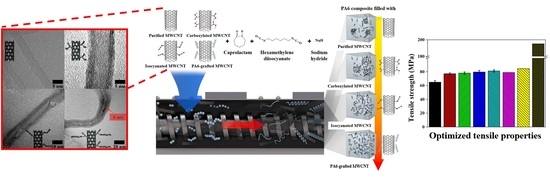
Enhanced Tensile Properties of Multi-Walled Carbon Nanotubes Filled Polyamide 6 Composites Based on Interface Modification and Reactive Extrusion
Abstract
1. Introduction
2. Experimental
2.1. Materials
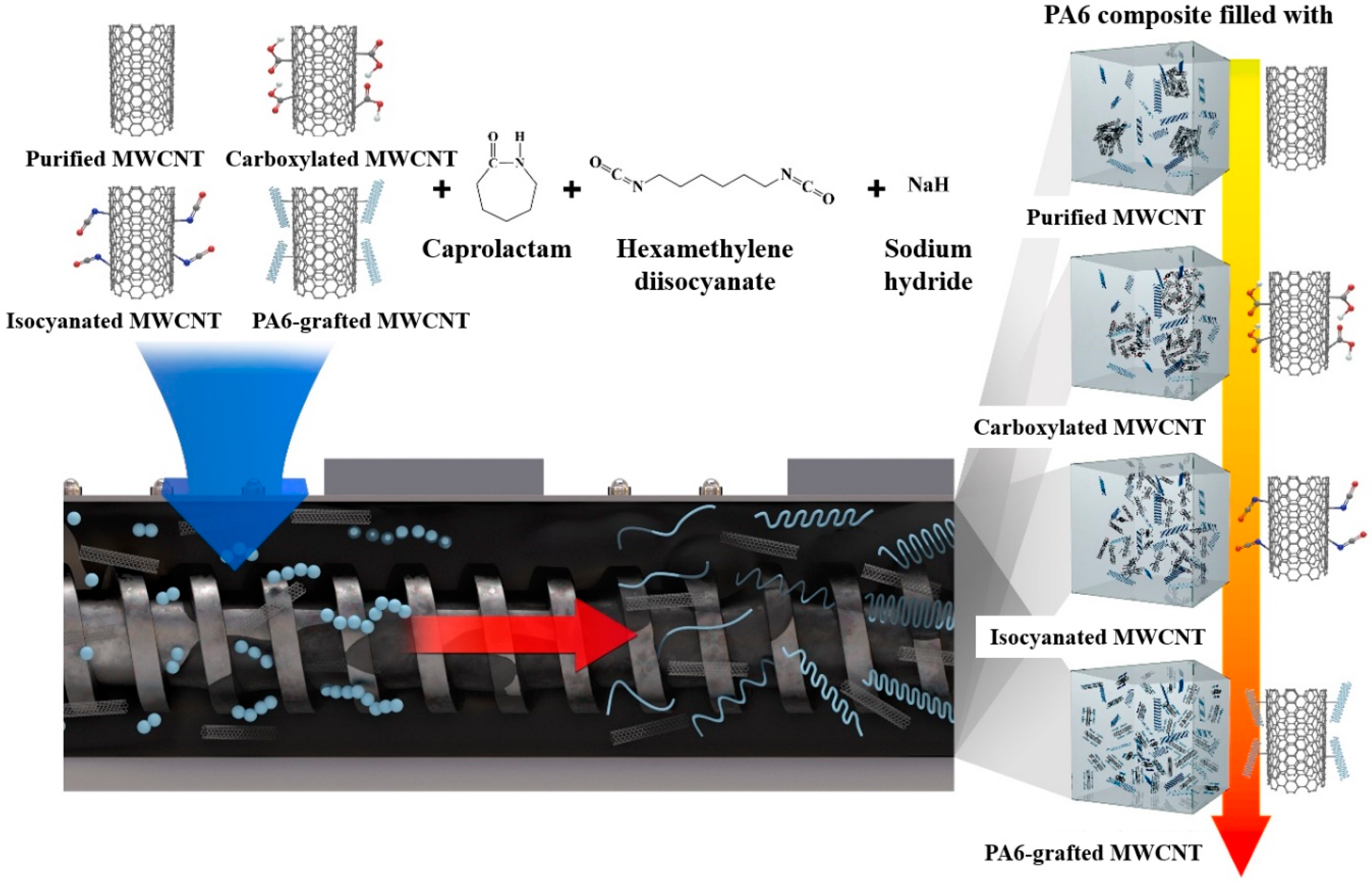
2.2. Surface Treatment of MWCNT
2.2.1. Carboxylated MWCNT
2.2.2. Isocyanated MWCNT
2.2.3. PA6 Grafted MWCNT
2.3. Composite Fabrication
2.4. Characterization
3. Micromechanics Modeling Approach
3.1. Modified Mori-Tanaka Method for Composite Stiffness Properties
3.2. Modified Mori-Tanaka Method for Composite Strength Properties
4. Results and Discussion
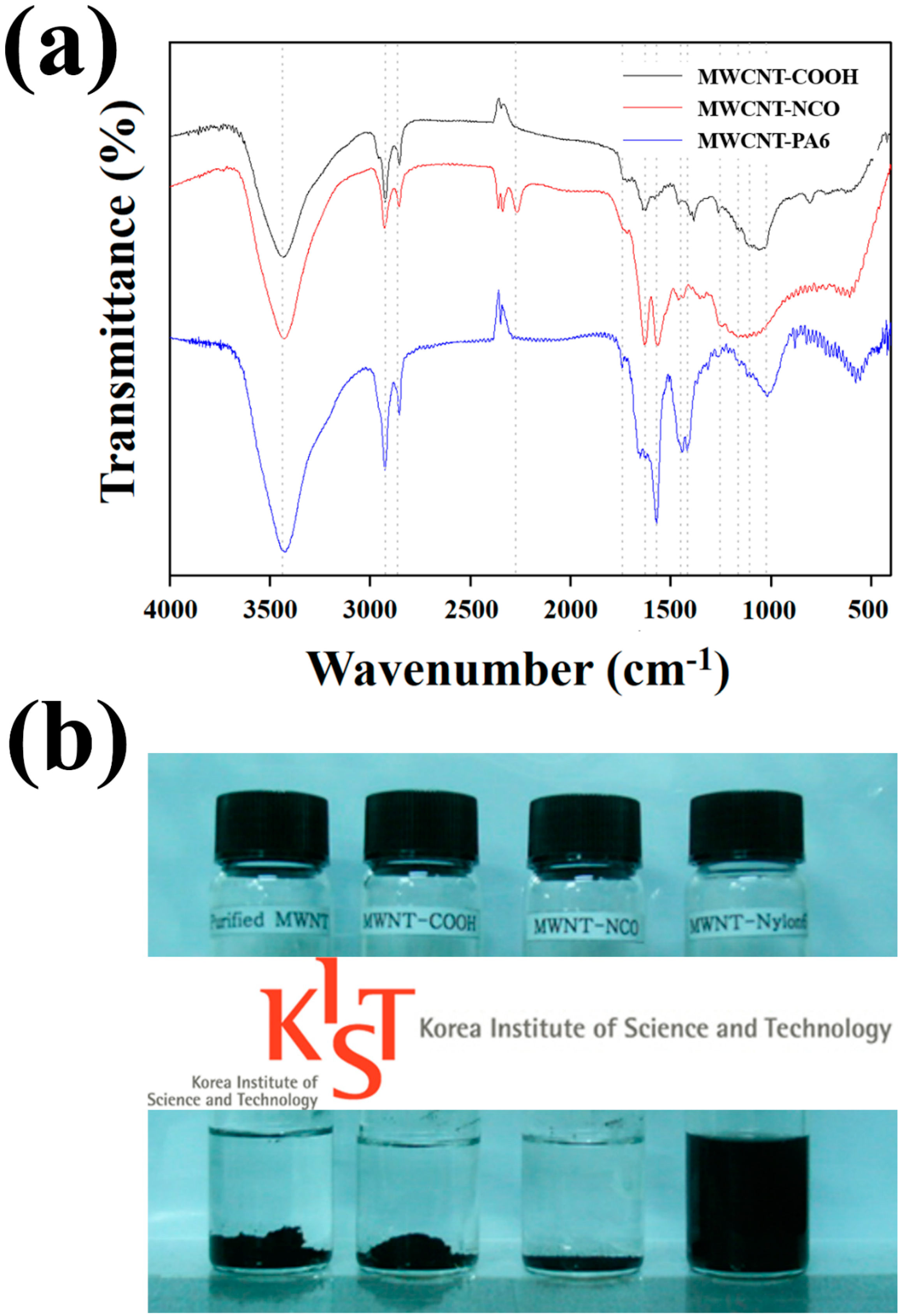
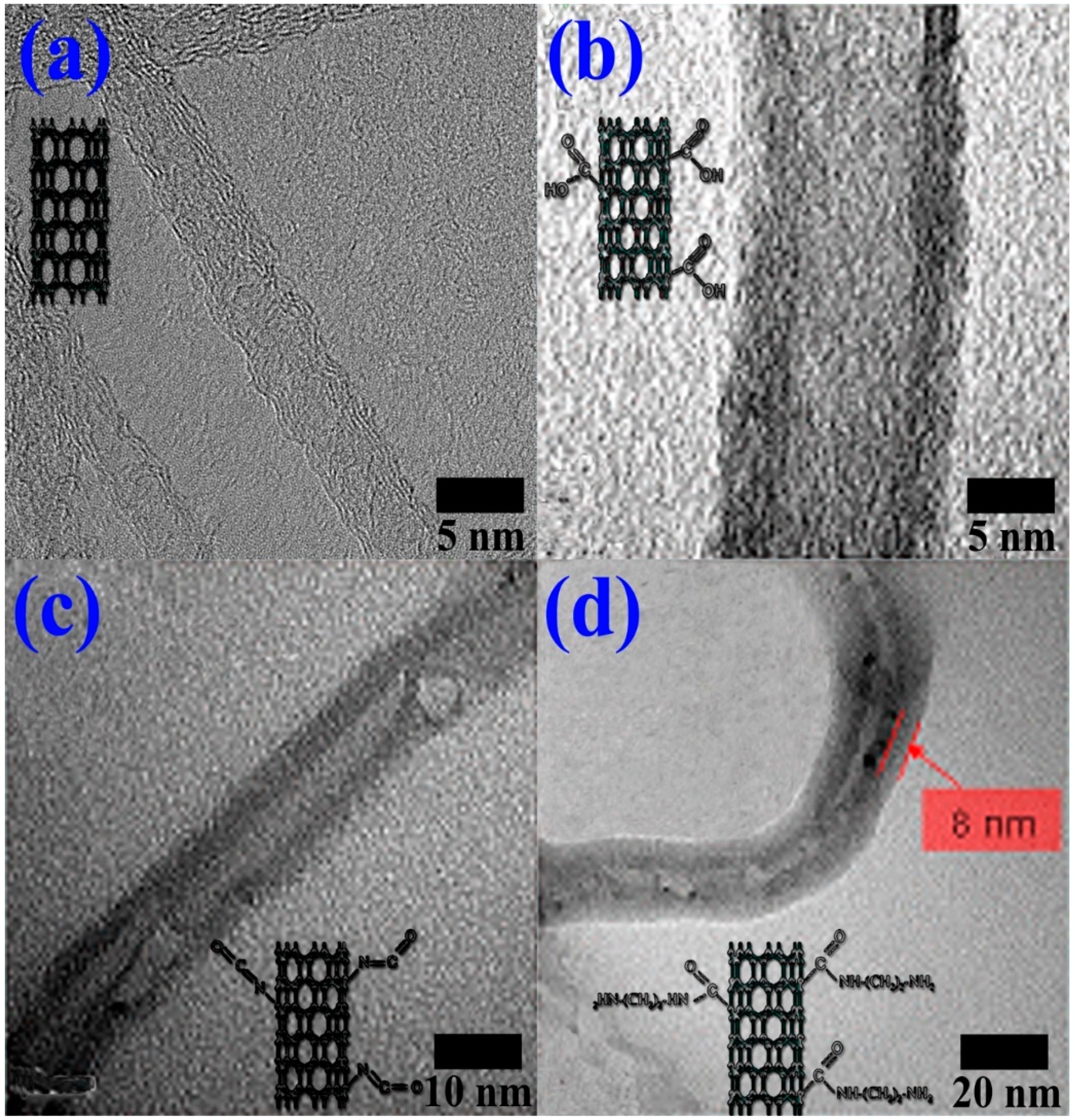
4.1. Surface Treatment of MWCNTs
4.2. Reactive Extrusion
4.3. MWCNT Dispersion of Composites
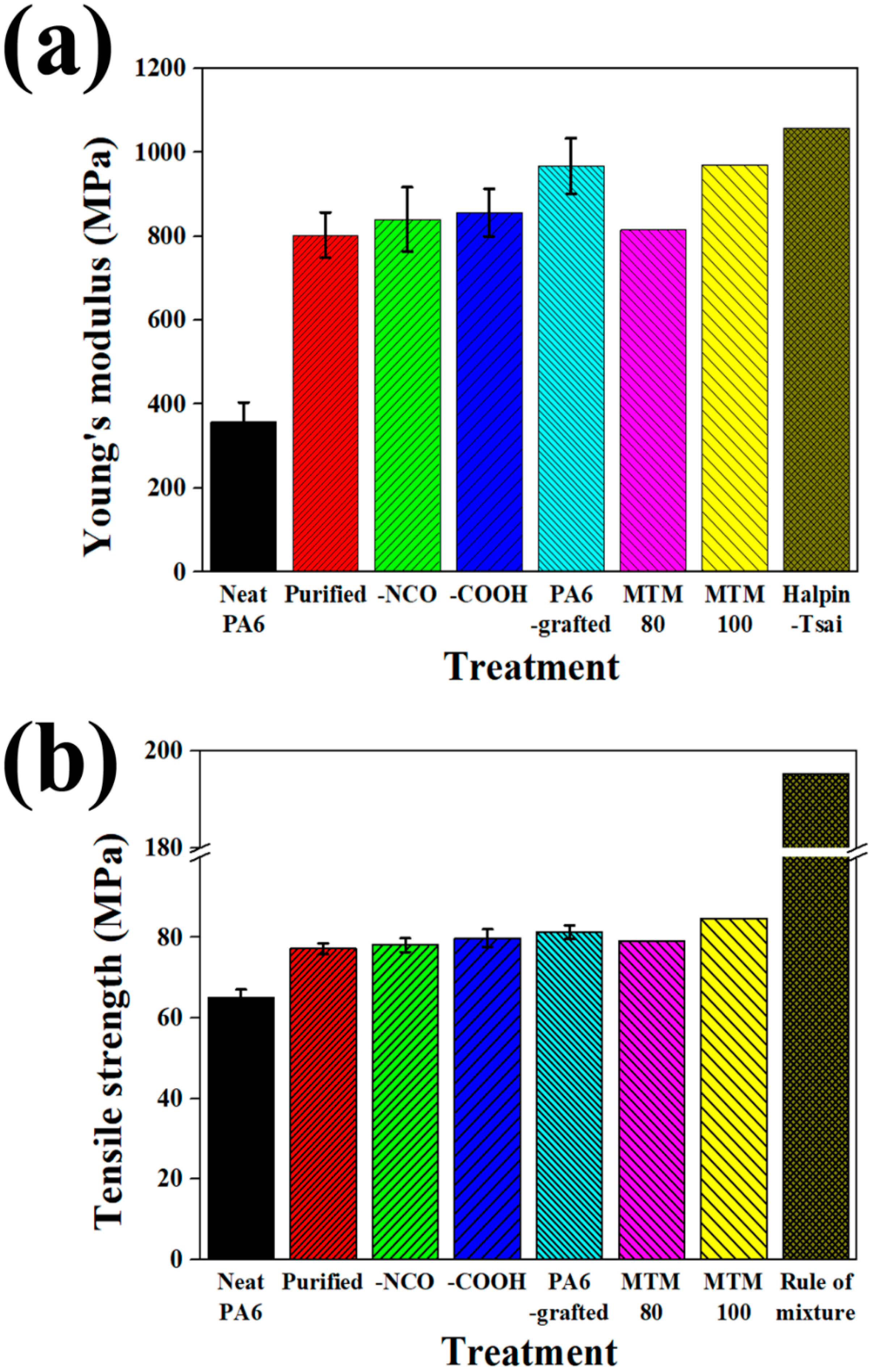
4.4. Tensile Properties of Composites
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Treacy, M.M.J.; Ebbesen, T.W.; Gibson, J.M. Exceptionally high Young’s modulus observed for individual carbon nanotubes. Nature 1996, 381, 678–680. [Google Scholar] [CrossRef]
- Wong, E.W.; Sheehan, P.E.; Lieber, C.M. Nanobeam mechanics: Elasticity, strength, and toughness of nanorods and nanotubes. Science 1997, 277, 1971–1975. [Google Scholar] [CrossRef]
- Spitalsky, Z.; Tasis, D.; Papagelis, K.; Galiotis, C. Carbon nanotube–polymer composites: Chemistry, processing, mechanical and electrical properties. Prog. Polym. Sci. 2010, 35, 357–401. [Google Scholar] [CrossRef]
