Bringing a New Flexible Mercaptoacetic Acid Linker to the Design of Coordination Polymers
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
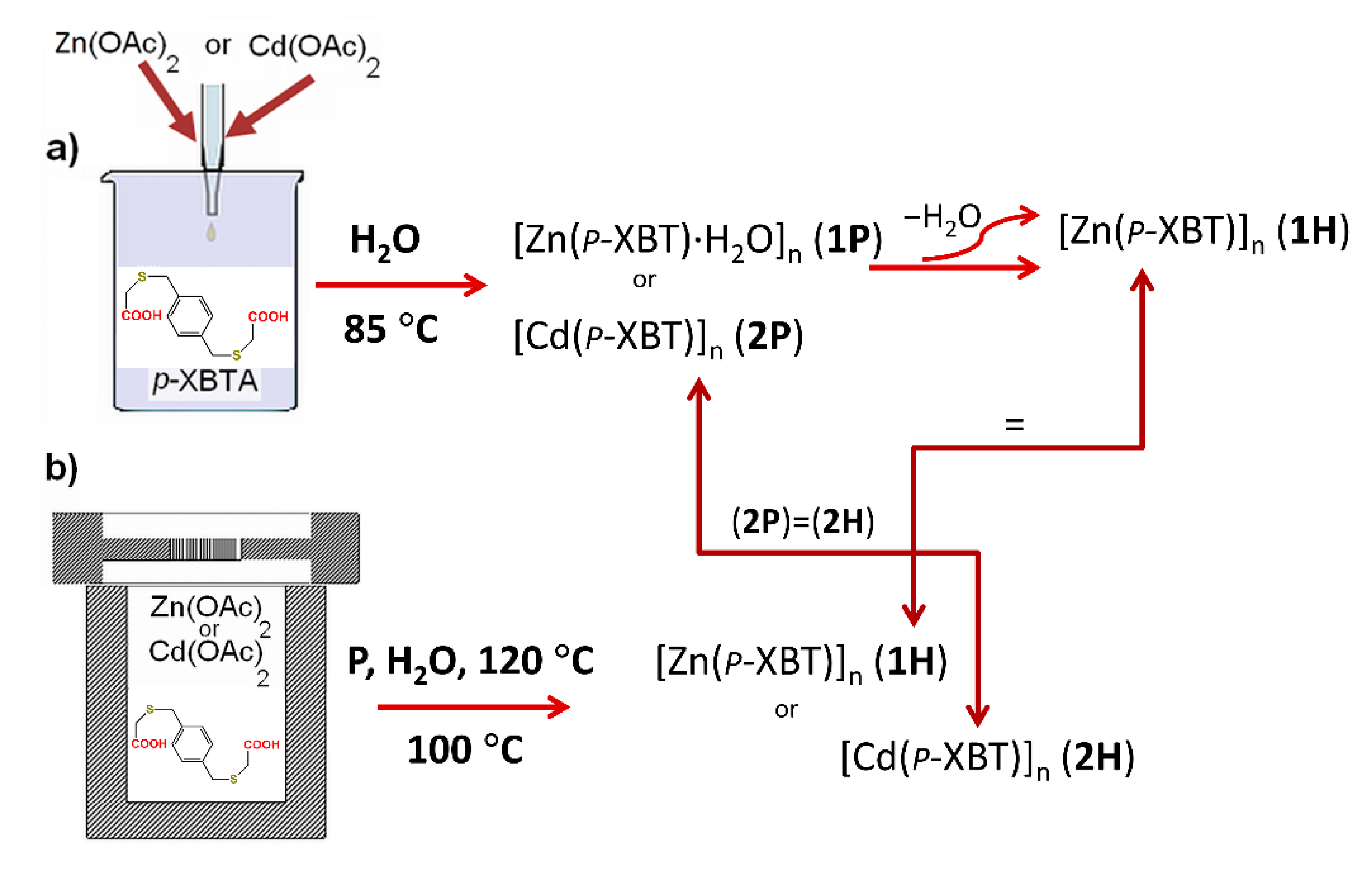
2.2. Precipitation Synthesis of 1P and 2P
2.3. Hydrothermal Synthesis of 1H and 2H
2.4. Single-Crystal X-ray Diffraction
3. Results and Discussion
3.1. FTIR Analysis
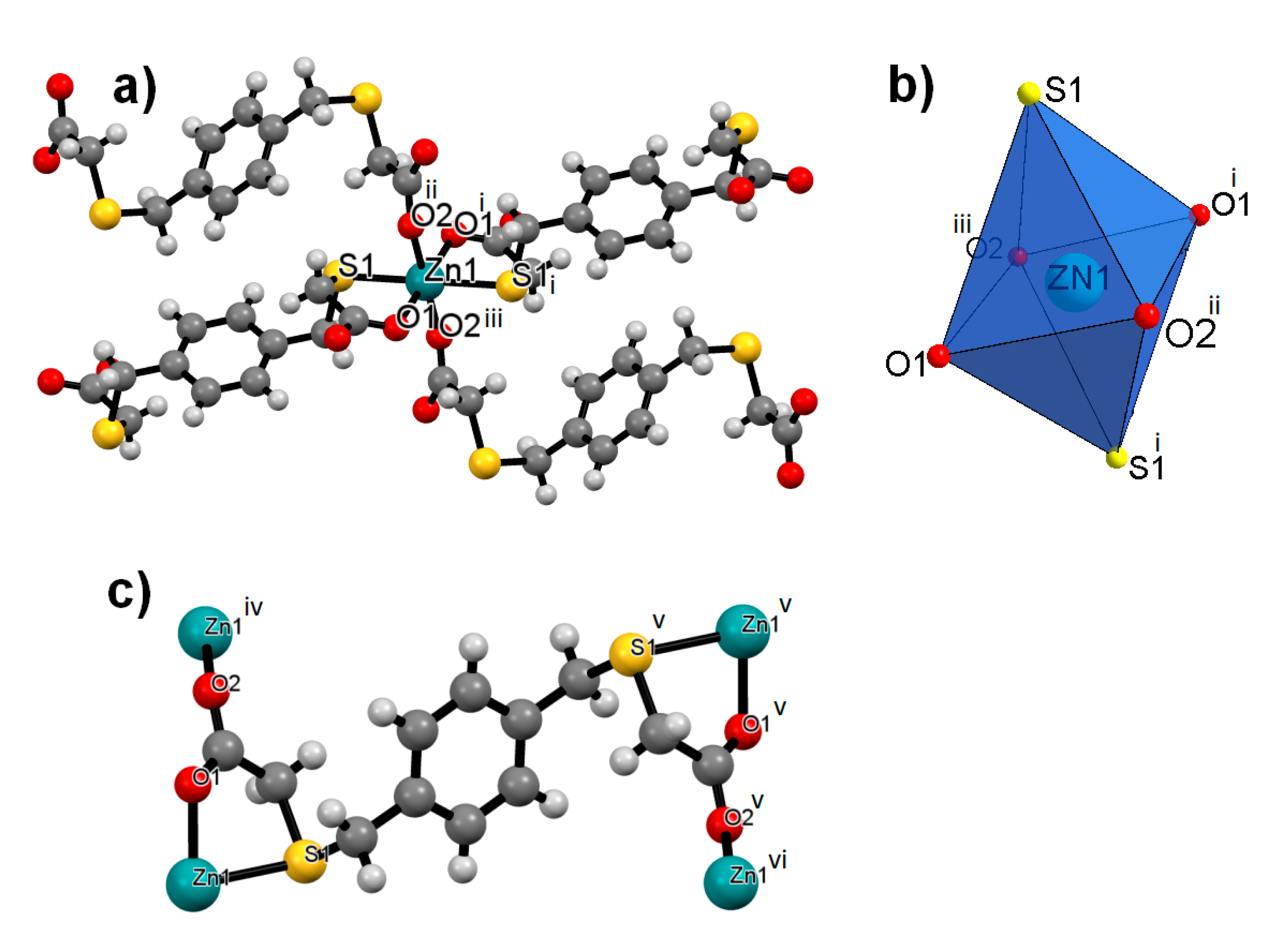
3.2. Crystal Structures
3.3. Powder X-ray Diffraction
3.4. Thermal Analysis
3.4.1. TG-DSC Analysis
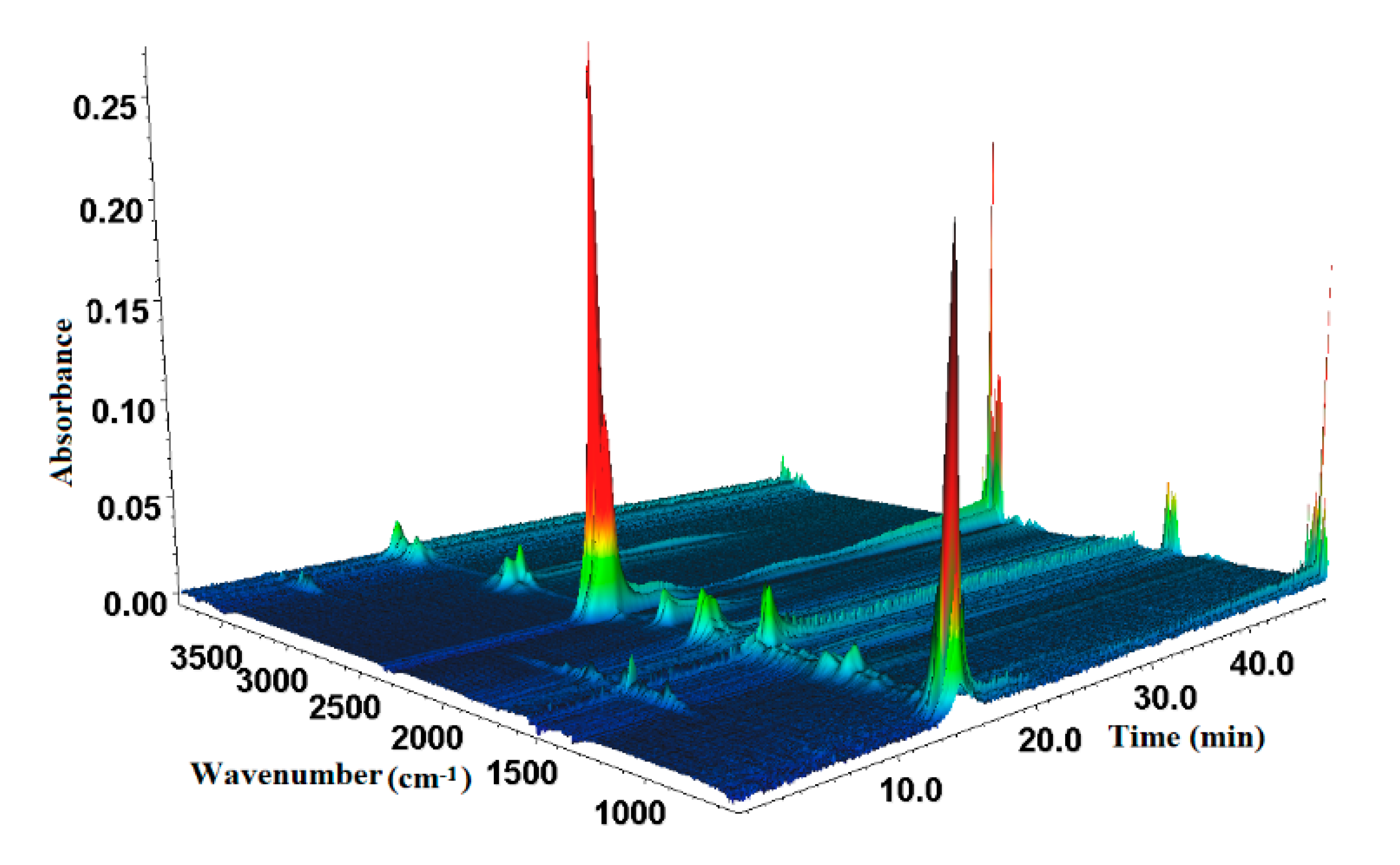
3.4.2. TG-FTIR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Biradha, K.; Ramanan, A.; Vittal, J.J. Coordination Polymers Versus Metal−Organic Frameworks. Cryst. Growth Des. 2009, 9, 2969–2970. [Google Scholar] [CrossRef]
