Effect of 1,2,4,5-Benzenetetracarboxylic Acid on Unsaturated Poly(butylene adipate-co-butylene itaconate) Copolyesters: Synthesis, Non-Isothermal Crystallization Kinetics, Thermal and Mechanical Properties
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Sample Preparation
2.3. Measurements
2.3.1. Nuclear Magnetic Resonance Spectroscopy (1H NMR)
2.3.2. Fourier Transform Infrared Spectroscopy (FT-IR)
2.3.3. Intrinsic Viscosity (I.V.)
2.3.4. Gel Permeation Chromatography (GPC)
2.3.5. Differential Scanning Calorimetry (DSC)
2.3.6. Thermogravimetric Analysis (TGA)
2.3.7. Dynamic Mechanical Analyzer (DMA)
2.3.8. X-ray Diffraction (XRD)
2.3.9. Tensile Test
2.3.10. Non-isothermal Crystallization Kinetic Procedures
3. Results and Discussion
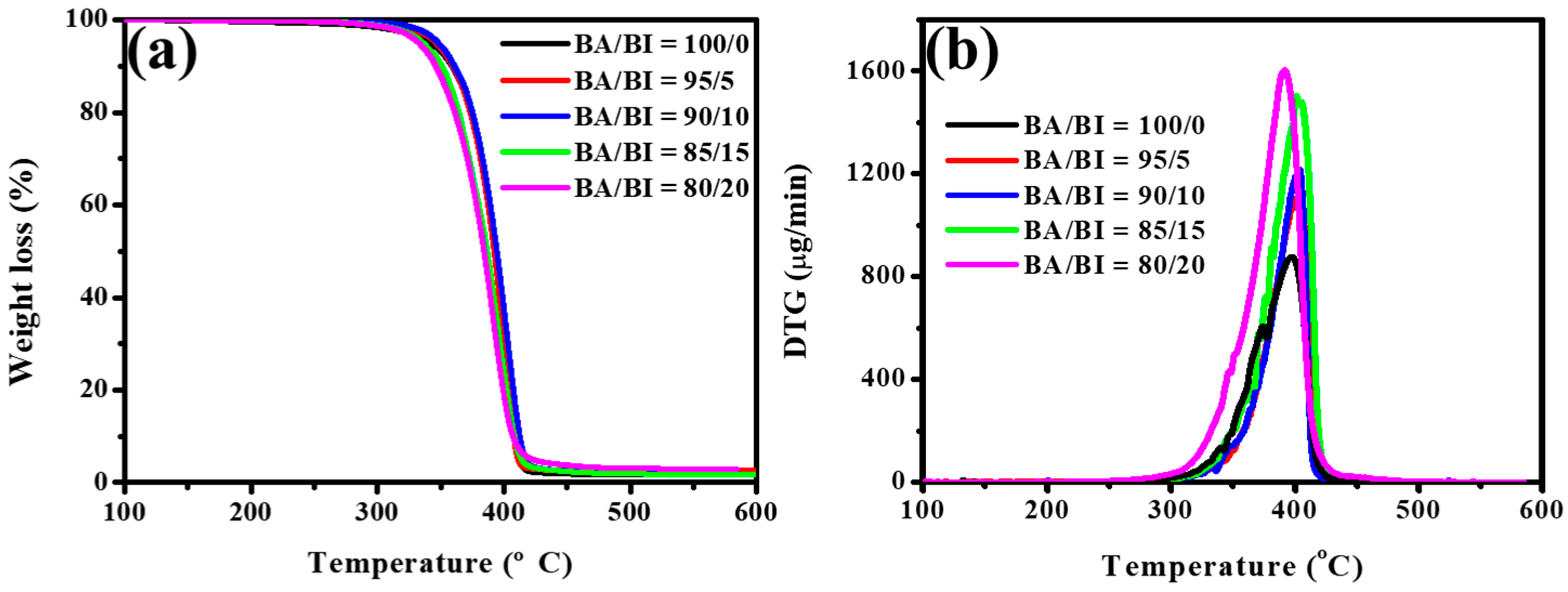
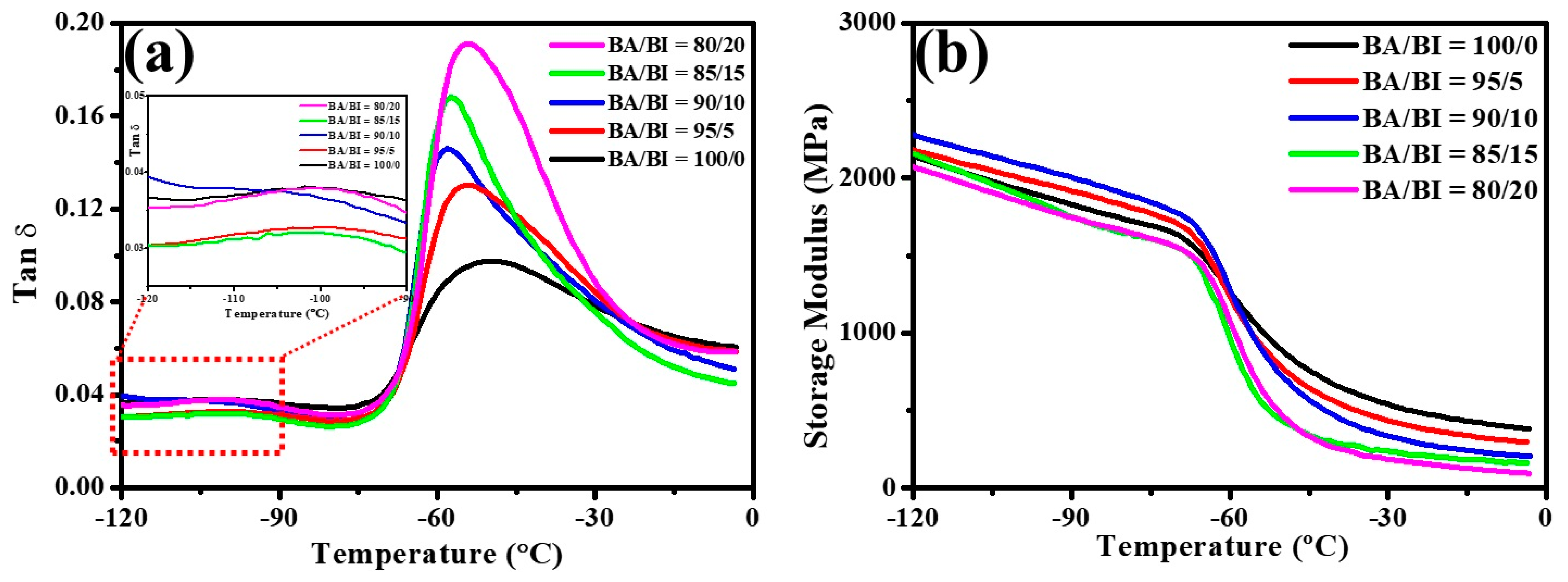
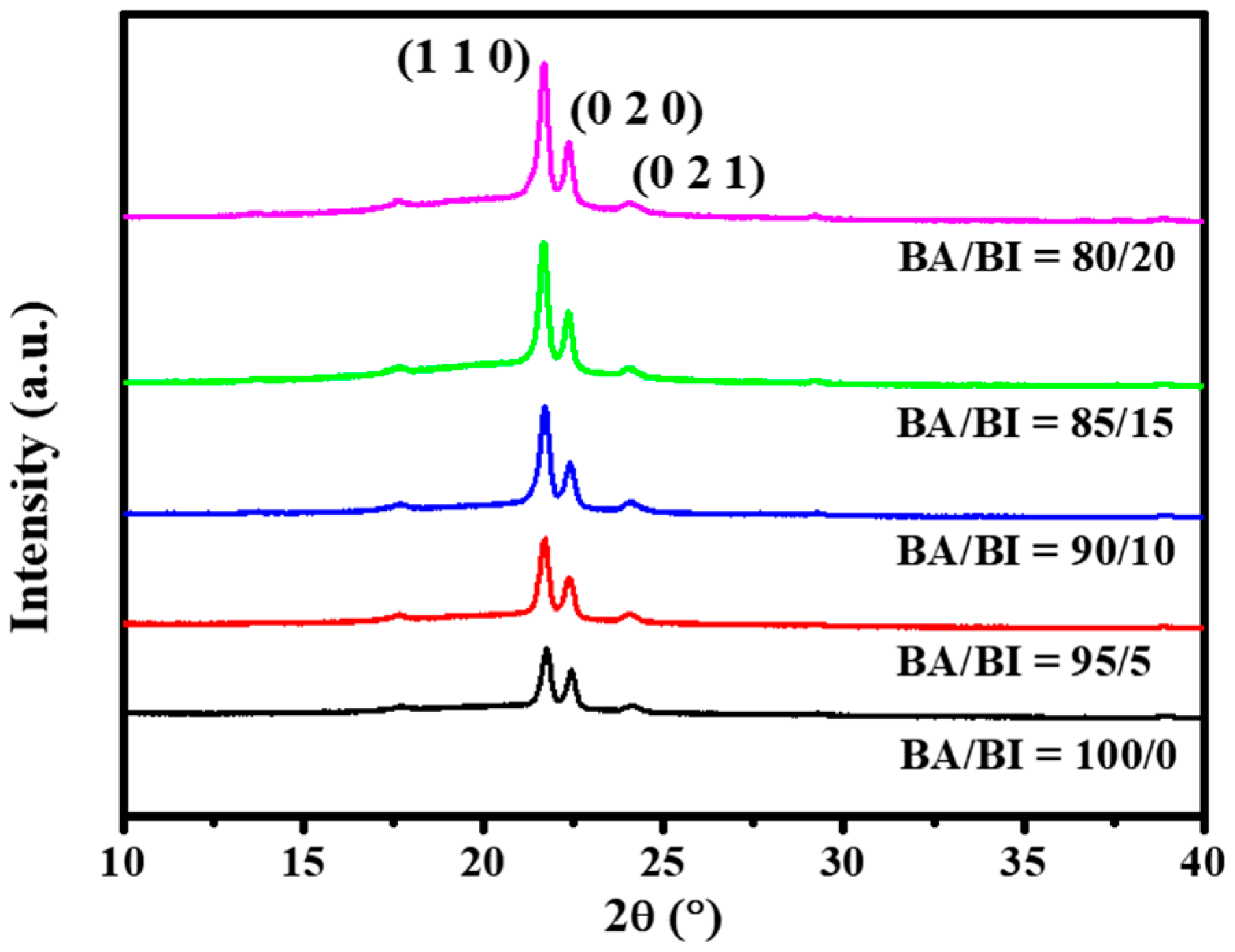
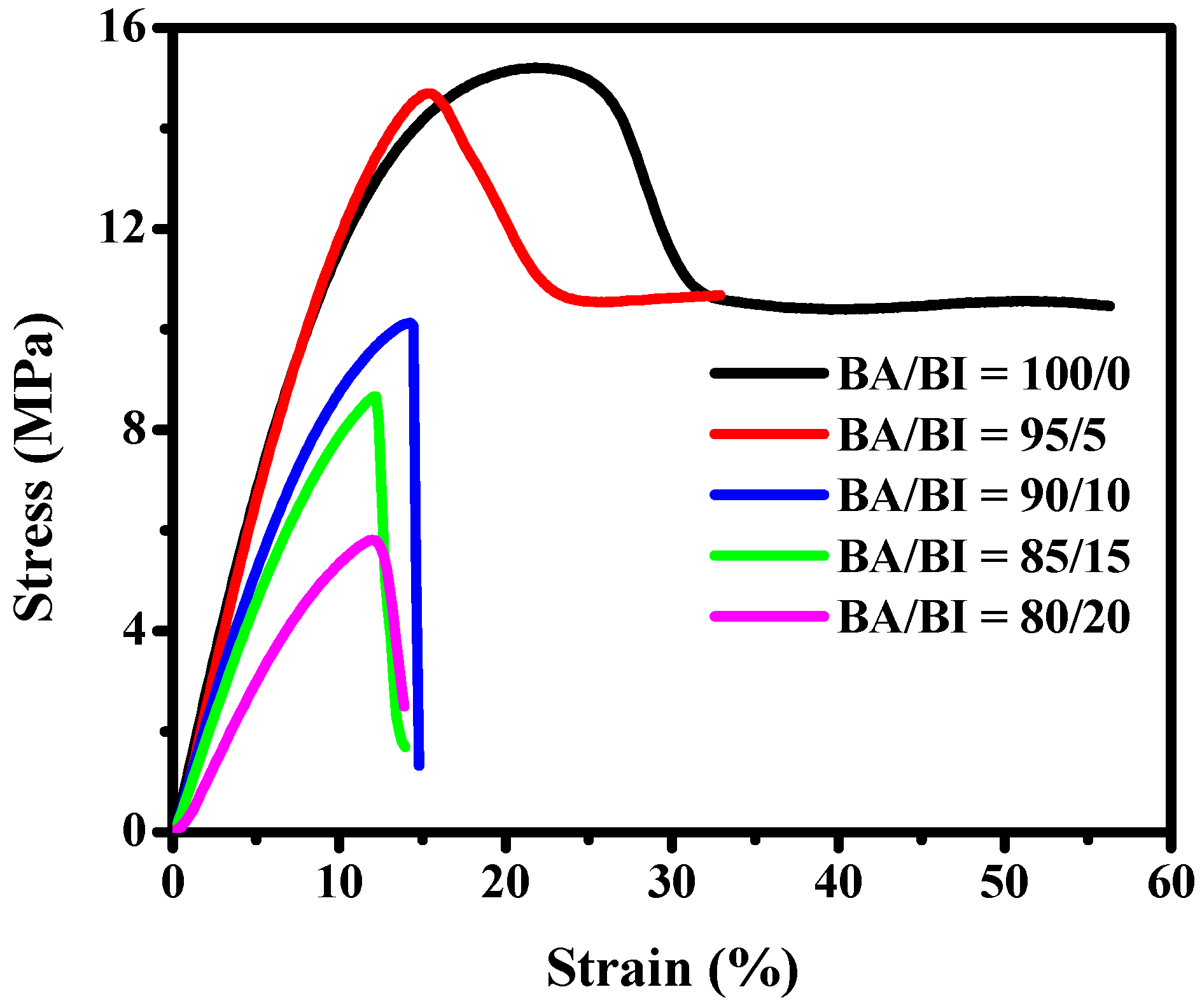
3.1. The Effect of the BA/BI ratio of PBABA Copolyesters with a BTCA Concentration of 0.1 mole%
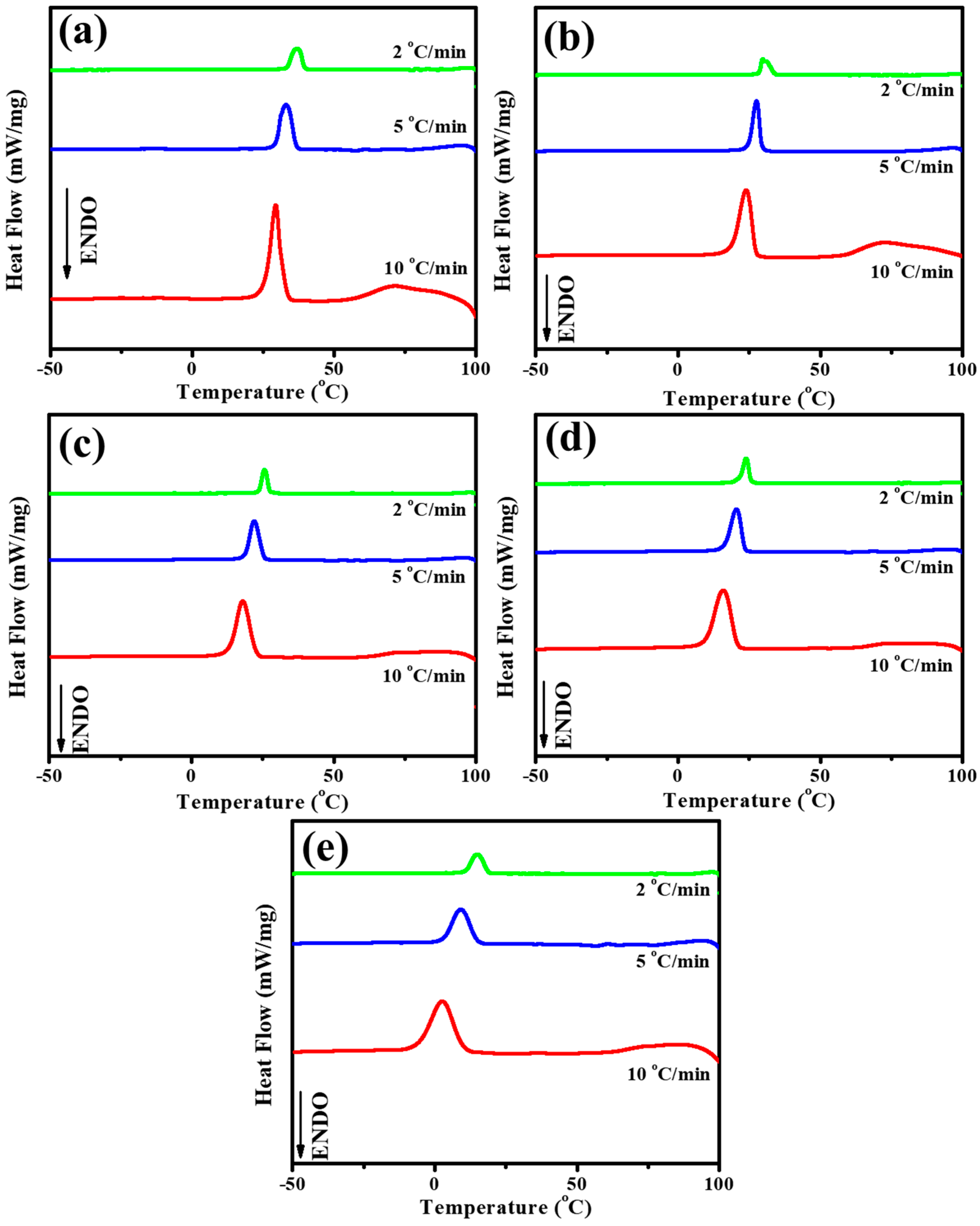
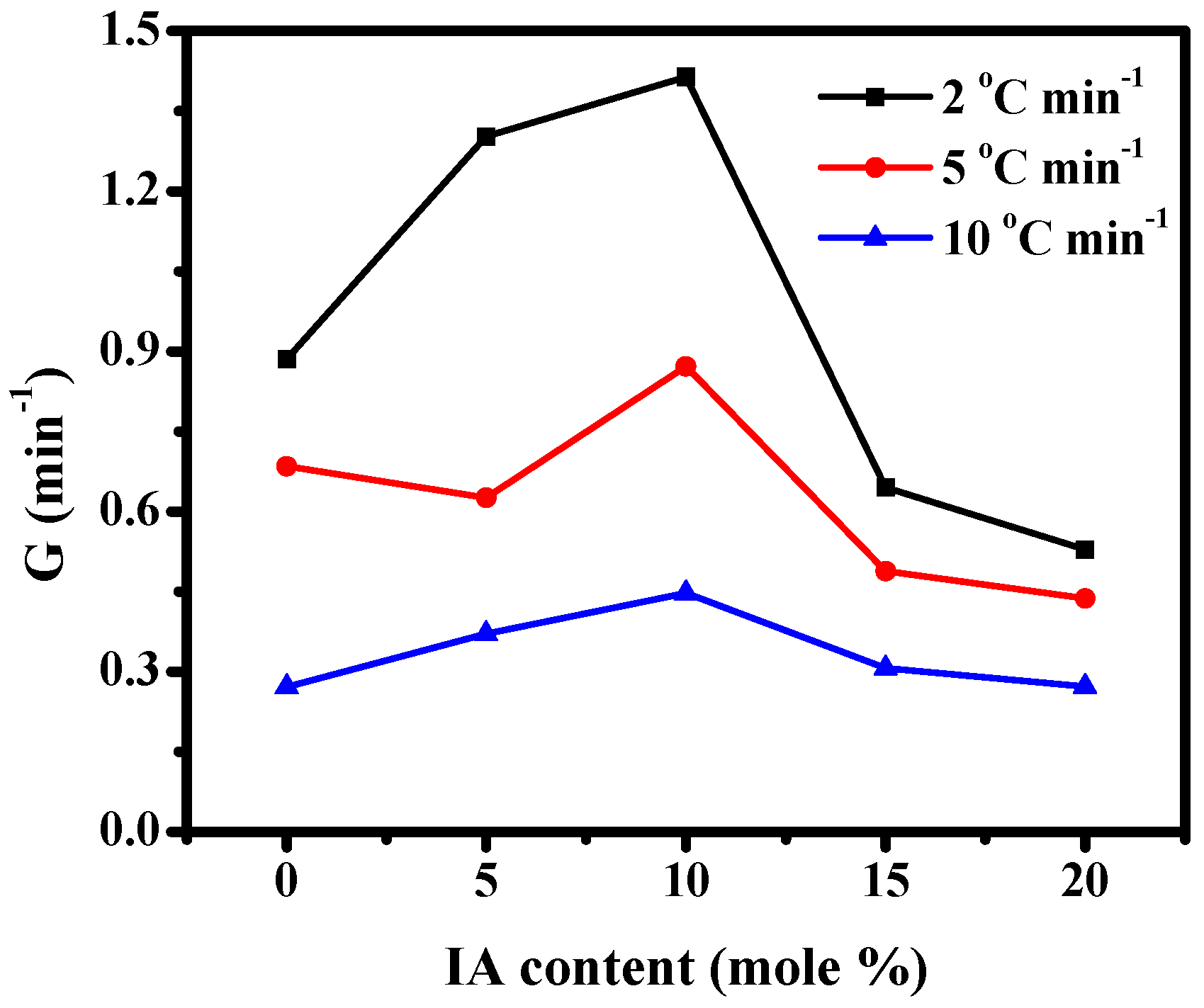
3.2. Non-Isothermal Crystallization Kinetics of PBABI Copolyesters
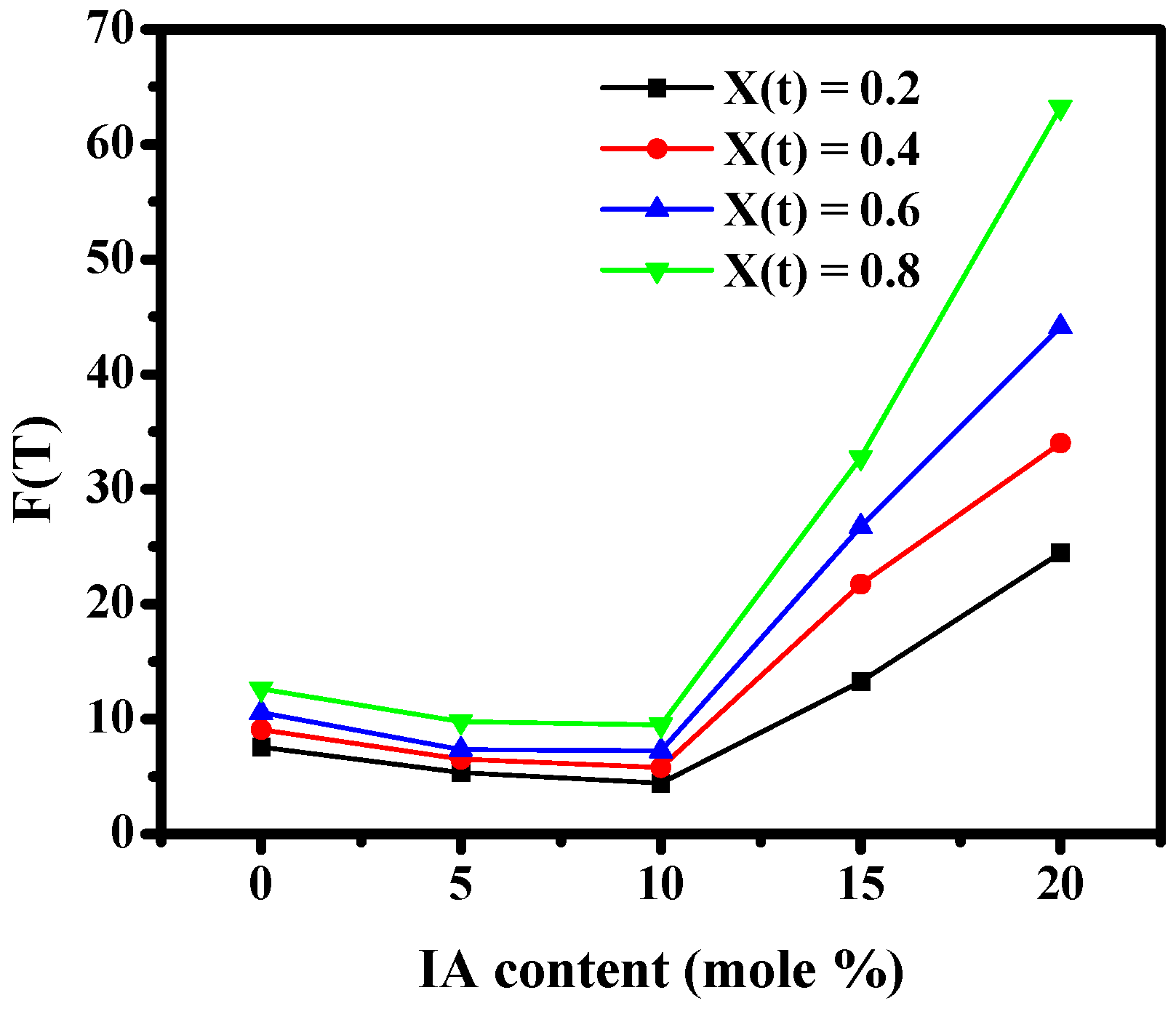
3.2.1. Non-Isothermal Crystallization Kinetics Based on Avrami Equation
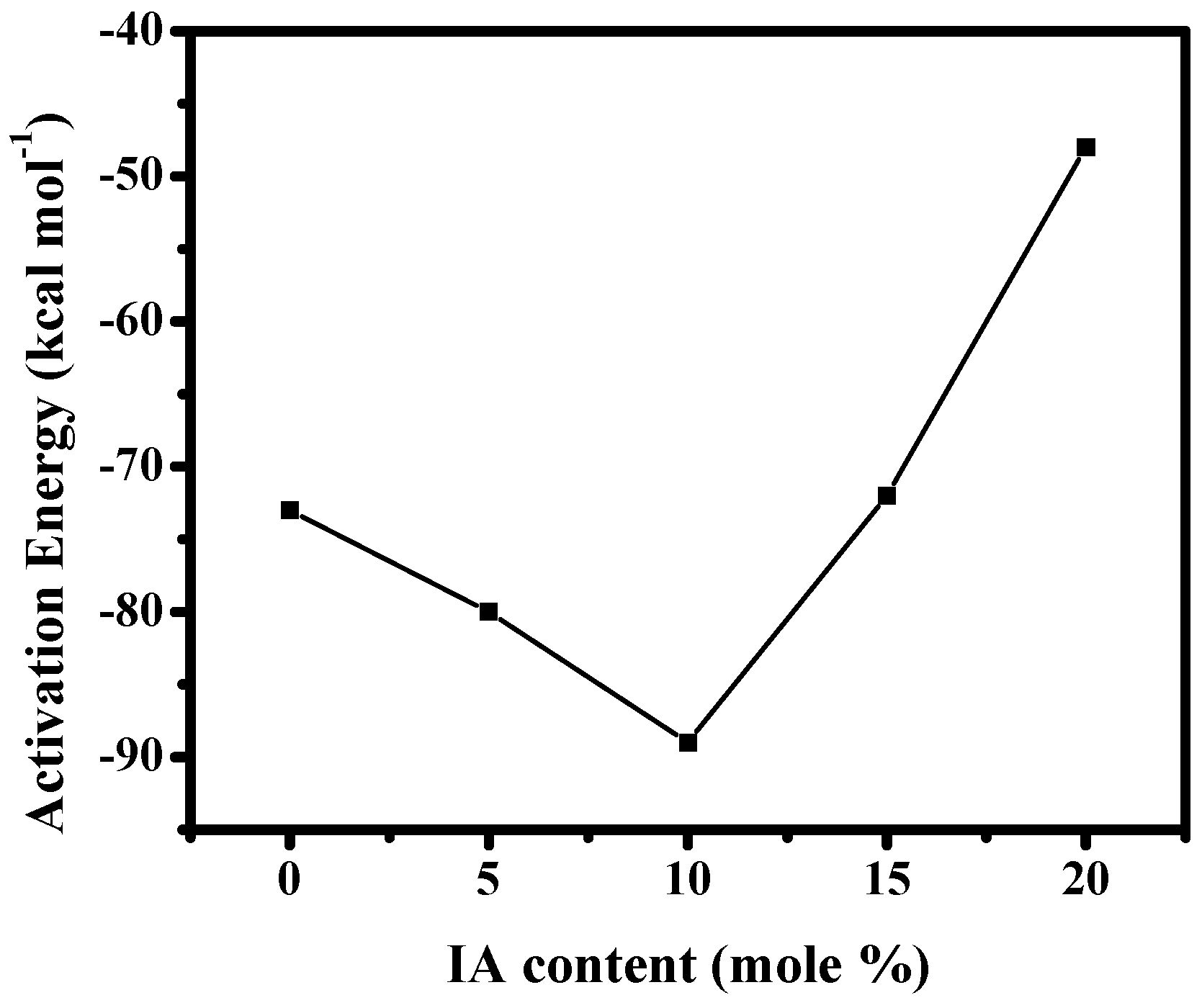
3.2.2. Non-Isothermal Crystallization Kinetics Based on Mo Equation
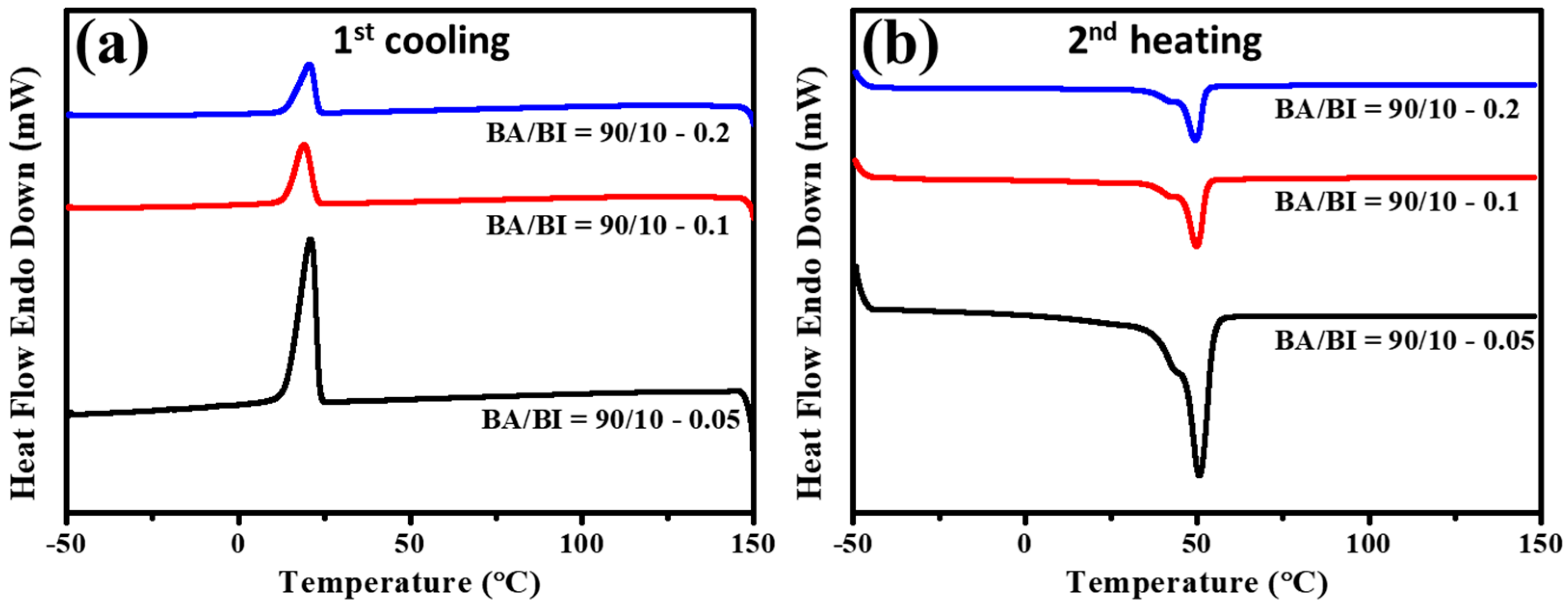
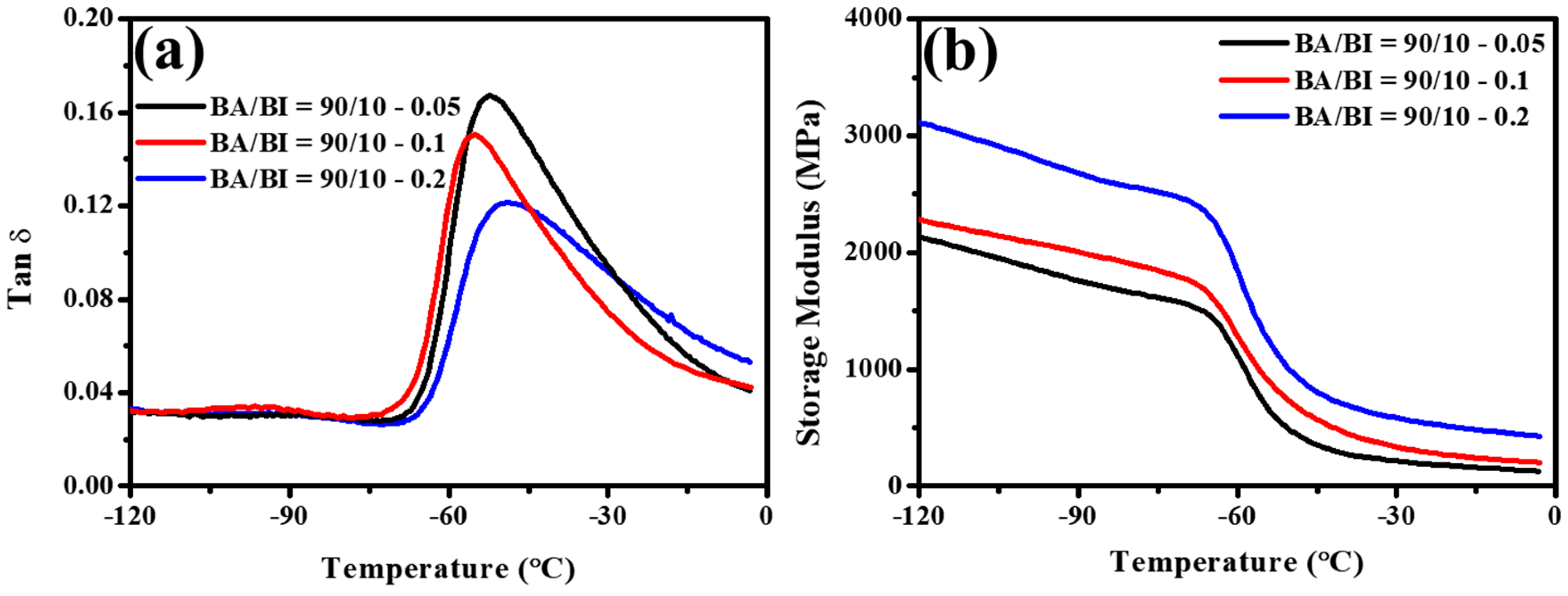
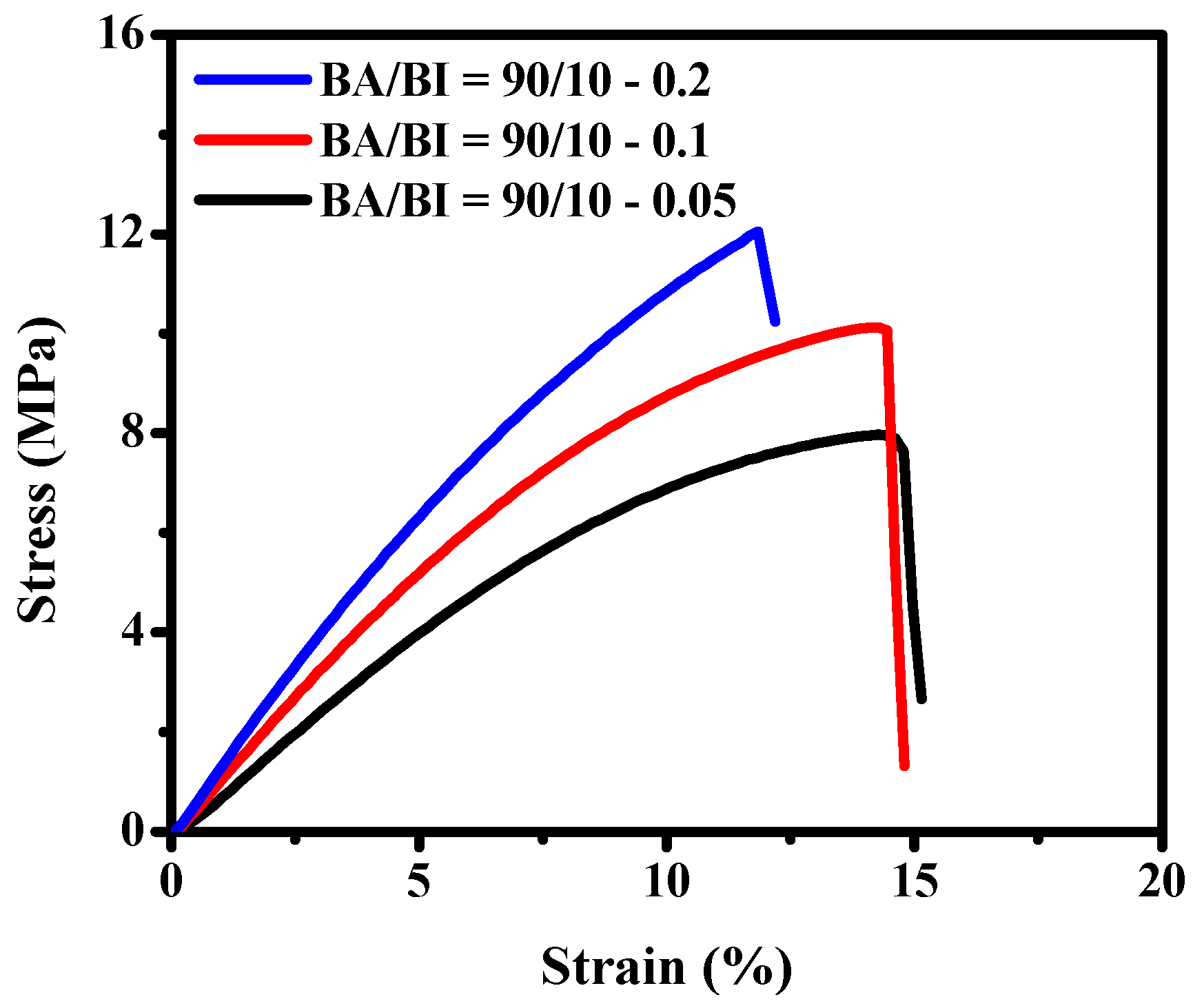
3.3. The Effect of Different BTCA Concentrations at BA/BI = 90/10 of PBABI Copolyesters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zia, K.M.; Noreen, A.; Zuber, M.; Tabasum, S.; Mujahid, M. Recent developments and future prospects on bio-based polyesters derived from renewable resources: A review. Int. J. Biol. Macromol. 2016, 82, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.J.A.; Shaver, M.P. Aliphatic polyester polymer stars: Synthesis, properties and applications in biomedicine and nanotechnology. Chem. Soc. Rev. 2011, 40, 1761–1776. [Google Scholar] [CrossRef] [PubMed]
- Vert, M.; Li, S.M.; Spenlehauer, G.; Guerin, P. Bioresorbability and biocompatibility of aliphatic polyesters. J. Mater. Sci. Mater. Med. 1992, 3, 432–446. [Google Scholar] [CrossRef]
