Design of Waterborne Asymmetric Block Copolymers as Thermoresponsive Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
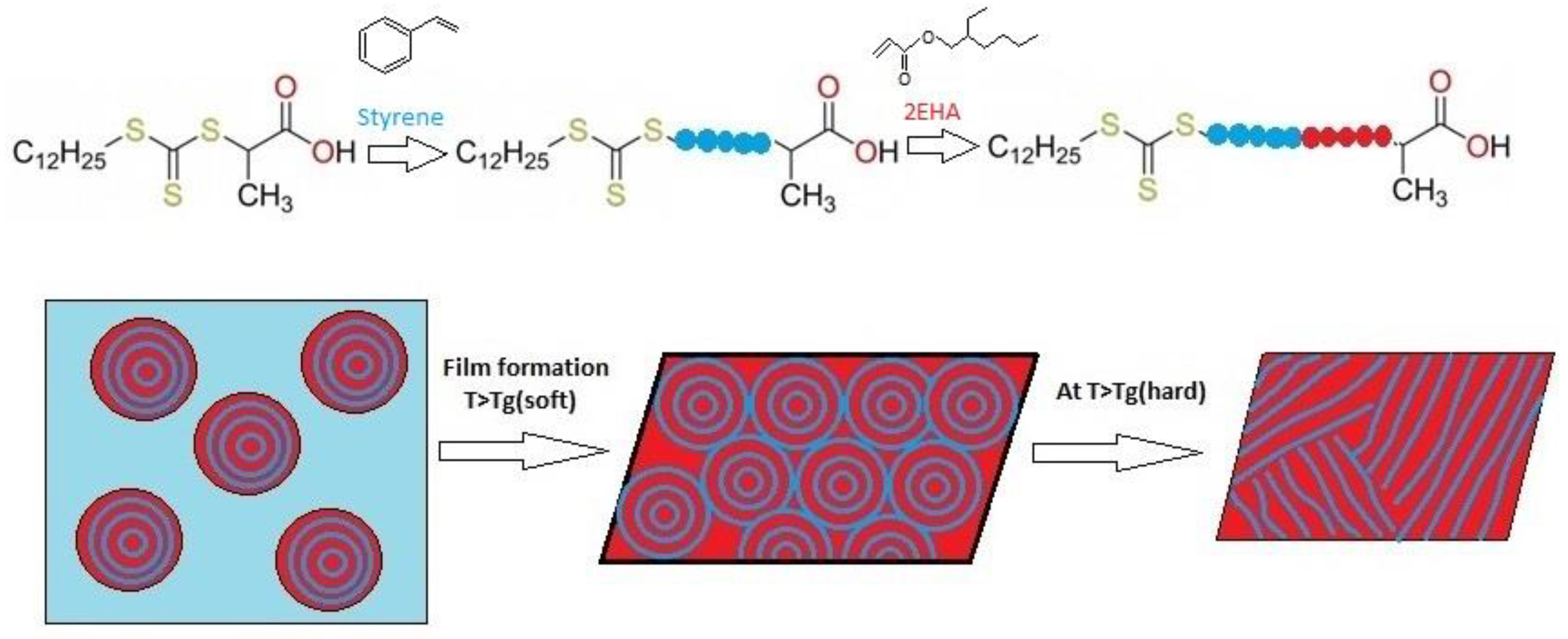
2.2.1. Synthesis of the First A Block: Batch Miniemulsion Polymerization
2.2.2. Synthesis of the Second Block: Semi-Batch Emulsion Polymerization
2.2.3. Characterization
3. Results
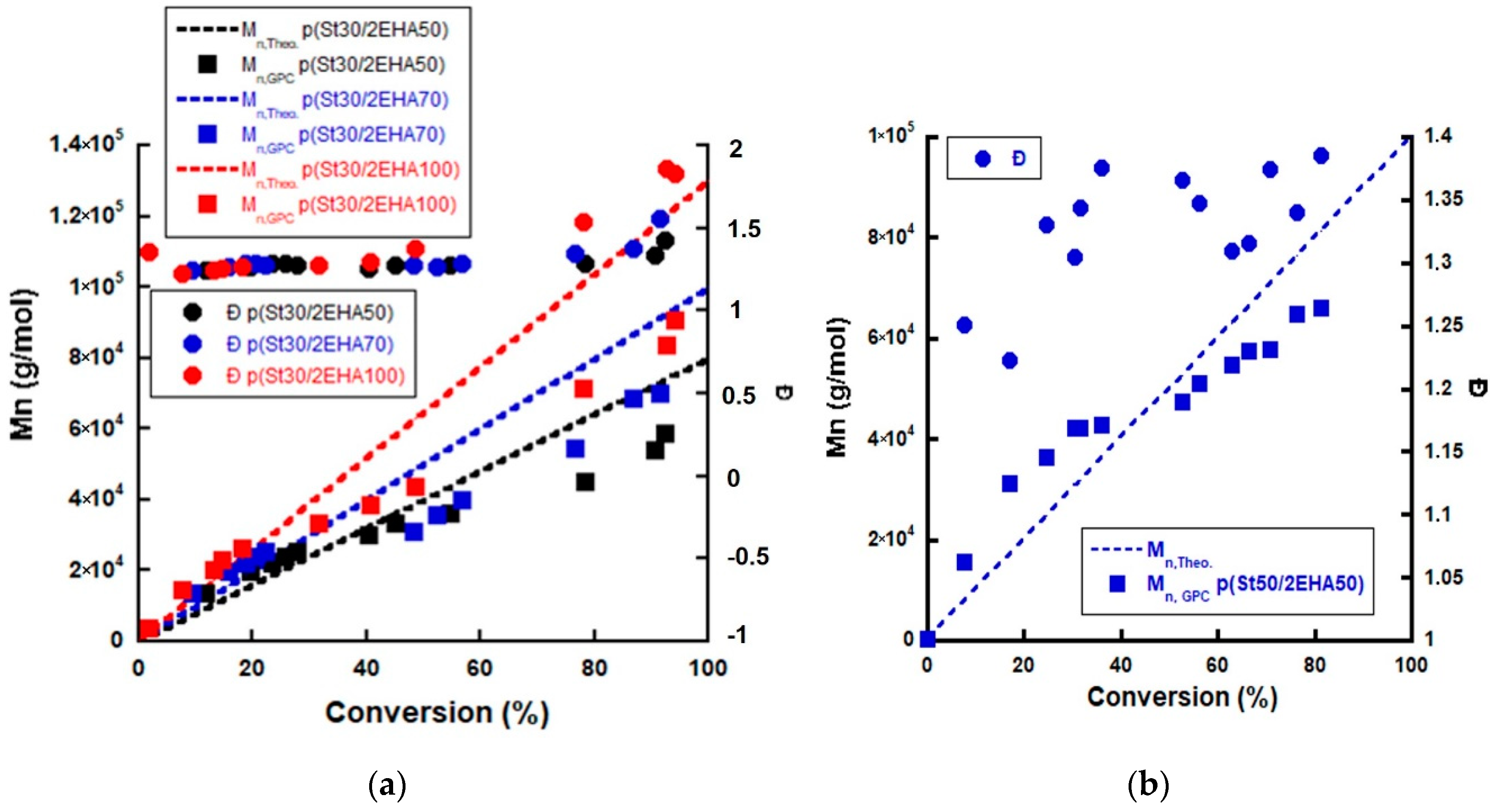
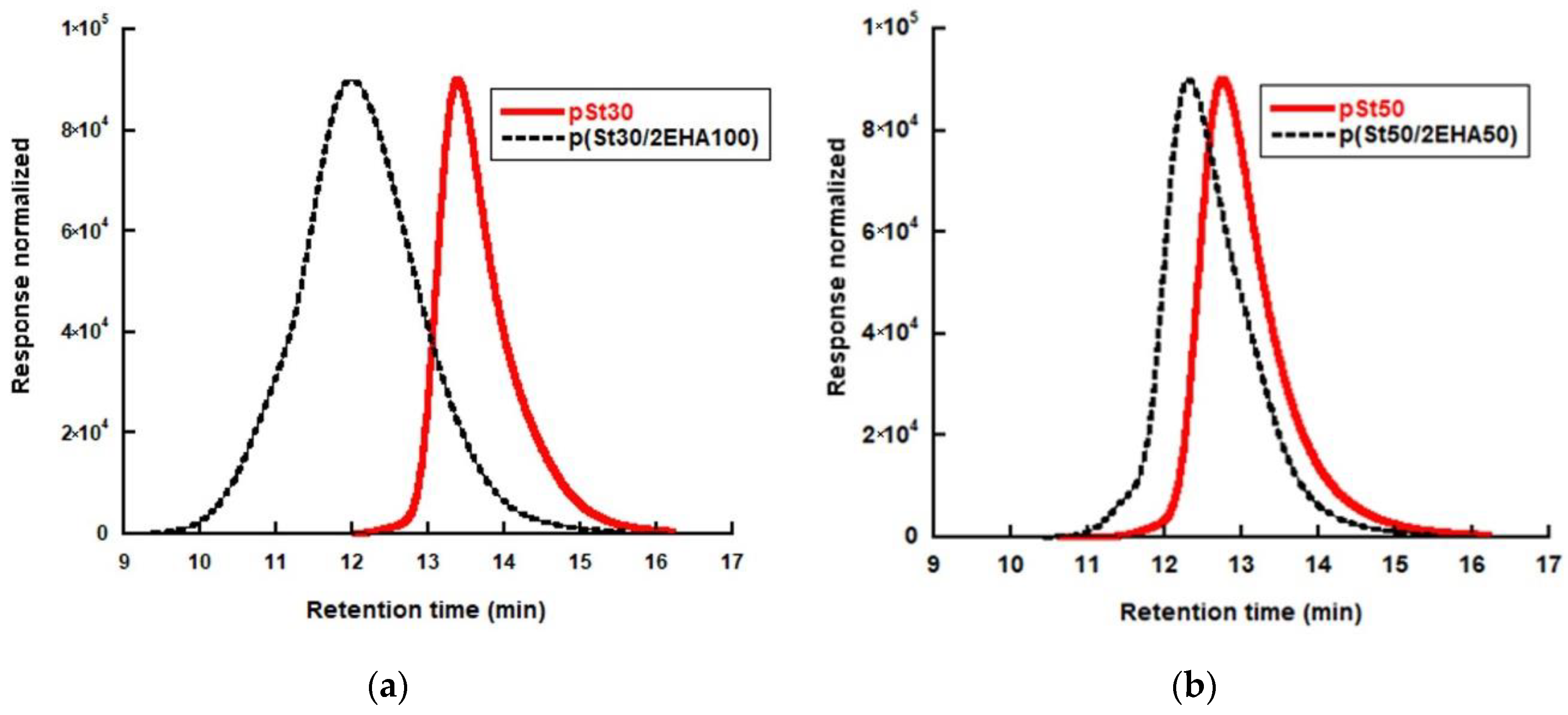
3.1. RAFT Mediated Miniemulsion Polymerization
3.2. AB Di-Block Copolymer Latex: Hard-Soft Domains
3.3. Thermal Properties of the Initial Homopolymers and Final Block Copolymers
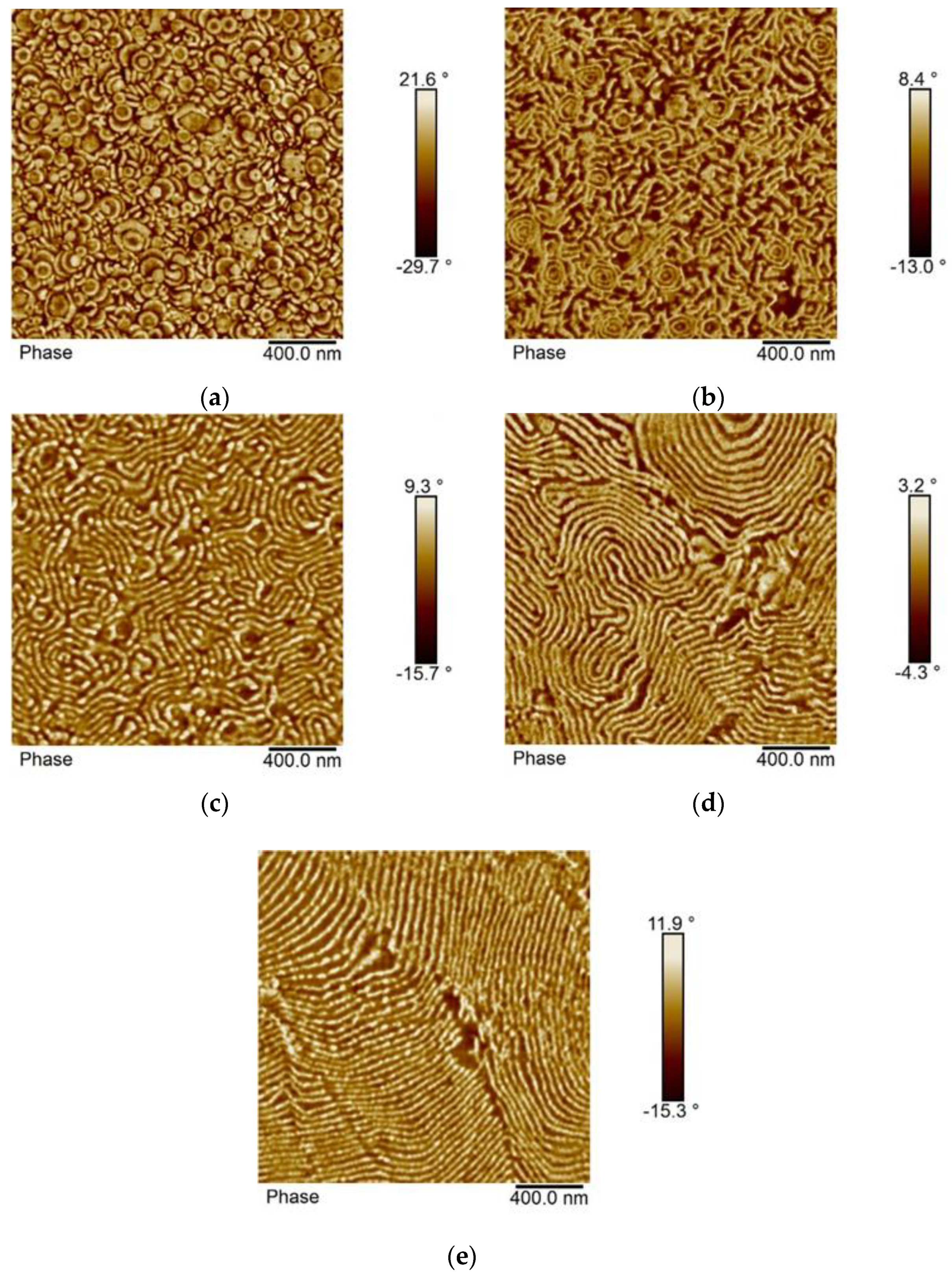
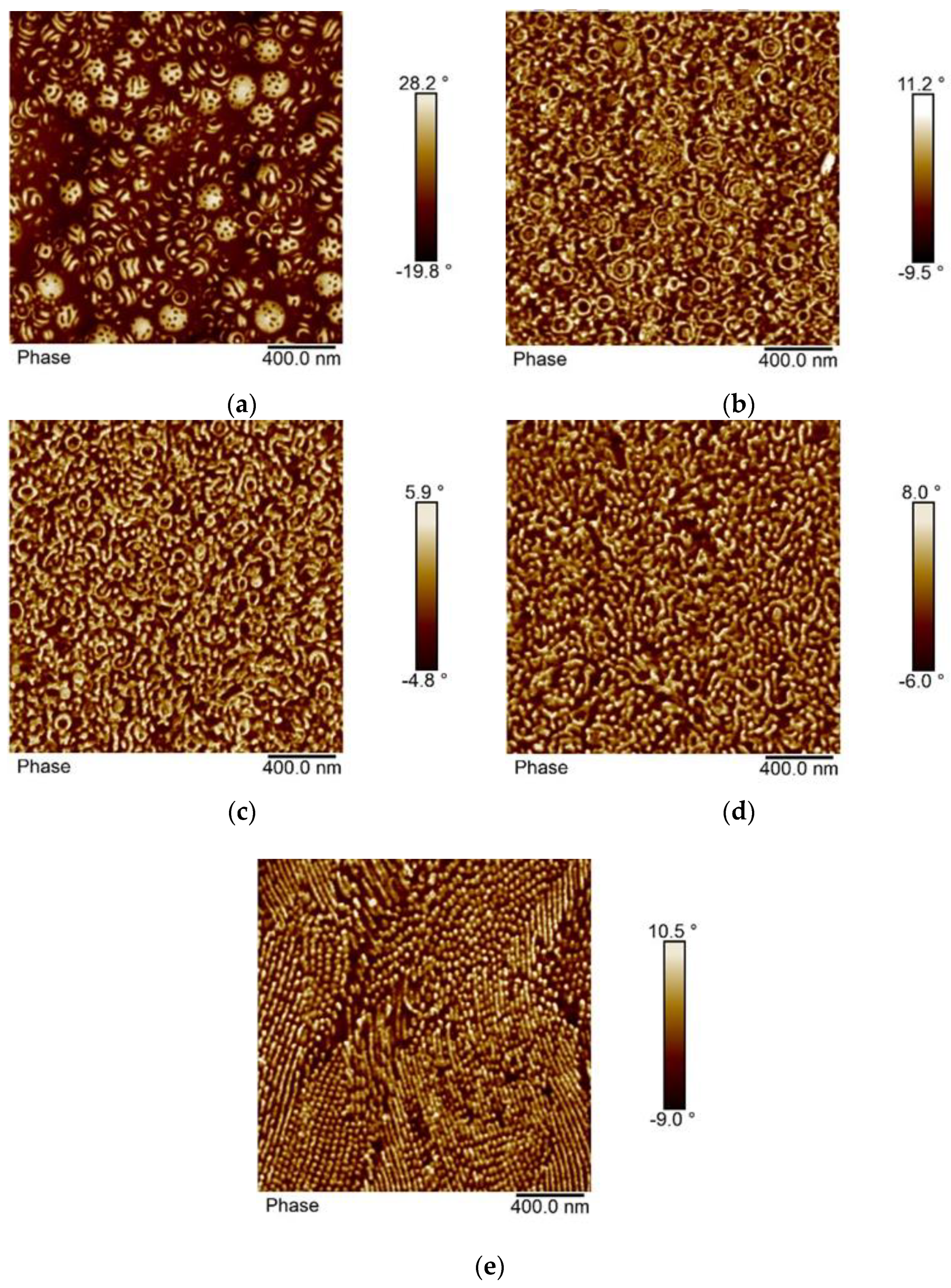
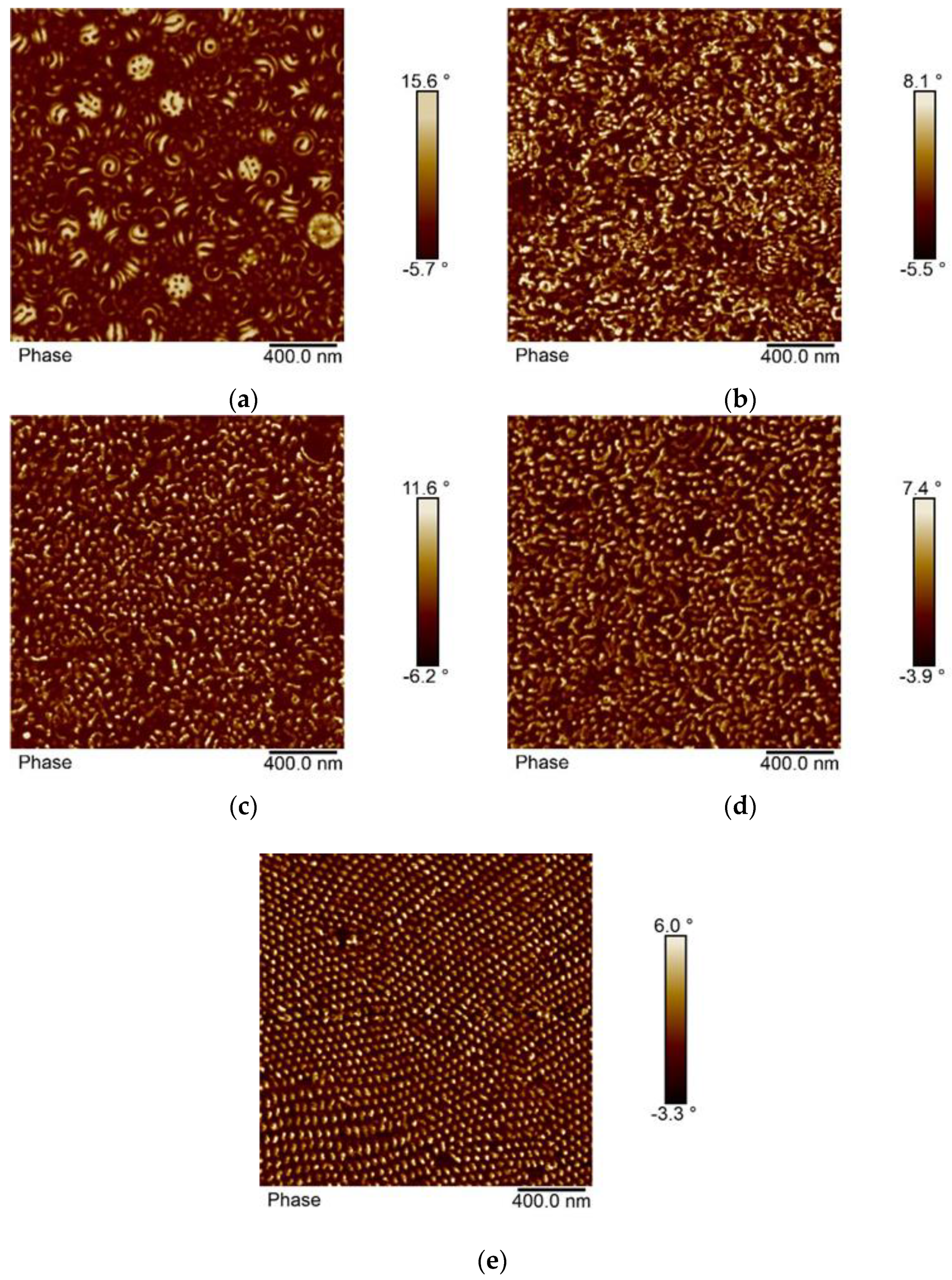
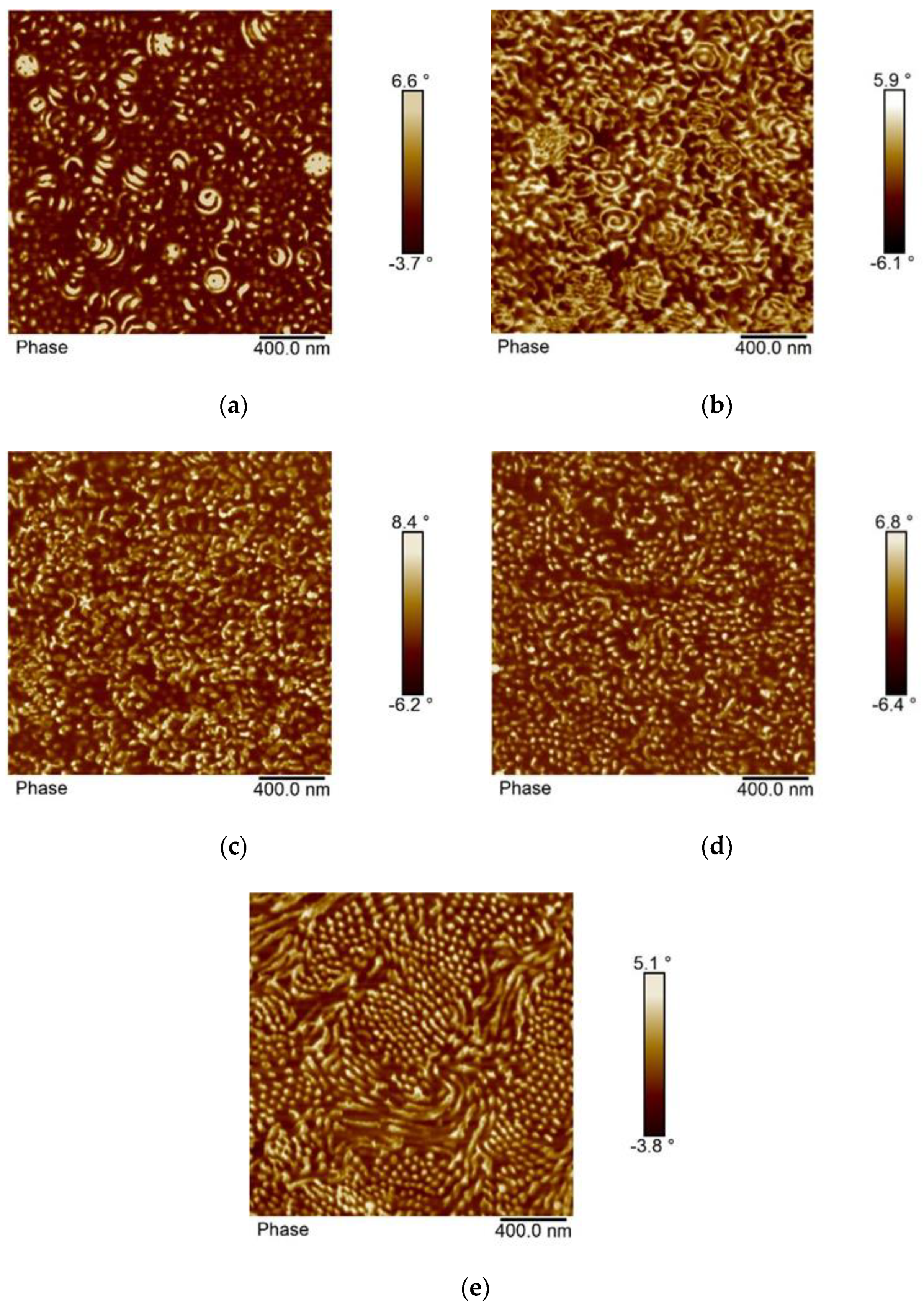
3.4. Morphology of the Block Copolymers
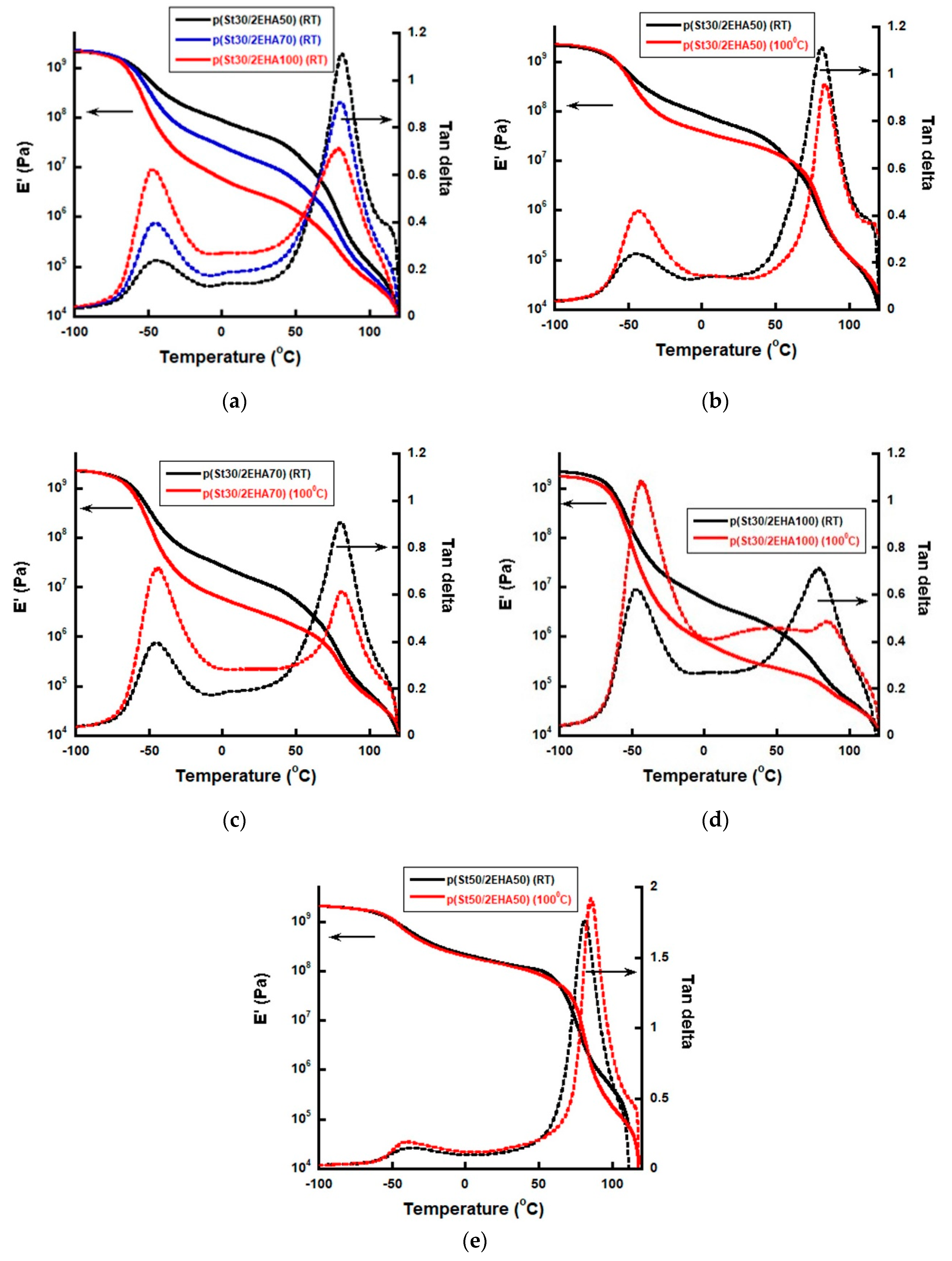
3.5. Viscoelastic Properties of AB Hard-Soft Block Copolymers
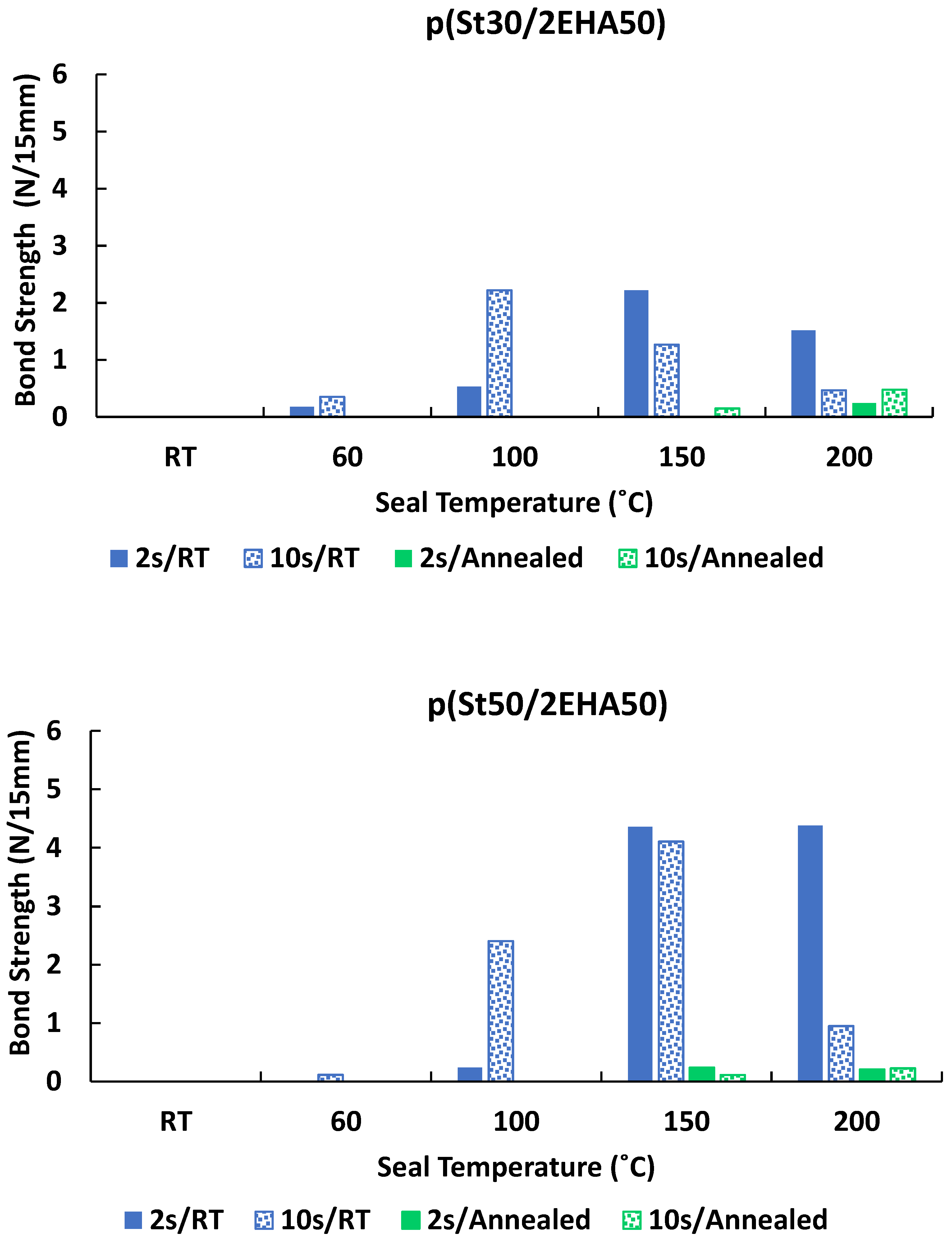
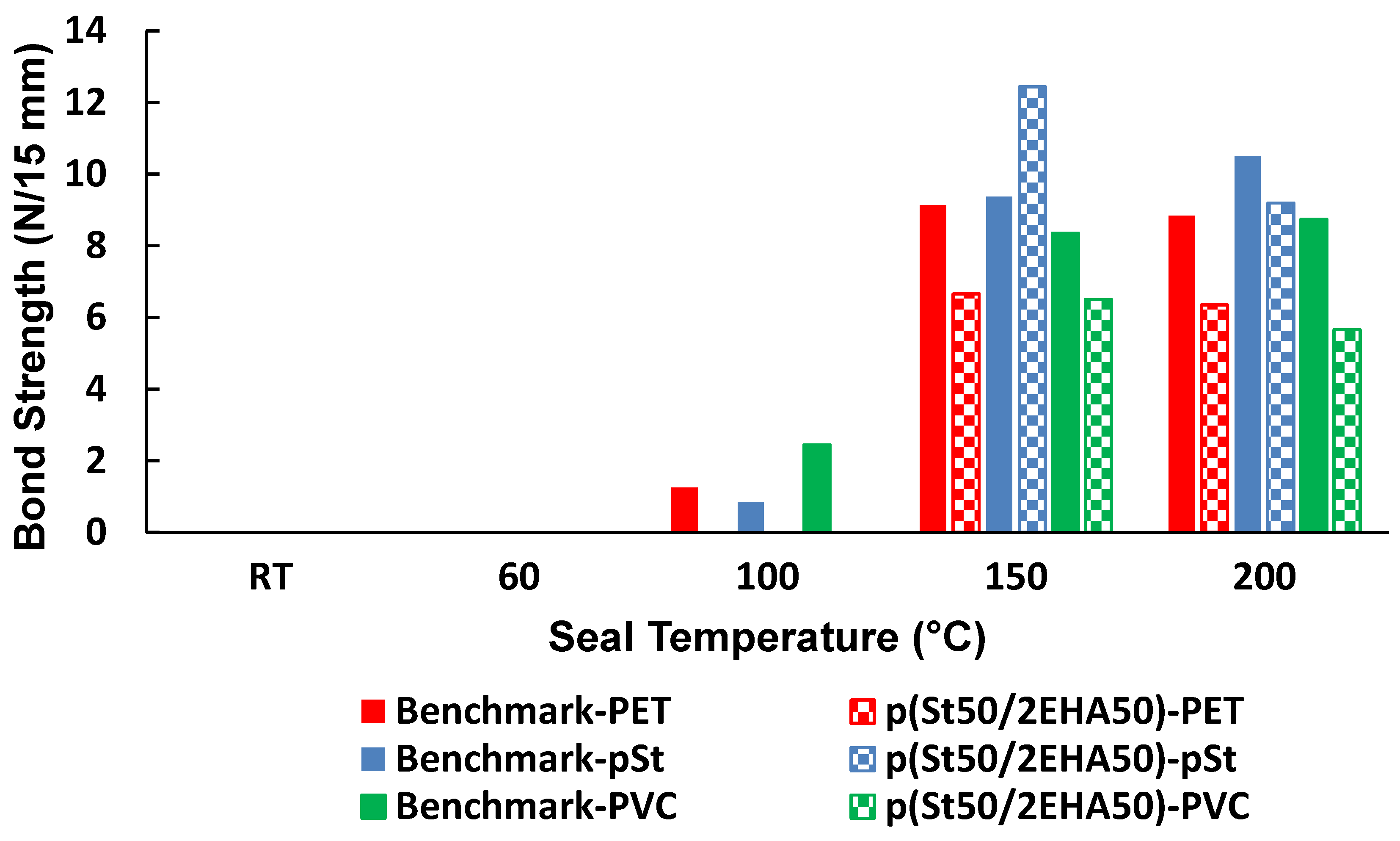
3.6. Heat Sealing Properties of the Block Copolymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nicolas, J.; Guillaneuf, Y.; Lefay, C.; Bertin, D.; Gigmes, D.; Charleux, B. Nitroxide-mediated polymerization: The Pivotal role of the kd value of the initiating alkoxyamine and the importance of the experimental conditions. Prog. Polym. Sci. 2013, 38, 63–235. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Atom Transfer Radical Polymerization (ATRP): Current status and future perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. RAFT polymerization and some of its applications. Chem. Asian J. 2013, 8, 1634–1644. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.R.; Carmean, R.N.; Sumerlin, B.S. Expanding the scope of RAFT polymerization: Recent advances and new horizons. Macromolecules 2015, 48, 5459–5469. [Google Scholar] [CrossRef]