- Tjong, S.C. Structural and mechanical properties of polymer nanocomposites. Mater. Sci. Eng. R Rep. 2006, 53, 73–197. [Google Scholar] [CrossRef]
- Roy, N.; Sengupta, R.; Bhowmick, A.K. Modifications of carbon for polymer composites and nanocomposites. Prog. Polym. Sci. 2012, 37, 781–819. [Google Scholar] [CrossRef]
- Jang, J.-U.; Lee, H.S.; Kim, J.W.; Kim, S.Y.; Kim, S.H.; Hwang, I.; Kang, B.J.; Kang, M.K. Facile and cost-effective strategy for fabrication of polyamide 6 wrapped multi-walled carbon nanotube via anionic melt polymerization of ε-caprolactam. Chem. Eng. J. 2019, 373, 251–258. [Google Scholar] [CrossRef]
- Wang, H.; Li, N.; Xu, Z.; Tian, X.; Mai, W.; Li, J.; Chen, C.; Chen, L.; Fu, H.; Zhang, X. Enhanced sheet-sheet welding and interfacial wettability of 3D graphene networks as radiation protection in gamma-irradiated epoxy composites. Compos. Sci. Technol. 2018, 157, 57–66. [Google Scholar] [CrossRef]
- Zhbanov, A.I.; Pogorelov, E.G.; Chang, Y.-C. Van der waals interaction between two crossed carbon nanotubes. ACS Nano 2010, 4, 5937–5945. [Google Scholar] [CrossRef]
- Li, W.; Shi, C.; Shan, M.; Guo, Q.; Xu, Z.; Wang, Z.; Yang, C.; Mai, W.; Niu, J. Influence of silanized low-dimensional carbon nanofillers on mechanical, thermomechanical, and crystallization behaviors of poly(L-lactic acid) composites—A comparative study. J. Appl. Polym. Sci. 2013, 130, 1194–1202. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Rana, S.; Cho, J.W.; Li, L.; Chan, S.H. Polymer nanocomposites based on functionalized carbon nanotubes. Prog. Polym. Sci. 2010, 35, 837–867. [Google Scholar] [CrossRef]
