Development and Evaluation of a Polyvinylalcohol -Cellulose Derivative-Based Film with Povidone-Iodine Predicted for Wound Treatment
Abstract
1. Introduction
2. Materials and Methods
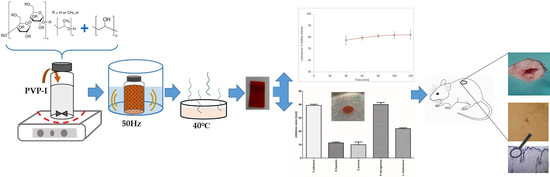
2.1. Film Preparation
2.2. Characterization of the Dried Films
2.2.1. Surface Morphology Observation
2.2.2. Mechanical Property Testing
2.2.3. Disintegrating Time
2.2.4. pH Film Studies
2.3. Fourier Transform Infrared (FTIR) Spectroscopy
2.4. In Vitro Iodine Release Studies
2.4.1. Determination of the Iodine Content in Povidone-Iodine
2.4.2. In Vitro Iodine Release Studies
2.5. Estimation of the Antimicrobial Activity of the Selected Polyvinyl Alcohol-Hydroxypropyl Methylcellulose Film (PVA/HPMC_B) with Incorporated PVP-I Using a Modified Disc Diffusion Method
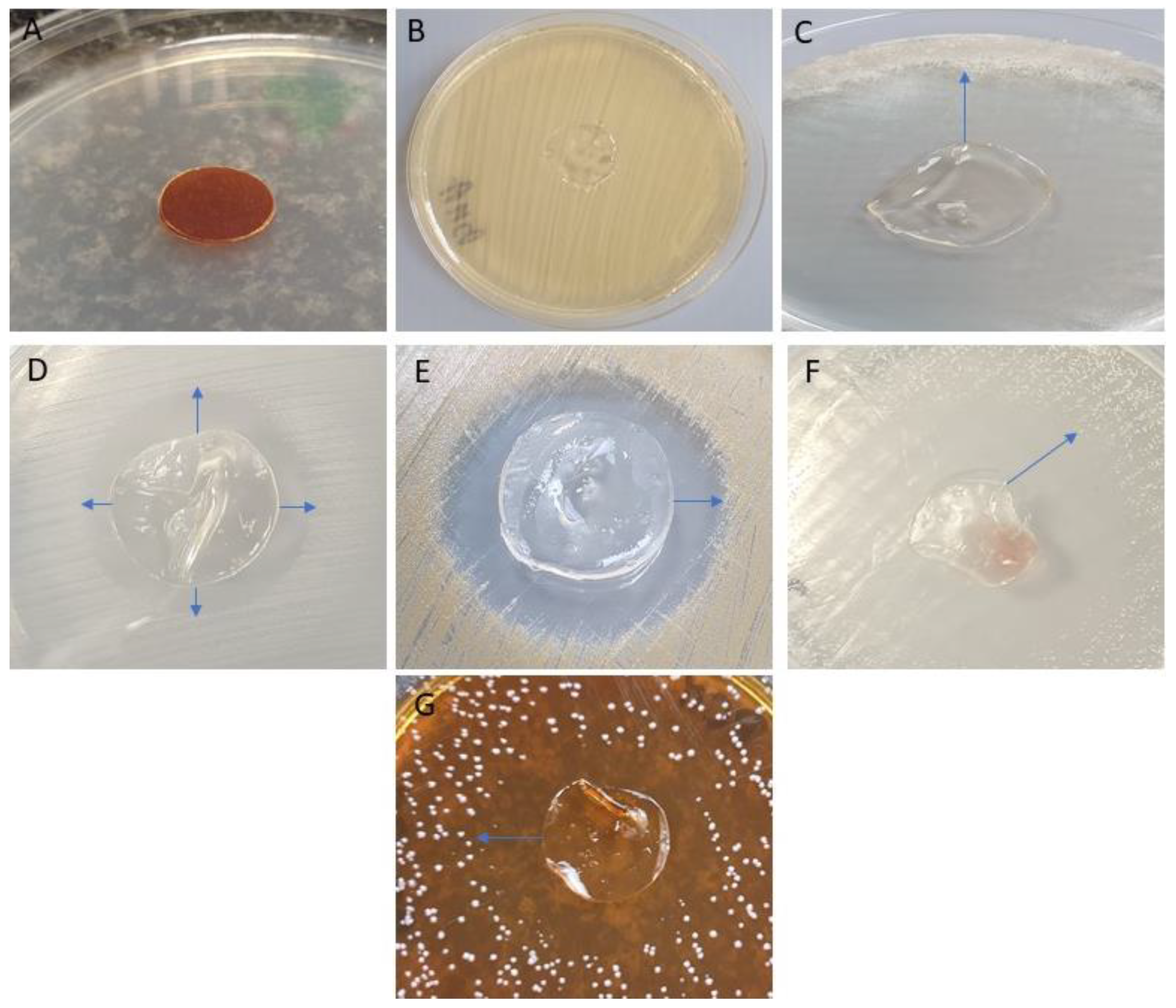
2.6. Assessment of Polymer Material after Subcutaneous Implantation in Rats
2.6.1. Animals and Experimental Design
2.6.2. Histopathological Analysis
2.6.3. Blood Biochemical Analysis
3. Results
3.1. Physicochemical Characteristics of the Polymer Film
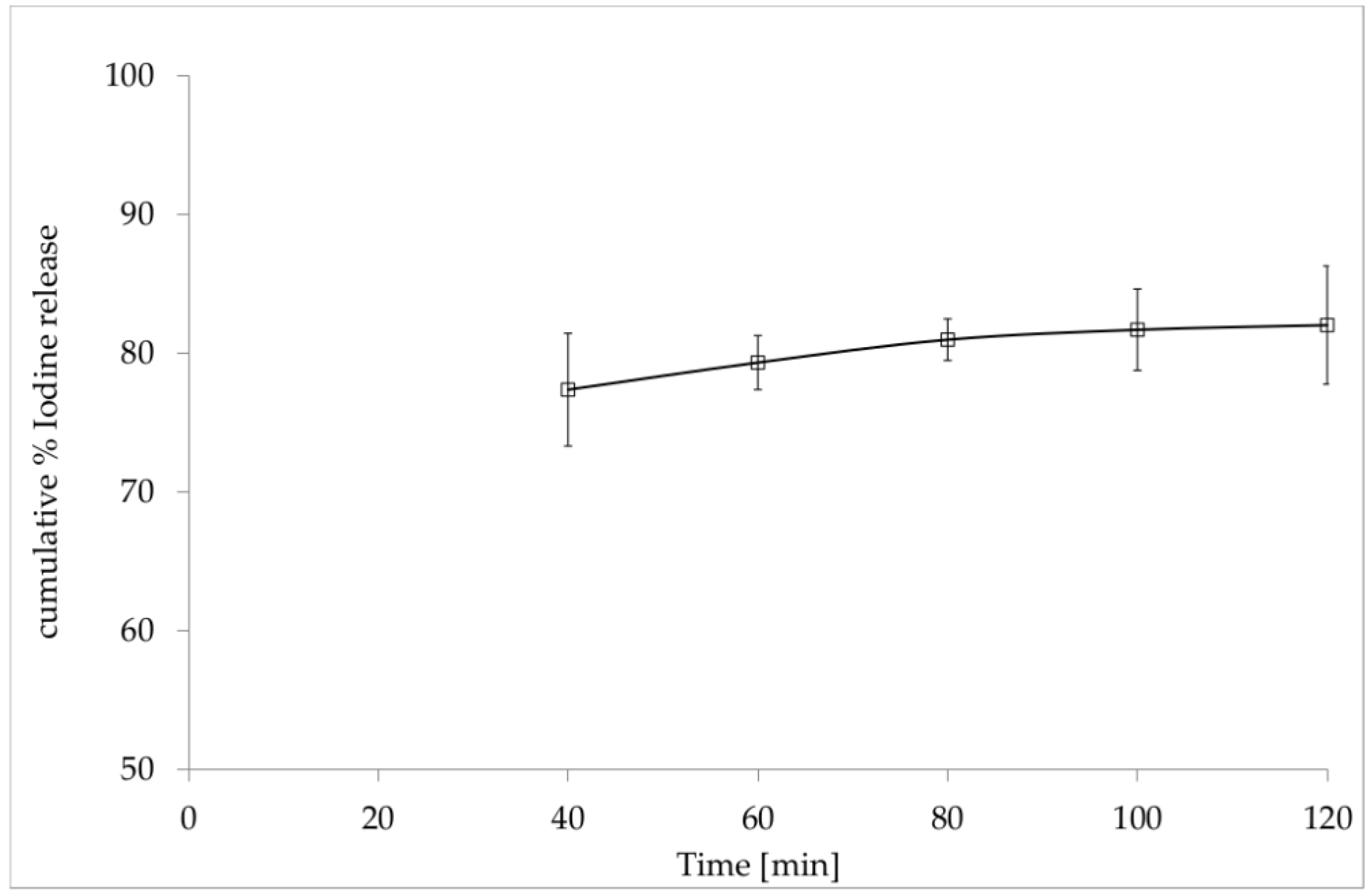
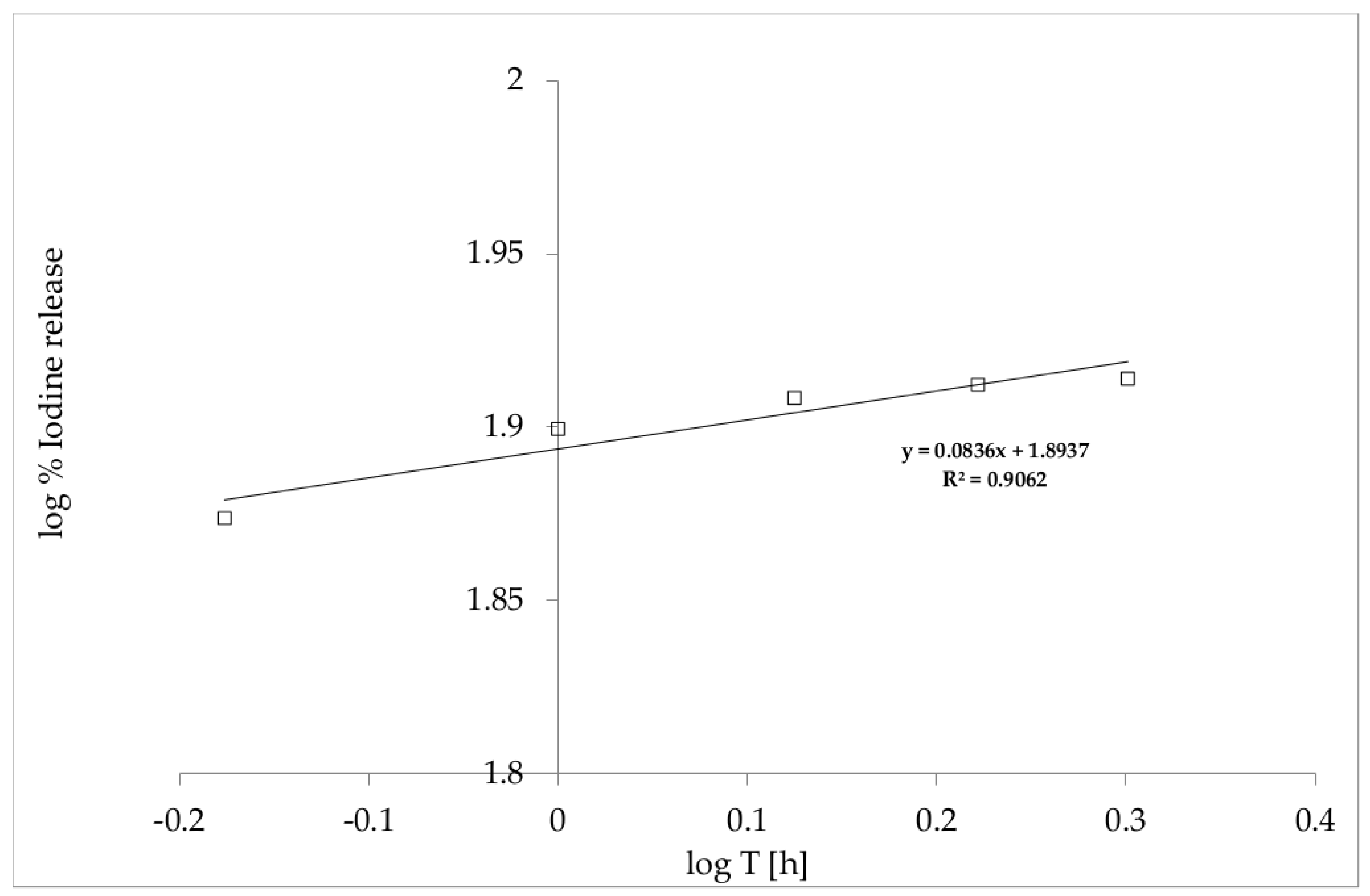
3.2. In Vitro Release of Iodine from the Film with Incorporated PVP-I
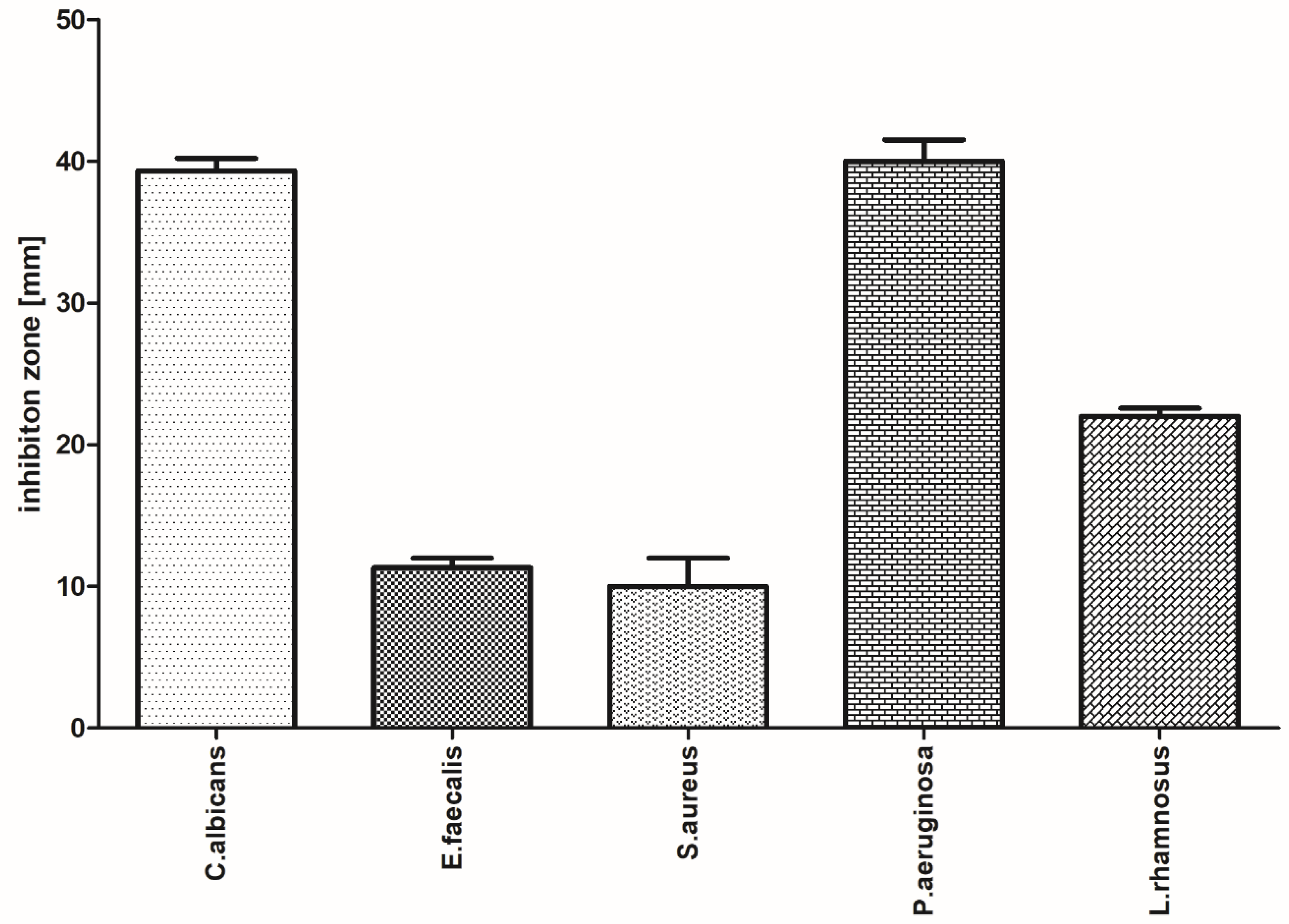
3.3. Evaluation of the Antimicrobial Activity of the Selected Film with Incorporated PVP-I Using a Modified Disc Diffusion Method
3.4. Assessment of Polymer Material after Subcutaneous Implantation in Rats
3.4.1. Microscopic Examination of Rat Skin Sections
3.4.2. Blood Biochemical and Morphological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sawicki, W.; Malejczyk, J. Histology; PZWL: Warsaw, Poland, 2014. [Google Scholar]