- Chong, B.Y.K.; Le, T.P.T.; Moad, G.; Rizzardo, E.; Thang, S.H. A more versatile route to block copolymers and other polymers of complex architecture by living radical polymerization: The RAFT process. Macromolecules 1999, 32, 2071–2074. [Google Scholar] [CrossRef]
- Jennings, J.; He, G.; Howdle, S.M.; Zetterlund, P.B. Block copolymer synthesis by controlled/living radical polymerization in heterogeneous systems. Chem. Soc. Rev. 2016, 45, 5055–5084. [Google Scholar] [CrossRef]
- McLeary, J.B.; Klumperman, B. RAFT mediated polymerisation in heterogeneous media. Soft Matter 2006, 2, 45–53. [Google Scholar] [CrossRef]
- Chiefari, J.; Chong, Y.K.B.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living free-radical polymerization by reversible addition–fragmentation chain transfer: The RAFT process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Keddie, D.J.; Moad, G.; Rizzardo, E.; Thang, S.H. RAFT agent design and synthesis. Macromolecules 2012, 45, 5321–5342. [Google Scholar] [CrossRef]
- Roshan, T.; Rizzardo, E.; Chiefari, J.; Krstina, J.; Moad, G.; Postma, A.; Thang, S.H. Living polymers by the use of trithiocarbonates as reversible addition–fragmentation chain transfer (RAFT) agents: ABA triblock copolymers by radical polymerization in two steps. Macromolecules 2000, 33, 243–245. [Google Scholar]
- Barner-Kowollik, C.; Quinn, J.F.; Nguyen, T.L.U.; Heuts, J.P.; Davis, T.P. Kinetic investigations of reversible addition fragmentation chain transfer polymerizations: Cumyl phenyldithioacetate mediated homopolymerizations of styrene and methyl methacrylate. Macromolecules 2001, 34, 7849–7857. [Google Scholar] [CrossRef]
- Donovan, M.S.; Sanford, T.A.; Lowe, A.B.; Sumerlin, B.S.; Mitsukami, Y.; Mccormick, C.L. RAFT polymerization of N,N-dimethylacrylamide in water. Macromolecules 2002, 35, 4570–4572. [Google Scholar] [CrossRef]
- Uzulina, I.; Kanagasabapathy, S.; Claverie, J. Reversible addition fragmentation transfer (RAFT) polymerization in emulsion. In Macromolecular Symposia; WILEY-VCH Verlag: Weinheim, Germany, 2000; Volume 150, pp. 33–38. [Google Scholar]
