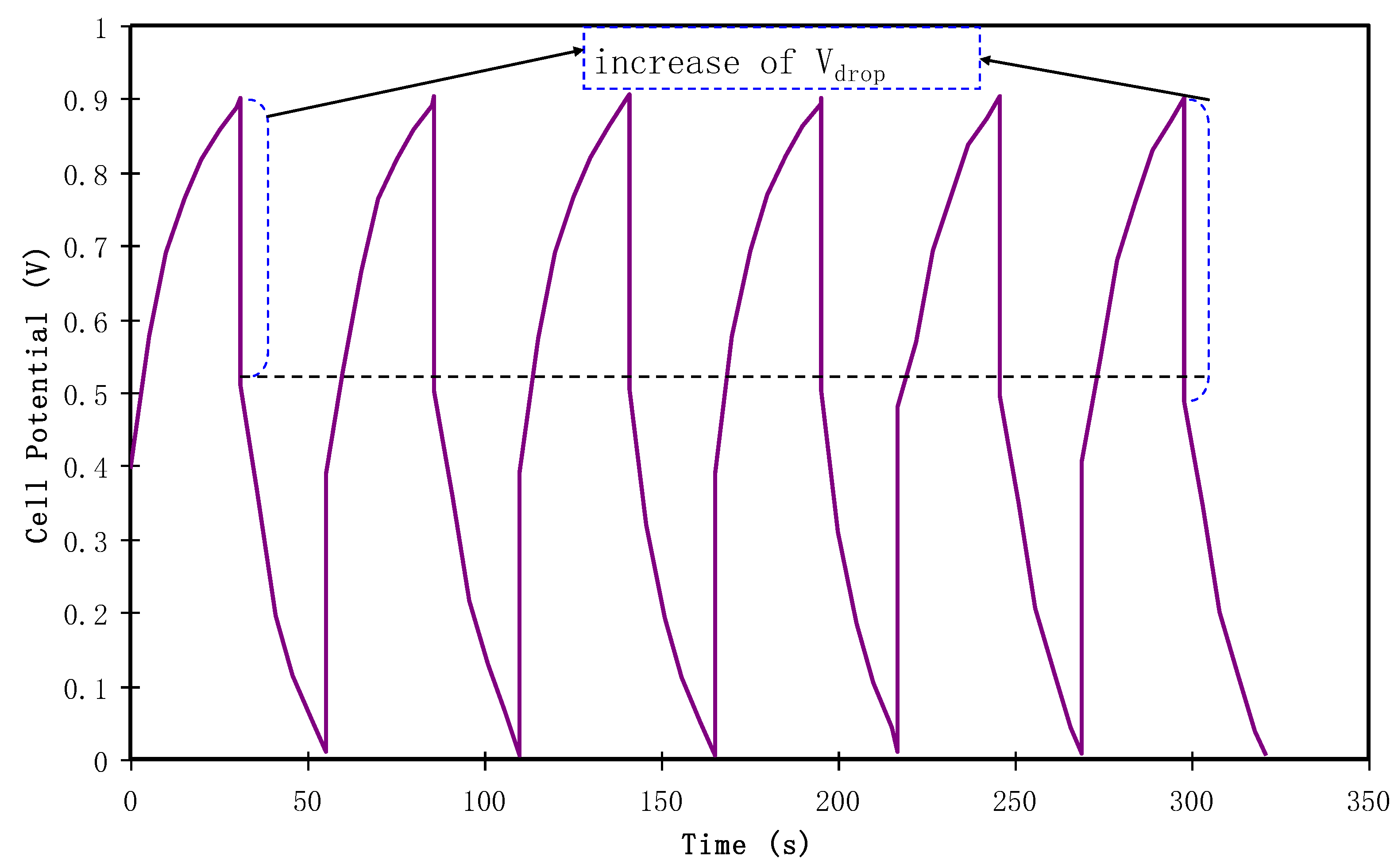
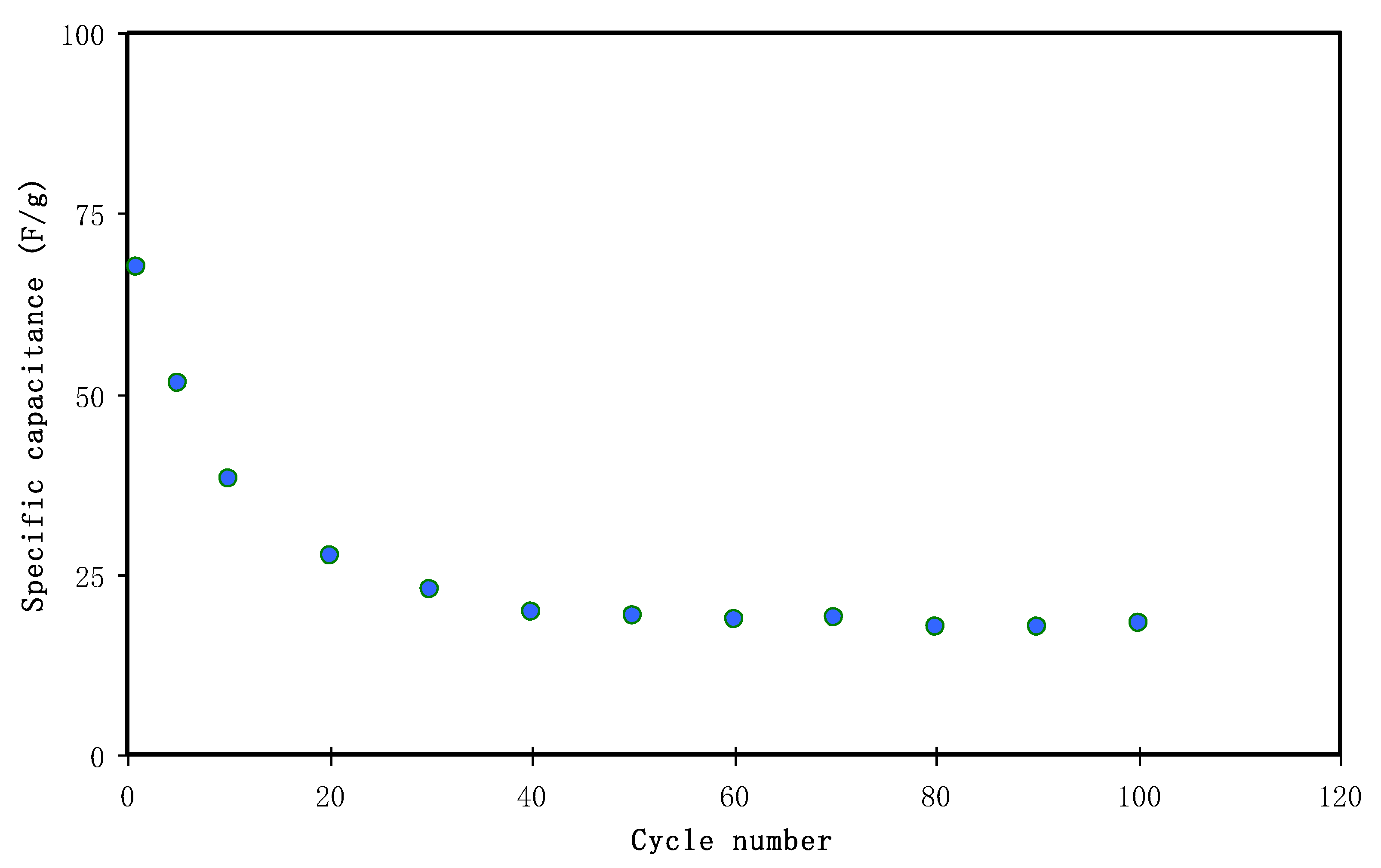
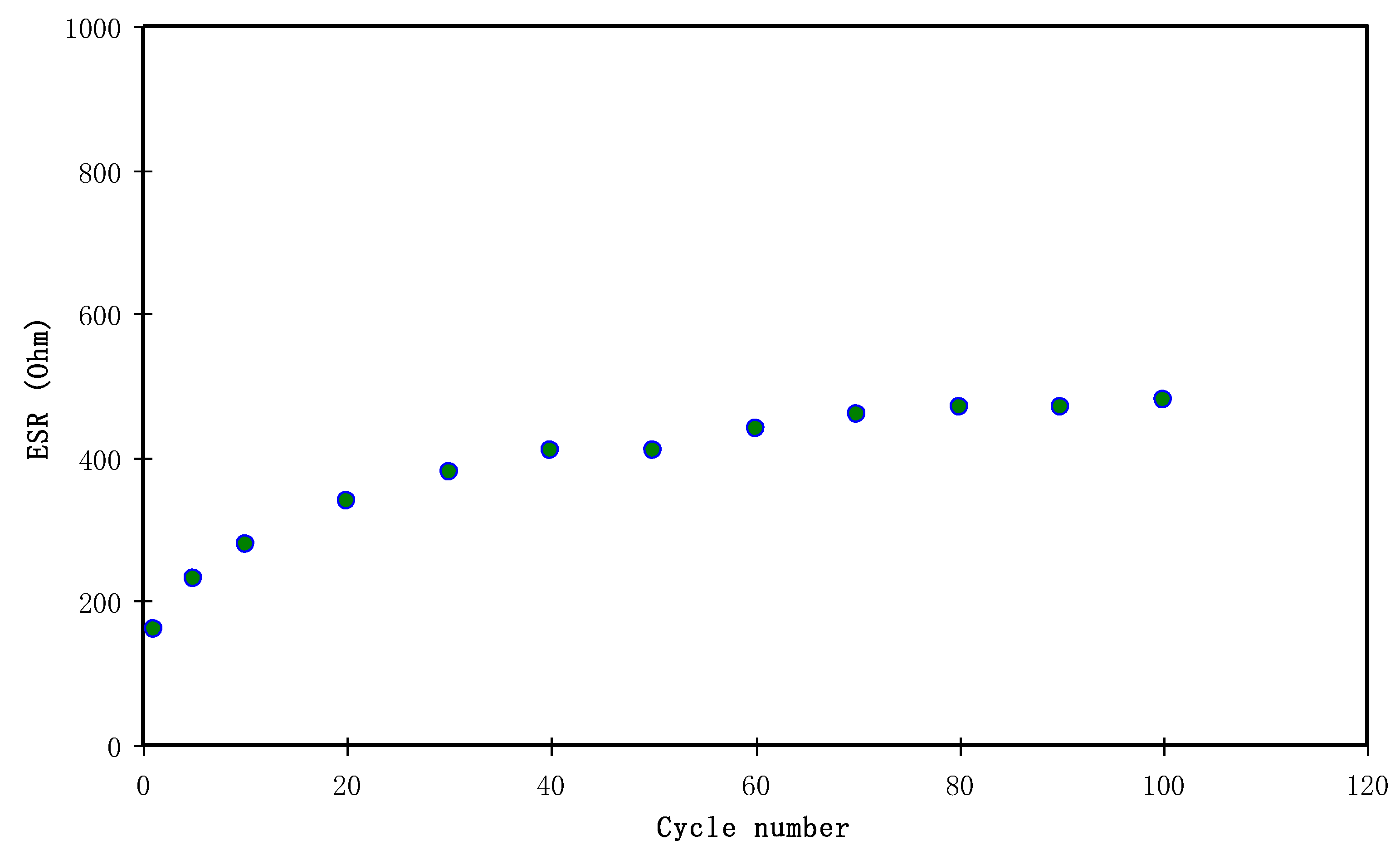
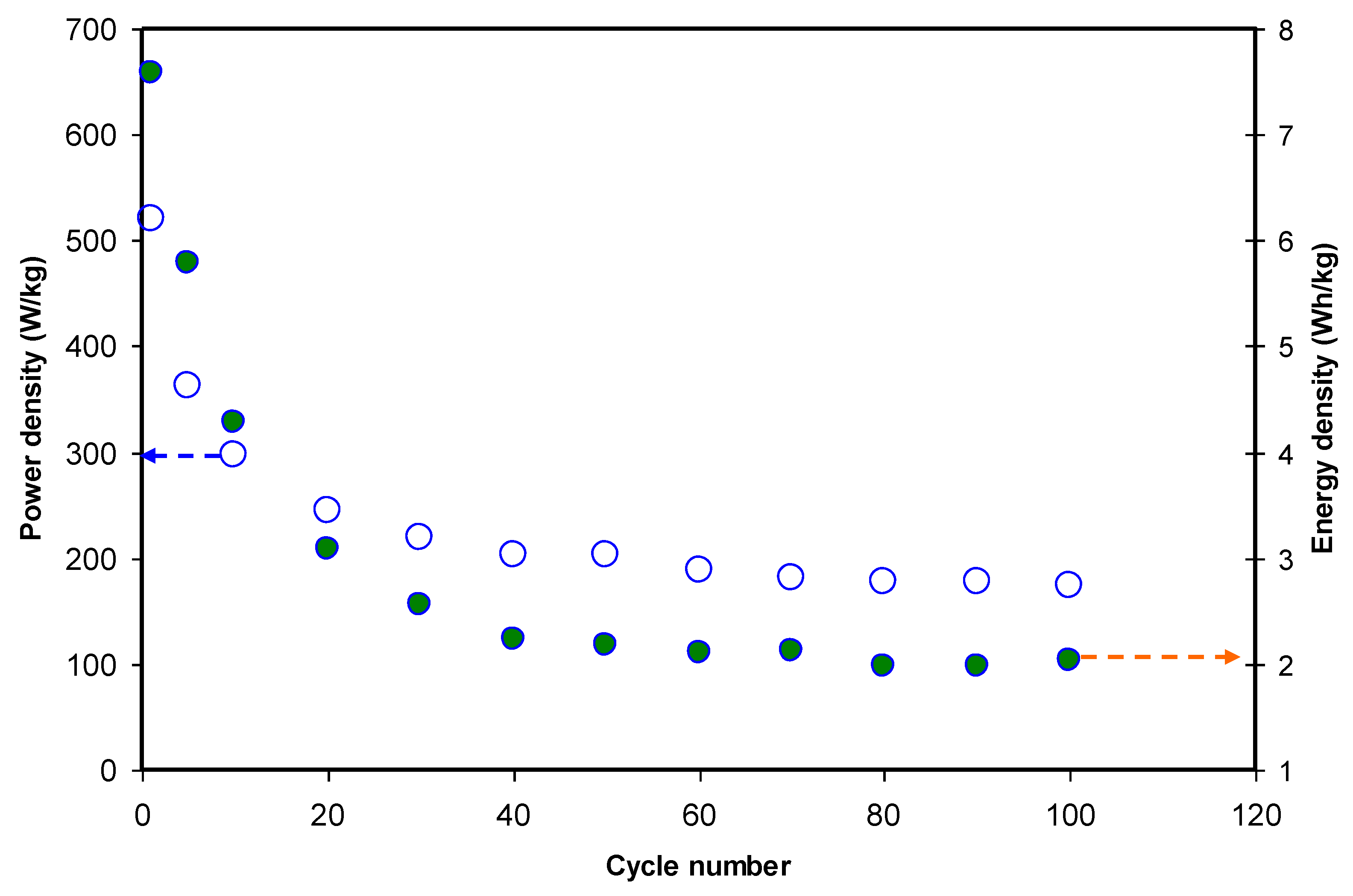
3.4.4. Charge-Discharge Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huo, P.; Ni, S.; Hou, P.; Xun, Z.; Liu, Y.; Gu, J. A cross linked soybean protein isolate gel polymer electrolyte based on neutral aqueous electrolyte for a high-energy-density supercapacitor. Polymers 2019, 11, 863. [Google Scholar] [CrossRef]
- Wang, J.A.; Lu, Y.T.; Lin, S.C.; Wang, Y.S.; Ma, C.C.M.; Hu, C.C. Designing a novel polymer electrolyte for improving the electrode/electrolyte interface in flexible all-solid-state electrical double-layer capacitors. ACS Appl. Mater. Interfaces 2018, 10, 17871–17882. [Google Scholar] [CrossRef] [PubMed]
- Kadir, M.F.Z.; Salleh, N.S.; Hamsan, M.H.; Aspanut, Z.; Majid, N.A.; Shukur, M.F. Biopolymeric electrolyte based on glycerolized methyl cellulose with NH4Br as proton source and potential application in EDLC. Ionics (Kiel) 2018, 24, 1651–1662. [Google Scholar] [CrossRef]
- Kamarudin, K.H.; Hassan, M.; Isa, M.I.N. Lightweight and flexible solid-state EDLC based on optimized CMC-NH4NO3 solid bio-polymer electrolyte. ASM Sci. J. 2018, 11, 29–36. [Google Scholar]
- Aziz, S.B.; Hamsan, M.H.; Karim, W.O.; Marif, A.S.; Abdulwahid, R.T.; Kadir, M.F.Z.; Brza, M.A. Study of impedance and solid-state double-layer capacitor behavior of proton (H+)-conducting polymer blend electrolyte-based CS:PS polymers. Ionics (Kiel) 2020. [Google Scholar] [CrossRef]
- Aziz, S.B.; Brza, M.A.; Hamsan, M.H.; Kadir, M.F.Z.; Muzakir, S.K.; Abdulwahid, R.T. Effect of ohmic-drop on electrochemical performance of EDLC fabricated from PVA: Dextran: NH4I based polymer blend electrolytes. J. Mater. Res. Technol. 2020, 9, 3734–3745. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hamsan, M.H.; Abdullah, R.M.; Abdulwahid, R.T.; Brza, M.A.; Marif, A.S.; Kadir, M.F.Z. Protonic EDLC cell based on chitosan (CS): Methylcellulose (MC) solid polymer blend electrolytes. Ionics (Kiel) 2020, 26, 1829–1840. [Google Scholar] [CrossRef]
- Wang, H.; Lin, J.; Shen, Z.X. Polyaniline (PANi) based electrode materials for energy storage and conversion. J. Sci. Adv. Mater. Devices 2016, 1, 225–255. [Google Scholar] [CrossRef]
- Kiamahalleh, M.V.; Zein, S.H.S.; Najafpour, G.; Sata, S.A.; Buniran, S. Multiwalled carbon nanotubes based nanocomposites for supercapacitors: A review of electrode materials. Nano 2012, 7, 1230002. [Google Scholar] [CrossRef]
- Shobana, V.; Parthiban, P.; Balakrishnan, K. Lithium based battery-type cathode material for hybrid supercapacitor. J. Chem. Pharm. Res. 2015, 7, 207–212. [Google Scholar]
- Minakshi, M.; Mitchell, D.R.G.; Jones, R.T.; Pramanik, N.C.; Jean-Fulcrand, A.; Garnweitner, G. A hybrid electrochemical energy storage device using sustainable electrode materials. Chem. Sel. 2020, 5, 1597–1606. [Google Scholar] [CrossRef]
- Minakshi, M.; Higley, S.; Baur, C.; Mitchell, D.R.G.; Jones, R.T.; Fichtner, M. Calcined chicken eggshell electrode for battery and supercapacitor applications. RSC Adv. 2019, 9, 26981–26995. [Google Scholar] [CrossRef]
- Minakshi, M.; Visbal, H.; Mitchell, D.R.G.; Fichtner, M. Bio-waste chicken eggshells to store energy. Dalt. Trans. 2018, 47, 16828–16834. [Google Scholar] [CrossRef]