- Douka, A.; Vouyiouka, S.; Papaspyridi, L.-M.; Papaspyrides, C.D. A review on enzymatic polymerization to produce polycondensation polymers: The case of aliphatic polyesters, polyamides and polyesteramides. Prog. Polym. Sci. 2018, 79, 1–25. [Google Scholar] [CrossRef]
- Vert, M. Aliphatic Polyesters: Great Degradable Polymers That Cannot Do Everything †. Biomacromolecules 2005, 6, 538–546. [Google Scholar] [CrossRef]
- Díaz, A.; Katsarava, R.; Puiggalí, J. Synthesis, Properties and Applications of Biodegradable Polymers Derived from Diols and Dicarboxylic Acids: From Polyesters to Poly(ester amide)s. Int. J. Mol. Sci. 2014, 15, 7064–7123. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, Z.; Liu, Q.; Wang, Z.; Jin, J. Synthesis and biodegradation of aliphatic polyesters from dicarboxylic acids and diols. J. Appl. Polym. Sci. 2003, 90, 982–990. [Google Scholar] [CrossRef]
- Zhou, C.; Wei, Z.; Yu, Y.; Shao, S.; Leng, X.; Wang, Y.; Li, Y. Biobased long-chain aliphatic polyesters of 1,12-dodecanedioic acid with a variety of diols: Odd-even effect and mechanical properties. Mater. Today Commun. 2019, 19, 450–458. [Google Scholar] [CrossRef]
- Dai, J.; Ma, S.; Wu, Y.; Zhu, J.; Liu, X. High bio-based content waterborne UV-curable coatings with excellent adhesion and flexibility. Prog. Org. Coat. 2015, 87, 197–203. [Google Scholar] [CrossRef]
- Dai, J.; Ma, S.; Liu, X.; Han, L.; Wu, Y.; Dai, X.; Zhu, J. Synthesis of bio-based unsaturated polyester resins and their application in waterborne UV-curable coatings. Prog. Org. Coat. 2015, 78, 49–54. [Google Scholar] [CrossRef]
- Fidanovski, B.Z.; Spasojevic, P.M.; Panic, V.V.; Seslija, S.I.; Spasojevic, J.P.; Popovic, I.G. Synthesis and characterization of fully bio-based unsaturated polyester resins. J. Mater. Sci. 2018, 53, 4635–4644. [Google Scholar] [CrossRef]
- Mehta, L.B.; Wadgaonkar, K.K.; Jagtap, R.N. Synthesis and characterization of high bio-based content unsaturated polyester resin for wood coating from itaconic acid: Effect of various reactive diluents as an alternative to styrene. J. Dispers. Sci. Technol. 2019, 40, 756–765. [Google Scholar] [CrossRef]
- Farmer, T.J.; Comerford, J.W.; Pellis, A.; Robert, T. Post-polymerization modification of bio-based polymers: Maximizing the high functionality of polymers derived from biomass: Post-polymerization modification of bio-based polymers. Polym. Int. 2018, 67, 775–789. [Google Scholar] [CrossRef]
