Synthesis, Characterization, and Analysis of Hybrid Carbon Nanotubes by Chemical Vapor Deposition: Application for Aluminum Removal
Abstract
1. Introduction
2. Experimental
2.1. Materials and Reagents
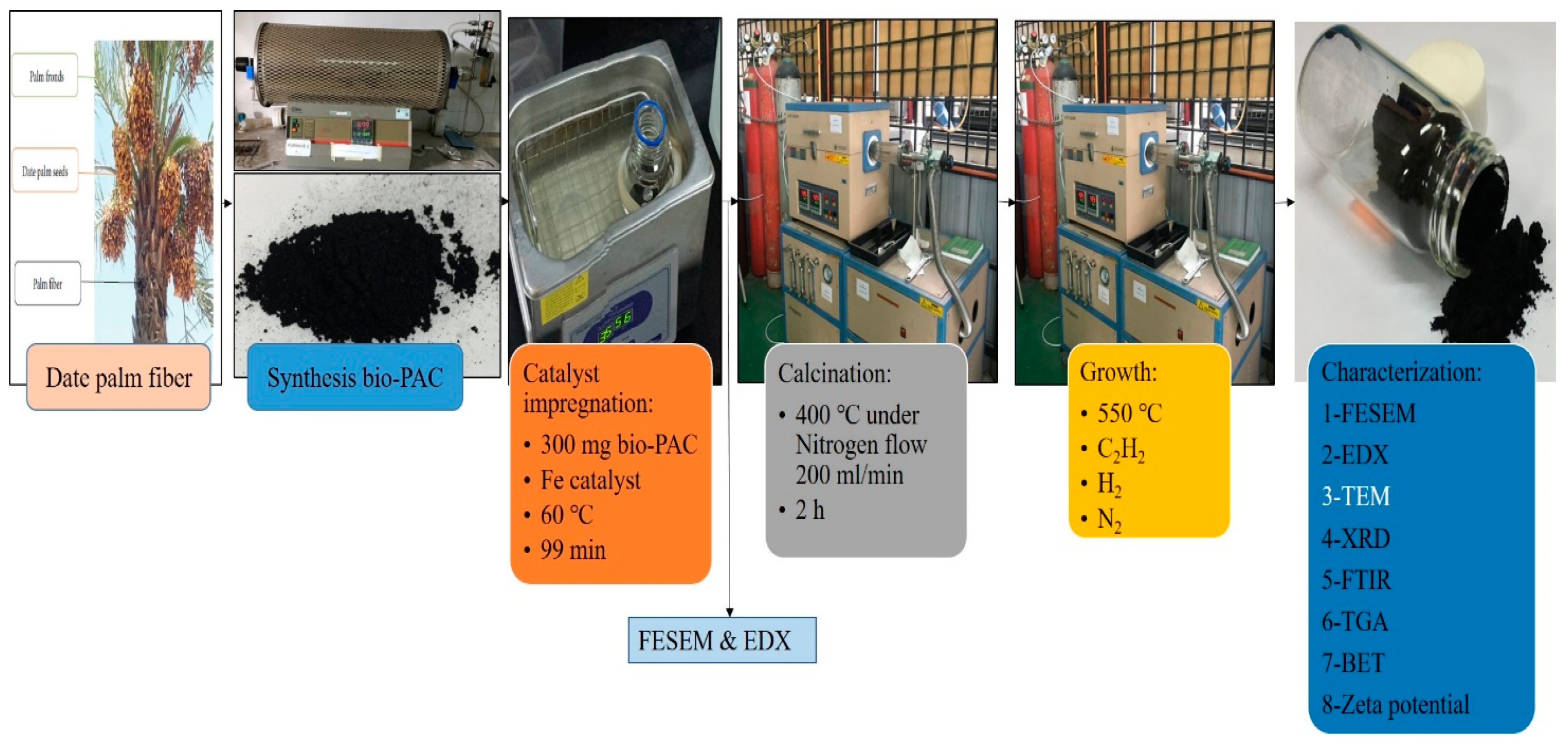
2.2. Synthesis of Hybrid CNTs
2.2.1. Catalyst Impregnation
2.2.2. CVD Growth
2.3. Characterizations
2.4. Adsorption Study
2.4.1. Experimental Design for Optimization of Al3+ Adsorption
2.4.2. Kinetic and Isotherm Adsorption
3. Results and Discussion
3.1. Characterization and Analysis
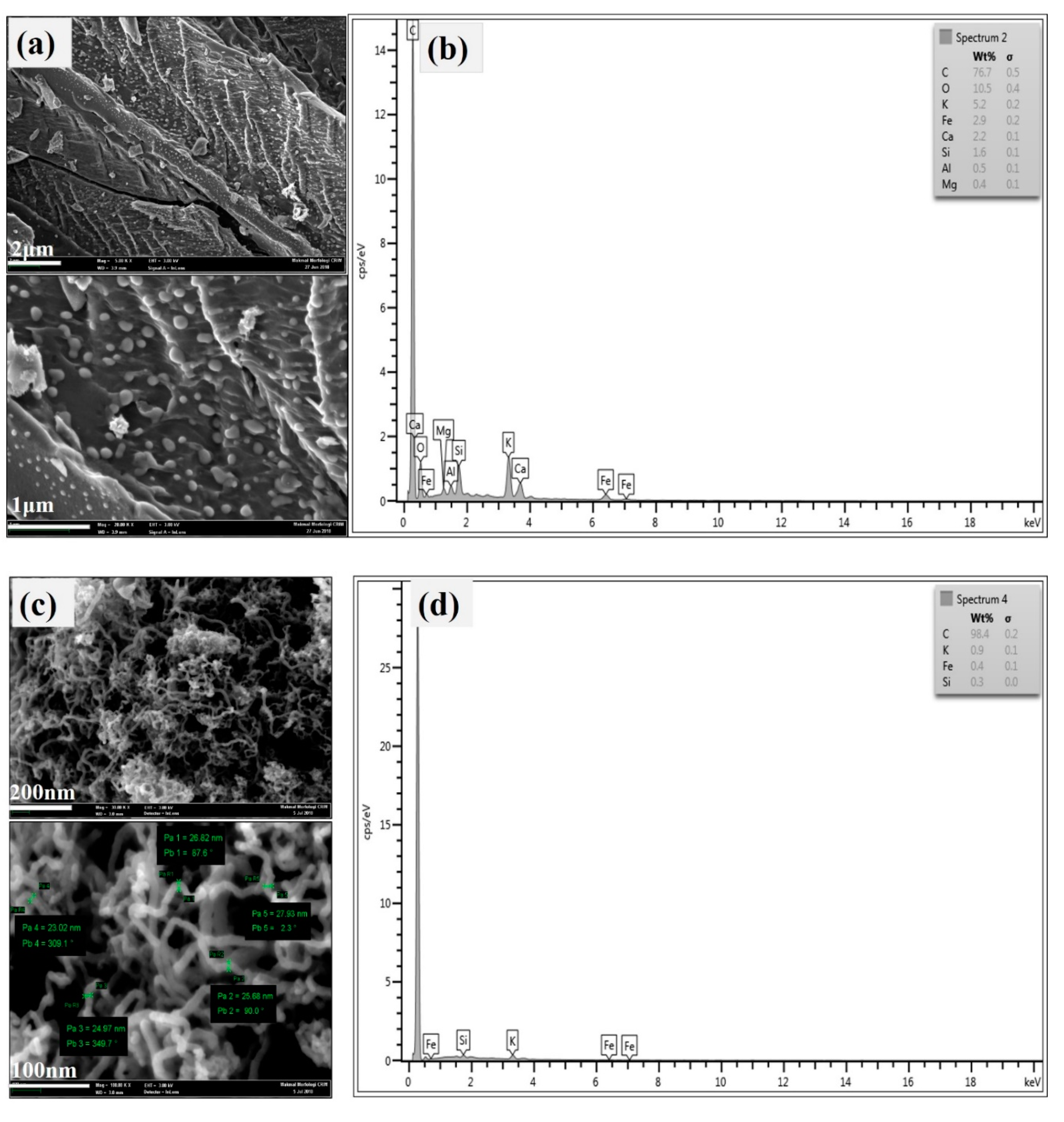
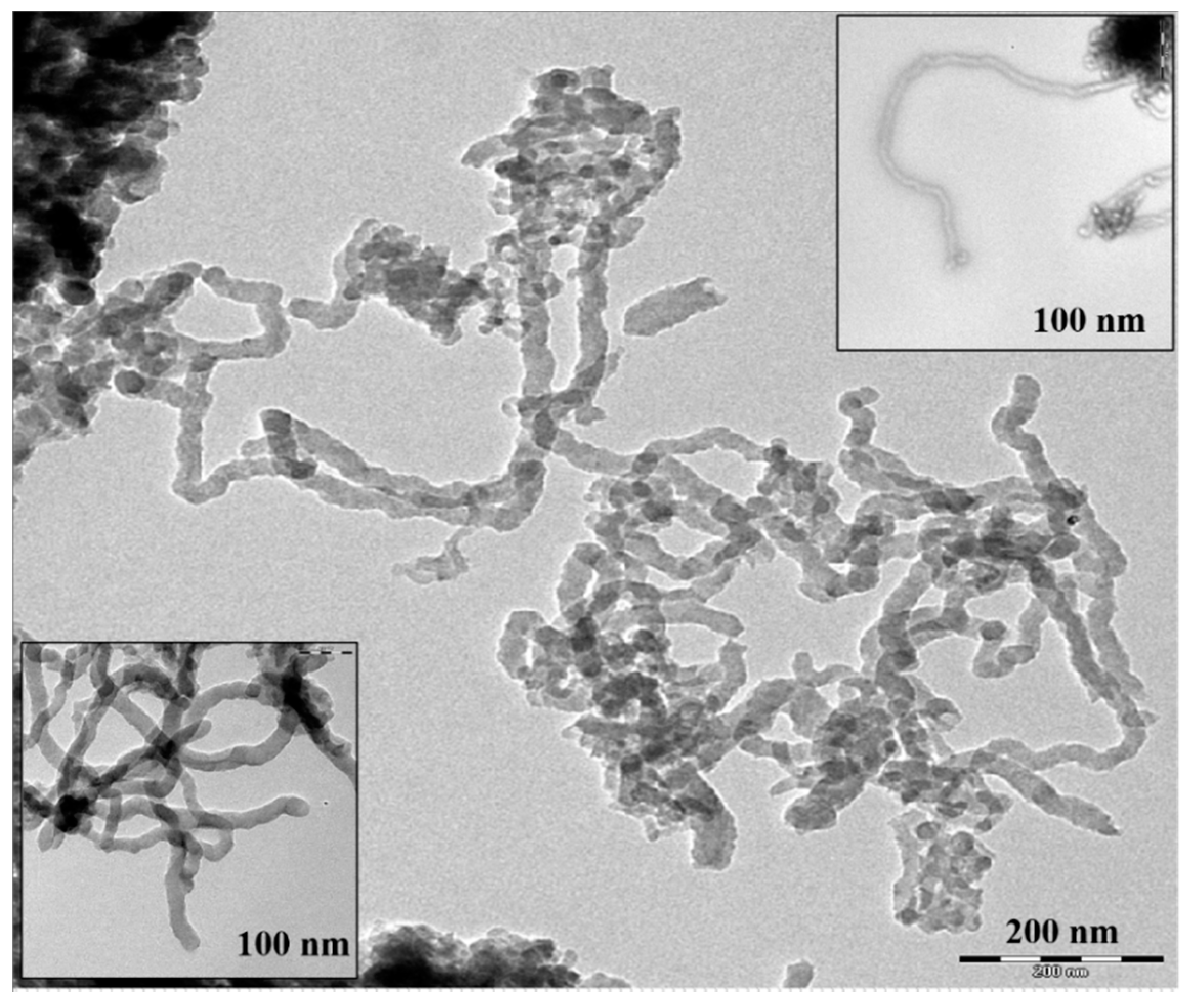
3.1.1. FESEM, EDX, and TEM of Hybrid CNTs
3.1.2. X-ray Diffraction
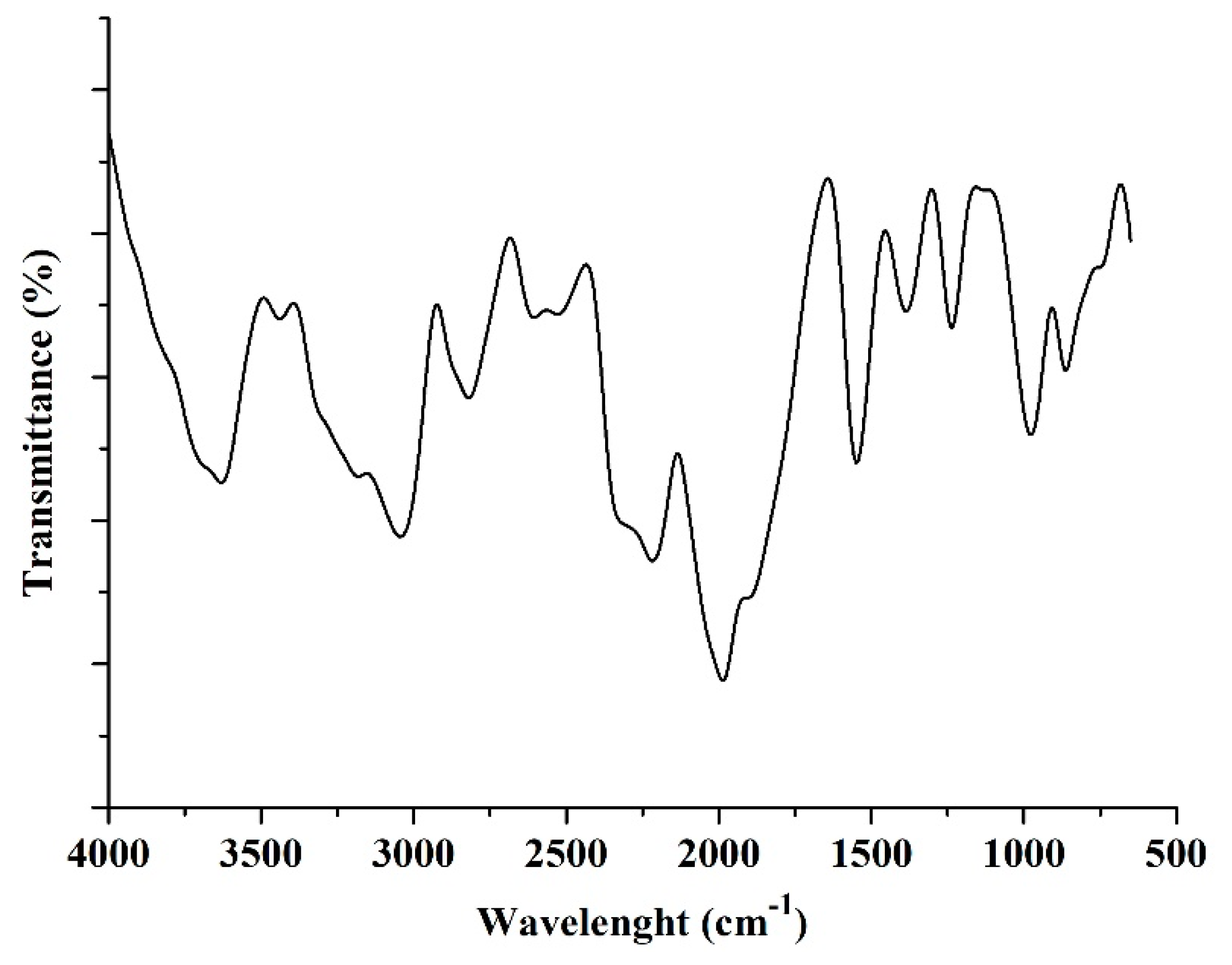
3.1.3. Fourier-Transform Infrared (FTIR) Analysis
3.1.4. Thermogravimetric Analysis (TGA)
3.1.5. Brunauer–Emmett–Teller (BET)
3.1.6. Zeta Potential
3.2. Application Studies
3.2.1. Optimization Study
3.2.2. Effect of Factors on Optimization of Adsorption of Al3+ by Hybrid CNTs
3.2.3. Adsorption Studies
Kinetics Studies
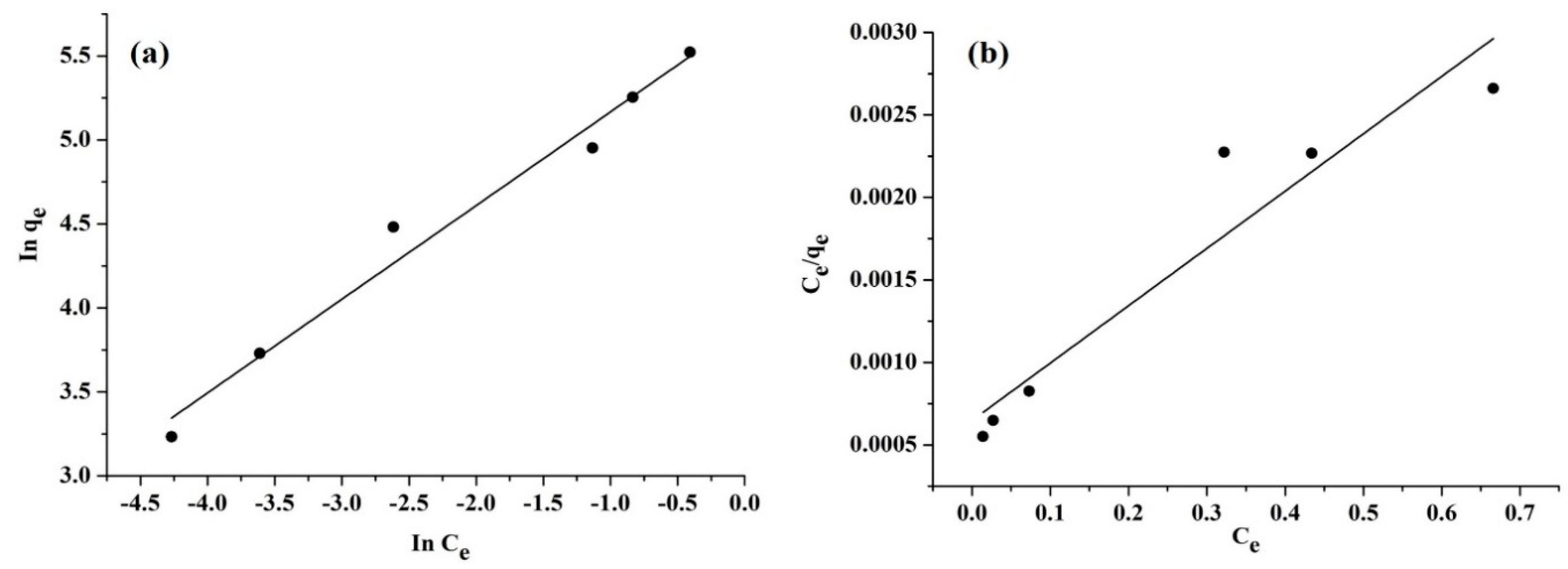
Isotherm Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Basheer, A.; M Hanafiah, M.; Abdulhakim Alsaadi, M.; Al-Douri, Y.; Malek, M.A.; Mohammed Aljumaily, M.; Saadi Fiyadh, S. Synthesis and characterization of natural extracted precursor date palm fibre-based activated carbon for aluminum removal by RSM optimization. Processes 2019, 7, 249. [Google Scholar] [CrossRef]
- Mishra, M.; Chauhan, M. Biosorption as a Novel Approach for Removing Aluminium from Water Treatment Plant Residual—A Review. In Water Quality Management; Springer: Berlin, Germany, 2018; pp. 93–99. [Google Scholar]
- Mohd-Salleh, S.N.A.; Mohd-Zin, N.S.; Othman, N. A Review of Wastewater Treatment using Natural Material and Its Potential as Aid and Composite Coagulant. Sains Malays. 2019, 48, 155–164. [Google Scholar] [CrossRef]
- Al-Muhtaseb, S.A.; El-Naas, M.H.; Abdallah, S. Removal of aluminum from aqueous solutions by adsorption on date-pit and BDH activated carbons. J. Hazard. Mater. 2008, 158, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Al-Raad, A.A.; Hanafiah, M.M.; Naje, A.S.; Ajeel, M.A.; O Basheer, A.; Ali Aljayashi, T.; Ekhwan Toriman, M. Treatment of Saline Water Using Electrocoagulation with Combined Electrical Connection of Electrodes. Processes 2019, 7, 242. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, X.; Guo, L.; Lan, J.; Zhang, L.; Cao, D. Heavy metal ion removal of wastewater by zeolite-imidazolate frameworks. Sep. Purif. Technol. 2018, 194, 462–469. [Google Scholar] [CrossRef]