- Dubal, D.P.; Ayyad, O.; Ruiz, V.; Gómez-Romero, P. Hybrid energy storage: The merging of battery and supercapacitor chemistries. Chem. Soc. Rev. 2015, 44, 1777–1790. [Google Scholar] [CrossRef] [PubMed]
- Orlins, S.; Guan, D. China’s toxic informal e-waste recycling: Local approaches to a global environmental problem. J. Clean. Prod. 2016, 114, 71–80. [Google Scholar] [CrossRef]
- Chai, M.N.; Isa, M.I.N. Solid biopolymer electrolytes based on carboxymethyl cellulose for use in coin cell proton batteries. J. Sustain. Sci. Manag. 2017, 2017, 42–48. [Google Scholar]
- Sarwat, F.; Ahmed, N.; Aman, A.; Qader, S.A.U. Optimization of growth conditions for the isolation of dextran producing Leuconostoc spp. from indigenous food sources. Pak. J. Pharm. Sci. 2013, 26, 793–797. [Google Scholar]
- Zhang, S.; Lee, K.H.; Frisbie, C.D.; Lodge, T.P. Ionic conductivity, capacitance, and viscoelastic properties of block copolymer-based ion gels. Macromolecules 2011, 44, 940–949. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, S.; Martinetti, L.; Lee, K.H.; McIntosh, L.D.; Frisbie, C.D.; Lodge, T.P. High toughness, high conductivity ion gels by sequential triblock copolymer self-assembly and chemical cross-linking. J. Am. Chem. Soc. 2013, 135, 9652–9655. [Google Scholar] [CrossRef]
- Tang, B.; White, S.P.; Frisbie, C.D.; Lodge, T.P. Synergistic increase in ionic conductivity and modulus of triblock copolymer ion gels. Macromolecules 2015, 48, 4942–4950. [Google Scholar] [CrossRef]
- Oh, H.; Seo, D.G.; Yun, T.Y.; Kim, C.Y.; Moon, H.C. Voltage-tunable multicolor, sub-1.5 V, flexible electrochromic devices based on ion gels. ACS Appl. Mater. Interfaces 2017, 9, 7658–7665. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.G.; Moon, H.C. Mechanically robust, highly ionic conductive gels based on random copolymers for bending durable electrochemical devices. Adv. Funct. Mater. 2018, 28, 1706948. [Google Scholar] [CrossRef]
- Kim, Y.M.; Seo, D.G.; Oh, H.; Moon, H.C. A facile random copolymer strategy to achieve highly conductive polymer gel electrolytes for electrochemical applications. J. Mater. Chem. C 2019, 7, 161–169. [Google Scholar] [CrossRef]
- Hwang, H.; Park, S.Y.; Kim, J.K.; Kim, Y.M.; Moon, H.C. Star-shaped block copolymers: Effective polymer gelators of high-performance gel electrolytes for electrochemical devices. ACS Appl. Mater. Interfaces 2019, 11, 4399–4407. [Google Scholar] [CrossRef]
- Barsbay, M.; Güner, A. Miscibility of dextran and poly(ethylene glycol) in solid state: Effect of the solvent choice. Carbohydr. Polym. 2007, 69, 214–223. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Shukur, M.F.; Aziz, S.B.; Kadir, M.F.Z. Dextran from Leuconostocmesenteroides-doped ammonium salt-based green polymer electrolyte. Bull. Mater. Sci. 2019, 42, 42–57. [Google Scholar] [CrossRef]
- Misenan, M.S.M.; Isa, M.I.N.; Khiar, A.S.A. Electrical and structural studies of polymer electrolyte based on chitosan/methyl cellulose blend doped with BMIMTFSI. Mater. Res. Express 2018, 5, 055304. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Shukur, M.F.; Kadir, M.F.Z. The effect of NH4NO3 towards the conductivity enhancement and electrical behavior in methyl cellulose-starch blend based ionic conductors. Ionics (Kiel) 2017, 23, 1137–1154. [Google Scholar] [CrossRef]
- Shukur, A.; Fadhlullah, M. Characterization of Ion Conducting Solid Biopolymer Electrolytes Based on Starch-Chitosan Blend and Application in Electrochemical Devices. Ph.D. Thesis, University of Malaya, Kuala Lumpur, Malaysia, 2015. [Google Scholar]