- Samanidou, V.; Deliyanni, E. Metal Organic Frameworks: Synthesis and Application; MDPI AG: Basel, Switzerland, 2020. [Google Scholar]
- Kaskel, S. The Chemistry of Metal-Organic Frameworks: Synthesis, Characterization, and Applications; Wiley: Weinheim, Germany, 2016. [Google Scholar]
- Liu, T.-F.; Lü, J.; Cao, R. Coordination polymers based on flexible ditopic carboxylate or nitrogen-donor ligands. CrystEngComm 2010, 12, 660–670. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, L.; Liu, B.; Cui, L.; Wang, Y.-Y.; Wu, B. Syntheses, Structures, and Luminescent Properties of Six New Zinc(II) Coordination Polymers Constructed by Flexible Tetracarboxylate and Various Pyridine Ligands. Cryst. Growth Des. 2013, 13, 3177–3187. [Google Scholar] [CrossRef]
- Ostasz, A.; Lyszczek, R.; Mazur, L.; Tarasiuk, B. Co-crystal formation between 2-amino-4,6-dimethylpyrimidine and new p-xylylene-bis(thioacetic) acid. CrystEngComm 2014, 16, 10262–10272. [Google Scholar] [CrossRef]
- Lyszczek, R.; Ostasz, A.; Bartyzel, A.; Lipke, A. Thermal, spectroscopic and luminescence investigations of lanthanide(III) coordination polymers based on V-shaped 4,4 ‘-sulfonyldibenzoic acid. J. Anal. Appl. Pyrol. 2015, 115, 370–378. [Google Scholar] [CrossRef]
- Gu, J.-Z.; Wen, M.; Liang, X.; Shi, Z.-F.; Kirillova, M.V.; Kirillov, A.M. Multifunctional Aromatic Carboxylic Acids as Versatile Building Blocks for Hydrothermal Design of Coordination Polymers. Crystals 2018, 8, 83. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Łyszczek, R.; Głuchowska, H.; Cristóvão, B.; Tarasiuk, B. New lanthanide biphenyl-4,4′-diacetates − hydrothermal synthesis, spectroscopic, magnetic and thermal investigations. Thermochim. Acta 2016, 645, 16–23. [Google Scholar] [CrossRef]
- Sienkiewicz-Gromiuk, J.; Rusinek, I.; Kurach, Ł.; Rzączyńska, Z. Thermal and spectroscopic (IR, XPS) properties of lanthanide(III) benzene-1,3,5-triacetate complexes. J. Therm. Anal. Calorim. 2016, 126, 327–342. [Google Scholar] [CrossRef]
- Lyszczek, R.; Mazur, L.; Ostasz, A.; Bartyzel, A.; Gluchowska, H. Lanthanide metal-organic frameworks: Structural, thermal and sorption properties. Adsorpt. Sci. Technol. 2017, 35, 677–683. [Google Scholar] [CrossRef]
- CrysAlis PRO; Agilent Technologies Ltd.: Yarnton, UK, 2013.