- Chen, J.; Yan, L.; Song, W.; Xu, D. Interfacial characteristics of carbon nanotube-polymer composites: A review. Compos. Part A Appl. Sci. Manuf. 2018, 114, 149–169. [Google Scholar] [CrossRef]
- Park, M.; Lee, H.; Jang, J.-U.; Park, J.H.; Kim, C.H.; Kim, S.Y.; Kim, J. Phenyl glycidyl ether as an effective noncovalent functionalization agent for multiwalled carbon nanotube reinforced polyamide 6 nanocomposite fibers. Compos. Sci. Technol. 2019, 177, 96–102. [Google Scholar] [CrossRef]
- Noh, Y.J.; Pak, S.Y.; Hwang, S.H.; Hwang, J.Y.; Kim, S.Y. Enhanced dispersion for electrical percolation behavior of multi-walled carbon nanotubes in polymer nanocomposites using simple powder mixing and in situ polymerization with surface treatment of the fillers. Compos. Sci. Technol. 2013, 89, 29–37. [Google Scholar] [CrossRef]
- Pak, S.Y.; Kim, H.M.; Kim, S.Y.; Youn, J.R. Synergistic improvement of thermal conductivity of thermoplastic composites with mixed boron nitride and multi-walled carbon nanotube fillers. Carbon 2012, 50, 4830–4838. [Google Scholar] [CrossRef]
- Yang, M.; Gao, Y.; Li, H.; Adronov, A. Functionalization of multiwalled carbon nanotubes with polyamide 6 by anionic ring-opening polymerization. Carbon 2007, 45, 2327–2333. [Google Scholar] [CrossRef]
- Yuen, S.-M.; Ma, C.-C.M.; Lin, Y.-Y.; Kuan, H.-C. Preparation, morphology and properties of acid and amine modified multiwalled carbon nanotube/polyimide composite. Compos. Sci. Technol. 2007, 67, 2564–2573. [Google Scholar] [CrossRef]