- Alam, T.M.; Otaigbe, J.U.; Rhoades, D.; Holland, G.P.; Cherry, B.R.; Kotula, P.G. Nanostructured polymer blends: Synthesis and structure. Polymer (Guildford) 2005, 46, 12468–12479. [Google Scholar] [CrossRef]
- Yan, S.; Zeng, S.; Su, X.; Yin, H.; Xiong, Y.; Xu, W. H3PO4-doped 1,2,4-triazole-polysiloxane proton conducting membrane prepared by sol-gel method. Solid State Ion. 2011, 198, 1–5. [Google Scholar] [CrossRef]
- Yusof, Y.M.; Shukur, M.F.; Illias, H.A.; Kadir, M.F.Z. Conductivity and electrical properties of corn starch-chitosan blend biopolymer electrolyte incorporated with ammonium iodide. Phys. Scr. 2014, 89, 035701–035711. [Google Scholar] [CrossRef]
- Buraidah, M.H.; Arof, A.K. Characterization of chitosan/PVA blended electrolyte doped with NH4I. J. Non Cryst. Solids 2011, 357, 3261–3266. [Google Scholar] [CrossRef]
- Kadir, M.F.Z.; Hamsan, M.H. Green electrolytes based on dextran-chitosan blend and the effect of NH4SCN as proton provider on the electrical response studies. Ionics (Kiel) 2018, 24, 2379–2398. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hamsan, M.H.; Karim, W.O.; Kadir, M.F.Z.; Brza, M.A.; Abdullah, O.G. High proton conducting polymer blend electrolytes based on chitosan: Dextran with constant specific capacitance and energy density. Biomolecules 2019, 9, 267. [Google Scholar] [CrossRef] [PubMed]
- Shamsudin, I.J.; Ahmad, A.; Hassan, N.H. Green polymer electrolytes based on chitosan and 1-butyl-3-methylimidazolium acetate. In Proceedings of the 2014 UKM FST Postgraduate Colloquium, Selangor, Malaysia, 9–11 April 2014; Volume 1614, pp. 393–398. [Google Scholar] [CrossRef]
- Göktepe, F.; Çelik, S.Ü.; Bozkurt, A. Preparation and the proton conductivity of chitosan/poly(vinyl phosphonic acid) complex polymer electrolytes. J. Non Cryst. Solids 2008, 354, 3637–3642. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abidin, Z.H.Z.; Kadir, M.F.Z. Innovative method to avoid the reduction of silver ions to silver nanoparticles (Ag+→Ago) in silver ion conducting based polymer electrolytes. Phys. Scr. 2015, 90, 35808. [Google Scholar] [CrossRef]
- Wan, Y.; Creber, K.A.M.; Peppley, B.; Bui, V.T. Chitosan-based solid electrolyte composite membranes. I. Preparation and characterization. J. Memb. Sci. 2006, 280, 666–674. [Google Scholar] [CrossRef]
- Aziz, S.B. Role of dielectric constant on ion transport: Reformulated Arrhenius equation. Adv. Mater. Sci. Eng. 2016, 2016, 2527013. [Google Scholar] [CrossRef]
- Aziz, S.B.; Brza, M.A.; Mishra, K.; Hamsan, M.H.; Karim, W.O.; Abdullah, R.M.; Kadir, M.F.Z.; Abdulwahid, R.T. Fabrication of high performance energy storage EDLC device from proton conducting methylcellulose: Dextran polymer blend electrolytes. J. Mater. Res. Technol. 2020, 9, 1137–1150. [Google Scholar] [CrossRef]
- Dos Santos Campos, F.; Cassimiro, D.L.; Crespi, M.S.; Almeida, A.E.; DaflonGremião, M.P. Preparation and characterisation of Dextran-70 hydrogel for controlled release of praziquantel. Braz. J. Pharm. Sci. 2013, 49, 75–83. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abidin, Z.H.Z.; Arof, A.K. Effect of silver nanoparticles on the DC conductivity in chitosan-silver triflate polymer electrolyte. Phys. B Condens. Matter. 2010, 405, 4429–4433. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, R.M. Crystalline and amorphous phase identification from the tanδ relaxation peaks and impedance plots in polymer blend electrolytes based on [CS:AgNt]x:PEO(x−1) (10 ≤ x ≤ 50). Electrochim. Acta 2018, 285, 30–46. [Google Scholar] [CrossRef]