- Ali, M.A.; Tateyama, S.; Oka, Y.; Kaneko, D.; Okajima, M.K.; Kaneko, T. Syntheses of high-performance biopolyamides derived from itaconic acid and their environmental corrosion. Macromolecules 2013, 46, 3719–3725. [Google Scholar] [CrossRef]
- Kumar, S.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Synthesis and characterization of itaconic-based epoxy resins. Polym. Adv. Technol. 2018, 29, 160–170. [Google Scholar] [CrossRef]
- Sanchez, I.C.; Eby, R.K. Crystallization of random copolymers. J. Res. Natl. Bur. Stand. Sect. Phys. Chem. 1973, 77A, 353. [Google Scholar] [CrossRef]
- Moore, O.B.; Hanson, P.-A.; Comerford, J.W.; Pellis, A.; Farmer, T.J. Improving the Post-polymerization Modification of Bio-Based Itaconate Unsaturated Polyesters: Catalyzing Aza-Michael Additions With Reusable Iodine on Acidic Alumina. Front. Chem. 2019, 7, 501. [Google Scholar] [CrossRef]
- Kim, J.; Kong, Y.P.; Niedzielski, S.M.; Singh, R.K.; Putnam, A.J.; Shikanov, A. Characterization of the crosslinking kinetics of multi-arm poly(ethylene glycol) hydrogels formed via Michael-type addition. Soft Matter 2016, 12, 2076–2085. [Google Scholar] [CrossRef]
- Poulopoulou, N.; Kantoutsis, G.; Bikiaris, D.N.; Achilias, D.S.; Kapnisti, M.; Papageorgiou, G.Z. Biobased Engineering Thermoplastics: Poly(butylene 2,5-furandicarboxylate) Blends. Polymers 2019, 11, 937. [Google Scholar] [CrossRef]
- Guidotti, G.; Genovese, L.; Soccio, M.; Gigli, M.; Munari, A.; Siracusa, V.; Lotti, N. Block Copolyesters Containing 2,5-Furan and trans-1,4-Cyclohexane Subunits with Outstanding Gas Barrier Properties. Int. J. Mol. Sci. 2019, 20, 2187. [Google Scholar] [CrossRef]
- Maniar, D.; Jiang, Y.; Woortman, A.J.J.; van Dijken, J.; Loos, K. Furan-Based Copolyesters from Renewable Resources: Enzymatic Synthesis and Properties. ChemSusChem 2019, 12, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Mincheva, R.; Xu, Y.; Raquez, J.-M.; Dubois, P. High Molecular Weight Poly(butylene succinate-co-butylene furandicarboxylate) Copolyesters: From Catalyzed Polycondensation Reaction to Thermomechanical Properties. Biomacromolecules 2012, 13, 2973–2981. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-Y.; Chen, C.-W.; Rwei, S.