- Fang, L.; Li, L.; Qu, Z.; Xu, H.; Xu, J.; Yan, N. A novel method for the sequential removal and separation of multiple heavy metals from wastewater. J. Hazard. Mater. 2018, 342, 617–624. [Google Scholar] [CrossRef]
- Ang, W.L.; Mohammad, A.W.; Johnson, D.; Hilal, N. Forward osmosis research trends in desalination and wastewater treatment: A review of research trends over the past decade. J. Water Process Eng. 2019, 31, 100886. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, L.; Liu, J.; Logan, B.E. Electrochemical technologies for wastewater treatment and resource reclamation. Environ. Sci. Water Res. Technol. 2016, 2, 800–831. [Google Scholar] [CrossRef]
- Thue, P.S.; dos Reis, G.S.; Lima, E.C.; Sieliechi, J.M.; Dotto, G.; Wamba, A.G.; Dias, S.L.; Pavan, F.A. Activated carbon obtained from sapelli wood sawdust by microwave heating for o-cresol adsorption. Res. Chem. Intermed. 2017, 43, 1063–1087. [Google Scholar] [CrossRef]
- Kizito, S.; Wu, S.; Kirui, W.K.; Lei, M.; Lu, Q.; Bah, H.; Dong, R. Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry. Sci. Total Environ. 2015, 505, 102–112. [Google Scholar] [CrossRef]
- Intani, K.; Latif, S.; Kabir, A.R.; Müller, J. Effect of self-purging pyrolysis on yield of biochar from maize cobs, husks and leaves. Bioresour. Technol. 2016, 218, 541–551. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, W.; Wu, X.; Gao, B. The properties of the self-compacting concrete with fly ash and ground granulated blast furnace slag mineral admixtures. J. Clean. Prod. 2015, 95, 66–74. [Google Scholar] [CrossRef]
- Fiyadh, S.S.; AlSaadi, M.A.; Jaafar, W.Z.B.; AlOmar, M.K.; Fayaed, S.S.; Mohd, N.S.B.; Hin, L.S.; El-Shafie, A. Review on heavy metal adsorption processes by carbon nanotubes. J. Clean. Prod. 2019, 230, 783–793. [Google Scholar] [CrossRef]
- Chen, G.; Liu, X.; Brookes, P.C.; Xu, J. Opportunities for phytoremediation and bioindication of arsenic contaminated water using a submerged aquatic plant: Vallisneria natans (Lour.) Hara. Int. J. Phytorem. 2015, 17, 249–255. [Google Scholar] [CrossRef]
- Murray, C.C.; Vatankhah, H.; McDonough, C.A.; Nickerson, A.; Hedtke, T.T.; Cath, T.Y.; Higgins, C.P.; Bellona, C.L. Removal of per-and polyfluoroalkyl substances using super-fine powder activated carbon and ceramic membrane filtration. J. Hazard. Mater. 2019, 366, 160–168. [Google Scholar] [CrossRef]
- Lee, S.; Ko, K.; Youk, J.; Lim, D.; Jeong, W. Preparation and Properties of Carbon Fiber/Carbon Nanotube Wet-Laid Composites. Polymers 2019, 11, 1597. [Google Scholar] [CrossRef]