- Gao, J.; Itkis, M.E.; Yu, A.; Bekyarova, E.; Zhao, B.; Haddon, R.C. Continuous spinning of a single-walled carbon nanotube−nylon composite fiber. J. Am. Chem. Soc. 2005, 127, 3847–3854. [Google Scholar] [CrossRef]
- Li, W.; Xu, Z.; Chen, L.; Shan, M.; Tian, X.; Yang, C.; Lv, H.; Qian, X. A facile method to produce graphene oxide-g-poly(L-lactic acid) as an promising reinforcement for PLLA nanocomposites. Chem. Eng. J. 2014, 237, 291–299. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Wang, L.; Wang, W.; Xu, Z. Effect of the molecular chains grafted on graphene nanosheets on the properties of poly(l-lactic acid) nanocomposites. Polym. Compos. 2015, 38, 5–12. [Google Scholar] [CrossRef]
- Kim, H.S.; Jang, J.-U.; Lee, H.; Kim, S.Y.; Kim, S.H.; Kim, J.; Jung, Y.C.; Yang, B.J. Thermal management in polymer composites: A review of physical and structural parameters. Adv. Eng. Mater. 2018, 20, 1800204. [Google Scholar] [CrossRef]
- Noh, Y.J.; Joh, H.-I.; Yu, J.; Hwang, S.H.; Lee, S.; Lee, C.H.; Kim, S.Y.; Youn, J.R. Ultra-high dispersion of graphene in polymer composite via solvent free fabrication and functionalization. Sci. Rep. 2015, 5, 9141. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Chen, L.; Teng, K.; Shi, J.; Qian, X.; Xu, Z.; Tian, X.; Hu, C.; Ma, M. Superior mechanical properties of epoxy composites reinforced by 3D interconnected graphene skeleton. ACS Appl. Mater. Interfaces 2015, 7, 11583–11591. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, J.H.; Yang, C.-M.; Kim, S.Y. Synergistic enhancement of thermal conductivity in composites filled with expanded graphite and multi-walled carbon nanotube fillers via melt-compounding based on polymerizable low-viscosity oligomer matrix. J. Alloy. Compd. 2017, 690, 274–280. [Google Scholar] [CrossRef]