-P. Influence of asymmetric substituent group 2-methyl-1,3-propanediol on bio-based poly(propylene furandicarboxylate) copolyesters. Soft Matter 2020, 16, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.; Cho, C.; Hsu, K.; He, C.; Kuo, C.; Chu, C.; Chen, Y.; Chen, C.; Rwei, S. Smart Wearable Textiles with Breathable Properties and Repeatable Shaping in In Vitro Orthopedic Support from a Novel Biomass Thermoplastic Copolyester. Macromol. Mater. Eng. 2019, 304, 1900103. [Google Scholar] [CrossRef]
- Cho, C.-J.; Chang, Y.-S.; Lin, Y.-Z.; Jiang, D.-H.; Chen, W.-H.; Lin, W.-Y.; Chen, C.-W.; Rwei, S.-P.; Kuo, C.-C. Green electrospun nanofiber membranes filter prepared from novel biomass thermoplastic copolyester: Morphologies and filtration properties. J. Taiwan Inst. Chem. Eng. 2020, 106, 206–214. [Google Scholar] [CrossRef]
- Hsu, K.-H.; Chen, C.-W.; Wang, L.-Y.; Chan, H.-W.; He, C.-L.; Cho, C.-J.; Rwei, S.-P.; Kuo, C.-C. Bio-based thermoplastic poly(butylene succinate-co-propylene succinate) copolyesters: Effect of glycerol on thermal and mechanical properties. Soft Matter 2019, 15, 9710–9720. [Google Scholar] [CrossRef]
- Chen, C.-W.; Hsu, T.-S.; Rwei, S.-P. Effect of Ethylenediaminetetraacetic Acid on Unsaturated Poly(Butylene Adipate-Co-Butylene Itaconate) Copolyester with Low-Melting Point and Controllable Hardness. Polymers 2019, 11, 611. [Google Scholar] [CrossRef]
- Chen, C.-W.; Hsu, T.-S.; Rwei, S.-P. Isothermal Kinetics of Poly(butylene adipate-co-butylene itaconate) Copolyesters with Ethylenediaminetetraacetic Acid. ACS Omega 2020, 5, 3080–3089. [Google Scholar] [CrossRef]
- Xiao, X.; Sui, C.; Han, L.; Liu, J.; Feng, B. Self-assembly of triorganotin(IV) moiety with 1,2,4,5-benzenetetracarboxylic acid: Syntheses, characterizations, and influence of solvent on the molecular structure (II). Heteroat. Chem. 2017, 28, e21356. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, L.; Wang, Y. New Hydrogen-bonded Supramolecular Hydrogels and Fibers Derived from 1,2,4,5-Benzenetetracarboxylic Acid and 4-Hydroxypyridine. Chem. Lett. 2006, 35, 548–549. [Google Scholar] [CrossRef]
- Karki, S.; Friščić, T.; Jones, W. Control and interconversion of cocrystal stoichiometry in grinding: stepwise mechanism for the formation of a hydrogen-bonded cocrystal. CrystEngComm 2009, 11, 470–481. [Google Scholar] [CrossRef]
- Tang, T.; Moyori, T.; Takasu, A. Isomerization-Free Polycondensations of Cyclic Anhydrides with Diols and Preparation of Polyester Gels Containing Cis or Trans Carbon Double Bonds via Photo-Cross-Linking and Isomerization in the Gels. Macromolecules 2013, 46, 5464–5472. [Google Scholar] [CrossRef]