- Puglisi, R.A.; Bongiorno, C.; Caccamo, S.; Fazio, E.; Mannino, G.; Neri, F.; Scalese, S.; Spucches, D.; La Magna, A. Chemical Vapor Deposition Growth of Silicon Nanowires with Diameter Smaller Than 5 nm. ACS Omega 2019, 4, 17967–17971. [Google Scholar] [CrossRef]
- Xiang, X.; Zhang, L.; Hima, H.I.; Li, F.; Evans, D.G. Co-based catalysts from Co/Fe/Al layered double hydroxides for preparation of carbon nanotubes. Appl. Clay Sci. 2009, 42, 405–409. [Google Scholar] [CrossRef]
- Aljumaily, M.M.; Alsaadi, M.A.; Hashim, N.A.; Alsalhy, Q.F.; Mjalli, F.S.; Atieh, M.A.; Al-Harrasi, A. PVDF-co-HFP/superhydrophobic acetylene-based nanocarbon hybrid membrane for seawater desalination via DCMD. Chem. Eng. Res. Des. 2018, 138, 248–259. [Google Scholar] [CrossRef]
- Alayan, H.M.; Alsaadi, M.A.; AlOmar, M.K.; Hashim, M.A. Growth and optimization of carbon nanotubes in powder activated carbon for an efficient removal of methylene blue from aqueous solution. Environ. Technol. 2019, 40, 2400–2415. [Google Scholar] [CrossRef]
- Yahyazadeh, A.; Khoshandam, B. Carbon nanotube synthesis via the catalytic chemical vapor deposition of methane in the presence of iron, molybdenum, and iron–molybdenum alloy thin layer catalysts. Results Phys. 2017, 7, 3826–3837. [Google Scholar] [CrossRef]
- Zhao, Y.; Choi, J.; Kim, P.; Fei, W.; Lee, C.J. Large-scale synthesis and characterization of super-bundle single-walled carbon nanotubes by water-assisted chemical vapor deposition. RSC Adv. 2015, 5, 30564–30569. [Google Scholar] [CrossRef]
- Chen, G.; Davis, R.C.; Kimura, H.; Sakurai, S.; Yumura, M.; Futaba, D.N.; Hata, K. The relationship between the growth rate and the lifetime in carbon nanotube synthesis. Nanoscale 2015, 7, 8873–8878. [Google Scholar] [CrossRef]
- Mamtm, A.; Ma’an, F.; Zahirah, A.; Yehya, M.; Mohammed, A. Optimisation of arsenic adsorption from water by carbon nanofibres grown on powdered activated carbon impregnated with nickel. J. Appl. Sci. 2009, 9, 3180–3183. [Google Scholar]
- Aljumaily, M.M.; Alsaadi, M.A.; Hashim, N.A.; Alsalhy, Q.F.; Das, R.; Mjalli, F. Embedded high-hydrophobic CNMs prepared by CVD technique with PVDF-co-HFP membrane for application in water desalination by DCMD. Desalin. Water Treat. 2019, 142, 37–48. [Google Scholar] [CrossRef]
- AlOmar, M.K.; Alsaadi, M.A.; Hayyan, M.; Akib, S.; Ibrahim, R.K.; Hashim, M.A. Lead removal from water by choline chloride based deep eutectic solvents functionalized carbon nanotubes. J. Mol. Liq. 2016, 222, 883–894. [Google Scholar] [CrossRef]