- Young, B.; Lowe, J.S.; Stevens, A.; Heath, J.W. Wheater Histologia Podręcznik I Atlas; Elsevier Urban&Partner: Wroclaw, Poland, 2013. [Google Scholar]
- Bryant, R.; Nix, D. Acute and Chronic Wounds; Elsevier Health Sciences: Amsterdam, Holland, 2006. [Google Scholar]
- Mir, M.; Najabat, M.; Barakullah, A.; Gulzar, A.; Arshad, M.; Maliha Asad, S.F. Synthetic polymeric biomaterials for wound healing: A review. Prog. Biomater. 2018, 7, 1–21. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Hunt, T.K.; Hopf, H.; Hussain, Z. Physiology of wound healing. Adv. Skin Wound Care 2000, 13, 6–11. [Google Scholar] [CrossRef]
- Kang, B.S.; Na, Y.C.; Jin, Y.W. Comparison of the Wound Healing Effect of Cellulose and Gelatin: An In Vivo Study. Arch. Plast. Surg. 2012, 39, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, S.; Morimoto, N.; Kimura, Y.; Taira, T.; Kitagawa, T.; Tomihata, K.; Tabata, Y.; Suzuki, S. Preparation of collagen/gelatin sponge scaffold for sustained release of bFGF. Tissue Eng. Part A 2008, 10, 1629–1638. [Google Scholar] [CrossRef]
- Jinno, C.; Morimoto, N.R.; Sakamoto, M.; Ogino, S.; Taira, T.; Suzuki, S.A. Comparison of Conventional Collagen Sponge and Collagen-Gelatin Sponge in Wound Healing. Biomed. Res. Int. 2016, 2016, 4567146. [Google Scholar] [CrossRef]
- Thomas, S. Wound Management and Dressings; Pharmaceutical Press: London, UK, 1990. [Google Scholar]
- Rheinecker, S.B. Wound management: The occlusive dressing. J. Athl. Train. 1995, 30, 143–146. [Google Scholar]
