Toxic Effects of Urethane Dimethacrylate on Macrophages Through Caspase Activation, Mitochondrial Dysfunction, and Reactive Oxygen Species Generation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture and Cell Treatment
2.3. Lactate dehydrogenase (LDH) Release Assay
2.4. Apoptosis and Necrosis Assay
2.5. Micronucleus (MN) Formation Assay
2.6. Single-Cell Gel Electrophoresis (Comet) Assay
2.7. Assays to Measure Caspase-3, -8, and -9 Activities
2.8. Mitochondrial Membrane Potential Assay
2.9. Measurement of Intracellular ROS Level
2.10. Statistical Analysis
3. Results
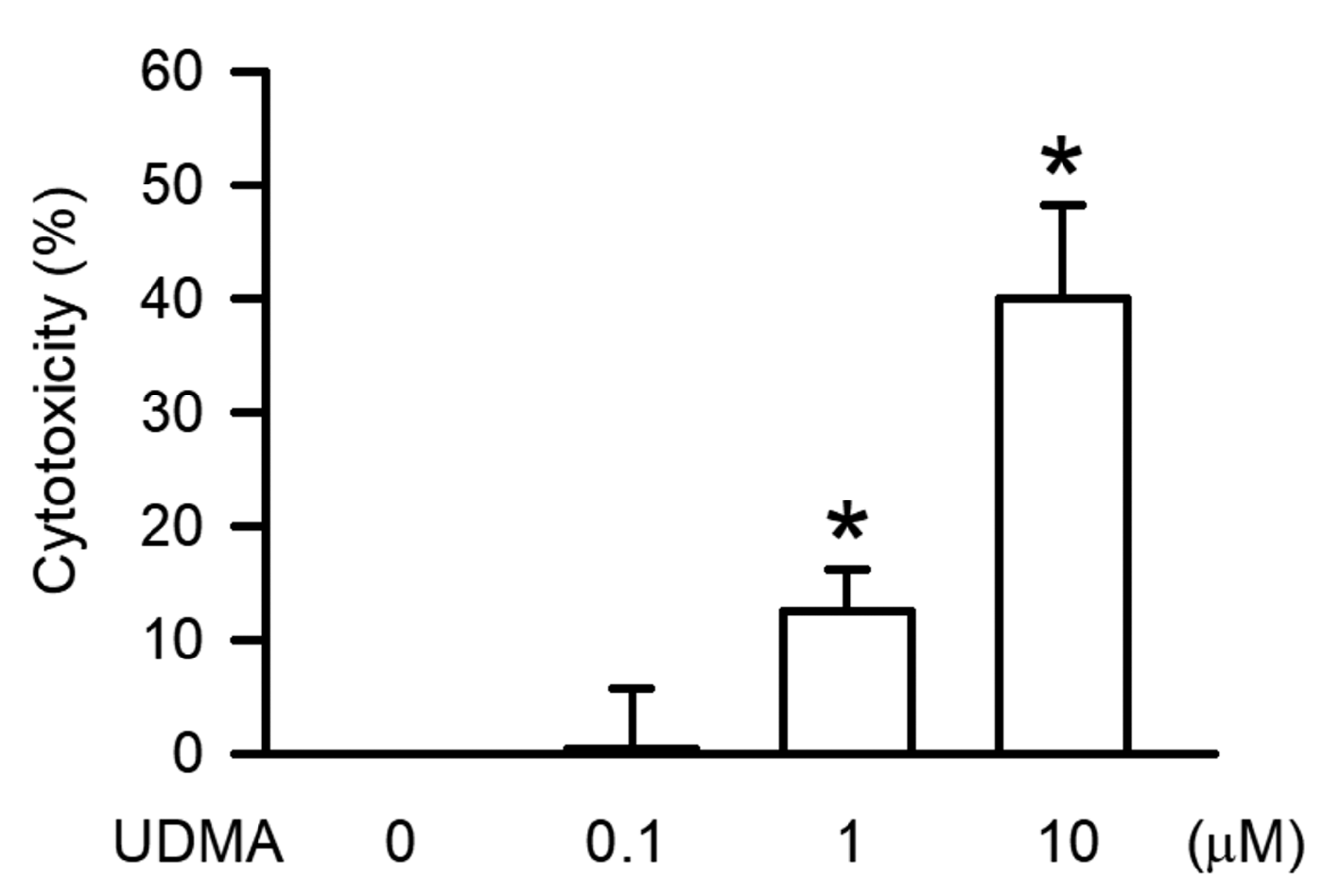
3.1. Effects of UDMA on Cytotoxicity of RAW264.7 Cells
3.2. Effects of UDMA on Necrosis and Apoptosis of RAW264.7 Cells
3.3. Effects of UDMA on MN Formation in RAW264.7 Cells
3.4. Effects of UDMA on DNA Damage in RAW264.7 Cells
3.5. Effects of UDMA on Caspase-3, -8, and -9 Activities in RAW264.7 Cells
3.6. Effects of UDMA on Mitochondrial Dysfunction in RAW264.7 Cells
3.7. Effects of UDMA on Intracellular ROS Generation in RAW264.7 Cells
3.8. Effects of NAC on UDMA-Induced Intracellular ROS Generation, Mitochondrial Dysfunction, and Cytotoxicity in RAW264.7 Cells
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Panpisut, P.; Khan, M.A.; Main, K.; Arshad, M.; Xia, W.; Petridis, H.; Young, A.M. Polymerization kinetics stability; volumetric changes; apatite precipitation; strontium release and fatigue of novel bone composites for vertebroplasty. PLoS ONE 2019, 14, e0207965. [Google Scholar] [CrossRef] [PubMed]
- Moszner, N.; Fischer, U.K.; Angermann, J.; Rheinberger, V. A partially aromatic urethane dimethacrylate as a new substitute for Bis-GMA in restorative composites. Dent. Mater. 2008, 24, 694–699. [Google Scholar] [CrossRef]
- Schulz, S.D.; Laquai, T.; Kümmerer, K.; Bolek, R.; Mersch-Sundermann, V.; Polydorou, O. Elution of Monomers from Provisional Composite Materials. Int. J. Polym. Sci. 2015, 2015, 617407. [Google Scholar] [CrossRef]
- Silva, G.A.; Lanza, L.D.; Lopes-Junior, N.; Moreira, A.; Alves, J.B. Direct pulp capping with a dentin bonding system in human teeth: A clinical and histological evaluation. Oper. Dent. 2006, 31, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Wisniewska-Jarosinska, M.; Poplawski, T.; Chojnacki, C.J.; Pawlowska, E.; Krupa, R.; Szczepanska, J.; Blasiak, J. Independent and combined cytotoxicity and genotoxicity of triethylene glycol dimethacrylate and urethane dimethacrylate. Mol Biol Rep. 2011, 38, 4603–4611. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.R.; Hakami-Tafreshi, R.; Tomasino-Perez, A.; Tayebi, L.; Lobner, D. Effects of dental composite resin monomers on dental pulp cells. Dent. Mater. J. 2019, 38, 579–583. [Google Scholar] [CrossRef]