- Yulianti, E.; Karo, A.K.; Susita, L. Synthesis of electrolyte polymer based on natural polymer chitosan by ion implantation technique. Procedia Chem. 2012, 4, 202–207. [Google Scholar] [CrossRef]
- Aziz, S.B.; Rasheed, M.A.; Hussein, A.M.; Ahmed, H.M. Fabrication of polymer blend composites based on [PVA-PVP](1−x):(Ag2S)x (0.01 ≤ x ≤ 0.03) with small optical band gaps: Structural and optical properties. Mater. Sci. Semicond. Process. 2017, 71, 197–203. [Google Scholar] [CrossRef]
- Rosli, N.H.A.; Chan, C.H.; Subban, R.H.Y.; Winie, T. Studies on the structural and electrical properties of hexanoyl chitosan/polystyrene-based polymer electrolytes. Phys. Procedia 2012, 25, 215–220. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abidin, Z.H.Z. Ion-transport study in nanocomposite solid polymer electrolytes based on chitosan: Electrical and dielectric analysis. J. Appl. Polym. Sci. 2015, 132, 1–10. [Google Scholar] [CrossRef]
- Hodge, R.M.; Edward, G.H.; Simon, G.P. Water absorption and states of water in semicrystallinepoly(vinyl alcohol) films. Polymer (Guildford) 1996, 37, 1371–1376. [Google Scholar] [CrossRef]
- Shukla, R.; Shukla, S.; Bivolarski, V.; Iliev, I.; Ivanova, I.; Goyal, A. Structural characterization of insoluble dextran produced by Leuconostocmesenteroides NRRL B-1149 in the presence of maltose. Food Technol. Biotechnol. 2011, 49, 291–296. [Google Scholar]
- Vettori, M.H.P.B.; Franchetti, S.M.M.; Contiero, J. Structural characterization of a new dextran with a low degree of branching produced by Leuconostocmesenteroides FT045B dextransucrase. Carbohydr. Polym. 2012, 88, 1440–1444. [Google Scholar] [CrossRef]
- Dumitraşcu, M.; Meltzer, V.; Sima, E.; Vîrgolici, M.G.; Albu, M.G.; Ficai, A.; Moise, V.; Minea, R.; Vancea, C.; Scǎrişoreanu, A.; et al. Characterization of electron beam irradiated collagenpolyvinylpyrrolidone (PVP) and collagen-dextran (DEX) blends. Dig. J. Nanomater. Biostruct. 2011, 6, 1793–1803. [Google Scholar]
- Aziz, S.B.; Marif, R.B.; Brza, M.A.; Hassan, A.N.; Ahmad, H.A.; Faidhalla, Y.A.; Kadir, M.F.Z. Structural, thermal, morphological and optical properties of PEO filled with biosynthesized Ag nanoparticles: New insights to band gap study. Results Phys. 2019, 13, 102220. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abidin, Z.H.Z. Electrical conduction mechanism in solid polymer electrolytes: New concepts to Arrhenius equation. J. Soft Matter. 2013, 2013, 323868. [Google Scholar] [CrossRef]
- Wei, D.; Sun, W.; Qian, W.; Ye, Y.; Ma, X. The synthesis of chitosan-based silver nanoparticles and their antibacterial activity. Carbohydr. Res. 2009, 344, 2375–2382. [Google Scholar] [CrossRef] [PubMed]
- Hafiza, M.N.; Isa, M.I.N. Solid polymer electrolyte production from 2-hydroxyethyl cellulose: Effect of ammonium nitrate composition on its structural properties. Carbohydr. Polym. 2017, 165, 123–131. [Google Scholar] [CrossRef]
- Monisha, S.; Mathavan, T.; Selvasekarapandian, S.; Benial, A.M.F.; Aristatil, G.; Mani, N.; Premalatha, M.; Vinothpandi, D. Investigation of bio polymer electrolyte based on cellulose acetate-ammonium nitrate for potential use in electrochemical devices. Carbohydr. Polym. 2017, 157, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.B.; Hamsan, M.H.; Brza, M.A.; Kadir, M.F.Z.; Abdulwahid, R.T.; Ghareeb, H.O.; Woo, H.J. Fabrication of energy storage EDLC device based on CS:PEO polymer blend electrolytes with high Li+ ion transference number. Results Phys. 2019, 15, 102584. [Google Scholar] [CrossRef]