- Dyson, M.; Young, S.; Pendle, C.L.; Webster, D.F.; Lang, S.M. Comparison of the effects of moist and dry conditions on dermal repair. J. Investig. Dermatol. 1988, 91, 434–439. [Google Scholar] [CrossRef]
- Atiyeh, B.S.; El Musa, K.A.; Dham, R. Scar quality and physiologic barrier function restoration after moist and moist exposed dressings of partial-thickness wounds. Dermatol. Surg. 2003, 29, 14–20. [Google Scholar]
- Atiyeh, B.S.; Amm, C.A.; El Musa, K.A. Improved scar quality following primary and secondary healing of cutaneous wounds. Aesthet. Plast. Surg. 2003, 27, 411–417. [Google Scholar] [CrossRef]
- Flanagan, M.; Marks-Maran, D.J. Wound Management; Churchill Livingstone: London, UK, 1997. [Google Scholar]
- Bing, J. Experimental observations on the use of a danish gelatine sponge preparation (>>spongostan<<) as an absorbable haemostatic agent. Acta Pharmacol. Toxicol. 1947, 3, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.K.; Krogsgaard, J.; Nielsen, K.M.; Nørgaard, E.B. A comparison between 2 absorbable haemostatic agents: Gelatin sponge (Spongostan) and oxidized regenerated cellulose (Surgicel). Int. J. Oral Surg. 1984, 13, 406–410. [Google Scholar] [CrossRef]
- Cegielski, M.; Izykowska, I.; Podhorska-Okolow, M.; Zabel, M.; Dziegiel, P. Development of foreign body giant cells in response to implantation of Spongostan as a scaffold for cartilage tissue engineering. In Vivo 2008, 22, 203–206. [Google Scholar] [PubMed]
- Gottardi, W. The uptake and release of molecular iodine by the skin: Chemical and bactericidal evidence of residual effects caused by povidone-iodine preparations. J. Hosp. Infect. 1995, 2, 9–18. [Google Scholar] [CrossRef]