- Fiyadh, S.S.; AlOmar, M.K.; Binti Jaafar, W.Z.; AlSaadi, M.A.; Fayaed, S.S.; Binti Koting, S.; Lai, S.H.; Chow, M.F.; Ahmed, A.N.; El-Shafie, A. Artificial Neural Network Approach for Modelling of Mercury Ions Removal from Water Using Functionalized CNTs with Deep Eutectic Solvent. Int. J. Mol. Sci. 2019, 20, 4206. [Google Scholar] [CrossRef]
- Pakade, V.E.; Tavengwa, N.T.; Madikizela, L.M. Recent advances in hexavalent chromium removal from aqueous solutions by adsorptive methods. RSC Adv. 2019, 9, 26142–26164. [Google Scholar] [CrossRef]
- Boscarino, S.; Filice, S.; Sciuto, A.; Libertino, S.; Scuderi, M.; Galati, C.; Scalese, S. Investigation of ZnO-decorated CNTs for UV Light Detection Applications. Nanomaterials 2019, 9, 1099. [Google Scholar] [CrossRef]
- Ionescu, M.I. Direct synthesis of carbon nanotubes on metallic foams as a cathode material with high mass load for lithium–air batteries. RSC Adv. 2017, 7, 30365–30369. [Google Scholar] [CrossRef]
- Minakshi, M.; Mitchell, D.R.; Baur, C.; Chable, J.; Barlow, A.J.; Fichtner, M.; Banerjee, A.; Chakraborty, S.; Ahuja, R. Phase evolution in calcium molybdate nanoparticles as a function of synthesis temperature and its electrochemical effect on energy storage. Nanoscale Adv. 2019, 1, 565–580. [Google Scholar] [CrossRef]
- Roe, D.P.; Xu, R.; Roberts, C.B. Influence of a carbon nanotube support and supercritical fluid reaction medium on Fe-catalyzed Fischer-Tropsch synthesis. Appl. Catal. A Gen. 2017, 543, 141–149. [Google Scholar] [CrossRef]
- Shen, D.; Liu, J.; Gan, L.; Huang, N.; Long, M. Green synthesis of Fe 3 O 4/cellulose/polyvinyl alcohol hybride aerogel and its application for dye removal. J. Polym. Environ. 2018, 26, 2234–2242. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Salmiati, S.; Hadibarata, T.; Kueh, A.B.H.; Salim, M.R.; Zaini, M.A.A. Silver Nanoparticles in the Water Environment in Malaysia: Inspection, characterization, removal, modeling, and future perspective. Sci. Rep. 2018, 8, 986. [Google Scholar] [CrossRef]
- Aljumaily, M.M.; Alsaadi, M.A.; Das, R.; Hamid, S.B.A.; Hashim, N.A.; AlOmar, M.K.; Alayan, H.M.; Novikov, M.; Alsalhy, Q.F.; Hashim, M.A. Optimization of the synthesis of superhydrophobic carbon nanomaterials by chemical vapor deposition. Sci. Rep. 2018, 8, 2778. [Google Scholar] [CrossRef]
- Minakshi, M.; Barmi, M.J.; Jones, R.T. Rescaling metal molybdate nanostructures with biopolymer for energy storage having high capacitance with robust cycle stability. Dalton Trans. 2017, 46, 3588–3600. [Google Scholar] [CrossRef]
- Ţucureanu, V.; Matei, A.; Avram, A.M. FTIR spectroscopy for carbon family study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef]