- Brännström, S.; Finnveden, M.; Johansson, M.; Martinelle, M.; Malmström, E. Itaconate based polyesters: Selectivity and performance of esterification catalysts. Eur. Polym. J. 2018, 103, 370–377. [Google Scholar] [CrossRef]
- Ina Schoon; Marcel Kluge; Steven Eschig; Tobias Robert Catalyst Influence on Undesired Side Reactions in the Polycondensation of Fully Bio-Based Polyester Itaconates. Polymers 2017, 9, 693. [CrossRef] [PubMed]
- Brännström, S.; Malmström, E.; Johansson, M. Biobased UV-curable coatings based on itaconic acid. J. Coat. Technol. Res. 2017, 14, 851–861. [Google Scholar] [CrossRef]
- Tang, T.; Takasu, A. Facile synthesis of unsaturated polyester-based double-network gels via chemoselective cross-linking using Michael addition and subsequent UV-initiated radical polymerization. RSC Adv. 2015, 5, 819–829. [Google Scholar] [CrossRef]
- Wu, M.C.; Woo, E. Effects of α-form or β-form nuclei on polymorphic crystalline morphology of poly(butylene adipate). Polym. Int. 2005, 54, 1681–1688. [Google Scholar] [CrossRef]
- Woo, E.M.; Wu, M.C. Thermal and X-ray analysis of polymorphic crystals, melting, and crystalline transformation in poly(butylene adipate). J. Polym. Sci. Part B Polym. Phys. 2005, 43, 1662–1672. [Google Scholar] [CrossRef]
- Gao, C.; Wang, J.; Han, S.; Hu, Z.; Liu, Y. Copolymerization modification of poly (butylene itaconate). In Proceedings of the AIP Conference Proceedings, Chongqing City, China, 27–28 May 2017; Volume 1864, p. 020221. [Google Scholar]
- Wang, H.; Gao, Z.; Yang, X.; Liu, K.; Zhang, M.; Qiang, X.; Wang, X. Epitaxial Crystallization Behavior of Poly(butylene adipate) on Orientated Poly(butylene succinate) Substrate. Polymers 2018, 10, 110. [Google Scholar] [CrossRef]
- Gan, Z.; Abe, H.; Doi, Y. Temperature-Induced Polymorphic Crystals of Poly(butylene adipate). Macromol. Chem. Phys. 2002, 203, 2369–2374. [Google Scholar] [CrossRef]
- Hou, C.; Li, H.; Sun, X.; Yan, S.; Wang, Y.; Chen, S. The dependence of the β-to-α phase transition behavior of poly(1,4-butylene adipate) on phase separated morphology in its blends with poly(vinylidene fluoride). Phys. Chem. Chem. Phys. 2018, 20, 15718–15724. [Google Scholar] [CrossRef] [PubMed]