- Erdogan, M.F.; Tatar, F.A.; Ünlütürk, U.; Cin, N.; Yysal, A.R. The effect of scrubbing hands with iodine-containing solutions on urinary iodine concentrations of the operating room staff. Thyroid 2013, 23, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Slots, J.; Jorgensen, M.G. Effective, safe, practical and affordable periodontal antimicrobial therapy: Where are we going, and are we there yet? Periodontology 2000 2002, 28, 298–312. [Google Scholar] [CrossRef]
- Heasman, P.A.; Heasman, L.; Stacey, F.; Mccracken, G.I. Local delivery of chlorhexidine gluconate (Perio-Chip®) in periodontal maintenance patients. J. Clin. Periodontol. 2001, 28, 90–95. [Google Scholar] [CrossRef]
- Hoang, T.; Jorgensen, M.G.; Keim, R.G.; Pattison, A.M.; Slots, J. Povidone-idione as a periodontal pocket di-sinfectant. J. Periodont. Res. 2003, 38, 311–318. [Google Scholar] [CrossRef]
- Walker, C.B.; Karpinia, K.; Baehni, P. Chemoterapeutics: Antibiotics and other antimicrobials. Periodontology 2000 2004, 36, 146–165. [Google Scholar] [CrossRef]
- Nair, S.C.; Anoop, K.R. Intraperiodontal pocket: An ideal route for local antimicrobial drug delivery. J. Adv. Pharm. Technol. Res. 2012, 3, 9–15. [Google Scholar]
- Preis, M.; Woertz, C.; Kleinebudde, P.; Breitkreutz, J. Oromucosal film preparations: Classification and characterization methods. Expert Opin. Drug Deliv. 2013, 10, 1303–1317. [Google Scholar] [CrossRef] [PubMed]
- Office for Registration of Medicinal Products, Medical Devices and Biocidal Products. Polish Pharmacopeia; Office for Registration of Medicinal Products, Medical Devices and Biocidal Products: Warsaw, Poland, 2014.