- ROSNAN, N.A.; Haan, T.Y.; Mohammad, A.W. The Effect of ZnO Loading for the Enhancement of PSF/ZnO-GO Mixed Matrix Membrane Performance. Sains Malays. 2018, 47, 2035–2045. [Google Scholar] [CrossRef]
- Gupta, V.K.; Agarwal, S.; Saleh, T.A. Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. J. Hazard. Mater. 2011, 185, 17–23. [Google Scholar] [CrossRef]
- Rodiles, X.; Reguero, V.; Vila, M.; Alemán, B.; Arévalo, L.; Fresno, F.; de la Peña O’Shea, V.; Vilatela, J. Carbon nanotube synthesis and spinning as macroscopic fibers assisted by the ceramic reactor tube. Sci. Rep. 2019, 9, 9239. [Google Scholar] [CrossRef]
- Misra, A.; Tyagi, P.K.; Rai, P.; Misra, D. FTIR Spectroscopy of multiwalled carbon nanotubes: A Simple approachto study the nitrogen doping. J. Nanosci. Nanotechnol. 2007, 7, 1820–1823. [Google Scholar] [CrossRef]
- Quan, C.; Li, A.; Gao, N. Synthesis of carbon nanotubes and porous carbons from printed circuit board waste pyrolysis oil. J. Hazard. Mater. 2010, 179, 911–917. [Google Scholar] [CrossRef]
- Bellanger, H.; Darmanin, T.; Taffin de Givenchy, E.; Guittard, F. Chemical and physical pathways for the preparation of superoleophobic surfaces and related wetting theories. Chem. Rev. 2014, 114, 2694–2716. [Google Scholar] [CrossRef]
- Hildago-Oporto, P.; Navia, R.; Hunter, R.; Coronado, G.; Gonzalez, M. Synthesis of carbon nanotubes using biochar as precursor material under microwave irradiation. J. Environ. Manag. 2019, 244, 83–91. [Google Scholar]
- Lawal, I.A.; Dolla, T.H.; Pruessner, K.; Ndungu, P. Synthesis and characterization of deep eutectic solvent functionalized CNT/ZnCo2O4 nanostructure: Kinetics, isotherm and regenerative studies on Eosin Y adsorption. J. Environ. Chem. Eng. 2019, 7, 102877. [Google Scholar] [CrossRef]
- Alayan, H.M.; Alsaadi, M.A.; Abo-Hamad, A.; AlOmar, M.K.; Aljumaily, M.M.; Das, R.; Hashim, M.A. Hybridizing carbon nanomaterial with powder activated carbon for an efficient removal of Bisphenol A from water: The optimum growth and adsorption conditions. Desalin. Water Treat. 2017, 95, 128–143. [Google Scholar]
- Lee, W.J.; Lee, J.M.; Kochuveedu, S.T.; Han, T.H.; Jeong, H.Y.; Park, M.; Yun, J.M.; Kwon, J.; No, K.; Kim, D.H. Biomineralized N-doped CNT/TiO2 core/shell nanowires for visible light photocatalysis. ACS Nano 2011, 6, 935–943. [Google Scholar] [CrossRef]
- Fraczek-Szczypta, A.; Dlugon, E.; Weselucha-Birczynska, A.; Nocun, M.; Blazewicz, M. Multi walled carbon nanotubes deposited on metal substrate using EPD technique. A spectroscopic study. J. Mol. Struct. 2013, 1040, 238–245. [Google Scholar] [CrossRef]