- Mitić, Ž.; Cakić, M.; Nikolić, G. Fourier-transform IR spectroscopic investigations of Cobalt(II)-dextran complexes by using D2O isotopic exchange. Spectroscopy 2010, 24, 269–275. [Google Scholar] [CrossRef][Green Version]
- Polu, A.R.; Kumar, R. AC impedance and dielectric spectroscopic studies of Mg2+ ion conducting PVA-PEG blended polymer electrolytes. Bull. Mater. Sci. 2011, 34, 1063–1067. [Google Scholar] [CrossRef]
- Aziz, S.B.; Woo, T.J.; Kadir, M.F.Z.; Ahmed, H.M. A conceptual review on polymer electrolytes and ion transport models. J. Sci. Adv. Mater. Devices 2018, 3, 1–17. [Google Scholar] [CrossRef]
- Samsudin, A.S.; Kuan, E.C.H.; Isa, M.I.N. Investigation of the potential of proton-conducting biopolymer electrolytes based methyl cellulose-glycolic acid. Int. J. Polym. Anal. Charact. 2011, 16, 477–485. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hamsan, M.H.; Kadir, M.F.Z.; Karim, W.O.; Abdullah, R.M. Development of polymer blend electrolyte membranes based on chitosan: Dextran with high ion transport properties for EDLC application. Int. J. Mol. Sci. 2019, 20, 3369. [Google Scholar] [CrossRef]
- Samsudin, A.S.; Isa, M.I.N. Characterization of carboxy methylcellulose doped with DTAB as new types of biopolymer electrolytes. Bull. Mater. Sci. 2012, 35, 1123–1131. [Google Scholar] [CrossRef]
- Sahli, N.B.; Bin Ali, A.M.M. Effect of lithium triflate salt concentration in methyl cellulose-based solid polymer electrolytes. In Proceedings of the CHUSER 2012—2012 IEEE Colloquium on Humanities, Science and Engineering, Kota Kinabalu, Malaysia, 3–4 December 2012; pp. 739–742. [Google Scholar] [CrossRef]
- Malathi, J.; Kumaravadivel, M.; Brahmanandhan, G.M.; Hema, M.; Baskaran, R.; Selvasekarapandian, S. Structural, thermal and electrical properties of PVA-LiCF3SO3 polymer electrolyte. J. Non Cryst. Solids 2010, 356, 2277–2281. [Google Scholar] [CrossRef]
- Mustafa, M.S.; Ghareeb, H.O.; Aziz, S.B.; Brza, M.A.; Al-Zangana, S.; Hadi, J.M.; Kadir, M.F.Z. Electrochemical characteristics of glycerolized PEO-based polymer electrolytes. Membranes 2020, 10, 116. [Google Scholar] [CrossRef]
- Aziz, S.B.; Karim, W.O.; Qadir, K.W.; Zafar, Q. Proton ion conducting solid polymer electrolytes based on chitosan incorporated with various amounts of barium titanate (BaTiO3). Int. J. Electrochem. Sci. 2018, 13, 6112–6125. [Google Scholar] [CrossRef]
- Singh, N.K.; Verma, M.L.; Minakshi, M. PEO nanocomposite polymer electrolyte for solid state symmetric capacitors. Bull. Mater. Sci. 2015, 38, 1577–1588. [Google Scholar] [CrossRef]
- Marf, A.S.; Abdullah, R.M.; Aziz, S.B. Structural, morphological, electrical and electrochemical properties of PVA: CS-based proton-conducting polymer blend electrolytes. Membranes 2020, 10, 71. [Google Scholar] [CrossRef]
- Vijaya, N.V.N.; Selvasekarapandian, S.S.S.; Malathi, J.M.J.; Iwai, Y.I.Y.; Nithya, H.N.H.; Kawamura, J.K.J. 1H NMR study on PVP-NH4Cl based-proton conducting polymer electrolyte. Indian J. Appl. Res. 2011, 3, 506–510. [Google Scholar] [CrossRef]