- Chang, H.H.; Chang, M.C.; Wang, H.H.; Huang, G.F.; Lee, Y.L.; Wang, Y.L.; Chan, C.P.; Yeung, S.Y.; Tseng, S.K.; Jeng, J.H. Urethane dimethacrylate induces cytotoxicity and regulates cyclooxygenase-2; hemeoxygenase and carboxylesterase expression in human dental pulp cells. Acta Biomater. 2014, 10, 722–731. [Google Scholar] [CrossRef]
- Oishi, Y.; Manabe, I. Macrophages in inflammation; repair and regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef]
- Howait, M.; Albassam, A.; Yamada, C.; Sasaki, H.; Bahammam, L.; Azuma, M.M.; Cintra, L.T.A.; Satoskar, A.R.; Yamada, S.; White, R.; et al. Elevated Expression of Macrophage Migration Inhibitory Factor Promotes Inflammatory Bone Resorption Induced in a Mouse Model of Periradicular Periodontitis. J. Immunol. 2019, 202, 2035–2043. [Google Scholar] [CrossRef]
- Tsai, P.K.; Wu, S.W.; Chiang, C.Y.; Lee, M.W.; Chen, H.Y.; Chen, W.Y.; Chen, C.J.; Yang, S.F.; Yeh, C.B.; Kuan, Y.H. Evaluation of cytotoxicity; apoptosis; and genotoxicity induced by indium chloride in macrophages through mitochondrial dysfunction and reactive oxygen species generation. Ecotoxicol. Environ. Saf. 2020, 15, 110348. [Google Scholar] [CrossRef]
- Liu, C.W.; Lin, H.W.; Yang, D.J.; Chen, S.Y.; Tseng, J.K.; Chang, T.J.; Chang, Y.Y. Luteolin inhibits viral-induced inflammatory response in RAW264.7 cells via suppression of STAT1/3 dependent NF-κB and activation of HO-1. Free Radic. Biol. Med. 2016, 95, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.M.; Chang, Y.C.; Lee, S.S.; Ho, Y.C.; Yang, M.L.; Lin, H.W.; Kuan, Y.H. Bisphenol A exhibits cytotoxic or genotoxic potential via oxidative stress-associated mitochondrial apoptotic pathway in murine macrophages. Food Chem. Toxicol. 2018, 122, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Van Landuyt, K.L.; Nawrot, T.; Geebelen, B.; De Munck, J.; Snauwaert, J.; Yoshihara, K.; Scheers, H.; Godderis, L.; Hoet, P.; Van Meerbeek, B. How much do resin-based dental materials release? A meta-analytical approach. Dent. Mater. 2011, 27, 723–747. [Google Scholar] [CrossRef]