- Ngulube, T.; Gumbo, J.; Masindi, V.; Maity, A. Preparation and characterisation of high performing magnesite-halloysite nanocomposite and its application in the removal of methylene blue dye. J. Mol. Struct. 2019, 1184, 389–399. [Google Scholar] [CrossRef]
- Gatabi, M.P.; Moghaddam, H.M.; Ghorbani, M. Point of zero charge of maghemite decorated multiwalled carbon nanotubes fabricated by chemical precipitation method. J. Mol. Liq. 2016, 216, 117–125. [Google Scholar] [CrossRef]
- Halder, G.; Sinha, K.; Dhawane, S. Defluoridation of wastewater using powdered activated carbon developed from Eichhornia crassipes stem: Optimization by response surface methodology. Desalin. Water Treat. 2015, 56, 953–966. [Google Scholar] [CrossRef]
- AlOmar, M.K.; Alsaadi, M.A.; Hayyan, M.; Akib, S.; Ibrahim, M.; Hashim, M.A. Allyl triphenyl phosphonium bromide based DES-functionalized carbon nanotubes for the removal of mercury from water. Chemosphere 2017, 167, 44–52. [Google Scholar] [CrossRef]
- Ahmad, N.S.; Radiman, S.; Yaacob, W.Z.W. Aggregation and Stability of Iron Oxide and Alumina Nanoparticles: Influences of pH and Humic Acid Concentration. Sains Malays. 2019, 48, 435–442. [Google Scholar] [CrossRef]
- Bradl, H.B. Adsorption of heavy metal ions on soils and soils constituents. J. Colloid Interface Sci. 2004, 277, 1–18. [Google Scholar] [CrossRef]
- Kumar, A.S.K.; Jiang, S.-J. Chitosan-functionalized graphene oxide: A novel adsorbent an efficient adsorption of arsenic from aqueous solution. J. Environ. Chem. Eng. 2016, 4, 1698–1713. [Google Scholar] [CrossRef]
- Ashrafi, A.; Rahbar-Kelishami, A.; Shayesteh, H. Highly efficient simultaneous ultrasonic assisted adsorption of Pb (II) by Fe3O4@ MnO2 core-shell magnetic nanoparticles: Synthesis and characterization, kinetic, equilibrium, and thermodynamic studies. J. Mol. Struct. 2017, 1147, 40–47. [Google Scholar] [CrossRef]
- Alvarez-Torrellas, S.; Boutahala, M.; Boukhalfa, N.; Munoz, M. Effective Adsorption of Methylene Blue dye onto Magnetic Nanocomposites. Modeling and Reuse Studies. Appl. Sci. 2019, 9, 4563. [Google Scholar] [CrossRef]
- Lakkaboyana, S.K.; Khantong, S.; Asmel, N.K.; Yuzir, A.; Yaacob, W.Z.W. Synthesis of Copper Oxide Nanowires-Activated Carbon (AC@ CuO-NWs) and Applied for Removal Methylene Blue from Aqueous Solution: Kinetics, Isotherms, and Thermodynamics. J. Inorg. Organomet. Polym. Mater. 2019, 29, 1–11. [Google Scholar] [CrossRef]
- Rong, J.; Rong, X.; Qiu, F.; Zhu, X.; Pan, J.; Zhang, T.; Yang, D. Facile preparation of glucose functionalized multi-wall carbon nanotubes and its application for the removal of cationic pollutants. Mater. Lett. 2016, 183, 9–13. [Google Scholar] [CrossRef]