- Ng, L.S.; Mohamad, A.A. Effect of temperature on the performance of proton batteries based on chitosan-NH4NO3-EC membrane. J. Memb. Sci. 2008, 325, 653–657. [Google Scholar] [CrossRef]
- Kadir, M.F.Z.; Arof, A.K. Application of PVA-chitosan blend polymer electrolyte membrane in electrical double layer capacitor. Mater. Res. Innov. 2011, 15, s217–s220. [Google Scholar] [CrossRef]
- Zhu, W.; Ou, X.; Lu, Z.; Chen, K.; Ling, Y.; Zhang, H. Enhanced performance of hierarchical CuS clusters applying TRGO as conductive carrier for supercapacitors. J. Mater. Sci. Mater. Electron. 2019, 30, 5760–5770. [Google Scholar] [CrossRef]
- Fattah, N.F.A.; Ng, H.M.; Mahipal, Y.K.; Numan, A.; Ramesh, S.; Ramesh, K. An approach to solid-state electrical double layer capacitors fabricated with graphene oxide-doped, ionic liquid-based solid copolymer electrolytes. Materials 2016, 9, 450. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.P.; Kumar, Y.; Hashmi, S.A. Ionic liquid incorporated PEO based polymer electrolyte for electrical double layer capacitors: A comparative study with lithium and magnesium systems. Solid State Ion. 2011, 190, 93–98. [Google Scholar] [CrossRef]
- Woo, H.J.; Liew, C.W.; Majid, S.R.; Arof, A.K. Poly(ε-caprolactone)-based polymer electrolyte for electrical double-layer capacitors. High Perform. Polym. 2014, 26, 637–640. [Google Scholar] [CrossRef]
- Yusof, Y.M.; Majid, N.A.; Kasmani, R.M.; Illias, H.A.; Kadir, M.F.Z. The effect of plasticization on conductivity and other properties of starch/chitosan blend biopolymer electrolyte incorporated with ammonium iodide. Mol. Cryst. Liq. Cryst. 2014, 603, 73–88. [Google Scholar] [CrossRef]
- Liew, C.; Ramesh, S. Electrical, structural, thermal and electrochemical properties of corn starch-based biopolymer electrolytes. Carbohydr. Polym. 2015, 124, 222–228. [Google Scholar] [CrossRef]
- Eftekhari, A. The mechanism of ultrafast supercapacitors. J. Mater. Chem. A 2018, 6, 2866–2876. [Google Scholar] [CrossRef]
- Eftekhari, A. Surface diffusion and adsorption in supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 3692–3701. [Google Scholar] [CrossRef]
- Teoh, K.H.; Lim, C.S.; Liew, C.W.; Ramesh, S.; Ramesh, S. Electric double-layer capacitors with corn starch-based biopolymer electrolytes incorporating silica as filler. Ionics (Kiel) 2015, 21, 2061–2068. [Google Scholar] [CrossRef]
- Liew, C.; Ramesh, S.; Arof, A.K. Enhanced capacitance of EDLCs (electrical double layer capacitors) based on ionic liquid-added polymer electrolytes. Energy 2016, 109, 546–556. [Google Scholar] [CrossRef]
- Emeléus, H.J.; Sharpe, A.G. Advances in Inorganic Chemistry and Radiochemistry, 1st ed.; Academic Press INC: New York, NY, USA, 1959. [Google Scholar] [CrossRef]
- Udawatte, G.K.; Perera, K.S.; Vidanapathirana, K.P. A gel polymer electrolyte based on PVdF and ZnCl2 for an electrochemical double layer capacitor. Ceylon J. Sci. 2018, 47, 331–336. [Google Scholar] [CrossRef]
- Subramaniam, C.K.; Ramya, C.S.; Ramya, K. Performance of EDLCs using Nafion and Nafion composites as electrolyte. J. Appl. Electrochem. 2011, 41, 197–206. [Google Scholar] [CrossRef]
- Shuhaimi, N.E.A.; Alias, N.A.; Majid, S.R.; Arof, A.K. Electrical double layer capacitor with proton conducting κ-carrageenanchitosan electrolytes. Funct. Mater. Lett. 2008, 1, 195–201. [Google Scholar] [CrossRef]
- Shuhaimi, N.E.A.; Teo, L.P.; Woo, H.J.; Majid, S.R.; Arof, A.K. Electrical double-layer capacitors with plasticized polymer electrolyte based on methyl cellulose. Polym. Bull. 2012, 69, 807–826. [Google Scholar] [CrossRef]