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Putz, H.; Brandenburg, K. Diamond—Crystal and Molecular Structure Visualization; Crystal Impact-GbR: Bonn, Germany, 2006. [Google Scholar]
- Blatov, V.A. Multipurpose crystallochemical analysis with the program package TOPOS. IUCr CompComm Newsl. 2006, 7, 4–38. [Google Scholar]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied topological analysis of crystal structures with the program package Topospro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Silverstein, R.; Webster, F.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; Wiley: Chichester, UK, 2004. [Google Scholar]
- Xiao, B.; Xiao, H.-Y.; Chen, M.-D. Study on the reactions of zinc carboxylate complexes with copper(II) or cobalt(II) ions. J. Coord. Chem. 2014, 67, 2455–2469. [Google Scholar] [CrossRef]
- Clegg, W.; Little, I.R.; Straughan, B.P. Zinc carboxylate complexes: Structural characterisation of some binuclear and linear trinuclear complexes. J. Chem. Soc. Dalton Trans. 1986, 1283–1288. [Google Scholar] [CrossRef]
- Stamatatos, T.C.; Katsoulakoua, E.; Nastopoulos, V.; Raptopoulou, C.P.; Manessi-Zoupa, E.; Perlepes, S.P. Cadmium Carboxylate Chemistry: Preparation, Crystal Structure, and Thermal and Spectroscopic Characterization of the One-dimensional Polymer [Cd(O2CMe)(O2CPh)(H2O)2]n. Z Naturforsch B 2003, 58, 1045–1054. [Google Scholar] [CrossRef]
- Palanisami, N.; Rajakannu, P.; Murugavel, R. Non-covalently aggregated zinc and cadmium complexes derived from substituted aromatic carboxylic acids: Synthesis, spectroscopy, and structural studies. Inorg. Chem. Commun. 2013, 405, 522–531. [Google Scholar] [CrossRef]
- Ye, B.-H.; Li, X.-Y.; Williams, I.D.; Chen, X.-M. Synthesis and Structural Characterization of Di- and Tetranuclear Zinc Complexes with Phenolate and Carboxylate Bridges. Correlations between 13C NMR Chemical Shifts and Carboxylate Binding Modes. Inorg. Chem. 2002, 41, 6426–6431. [Google Scholar] [CrossRef]
- Vaz, J.L.L.; Duc, G.; Petit-Ramel, M.; Faure, R.; Vittori, O. Cd(II) complexes with phthalic acids: Solution study and crystal structure of cadmium(II) phthalate hydrate. Can. J. Chem. 1996, 74, 359–364. [Google Scholar] [CrossRef]
- Materazzi, S. Thermogravimetry—Infrared Spectroscopy (TG-FTIR) Coupled Analysis. Appl. Spectrosc. Rev. 1997, 32, 385–404. [Google Scholar] [CrossRef]
- Risoluti, R.; Fabiano, M.A.; Gullifa, G.; Ciprioti, S.V.; Materazzi, S. FTIR-evolved gas analysis in recent thermoanalytical investigations. Appl. Spectrosc. Rev. 2017, 52, 39–72. [Google Scholar] [CrossRef]
- Gao, N.; Li, A.; Quan, C.; Du, L.; Duan, Y. TG–FTIR and Py–GC/MS analysis on pyrolysis and combustion of pine sawdust. J. Anal. Appl. Pyrolysis 2013, 100, 26–32. [Google Scholar] [CrossRef]
- Meng, A.; Zhou, H.; Qin, L.; Zhang, Y.; Li, Q. Quantitative and kinetic TG-FTIR investigation on three kinds of biomass pyrolysis. J. Anal. Appl. Pyrolysis 2013, 104, 28–37. [Google Scholar] [CrossRef]
- Łyszczek, R.; Bartyzel, A.; Głuchowska, H.; Mazur, L.; Sztanke, M.; Sztanke, K. Thermal investigations of biologically important fused azaisocytosine-containing congeners and the crystal structure of one representative. J. Anal. Appl. Pyrolysis 2018, 135, 141–151. [Google Scholar] [CrossRef]
- Thermo Scientific™ Inc. OMNIC™ Specta Software; Thermo Scientific™ Inc.: Waltham, MA, USA, 2013. [Google Scholar]