- Lin, Y.-F.; Chang, C.-Y. Magnetic mesoporous iron oxide/carbon aerogel photocatalysts with adsorption ability for organic dye removal. RSC Adv. 2014, 4, 28628–28631. [Google Scholar] [CrossRef]
- Ibrahim, R.K.; El-Shafie, A.; Hin, L.S.; Mohd, N.S.B.; Aljumaily, M.M.; Ibraim, S.; AlSaadi, M.A. A clean approach for functionalized carbon nanotubes by deep eutectic solvents and their performance in the adsorption of methyl orange from aqueous solution. J. Environ. Manag. 2019, 235, 521–534. [Google Scholar] [CrossRef]
- Madrakian, T.; Afkhami, A.; Ahmadi, M.; Bagheri, H. Removal of some cationic dyes from aqueous solutions using magnetic-modified multi-walled carbon nanotubes. J. Hazard. Mater. 2011, 196, 109–114. [Google Scholar] [CrossRef]



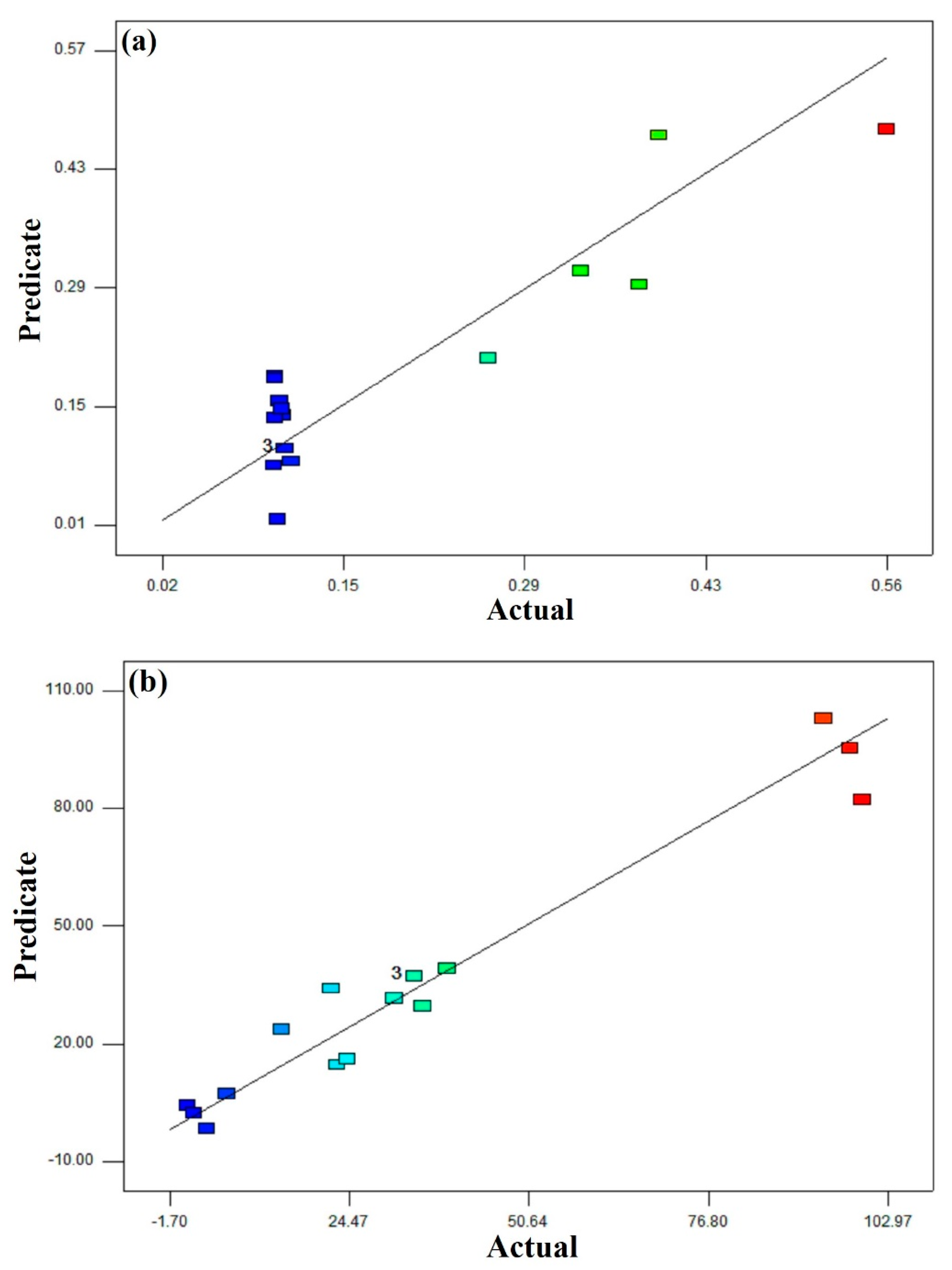


| Run | Dosage (mg/g) | pH | Time (min) | Response Removal (%) | Response Capacity (mg/g) |
|---|---|---|---|---|---|
| 1 | 5 | 11 | 10 | 93.62 | 93.62 |
| 2 | 20 | 3 | 120 | 96.74 | 24.185 |
| 3 | 12.50 | 7 | 65 | 84.68 | 33.872 |
| 4 | 12.50 | 7 | 65 | 84.68 | 33.872 |
| 5 | 12.50 | 7 | 10 | 87.86 | 35.144 |
| 6 | 5 | 7 | 65 | 99.26 | 99.26 |
| 7 | 20 | 11 | 120 | 7.08 | 1.77 |
| 8 | 12.50 | 7 | 120 | 77.52 | 31.008 |
| 9 | 12.50 | 3 | 65 | 9.08 | 3.632 |
| 10 | 20 | 3 | 10 | 3.16 | 0.79 |
| 11 | 12.50 | 7 | 65 | 84.68 | 33.872 |
| 12 | 20 | 7 | 65 | 87.14 | 21.785 |
| 13 | 12.50 | 11 | 65 | 96.84 | 38.736 |
| 14 | 5 | 3 | 10 | 6.54 | 6.54 |
| 15 | 5 | 3 | 120 | 14.54 | 14.54 |
| 16 | 20 | 11 | 10 | 90.58 | 22.645 |
| 17 | 5 | 11 | 120 | 97.52 | 97.52 |
| Materials | BET Surface Area (m2/g) | Pore-Volume (m3/g) | Pore Size (nm) | References |
|---|---|---|---|---|
| Hybrid CNTs | 71.24 | 0.230 | 12 | This work |
| Synthesized CNTs | 57.35 | 0.01 | 10 | [45] |
| CNT/ZnCo2O4 | 67.60 | 1.103 | - | [46] |
| CNMH | 164.60 | 0.29 | 9 | [47] |
| CNT/TiO2 | 51.44 | 0.67 | 9.33 | [48] |
| Sample | pH | Zeta Potential (MV) |
|---|---|---|
| 1 | 3 | 0.111 |
| 2 | 5 | −5.255 |
| 3 | 7 | −7.602 |
| 4 | 9 | −10.558 |
| 5 | 11 | −23 |
| Source | Sum of Squares | df | Mean Square | F Value | P−Value Prob > F |
|---|---|---|---|---|---|
| Model | 0.27 | 9 | 0.030 | 3.99 | 0.0409 |
| A-Dose | 8.642 × 10−3 | 1 | 8.642 × 10−3 | 1.14 | 0.3209 |
| B-pH | 0.074 | 1 | 0.074 | 9.81 | 0.0165 |
| C-Contact Time | 9.866 × 10−3 | 1 | 9.866 × 10−3 | 1.30 | 0.2913 |
| AB | 8.801 × 10−3 | 1 | 8.801 × 10−3 | 1.16 | 0.3168 |
| AC | 4.389 × 10−4 | 1 | 4.389 × 10−4 | 0.058 | 0.8167 |
| BC | 0.092 | 1 | 0.092 | 12.16 | 0.0102 |