- Huang, F.M.; Kuan, Y.H.; Lee, S.S.; Chang, Y.C. Cytotoxicity and genotoxicity of Triethyleneglycol-Dimethacrylate in macrophages Involved in DNA damage and caspases activation. Environ. Toxicol. 2015, 30, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Löfroth, M.; Ghasemimehr, M.; Falk, A.; Vult von Steyern, P. Bisphenol A in dental materials - existence, leakage and biological effects. Heliyon. 2019, 5, e01711. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.; Spratt, D.A.; Pratten, J.; Gulabivala, K.; Mordan, N.J.; Young, A.M. Chlorhexidine-releasing methacrylate dental composite materials. Biomaterials. 2005, 26, 7145–7153. [Google Scholar] [CrossRef]
- Huang, F.M.; Chang, Y.C.; Lee, S.S.; Yeh, C.H.; Lee, K.G.; Huang, Y.C.; Chen, C.J.; Chen, W.Y.; Pan, P.H.; Kuan, Y.H. BisGMA-induced cytotoxicity and genotoxicity in macrophages are attenuated by wogonin via reduction of intrinsic caspase pathway activation. Environ. Toxicol. 2016, 31, 176–184. [Google Scholar] [CrossRef]
- Reichl, F.X.; Esters, M.; Simon, S.; Seiss, M.; Kehe, K.; Kleinsasser, N.; Folwaczny, M.; Glas, J.; Hickel, R. Cell death effects of resin-based dental material compounds and mercurials in human gingival fibroblasts. Arch. Toxicol. 2006, 80, 370–377. [Google Scholar] [CrossRef]
- Poplawski, T.; Loba, K.; Pawlowska, E.; Szczepanska, J.; Blasiak, J. Genotoxicity of urethane dimethacrylate; a tooth restoration component. Toxicol. In Vitro. 2010, 24, 854–862. [Google Scholar] [CrossRef]
- Neves, S.O.; Magalhães, L.M.D.; Corrêa, J.D.; Dutra, W.O.; Gollob, K.J.; Silva, T.A.; Horta, M.C.R.; Souza, P.E.A. Composite-derived monomers affect cell viability and cytokine expression in human leukocytes stimulated with Porphyromonas gingivalis. J. Appl. Oral. Sci. 2019, 27, e20180529. [Google Scholar] [CrossRef]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis; necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.Y.; Wang, J.S.; Chao, M.W. Causation by Diesel Exhaust Particles of Endothelial Dysfunctions in Cytotoxicity, Pro-inflammation, Permeability, and Apoptosis Induced by ROS Generation. Cardiovasc. Toxicol. 2017, 17, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Gallorini, M.; Cataldi, A.; di Giacomo, V. HEMA-induced cytotoxicity: Oxidative stress, genotoxicity and apoptosis. Int. Endod. J. 2014, 47, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old; new and emerging functions of caspases. Cell Death Differ. 2015, 22, 526–539. [Google Scholar] [CrossRef]