- Monteiro, M.J.; Hodgson, M.; De Brouwer, H.J. The influence of RAFT on the rates and molecular weight distributions of styrene in seeded emulsion polymerizations. Polym. Sci. Part A Polym. Chem. 2000, 38, 3864–3874. [Google Scholar] [CrossRef]
- Asua, J.M. Miniemulsion polymerization. Prog. Polym. Sci. 2002, 27, 1283–1346. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, X.J. Reversible addition–fragmentation transfer (RAFT) copolymerization of methyl methacrylate and styrene in miniemulsion. Polym. Sci. Part A Polym. Chem. 2004, 42, 6248–6258. [Google Scholar] [CrossRef]
- Bowes, A.; Mcleary, J.B.; Sanderson, R.D. AB and ABA type butyl acrylate and styrene block copolymers via raft-mediated miniemulsion polymerization. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 588–604. [Google Scholar] [CrossRef]
- Butte, A.; Storti, G.; Morbidelli, M. Miniemulsion living free radical polymerization of styrene. Macromolecules 2001, 34, 5885–5896. [Google Scholar] [CrossRef]
- Wei, R.; Luo, Y.; Li, Z. Synthesis of structured nanoparticles of styrene/butadiene block copolymers via RAFT seeded emulsion polymerization. Polymer 2010, 51, 3879–3886. [Google Scholar] [CrossRef]
- Froimowicz, P.; Van Heukelum, B.; Scholten, C.; Greiner, K.; Araujo, O.; Landfester, K.J. Highly symmetric poly (styrene)-block-poly (butadiene-stat-styrene)-block-poly (styrene) copolymer prepared in a non-stop one-pot RAFT polymerization in miniemulsion. Polym. Sci. Part A Polym. Chem. 2014, 52, 883–889. [Google Scholar] [CrossRef]
- Yang, L.; Han, Q.; Song, Q.; Li, H.; Zhao, Q.; Shen, Y.; Luo, Y. Control over ABA-type triblock copolymer latex morphology in RAFT miniemulsion polymerization and mechanical properties of the latex films. Colloid Polym. Sci. 2017, 295, 891–902. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Q.; Zhan, X.; Chen, F.; Rao, G.; Xiong, J. Preparation, kinetics and microstructures of well-defined PS-b-PS/Bd diblock copolymers via RAFT miniemulsion polymerization. J. Polym. Res. 2013, 20, 1–13. [Google Scholar] [CrossRef]