| A2 | 2.525 × 10−4 | 1 | 2.525 × 10−4 | 0.033 | 0.8603 |
| B2 | 0.040 | 1 | 0.040 | 5.32 | 0.0544 |
| C2 | 6.937 × 10−4 | 1 | 6.937E × 10−4 | 0.092 | 0.7710 |
| Source | Sum of Squares | df | Mean Square | F Value | P − Value Prob > F |
|---|---|---|---|---|---|
| Model | 15582.14 | 9 | 1731.35 | 14.14 | 0.0010 |
| A-Dose | 5774.65 | 1 | 5774.65 | 47.17 | 0.0002 |
| B-pH | 4186.28 | 1 | 4186.28 | 34.19 | 0.0006 |
| C-Contact Time | 10.58 | 1 | 10.58 | 0.086 | 0.7774 |
| AB | 3638.90 | 1 | 3638.90 | 29.72 | 0.0010 |
| AC | 11 | 1 | 11 | 0.090 | 0.7731 |
| BC | 292.46 | 1 | 292.46 | 2.39 | 0.1661 |
| A2 | 1181.14 | 1 | 1181.14 | 9.65 | 0.0172 |
| B2 | 901.39 | 1 | 901.39 | 7.36 | 0.0301 |
| C2 | 111.47 | 1 | 111.47 | 0.91 | 0.3718 |
| Pseudo-First-Order ln(qc − qt) vs time (t) | Pseudo-Second-Order (t/qc vs t) | Intraparticle (qc vs t0.5) | |||
|---|---|---|---|---|---|
| Dose mg | pH | C0 mg/L | R2 | R2 | R2 |
| 13.5 | 7 | 3 | 0.9091 | 0.9583 | 0.8769 |
| 13.5 | 7 | 5 | 0.7084 | 0.999 | 0.522 |
| 13.5 | 7 | 10 | 0.8275 | 0.8736 | 0.874 |
| Absorbent | Pollutant | Capacity (mg/g) | References |
|---|---|---|---|
| Hybrid CNTs | Al3+ | 347.88 | This study |
| MWCNT | RhB | 568.181 | [60] |
| mesoporous composite γ-Fe2O3/α-Fe2O3/CA | RhB | 165 | [61] |
| Pn,n-CNTs | MO | 263.14 | [62] |
| magnetic-modified multi-walled carbon nanotubes | MB | 48.1 | [63] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basheer, A.O.; Hanafiah, M.M.; Alsaadi, M.A.; Wan Yaacob, W.Z.; Al-Douri, Y. Synthesis, Characterization, and Analysis of Hybrid Carbon Nanotubes by Chemical Vapor Deposition: Application for Aluminum Removal. Polymers 2020, 12, 1305. https://doi.org/10.3390/polym12061305
Basheer AO, Hanafiah MM, Alsaadi MA, Wan Yaacob WZ, Al-Douri Y. Synthesis, Characterization, and Analysis of Hybrid Carbon Nanotubes by Chemical Vapor Deposition: Application for Aluminum Removal. Polymers. 2020; 12(6):1305. https://doi.org/10.3390/polym12061305
Chicago/Turabian StyleBasheer, Alfarooq O., Marlia M. Hanafiah, Mohammed Abdulhakim Alsaadi, Wan Zuhairi Wan Yaacob, and Y. Al-Douri. 2020. "Synthesis, Characterization, and Analysis of Hybrid Carbon Nanotubes by Chemical Vapor Deposition: Application for Aluminum Removal" Polymers 12, no. 6: 1305. https://doi.org/10.3390/polym12061305
APA StyleBasheer, A. O., Hanafiah, M. M., Alsaadi, M. A., Wan Yaacob, W. Z., & Al-Douri, Y. (2020). Synthesis, Characterization, and Analysis of Hybrid Carbon Nanotubes by Chemical Vapor Deposition: Application for Aluminum Removal. Polymers, 12(6), 1305. https://doi.org/10.3390/polym12061305