- Voss, A.K.; Strasser, A. The essentials of developmental apoptosis. F1000Res. 2020, 9, F1000. [Google Scholar] [CrossRef] [PubMed]
- Connolly, P.; Garcia-Carpio, I.; Villunger, A. Cell-Cycle Cross Talk with Caspases and Their Substrates. Cold Spring Harb. Perspect. Biol. 2020, 12, a036475. [Google Scholar] [CrossRef]
- Vakifahmetoglu-Norberg, H.; Ouchida, A.T.; Norberg, E. The role of mitochondria in metabolism and cell death. Biochem. Biophys. Res. Commun. 2017, 482, 426–431. [Google Scholar] [CrossRef]
- Spinelli, J.B.; Haigis, M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef]
- Kausar, S.; Wang, F.; Cui, H. The Role of Mitochondria in Reactive Oxygen Species Generation and Its Implications for Neurodegenerative Diseases. Cells 2018, 7, 274. [Google Scholar] [CrossRef]
- Chang, H.H.; Chang, M.C.; Lin, L.D.; Lee, J.J.; Wang, T.M.; Huang, C.H.; Yang, T.T.; Lin, H.J.; Jeng, J.H. The mechanisms of cytotoxicity of urethane dimethacrylate to Chinese hamster ovary cells. Biomaterials 2010, 31, 6917–6925. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-Y.; Chiang, C.-Y.; Chiang, Y.-W.; Lee, M.-W.; Lee, C.-Y.; Chen, H.-Y.; Lin, H.-W.; Kuan, Y.-H. Toxic Effects of Urethane Dimethacrylate on Macrophages Through Caspase Activation, Mitochondrial Dysfunction, and Reactive Oxygen Species Generation. Polymers 2020, 12, 1398. https://doi.org/10.3390/polym12061398
Chang C-Y, Chiang C-Y, Chiang Y-W, Lee M-W, Lee C-Y, Chen H-Y, Lin H-W, Kuan Y-H. Toxic Effects of Urethane Dimethacrylate on Macrophages Through Caspase Activation, Mitochondrial Dysfunction, and Reactive Oxygen Species Generation. Polymers. 2020; 12(6):1398. https://doi.org/10.3390/polym12061398
Chicago/Turabian StyleChang, Chih-Yang, Chen-Yu Chiang, Yun-Wei Chiang, Min-Wei Lee, Chien-Ying Lee, Hung-Yi Chen, Hui-Wen Lin, and Yu-Hsiang Kuan. 2020. "Toxic Effects of Urethane Dimethacrylate on Macrophages Through Caspase Activation, Mitochondrial Dysfunction, and Reactive Oxygen Species Generation" Polymers 12, no. 6: 1398. https://doi.org/10.3390/polym12061398
APA StyleChang, C.-Y., Chiang, C.-Y., Chiang, Y.-W., Lee, M.-W., Lee, C.-Y., Chen, H.-Y., Lin, H.-W., & Kuan, Y.-H. (2020). Toxic Effects of Urethane Dimethacrylate on Macrophages Through Caspase Activation, Mitochondrial Dysfunction, and Reactive Oxygen Species Generation. Polymers, 12(6), 1398. https://doi.org/10.3390/polym12061398