- Wei, R.; Luo, Y.; Zeng, W.; Wang, F.; Xu, S. Styrene–Butadiene–Styrene triblock copolymer latex via reversible addition–fragmentation chain transfer miniemulsion polymerization. Ind. Eng. Chem. Res. 2012, 51, 15530–15535. [Google Scholar] [CrossRef]
- Siljanovska Petreska, G.; Auschra, C.; Paulis, M. Confinement driven crystallization of ABA crystalline-soft-crystalline block copolymers synthesized via RAFT mediated miniemulsion polymerization. Polymer 2018, 158, 327–337. [Google Scholar] [CrossRef]
- Siljanovska Petreska, G.; Arbe, A.; Auschra, C.; Paulis, M. Mechanical and morphological properties of waterborne ABA hard-soft-hard block copolymers synthesized by means of RAFT miniemulsion polymerization. Polymers 2019, 11, 1259. [Google Scholar] [CrossRef]
- Such, C.H.; Rizzardo, E.; Serelis, A.K.; Hawkett, B.S.; Gilbert, R.G.; Ferguson, C.J.; Hughes, R.J.; Olejnik, E. Aqueous Dispersions of Polymer Particles. WO2003055919A1, 10 July 2003. [Google Scholar]
- Hawkett, B.S.; Such, C.H.; Nguyen, D.N.; Farrugia, J.M.; Mackinnon, O.M. Polymerisation Process and Polymer Product. WO2006037161A1, 13 April 2006. [Google Scholar]
- Strandman, S.; Zhu, X.X. Thermo-responsive block copolymers with multiple phase transition temperatures in aqueous solutions. Prog. Polym. Sci. 2015, 42, 154–176. [Google Scholar] [CrossRef]
- Makvandi, P.; Ali, G.W.; Della Sala, F.; Abdel-Fattah, W.I.; Borzacchiello, A. Biosynthesis and characterization of antibacterial thermosensitive hydrogels based on corn silk extract, hyaluronic acid and nanosilver for potential wound healing. Carbohydr. Polym. 2019, 223, 115023. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lu, W.; Kang, N.G.; Mays, J.; Hong, K. Thermoplastic elastomers based on block, graft, and star copolymers. In Elastomers; Cankaya, N., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Kamiya, D.; Maeda, K.; Ota, H. Heat-Sensitive Adhesive Sheet. US 6368707B1, 9 April 2002. [Google Scholar]