- Mazumdar, N.; Chikindas, M.L.; Uhrich, K. Slow Release Polymer-Iodine Tablets for Disinfection of Untreated Surface Water. J. Appl. Polym. Sci. 2010, 117, 329–334. [Google Scholar] [CrossRef]
- Gouda, R.; Baishya, H.; Qing, Z. Application of mathematical models in drug release kinetics of Carbidopa and Levodopa ER tablets. J. Dev. Drugs 2017, 6, 171. [Google Scholar]
- Papadopoulou, E.L.; Valentini, P.; Mussino, F. Antibacterial bioelastomers with sustained povidone-iodine releas. Chem. Eng. J. 2018, 347, 19–26. [Google Scholar] [CrossRef]
- Lim, S.J.; Lee, J.H.; Piao, M.G.; Lee, M.K.; Oh, D.H.; Hwang, D.H.; Quan, Q.Z.; Yong, C.S.; Choi, H.G. Effect of sodium carboxymethylcellulose and fucidic acid on the gel characterization of polyvinylalcohol-based wound dressing. Arch. Pharm. Res. 2010, 33, 1073–1081. [Google Scholar] [CrossRef]
- Cazon, P.; Velazquez, G.; Vazquez, M. Characterization of mechanical and barrier properties of bacterial cellulose, glycerol and polyvinyl alcohol (PVOH) composite films with eco-friendly UV-protective properties. Food Hydrocoll. 2020, 99, 105323. [Google Scholar] [CrossRef]
- Sreeja, S.N.; Smijesh, N.A.; Sreedhanya, S.; Reji, P.; Muneera, C.I. Organic dye impregnated poly(vinyl alcohol) nanocomposite as an efficient optical limiter: Structure, morphology and photophysical properties. J. Mater. Chem. C 2013, 1, 3851–3861. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Kamoun, E.A.; Chen, X.; Eldin, M.S.M.; Kenawy, E.R.S. Crosslinked poly (Vinyl alcohol) hydrogels for wound dressing applications: A review of remarkably blended polymers. Arab. J. Chem. 2015, 8, 1–14. [Google Scholar] [CrossRef]
- Mishra, A.; Chaudhary, N. Study of povidone iodine loaded hydrogels as wound dressing material. Trends Biomater. Artif. Organs 2010, 23, 122–128. [Google Scholar]
- Shitole, A.A.; Raut, P.W.; Khandwekar, A.; Sharma, N.; Baruach, M. Design and engineering of polyvinyl alcohol based biomimetic hydrogels for wound healing and repair. J. Polym. Res. 2019, 26, 201. [Google Scholar] [CrossRef]