- Kim, S.Y.; Noh, Y.J.; Yu, J. Improved thermal conductivity of polymeric composites fabricated by solvent-free processing for the enhanced dispersion of nanofillers and a theoretical approach for composites containing multiple heterogeneities and geometrized nanofillers. Compos. Sci. Technol. 2014, 101, 79–85. [Google Scholar] [CrossRef]
- Kim, S.Y.; Noh, Y.J.; Yu, J. Prediction and experimental validation of electrical percolation by applying a modified micromechanics model considering multiple heterogeneous inclusions. Compos. Sci. Technol. 2015, 106, 156–162. [Google Scholar] [CrossRef]
- Kim, S.Y.; Noh, Y.J.; Yu, J. Thermal conductivity of graphene nanoplatelets filled composites fabricated by solvent-free processing for the excellent filler dispersion and a theoretical approach for the composites containing the geometrized fillers. Compos. Part A Appl. Sci. Manuf. 2015, 69, 219–225. [Google Scholar] [CrossRef]
- Noh, Y.J.; Kim, S.Y. Synergistic improvement of thermal conductivity in polymer composites filled with pitch based carbon fiber and graphene nanoplatelets. Polym. Test. 2015, 45, 132–138. [Google Scholar] [CrossRef]
- Noh, Y.J.; Kim, H.S.; Ku, B.-C.; Khil, M.-S.; Kim, S.Y. Thermal conductivity of polymer composites with geometric characteristics of carbon allotropes. Adv. Eng. Mater. 2016, 18, 1127–1132. [Google Scholar] [CrossRef]