- Bauer, G.; Schumacher, K.-J.; Fricke, H.; Wistuba, E.; Neumann, H.J. Use of Aqueous Dispersions as Heat-Seal Adhesives. US 5385967A, 31 January 1995. [Google Scholar]
- Klesse, W.; Markert, G.; Weber, M. Multiple Phase Synthetic Resin Dispersion. US 5306 743, 26 April 1994. [Google Scholar]
- Magrum, G.R. Heat Reactivatable Adhesive. US 5837089A, 17 November 1998. [Google Scholar]
- Moncla, B.M.; Eckersley, S.T.; Czerepinksi, R.G.; Diehl, C.F.; Kalinowski, M.J.; Schmidt, D.C. Aqueous Polymer Dispersions and Products from those Dispersions. US 8,383,723 B2, 26 February 2013. [Google Scholar]
- Hsu, S.-J.R.; Bauer, L.H.; Horvath, S.A. Emulsion Copolymers for Heat Seal Adhesive. WO 2011/017388 A2, 10 February 2011. [Google Scholar]
- Bauer, L.H.; Hsu, S.-J.R.; Stanislawczyk, V.; Fish, W.; Horvath, S.A. Waterborne Polymers for Heat Seal Adhesive. US 2017/0009111A1, 12 January 2017. [Google Scholar]
- Hermes, F.; Sturm, D.; Golditz, C.; Wicke, M.; Jung, H.; Hartmann, J.; Keller, B. Aqueous Binders for Heat-Sealing Applications. US 2015/0191619, 9 July 2015. [Google Scholar]
- Rigney, J.; Reinhold, F. Effect of polymer design and formulation on the performance of water-based acrylic dispersions for heat seal lacquers. In Proceedings of the SPE ANTAC Anaheim 2017, Anaheim, CA, USA, 8–10 May 2017; pp. 1278–1282. [Google Scholar]
- Taylor, J.W.; Winnik, M.A. Functional latex and thermoset latex films. J. Coat. Technol. Res. 2004, 1, 163–190. [Google Scholar] [CrossRef]
- Thomas, D.B.; Convertine, A.J.; Myrick, L.J.; Scales, C.W.; Smith, A.E.; Lowe, A.B.; Vasilieva, Y.A.; Ayres, N.; McCormick, C.L. Kinetics and molecular weight control of the polymerization of acrylamide via RAFT. Macromolecules 2004, 37, 8941–8950. [Google Scholar] [CrossRef]