- Neres Santos, A.M.; Duarte Moreira, A.P.; Piler Carvalho, C.W.; Ribeiro, E.; McGuinness, G.B.; Fernandes Mendes, M.; Nunes Oliveira, R. Physically Cross-Linked Gels of PVA with Natural Polymers as Matrices for Manuka Honey Release in Wound-Care Applications. Materials 2019, 12, 559. [Google Scholar] [CrossRef]
- Shyna, S.; Shantikrishna, A.; Prabha, D.; Nair, P.D.; Thomas, L.V. A nonadherent chitosan-polyvinyl alcohol absorbent wound dressing prepared via controlled freeze-dry technology. Int. J. Biol. Macromol. 2020, 150, 129–140. [Google Scholar]
- Lee, J.H.; Lim, S.; Oh, D.H.; Ku, S.K.; Li, D.X.; Yong, C.S.; Choi, H.G. Wound healing evaluation of sodium fucidate-loaded polyvinylalcohol/sodium carboxymethylcellulose-based wound dressing. Arch. Pharm. Res. 2010, 33, 1083–1089. [Google Scholar] [CrossRef]
- Oliveira, R.N.; Moreira, A.P.D.; da Silva Moreira Thiré, R.M.; Quilty, B.; Passos, T.M.; Simon, P.; Mancuni, M.C.; McGuinness, G.B. Absorbent polyvinyl alcohol–sodium carboxymethyl cellulose hydrogels for propolis delivery in wound healing applications. Polym. Eng. Sci. 2017, 57, 1224–1233. [Google Scholar] [CrossRef]
- Jamal Mohamed, A.; Perinbam, K.; Vahitha, V.; Devanesan, S.; Janakiraman, K.K. Povidone iodine loaded film-forming topical gel and evaluationon its chemical stability. Int. J. Res. Pharm. Sci. 2019, 11, 148–153. [Google Scholar]
- Chen, S.; Chen, J.W.; Guo, B.; Xu, C.C. Preoperative Antisepsis with Chlorhexidine Versus Povidone-Iodine for the Prevention of Surgical Site Infection: A Systematic Review and Meta-analysis. World J. Surg. 2020, 29, 1–13. [Google Scholar] [CrossRef]
- Ciulla, T.A.; Starr, M.B.; Masket, S. Bacterial endophthalmitis prophylaxis for cataract surgery: An evidence-based update. Ophthalmology 2002, 109, 13–24. [Google Scholar] [CrossRef]
- Gottardi, W. Iodine and iodine compounds. In Disinfection, Sterilization and Preservation; Block, S.S., Ed.; Lea and Febiger: Philadelphia, PA, USA, 1991; pp. 152–166. [Google Scholar]