- Yu, J.; Cha, J.E.; Kim, S.Y. Thermally conductive composite film filled with highly dispersed graphene nanoplatelets via solvent-free one-step fabrication. Compos. Part B Eng. 2017, 110, 171–177. [Google Scholar] [CrossRef]
- Yu, J.; Lacy, T.E.; Toghiani, H.; Pittman, C.U. Classical micromechanics modeling of nanocomposites with carbon nanofibers and interphase. J. Compos. Mater. 2011, 45, 2401–2413. [Google Scholar] [CrossRef]
- Jung, H.; Choi, H.K.; Yu, J. Prediction and experimental validation of composite strength by applying modified micromechanics for composites containing multiple distinct heterogeneities. Compos. Part B Eng. 2016, 91, 1–7. [Google Scholar] [CrossRef]
- Davé, R.S.; Kruse, R.L.; Stebbins, L.R.; Udipi, K. Polyamides from lactams via anionic ring-opening polymerization: 2. Kinetics. Polymer 1997, 38, 939–947. [Google Scholar] [CrossRef]
- Ricco, L.; Russo, S.; Orefice, G.; Riva, F. Anionic poly(ε-caprolactam): relationships among conditions of synthesis, chain regularity, reticular order, and polymorphism. Macromolecules 1999, 32, 7726–7731. [Google Scholar] [CrossRef]
- Wittmer, V.P.; Gerrens, H. Über die anionische schnellpolymerisation von caprolactam. Macromol. Chem. Phys. 1965, 89, 27–43. [Google Scholar] [CrossRef]
- Zhang, W.D.; Shen, L.; Phang, I.Y.; Liu, T. Carbon nanotubes reinforced nylon-6 composite prepared by simple melt-compounding. Macromolecules 2004, 37, 256–259. [Google Scholar] [CrossRef]
- Fornes, T.D.; Yoon, P.J.; Keskkula, H.; Paul, D.R. Nylon 6 nanocomposites: The effect of matrix molecular weight. Polymer 2001, 42, 09929–09940. [Google Scholar] [CrossRef]