- Barner-Kowollik, C.; Quinn, J.F.; Morsley, D.R.; Davis, T.P. Modeling the reversible addition–fragmentation chain transfer process in cumyl dithiobenzoate-mediated styrene homopolymerizations: Assessing rate coefficients for the addition–fragmentation equilibrium. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 1353–1365. [Google Scholar] [CrossRef]
- Monteiro, M.J.; De Brouwer, H. Intermediate radical termination as the mechanism for retardation in reversible addition–fragmentation chain transfer polymerization. Macromolecules 2001, 34, 349–352. [Google Scholar] [CrossRef]
- Kwak, Y.; Goto, A.; Tsujii, Y.; Murata, Y.; Komatsu, K.; Fukuda, T. A kinetic study on the rate retardation in radical polymerization of styrene with addition–fragmentation chain transfer. Macromolecules 2002, 35, 3026–3029. [Google Scholar] [CrossRef]
- Wang, A.R.; Zhu, S. Calculations of monomer conversion and radical concentration in reversible addition-fragmentation chain transfer radical polymerization. Macromol. Theory Simul. 2003, 12, 663–668. [Google Scholar] [CrossRef]
- Coote, M.L. Ab initio study of the addition–fragmentation equilibrium in raft polymerization: When is polymerization retarded? Macromolecules 2004, 37, 5023–5031. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Radical addition-fragmentation chemistry in polymer synthesis. Polymer 2008, 49, 1079–1131. [Google Scholar] [CrossRef]
- Derboven, P.; Van Steenberge, P.H.M.; Reyniers, M.F.; Barner-Kowollik, C.; D’hooge, D.R.; Marin, G.B. Chain transfer degeneratuce RAFT polymerization revisited: A comparative study of literature methods. Macromol. Theory Simul. 2016, 25, 104–115. [Google Scholar] [CrossRef]
- Koiry, B.P.; Singha, N.K. Copper mediated controlled radical copolymerization of styrene and 2-ethylhexyl acrylate and determination of their reactivity ratios. Front. Chem. 2014, 2, 91–98. [Google Scholar] [CrossRef][Green Version]
- Plessis, C.; Arzamendi, G.; Leiza, J.R.; Schoonbrood, H.A.S.; Charmot, D.; Asua, J.M. Seeded semibatch emulsion polymerization of n-butyl acrylate. kinetics and structural properties. Macromolecules 2000, 33, 5041–5047. [Google Scholar] [CrossRef]