- Kirillov, A.M.; Coelho, J.A.; Kirillova, M.V.; da Silva, M.F.C.G.; Nesterov, D.S.; Gruenwald, K.R.; Haukka, M.; Pombeiro, A.J. Bringing an “old” biological buffer to coordination chemistry: New 1D and 3D coordination polymers with [Cu4(Hbes)4] cores for mild hydrocarboxylation of alkanes. Inorg. Chem. 2010, 49, 6390–6392. [Google Scholar] [CrossRef]
- Kirillova, M.V.; Kirillov, A.M.; Martins, A.N.; Graiff, C.; Tiripicchio, A.; Pombeiro, A.J. Topologically unique heterometallic CuII/Li coordination polymers self-assembled from N,N-bis(2-Hydroxyethyl)-2-aminoethanesulfonic acid biobuffer: Versatile catalyst precursors for mild hydrocarboxylation of alkanes to carboxylic acids. Inorg. Chem. 2012, 51, 5224–5234. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Karabach, Y.Y.; Kirillova, M.V.; Haukka, M.; Pombeiro, A.J.L. Topologically unique 2D heterometallic CuII/Mg coordination polymer: Synthesis, structural features, and catalytic use in alkane hydrocarboxylation. Cryst. Growth Des. 2012, 12, 1069–1074. [Google Scholar] [CrossRef]
- Dias, S.S.P.; Kirillova, M.V.; André, V.; Kłak, J.; Kirillov, A.M. New tricopper(II) cores self-assembled from aminoalcohol biobuffers and homophthalic acid: Synthesis, structural and topological features, magnetic properties and mild catalytic oxidation of cyclic and linear C5–C8 alkanes. Inorg. Chem. Front. 2015, 2, 525–537. [Google Scholar] [CrossRef]
- Gu, J.Z.; Wen, M.; Cai, Y.; Shi, Z.; Nesterov, D.S.; Kirillova, M.V.; Kirillov, A.M. Cobalt(II) Coordination Polymers Assembled from Unexplored Pyridine-Carboxylic Acids: Structural Diversity and Catalytic Oxidation of Alcohols. Inorg. Chem. 2019, 58, 5875–5885. [Google Scholar] [CrossRef] [PubMed]
- Morsali, A.; Hashemi, L. Main Group Metal Coordination Polymers: Structures and Nanostructures; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- MacGillivray, L.R.; Lukehart, C.M. (Eds.) Metal-Organic Framework Materials; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]





| Compound | [Zn(p-XBT)]n (1H) | [Cd(p-XBT)]n (2H) |
|---|---|---|
| Chemical formula | C12H12O4S2Zn | C12H12O4S2Cd |
| Formula weight | 349.71 | 396.74 |
| Crystal system | monoclinic | monoclinic |
| Space group | P21/c | P21/c |
| a (Å) | 9.4845(6) | 8.9996(5) |
| b (Å) | 8.3523(8) | 8.5045(11) |
| c (Å) | 7.9804(4) | 8.6568(6) |
| β (°) | 90.502(5) | 90.454(6) |
| V (Å3) | 632.16(8) | 662.55(10) |
| Z | 2 | 2 |
| T (K) | 100(2) | 100(2) |
| Color and shape | colorless plate | colorless plate |
| Dcalc (g cm−3) | 1.837 | 1.989 |
| μ (mm−1) | 2.278 | 1.968 |
| Refl. collected | 2546 | 2795 |
| Refl. unique | 1444 | 1507 |
| R(int) | 0.0281 | 0.0285 |
| Refl. obs. (I > 2σ(I)) | 1259 | 1253 |
| Refined parameters | 112 | 112 |
| GooF on F2 | 0.995 | 0.973 |
| R1 (I > 2σ(I)) | 0.0288 | 0.0272 |
| wR2 (all data) | 0.0705 | 0.0625 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostasz, A.; Kirillov, A.M. Bringing a New Flexible Mercaptoacetic Acid Linker to the Design of Coordination Polymers. Polymers 2020, 12, 1329. https://doi.org/10.3390/polym12061329
Ostasz A, Kirillov AM. Bringing a New Flexible Mercaptoacetic Acid Linker to the Design of Coordination Polymers. Polymers. 2020; 12(6):1329. https://doi.org/10.3390/polym12061329
Chicago/Turabian StyleOstasz, Agnieszka, and Alexander M. Kirillov. 2020. "Bringing a New Flexible Mercaptoacetic Acid Linker to the Design of Coordination Polymers" Polymers 12, no. 6: 1329. https://doi.org/10.3390/polym12061329
APA StyleOstasz, A., & Kirillov, A. M. (2020). Bringing a New Flexible Mercaptoacetic Acid Linker to the Design of Coordination Polymers. Polymers, 12(6), 1329. https://doi.org/10.3390/polym12061329