- Koning, C.; Duin, M.V.; Pagnoulle, C.; Jerome, R. Strategies for compatibilization of polymer blends. Prog. Polym. Sci. 1998, 23, 707–757. [Google Scholar] [CrossRef]
- Liu, T.; Phang, I.Y.; Shen, L.; Chow, S.Y.; Zhang, W.D. Morphology and mechanical properties of multiwalled carbon nanotubes reinforced nylon-6 composites. Macromolecules 2004, 37, 7214–7222. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.; Jang, J.-u.; Park, J.H.; Yu, J.; Kim, S.Y. Enhanced Tensile Properties of Multi-Walled Carbon Nanotubes Filled Polyamide 6 Composites Based on Interface Modification and Reactive Extrusion. Polymers 2020, 12, 997. https://doi.org/10.3390/polym12050997
Park M, Jang J-u, Park JH, Yu J, Kim SY. Enhanced Tensile Properties of Multi-Walled Carbon Nanotubes Filled Polyamide 6 Composites Based on Interface Modification and Reactive Extrusion. Polymers. 2020; 12(5):997. https://doi.org/10.3390/polym12050997
Chicago/Turabian StylePark, Min, Ji-un Jang, Jong Hyuk Park, Jaesang Yu, and Seong Yun Kim. 2020. "Enhanced Tensile Properties of Multi-Walled Carbon Nanotubes Filled Polyamide 6 Composites Based on Interface Modification and Reactive Extrusion" Polymers 12, no. 5: 997. https://doi.org/10.3390/polym12050997
APA StylePark, M., Jang, J.-u., Park, J. H., Yu, J., & Kim, S. Y. (2020). Enhanced Tensile Properties of Multi-Walled Carbon Nanotubes Filled Polyamide 6 Composites Based on Interface Modification and Reactive Extrusion. Polymers, 12(5), 997. https://doi.org/10.3390/polym12050997