- Hamzehlou, S.; Reyes, Y.; Hutchinson, R.; Leiza, J.R. Copolymerization of n-butyl acrylate and styrene: Terminal vs penultimate model. Macromol. Chem. Phys. 2014, 215, 1668–1678. [Google Scholar] [CrossRef]
- Ballard, N.; Asua, J.M. Progress in Polymer Science Radical polymerization of acrylic monomers: An overview. Prog. Polym. Sci. 2018, 79, 40–60. [Google Scholar] [CrossRef]
- Mehravar, E.; Leiza, J.R.; Asua, J.M. Performance of latexes containing nano-sized crystalline domains formed by comb-like polymers. Polymer 2016, 96, 121–129. [Google Scholar] [CrossRef]
- Bates, F.S.; Fredrickson, G.H. Block copolymers-designer soft materials. Phys. Today 1999, 52, 32–38. [Google Scholar] [CrossRef]
- Ku, K.H.; Yang, H.; Shin, J.M.; Kim, B.J. Aspect ratio effect of nanorod surfactants on the shape and internal morphology of block copolymer particles. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 188–192. [Google Scholar] [CrossRef]
- Ku, K.H.; Shin, J.M.; Kim, M.P.; Lee, C.H.; Seo, M.K.; Yi, G.R.; Jang, S.G.; Kim, B.J. Size-controlled nanoparticle-guided assembly of block copolymers for convex lens-shaped particles. J. Am. Chem. Soc. 2014, 136, 9982–9989. [Google Scholar] [CrossRef]
- Jeon, S.J.; Yi, G.R.; Yang, S.M. Cooperative assembly of block copolymers with deformable interfaces: Toward nanostructured particles. Adv. Mater. 2008, 20, 4103–4108. [Google Scholar] [CrossRef]
- Jang, S.G.; Audus, D.J.; Klinger, D.; Krogstad, D.V.; Kim, B.J.; Cameron, A.; Kim, S.W.; Delaney, K.T.; Hur, S.M.; Killops, K.L.; et al. Striped, ellipsoidal particles by controlled assembly of diblock copolymers. Am. Chem. Soc. 2013, 135, 6649–6657. [Google Scholar] [CrossRef] [PubMed]


| Reactions | Conversion (%) | Mn,GPC (g/mol) | Ð | dd (nm) | dp (nm) | Conversion (%) | dp (nm) | Mn,GPC (g/mol) | Ð |
|---|---|---|---|---|---|---|---|---|---|
| pSt Homopolymers | P(St/2EHA) Block Copolymers | ||||||||