- Minke, R.; Blackwell, J. Polymorphic structures of poly(tetramethylene adipate). J. Macromol. Sci. Part B 1979, 16, 407–417. [Google Scholar] [CrossRef]
- Noguchi, K.; Kondo, H.; Ichikawa, Y.; Okuyama, K.; Washiyama, J. Molecular and crystal structure of poly(tetramethylene adipate) α form based on synchrotron X-ray fiber diffraction. Polymer 2005, 46, 10823–10830. [Google Scholar] [CrossRef]
- Panic, V.V.; Seslija, S.I.; Popovic, I.G.; Spasojevic, V.D.; Popovic, A.R.; Nikolic, V.B.; Spasojevic, P.M. Simple one-pot synthesis of fully biobased unsaturated polyester resins based on itaconic acid. Biomacromolecules 2017, 18, 3881–3891. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, H.; Wan, X.; Zhang, D.; Zhou, Q.-F.; Woo, E.M.; Turner, S.R. Effect of rod-like imide unit on crystallization of copoly(ethylene terephthalate-imide). Polymer 2002, 43, 7377–7387. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, H.; Wan, X.; Zhang, D.; Zhou, Q.-F.; Woo, E.M.; Turner, S.R. Crystallization kinetics of new copoly(ethylene terephthalate-imide)s. Polymer 2002, 43, 3683–3690. [Google Scholar] [CrossRef]
- Müller, A.J.; Michell, R.M.; Lorenzo, A.T. Isothermal Crystallization Kinetics of Polymers. In Polymer Morphology; Guo, Q., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 181–203. ISBN 978-1-118-89275-6. [Google Scholar]
- Avrami, M. Kinetics of Phase Change. II Transformation-Time Relations for Random Distribution of Nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Lu, X.F.; Hay, J.N. Isothermal crystallization kinetics and melting behaviour of poly(ethylene terephthalate). Polymer 2001, 42, 9423–9431. [Google Scholar] [CrossRef]
- Keridou, I.; del Valle, L.J.; Funk, L.; Turon, P.; Franco, L.; Puiggalí, J. Non-Isothermal Crystallization Kinetics of Poly(4-Hydroxybutyrate) Biopolymer. Molecules 2019, 24, 2840. [Google Scholar] [CrossRef]
- Xie, W.-J.; Zhou, X.-M. Non-isothermal crystallization kinetics and characterization of biodegradable poly(butylene succinate-co-neopentyl glycol succinate) copolyesters. Mater. Sci. Eng. C 2015, 46, 366–373. [Google Scholar] [CrossRef]
- Yarici, T.; Kodal, M.; Ozkoc, G. Non-isothermal crystallization kinetics of Poly(Butylene succinate) (PBS) nanocomposites with different modified carbon nanotubes. Polymer 2018, 146, 361–377. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetics of non-isothermal crystallization. Polymer 1971, 12, 150–158. [Google Scholar] [CrossRef]