- Arof, A.K.; Shuhaimi, N.E.A.; Alias, N.A.; Kufian, M.Z.; Majid, S.R. Application of chitosan/iota-carrageenan polymer electrolytes in electrical double layer capacitor (EDLC). J. Solid State Electrochem. 2010, 14, 2145–2152. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Shukur, M.F.; Aziz, S.B.; Yusof, Y.M.; Kadir, M.F.Z. Influence of NH4Br as an ionic source on the structural/electrical properties of dextran-based biopolymer electrolytes and EDLC application. Bull. Mater. Sci. 2019, 43, 30. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Aziz, S.B.; Azha, M.A.S.; Azli, A.A.; Shukur, M.F.; Yusof, Y.M.; Muzakir, S.K.; Manan, N.S.A.; Kadir, M.F.Z. Solid-state double layer capacitors and protonic cell fabricated with dextran from Leuconostocmesenteroides based green polymer electrolyte. Mater. Chem. Phys. 2019, 241, 122290. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hamsan, M.H.; Abdullah, R.M.; Kadir, M.F.Z. A promising polymer blend electrolytes based on Chitosan: Methyl cellulose for EDLC application with high specific capacitance and energydensity. Molecules 2019, 24, 2503. [Google Scholar] [CrossRef]
- Suleman, M.; Deraman, M.; Othman, M.A.R.; Omar, R.; Hashim, M.A.; Basri, N.H.; Nor, N.S.M.; Dolah, B.N.M.; Hanappi, M.F.Y.M.; Hamdan, E.; et al. Electric double-layer capacitors with tea waste derived activated carbon electrodes and plastic crystal based flexible gel polymer electrolytes. J. Phys. Conf. Ser. 2016, 739. [Google Scholar] [CrossRef]
- Lim, C.; Teoh, K.H.; Liew, C.; Ramesh, S. Electric double layer capacitor based on activated carbon electrode and biodegradable composite polymer electrolyte. Ionics (Kiel) 2014, 20, 251–258. [Google Scholar] [CrossRef]
- Ajina, A.; Isa, D. Symmetrical supercapacitor using coconut shell-based activated carbon. Pertanika J. Sci. Technol. 2010, 18, 351–363. [Google Scholar]
- Yuhanees, M.Y. Characteristics of Corn Starch/Chitosan Blend Green Polymer Electrolytes Complexed with Ammonium Iodide and Its Application in Energy Devices. Ph.D. Thesis, University of Malaya, Kuala Lumpur, Malaysia, 2017. [Google Scholar]
- Lim, C.S.; Teoh, K.H.; Liew, C.W.; Ramesh, S. Capacitive behavior studies on electrical double layer capacitor using poly (vinyl alcohol)-lithium perchlorate based polymer electrolyte incorporated with TiO2. Mater. Chem. Phys. 2014, 143, 661–667. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hamsan, M.H.; Brza, M.A.; Kadir, M.F.Z.; Muzakir, S.K.; Abdulwahidad, R.T. Effect of glycerol on EDLC characteristics of chitosan: Methylcellulose polymer blend electrolytes. J. Mater. Res. Technol. 2020, 9, 8355–8366. [Google Scholar] [CrossRef]
- Buraidah, M.H.B.A. Ionic Conductivity and Related Studies on Chitosan-Based Electrolytes with Application in Solar Cells. Ph.D. Thesis, University of Malaya, Kuala Lumpur, Malaysia, 2012. [Google Scholar]
- Jenkins, H.D.B.; Morris, D.F.C. A new estimation of the lattice energies of the ammonium halides and the proton affinity of gaseous ammonia. Mol. Phys. 1976, 32, 231–236. [Google Scholar] [CrossRef]



 and capacitor represented by
and capacitor represented by 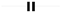 .
.
 and capacitor represented by
and capacitor represented by 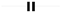 .
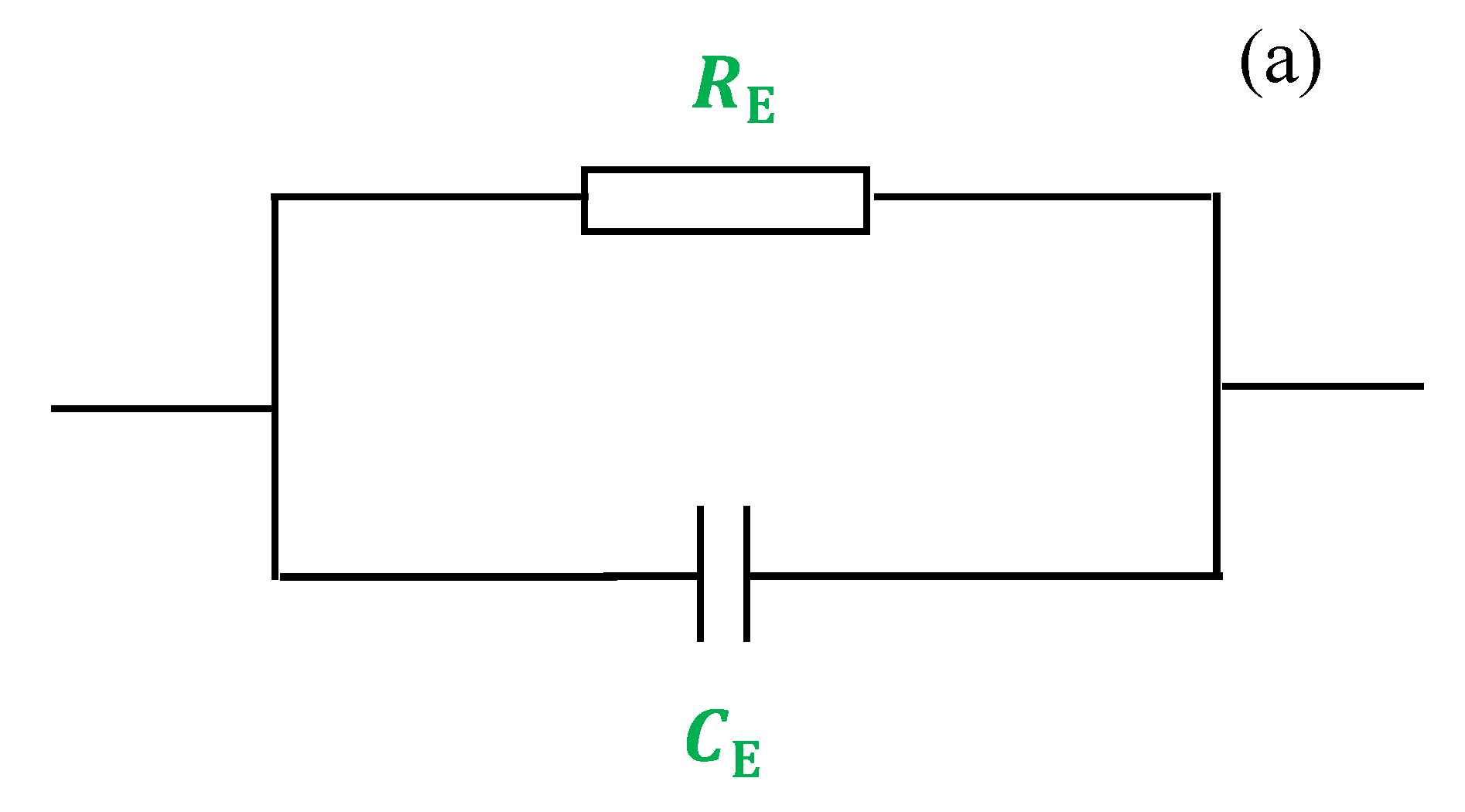
.