- Pinna, A.; Donadu, M.G.; Usai, D.; Dore, S.; D’Amico-Ricci, G.; Boscia, F.; Zanetti, S. In vitro antimicrobial activity of a new ophthalmic solution containing povidone-iodine 0.6% (IODIM). Acta Opthalmol. 2020, 98, 178–180. [Google Scholar] [CrossRef]
- Kaewiad, K.; Nakpheng, T.; Srichana, T. Dental floss impregnated with povidone-iodine coated with Eudragit L-100 as an antimicrobial delivery system against periodontal-associated pathogens. J. Med. Microbiol. 2020, 69, 298–308. [Google Scholar] [CrossRef]
- Prsehaw, P.M.; Seymour, R.A.; Haesman, P.A. Current concepts in periodontal pathogenesis. Dent. Update 2004, 31, 570–572. [Google Scholar] [CrossRef]
- Colombo, A.P.; Magalhaes, C.B.; Hartenbach, F.A.; do Souto, R.M.; da Silva-Boghossian, C.M. Periodontal-disease-associated Biofilm: A Reservoir for Pathogens of Medical Importance. Microb. Pathog. 2016, 94, 27–34. [Google Scholar]
- Pinheiro, E.T.; Mayer, M.P.A. Enterococcus faecalis in Oral Infections. J. Interdiscip. Med. Dent. Sci. 2015, 3160. [Google Scholar]
- Ga-Yeon, K.; Chong Heon, L. Antimicrobial susceptibility and pathogenic genes of Staphylococcus aureus isolated from the oral cavity of patients with periodontitis. J. Periodontal Implant Sci. 2015, 45, 223–228. [Google Scholar]
- Nam, S.Y.; Nho, Y.C.; Hong, S.H.; Chae, G.T.; Jang, H.S.; Suh, T.S.; Ahn, W.S.; Ryu, K.E.; Chun, H.J. Evaluations of poly (vinyl alcohol)/alginate hydrogels cross-linked by γ-ray irradiation technique. Macromol. Res. 2004, 12, 219–224. [Google Scholar] [CrossRef]
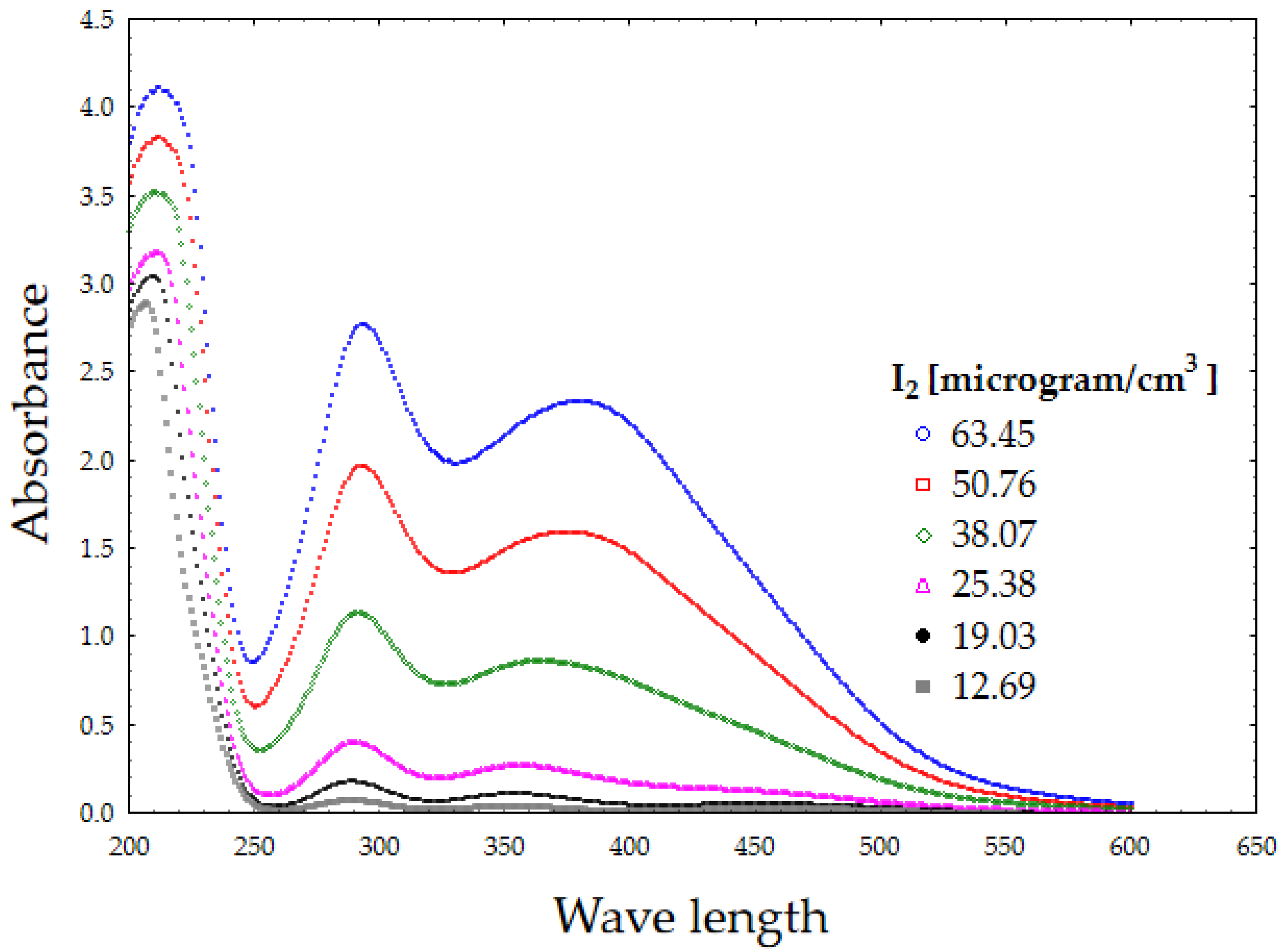
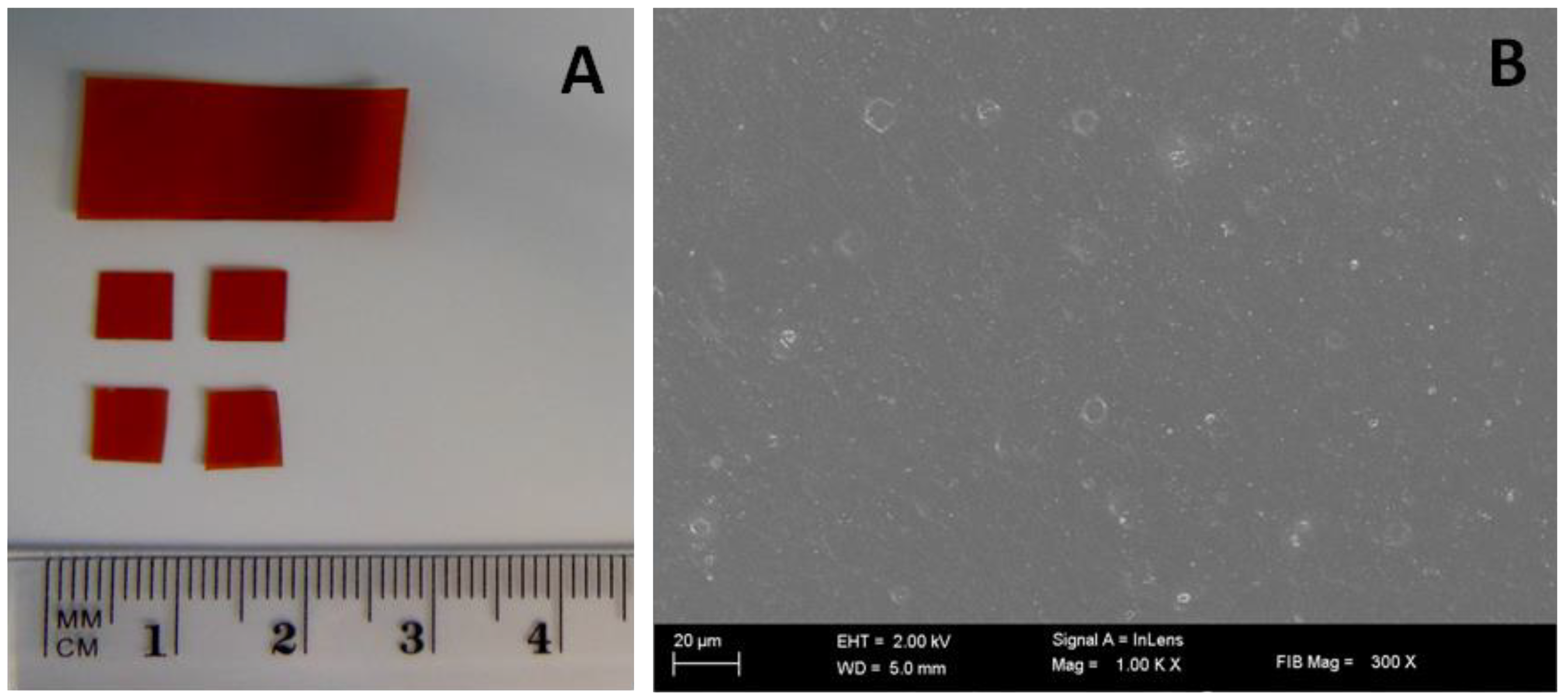
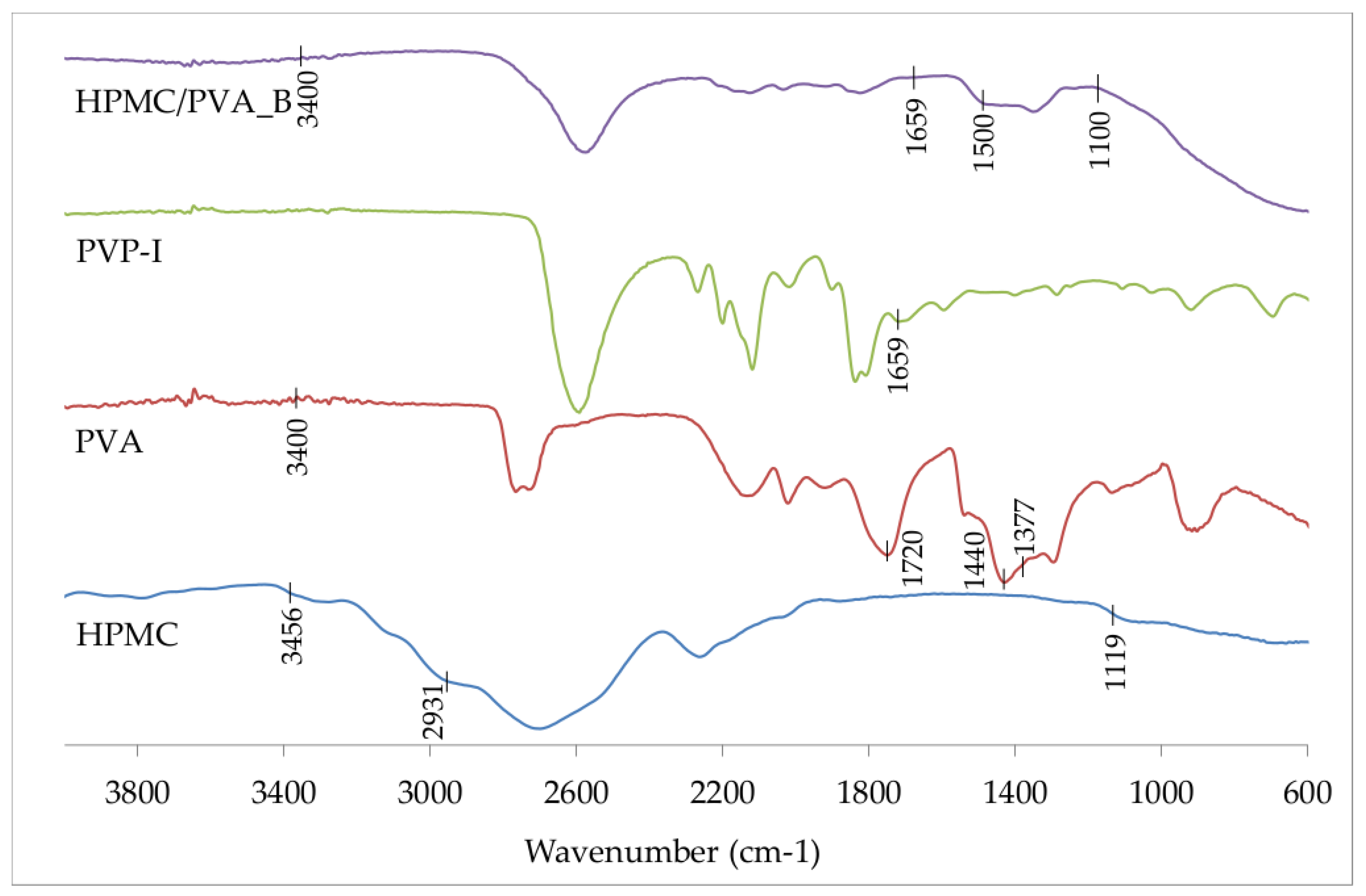



| Batch Code | PVA | CMC | HPMC | GLY | PVP-I |
|---|---|---|---|---|---|
| [mg per Film] | |||||
| PVA/CMC_A | 63.2 | 40.5 | - | 22.8 | 12.0 |
| PVA/HPMC_A | 63.2 | - | 40.5 | 22.8 | 12.5 |
| PVA/CMC_B | 88.5 | 15.2 | - | 22.8 | 12.0 |
| PVA/HPMC_B | 88.5 | - | 15.2 | 22.8 | 12.0 |
| Physical Characteristics | PVA/HPMC_B |
|---|---|
| Thickness (mm) | 0.46 ± 0.003 |