- Liu, T.; Mo, Z.; Wang, S.; Zhang, H. Nonisothermal melt and cold crystallization kinetics of poly(aryl ether ether ketone ketone). Polym. Eng. Sci. 1997, 37, 568–575. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Dobreva, A.; Alonso, M.; Gonzalez, M.; Gonzalez, A.; de Saja, J.A. A non-isothermal differential scanning calorimetry method for the determination of specific surface energies in polymer crystals. Thermochim. Acta 1995, 258, 197–204. [Google Scholar] [CrossRef]
- Fisher, J.P.; Timmer, M.D.; Holland, T.A.; Dean, D.; Engel, P.S.; Mikos, A.G. Photoinitiated Cross-Linking of the Biodegradable Polyester Poly(propylene fumarate). Part I. Determination of Network Structure. Biomacromolecules 2003, 4, 1327–1334. [Google Scholar] [CrossRef]
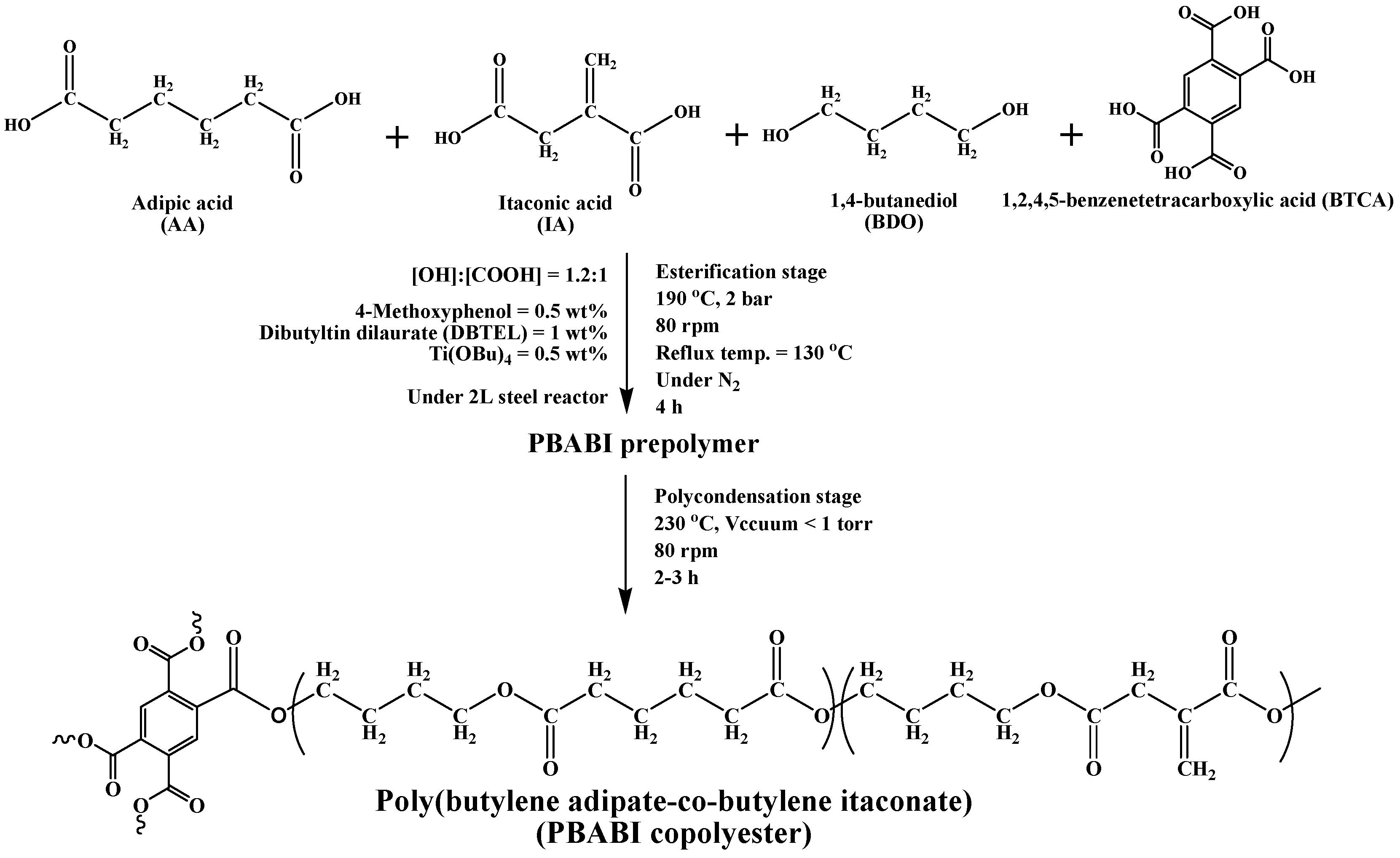
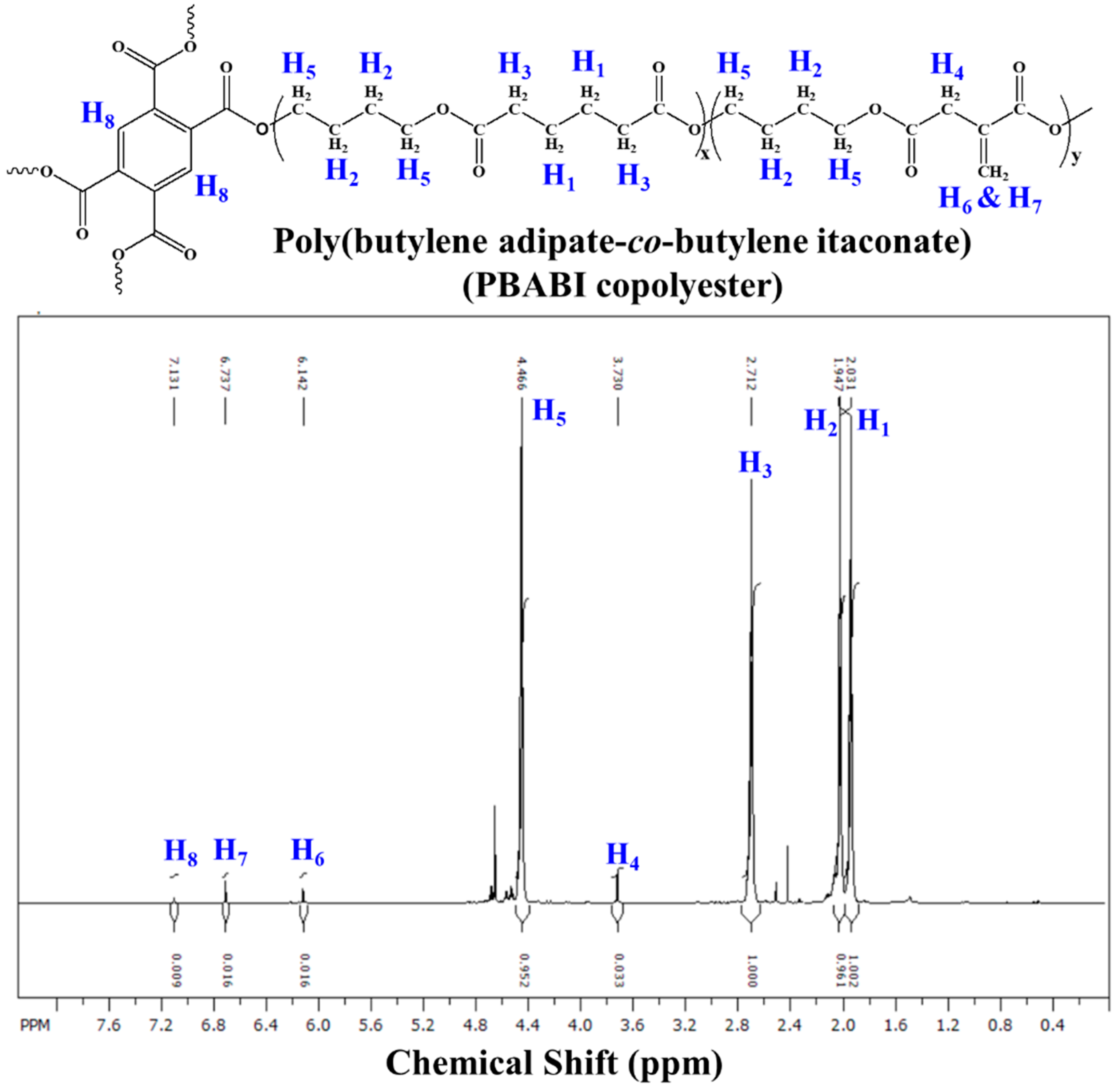
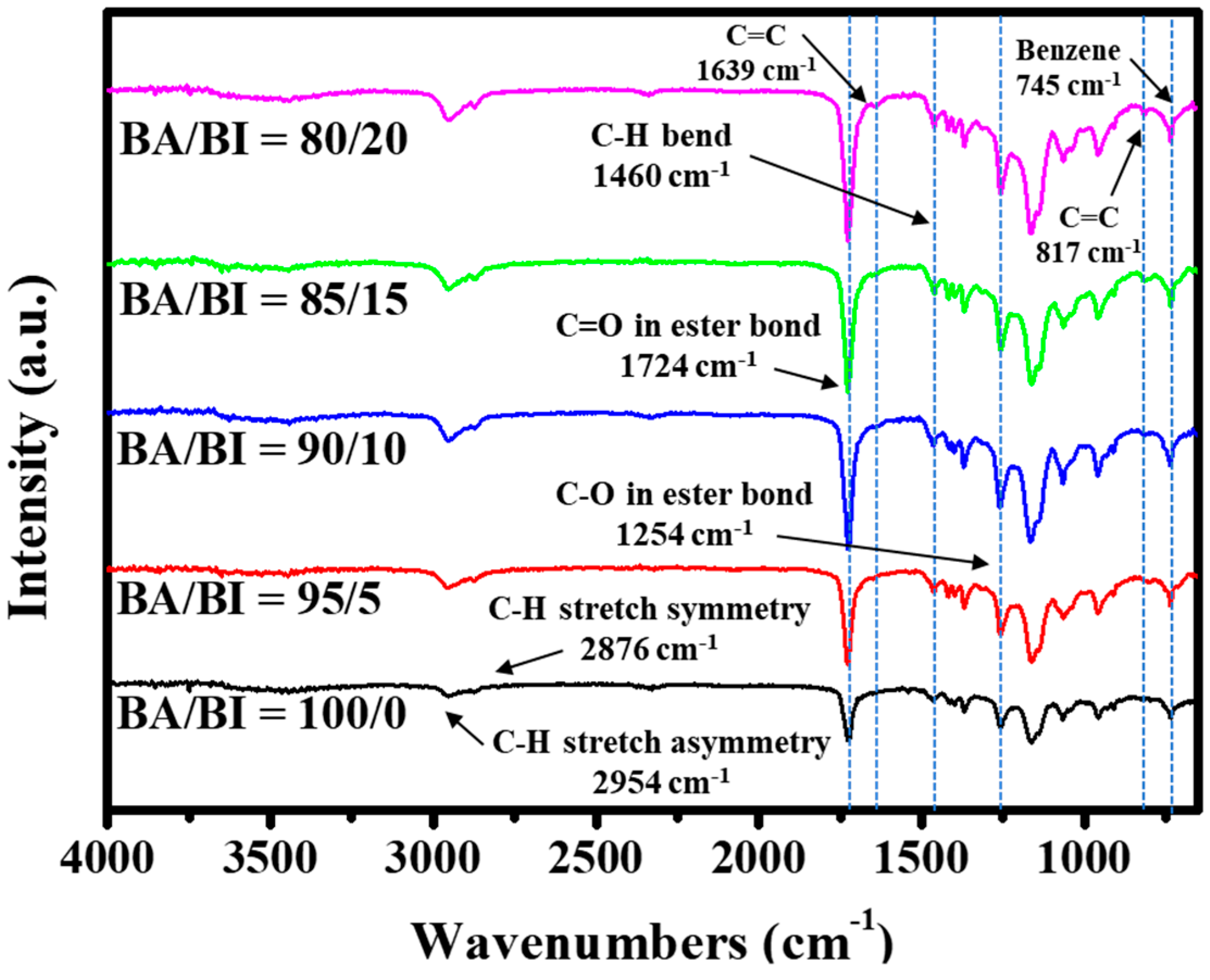
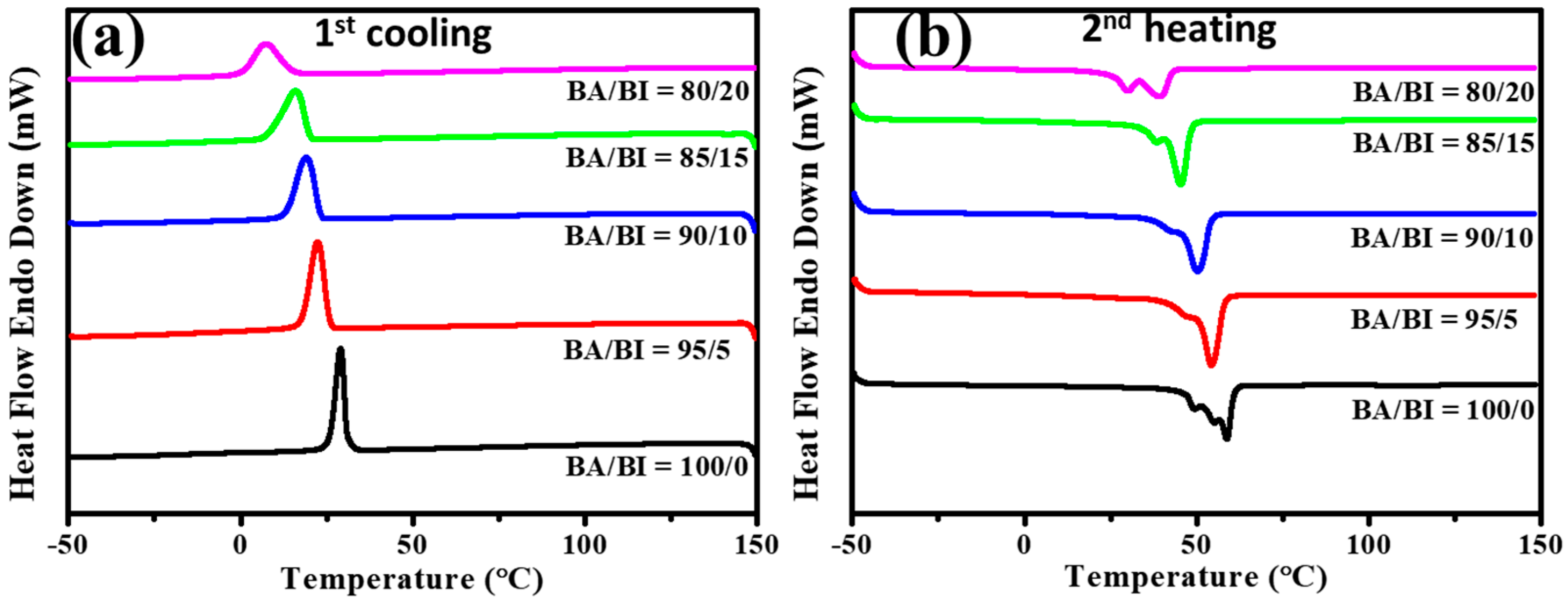
| Sample | I.V. (dL g−1) | Mn (g mole−1) | Mw (g mole−1) | Mw/Mn (PDI) |
|---|---|---|---|---|
| BA/BI = 100/0 | 0.75 | 16,787 | 36,064 | 2.15 |
| BA/BI = 95/5 | 1.17 | 26,281 | 64,126 | 2.44 |
| BA/BI = 90/10 | 1.23 | 31,970 | 80,564 | 2.52 |
| BA/BI = 85/15 | 1.27 | 34,791 | 134,641 | 3.87 |
| BA/BI = 80/20 | 1.25 | 39,024 | 161,950 | 4.15 |
| Sample | *Tg | Tc | ΔHc | Tm | ΔHm | Td-5% | #Xc |
|---|---|---|---|---|---|---|---|
| (°C) | (°C) | (mJ mg−1) | (°C) | (mJ mg−1) | (°C) | (%) | |
| BA/BI = 100/0 | −51.1 | 29.0 | −60.6 | 49.0 | 57.3 | 341.2 | 42.4 |
| BA/BI = 95/5 | −54.6 | 22.5 | −59.4 | 47.4 | 56.0 | 346.5 | 41.6 |
| BA/BI = 90/10 | −58.1 | 18.9 | −57.6 | 42.6 | 52.7 | 348.1 | 40.3 |
| BA/BI = 85/15 | −57.6 | 15.8 | −57.3 | 38.4 | 52.2 | 336.1 | 39.9 |
| BA/BI = 80/20 | −54.6 | 7.2 | −47.5 | 29.8 | 45.0 | 332.6 | 34.2 |
| Sample | Yield Strength (MPa) | Elongation (%) | Young’s Modulus (MPa) |
|---|---|---|---|
| BA/BI = 100/0 | 15.19 ± 1.15 | 56.13 ± 3.99 | 162.95 ± 12.29 |
| BA/BI = 95/5 | 14.69 ± 1.01 | 32.73 ± 3.45 | 158.45 ± 10.45 |
| BA/BI = 90/10 | 10.12 ± 0.36 | 14.79 ± 2.23 | 96.08 ± 7.82 |
| BA/BI = 85/15 | 8.66 ± 0.33 | 13.78 ± 1.14 | 78.72 ± 6.26 |
| BA/BI = 80/20 | 5.81 ± 0.19 | 13.91 ± 1.04 | 32.19 ± 1.45 |
| Sample | ϕ (°C min−1) | * To (°C) | * Tp (°C) | n | K (min-n) | t1/2 (min) | G (min−1) |