| p(St50/2EHA50) | 72.0 | 36,121 | 1.37 | 98.6 | 115.3 | 81.2 | 150.4 | 65,980 | 1.38 |
| p(St30/2EHA50) | 74.2 | 22,364 | 1.27 | 100.6 | 121.1 | 92.5 | 160.6 | 58,611 | 1.42 |
| p(St30/2EHA70) | 74.2 | 22,364 | 1.27 | 100.6 | 121.1 | 91.4 | 165.0 | 70,103 | 1.55 |
| p(St30/2EHA100) | 78.1 | 23,509 | 1.27 | 89.9 | 126.2 | 94.2 | 171.3 | 90,834 | 1.83 |
| Material Code | Tg1 (°C) | Tg2 (°C) |
|---|---|---|
| DSC analysis of the samples dried at 23 °C | ||
| pSt30 | - | 67.3 |
| pSt50 | - | 61.0 |
| p(St30/2EHA50) | −60.5 | 79.6 |
| p(St30/2EHA70) | −60.5 | 78.3 |
| p(St30/2EHA100) | −61.6 | 79.5 |
| p(St50/2EHA50) | −50.4 | 69.9 |
| Material Code | Mole Fraction | Weight Fraction | Theoretical Morphology | ||
|---|---|---|---|---|---|
| % St | %2EHA + SA | %St + SA | %2EHA | ||
| p(St50/2EHA50) | 63.5 | 36.5 | 48.8 | 51.2 | L |
| p(St30/2EHA50) | 48.9 | 51.1 | 34.7 | 65.3 | C |
| p(St30/2EHA70) | 40.4 | 59.6 | 27.6 | 72.4 | C |
| p(St30/2EHA100) | 32.4 | 67.6 | 21.1 | 78.9 | C |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siljanovska Petreska, G.; van Sluijs, C.; Auschra, C.; Paulis, M. Design of Waterborne Asymmetric Block Copolymers as Thermoresponsive Materials. Polymers 2020, 12, 1253. https://doi.org/10.3390/polym12061253
Siljanovska Petreska G, van Sluijs C, Auschra C, Paulis M. Design of Waterborne Asymmetric Block Copolymers as Thermoresponsive Materials. Polymers. 2020; 12(6):1253. https://doi.org/10.3390/polym12061253
Chicago/Turabian StyleSiljanovska Petreska, Gordana, Christof van Sluijs, Clemens Auschra, and Maria Paulis. 2020. "Design of Waterborne Asymmetric Block Copolymers as Thermoresponsive Materials" Polymers 12, no. 6: 1253. https://doi.org/10.3390/polym12061253
APA StyleSiljanovska Petreska, G., van Sluijs, C., Auschra, C., & Paulis, M. (2020). Design of Waterborne Asymmetric Block Copolymers as Thermoresponsive Materials. Polymers, 12(6), 1253. https://doi.org/10.3390/polym12061253