| Mass (g) | 0.1385 ± 0.0043 |
| Disintegration time (min) | 110 ± 8.37 |
| pH | 6.33 ± 0.32 |
| Group | Altp [U/L] | Alt [[U/L] | Crea [mg/dL] | Bun [mg/dL] | Alb [g/dL] | Glc [mg/dL] | CRP [mg/L] |
|---|---|---|---|---|---|---|---|
| Spongostan | 38.2 | 93.0 | 0.596 | 17.20 | 4.96 | 92.6 | 1.45 |
| PVA/HPMC_B/C | 41.4 | 38.8 | 0.590 | 15.68 | 5.01 | 85.7 | 1.69 |
| PVA/HPMC_B/T | 43.0 | 38.4 | 0.614 | 16.08 | 4.93 | 85.6 | 1.59 |
| Group | RBC (106/μL) | WBC (103/μL) | HGB (g/dL) | MPV (%) |
|---|---|---|---|---|
| Spongostan | 8.06 | 10.012 | 15.07 | 40.11 |
| PVA/HPMC_B/C | 8.15 | 10.108 | 15.22 | 40.20 |
| PVA/HPMC_B/T | 8.45 | 10.750 | 14.83 | 40.41 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kida, D.; Gładysz, O.; Szulc, M.; Zborowski, J.; Junka, A.; Janeczek, M.; Lipińska, A.; Skalec, A.; Karolewicz, B. Development and Evaluation of a Polyvinylalcohol -Cellulose Derivative-Based Film with Povidone-Iodine Predicted for Wound Treatment. Polymers 2020, 12, 1271. https://doi.org/10.3390/polym12061271
Kida D, Gładysz O, Szulc M, Zborowski J, Junka A, Janeczek M, Lipińska A, Skalec A, Karolewicz B. Development and Evaluation of a Polyvinylalcohol -Cellulose Derivative-Based Film with Povidone-Iodine Predicted for Wound Treatment. Polymers. 2020; 12(6):1271. https://doi.org/10.3390/polym12061271
Chicago/Turabian StyleKida, Dorota, Olimpia Gładysz, Małgorzata Szulc, Jacek Zborowski, Adam Junka, Maciej Janeczek, Anna Lipińska, Aleksandra Skalec, and Bożena Karolewicz. 2020. "Development and Evaluation of a Polyvinylalcohol -Cellulose Derivative-Based Film with Povidone-Iodine Predicted for Wound Treatment" Polymers 12, no. 6: 1271. https://doi.org/10.3390/polym12061271
APA StyleKida, D., Gładysz, O., Szulc, M., Zborowski, J., Junka, A., Janeczek, M., Lipińska, A., Skalec, A., & Karolewicz, B. (2020). Development and Evaluation of a Polyvinylalcohol -Cellulose Derivative-Based Film with Povidone-Iodine Predicted for Wound Treatment. Polymers, 12(6), 1271. https://doi.org/10.3390/polym12061271