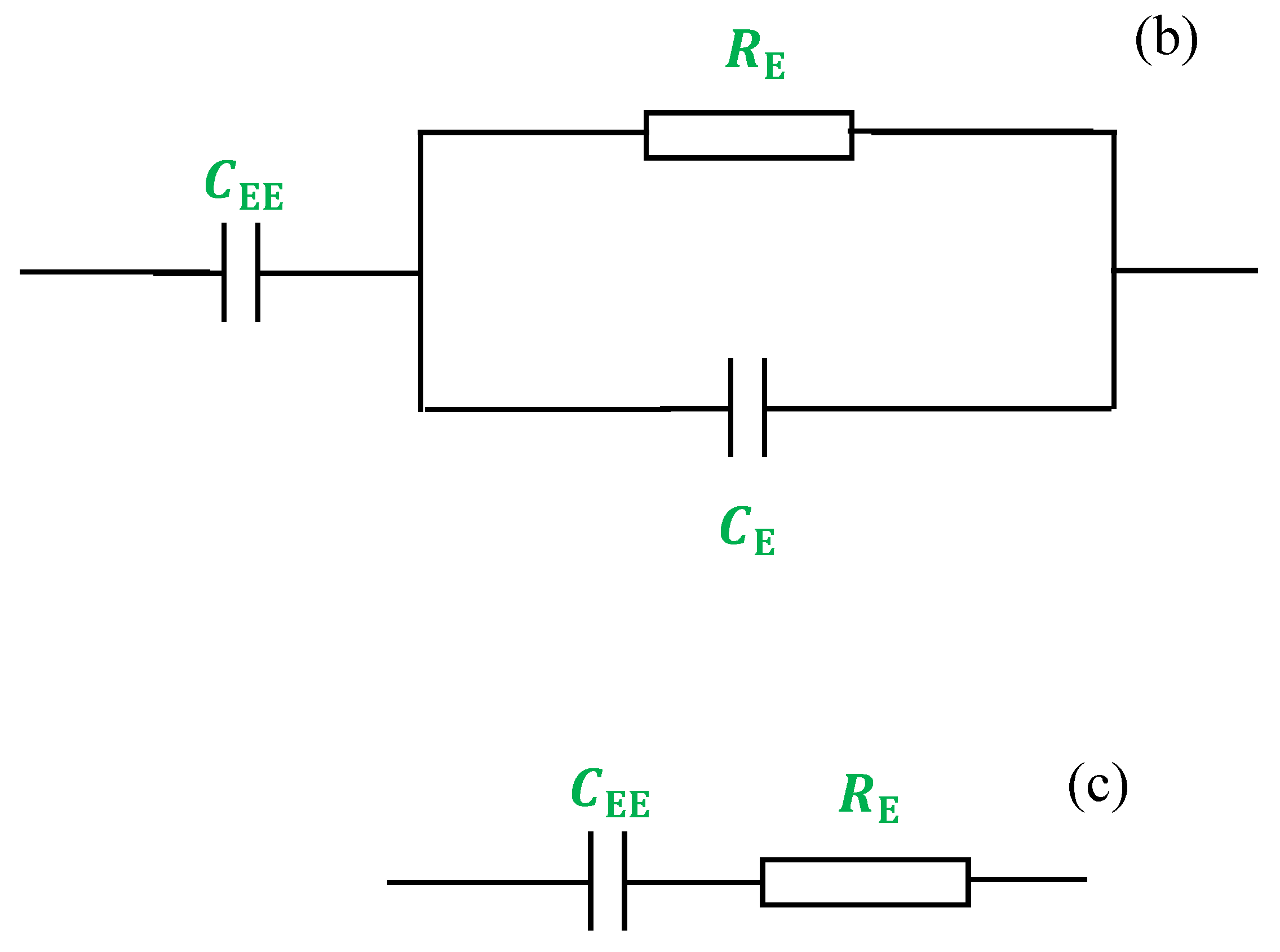
| Sample Designation | DCConductivity (S/cm) |
|---|---|
| CSDPE0 | 5.01 × 10−10 |
| CSDPE1 | 2.73 × 10−7 |
| CSDPE2 | 1.27 × 10−5 |
| CSDPE3 | 5.62 × 10−4 |
| CSDPE4 | 5.16 × 10−3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
B. Aziz, S.; Hamsan, M.H.; M. Nofal, M.; Karim, W.O.; Brevik, I.; Brza, M.A.; Abdulwahid, R.T.; Al-Zangana, S.; Kadir, M.F.Z. Structural, Impedance and Electrochemical Characteristics of Electrical Double Layer Capacitor Devices Based on Chitosan: Dextran Biopolymer Blend Electrolytes. Polymers 2020, 12, 1411. https://doi.org/10.3390/polym12061411
B. Aziz S, Hamsan MH, M. Nofal M, Karim WO, Brevik I, Brza MA, Abdulwahid RT, Al-Zangana S, Kadir MFZ. Structural, Impedance and Electrochemical Characteristics of Electrical Double Layer Capacitor Devices Based on Chitosan: Dextran Biopolymer Blend Electrolytes. Polymers. 2020; 12(6):1411. https://doi.org/10.3390/polym12061411
Chicago/Turabian StyleB. Aziz, Shujahadeen, Muhamad H. Hamsan, Muaffaq M. Nofal, Wrya O. Karim, Iver Brevik, Mohamad. A. Brza, Rebar T. Abdulwahid, Shakhawan Al-Zangana, and Mohd F. Z. Kadir. 2020. "Structural, Impedance and Electrochemical Characteristics of Electrical Double Layer Capacitor Devices Based on Chitosan: Dextran Biopolymer Blend Electrolytes" Polymers 12, no. 6: 1411. https://doi.org/10.3390/polym12061411
APA StyleB. Aziz, S., Hamsan, M. H., M. Nofal, M., Karim, W. O., Brevik, I., Brza, M. A., Abdulwahid, R. T., Al-Zangana, S., & Kadir, M. F. Z. (2020). Structural, Impedance and Electrochemical Characteristics of Electrical Double Layer Capacitor Devices Based on Chitosan: Dextran Biopolymer Blend Electrolytes. Polymers, 12(6), 1411. https://doi.org/10.3390/polym12061411