|---|---|---|---|---|---|---|---|
| BA/BI = 100/0 | 2 | 40.36 | 37.15 | 5.76 | 0.3449 | 1.1288 | 0.8859 |
| 5 | 37.63 | 33.07 | 4.28 | 0.1372 | 1.4600 | 0.6849 | |
| 10 | 35.31 | 29.46 | 5.19 | 0.0008 | 3.6816 | 0.2716 | |
| BA/BI = 95/5 | 2 | 34.24 | 29.63 | 3.54 | 1.7681 | 0.7676 | 1.3028 |
| 5 | 31.16 | 27.53 | 5.92 | 0.0271 | 1.5975 | 0.6260 | |
| 10 | 28.06 | 24.01 | 3.99 | 0.0133 | 2.6935 | 0.3713 | |
| BA/BI = 90/10 | 2 | 27.67 | 25.68 | 3.28 | 2.1618 | 0.7070 | 1.4145 |
| 5 | 25.97 | 21.99 | 3.94 | 0.4035 | 1.1472 | 0.8717 | |
| 10 | 23.98 | 17.98 | 5.24 | 0.0102 | 2.2370 | 0.4470 | |
| BA/BI = 85/15 | 2 | 25.92 | 23.82 | 5.92 | 0.0515 | 1.5514 | 0.6446 |
| 5 | 24.14 | 20.48 | 5.94 | 0.0098 | 2.0482 | 0.4882 | |
| 10 | 21.77 | 15.97 | 5.62 | 0.0009 | 3.2631 | 0.3065 | |
| BA/BI = 80/20 | 2 | 19.09 | 15.01 | 4.68 | 0.0352 | 1.8904 | 0.5290 |
| 5 | 15.75 | 9.19 | 4.21 | 0.0213 | 2.2869 | 0.4373 | |
| 10 | 11.98 | 2.74 | 4.11 | 0.0033 | 3.6732 | 0.2722 |
| X (t) | BA/BI = 100/0 | BA/BI = 95/5 | BA/BI = 90/10 | BA/BI = 85/15 | BA/BI = 80/20 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| a | F(T) | a | F(T) | a | F(T) | a | F(T) | a | F(T) | |
| 0.2 | 1.23 | 7.56 | 1.09 | 5.33 | 1.36 | 4.41 | 2.11 | 13.27 | 2.46 | 24.46 |
| 0.4 | 1.25 | 9.09 | 1.14 | 6.51 | 1.45 | 5.78 | 2.13 | 21.73 | 2.35 | 34.01 |
| 0.6 | 1.25 | 10.55 | 1.18 | 7.33 | 1.52 | 7.24 | 2.13 | 26.73 | 2.29 | 44.13 |
| 0.8 | 1.27 | 12.63 | 1.29 | 9.77 | 1.62 | 9.49 | 2.16 | 32.76 | 2.31 | 63.21 |
| BA/BI = 95/5 | BA/BI = 90/10 | BA/BI = 85/15 | BA/BI = 80/20 | |
|---|---|---|---|---|
| ϕ | 0.3203 | 0.2869 | 0.4232 | 0.9581 |
| Sample | H1 | H2 | H3 | H4 | H5 | H6 | H7 | H8 |
|---|---|---|---|---|---|---|---|---|
| BA/BI = 90/10–0.05 | 1.893 (1.003) | 1.975 (0.986) | 2.654 (1.000) | 3.678 (0.033) | 4.415 (1.021) | 6.085 (0.019) | 6.678 (0.017) | 7.073 (0.010) |
| BA/BI = 90/10–0.1 | 1.897 (1.004) | 1.971 (0.985) | 2.658 (1.000) | 3.683 (0.045) | 4.419 (1.029) | 6.095 (0.030) | 6.684 (0.024) | 7.072 (0.011) |
| BA/BI = 90/10–0.2 | 1.916 (1.007) | 1.998 (1.026) | 2.677 (1.000) | 3.706 (0.036) | 4.438 (1.024) | 6.102 (0.024) | 6.706 (0.021) | 7.102 (0.014) |
| Sample | * Tg | Tc (onset) | Tc (peak) | ΔHc | Tm | ΔHm |
|---|---|---|---|---|---|---|
| (°C) | (°C) | (°C) | (mJ mg−1) | (°C) | (mJ mg−1) | |
| BA/BI = 90/10–0.05 | −52.4 | 24.5 | 21.1 | −56.5 | 43.4, 50.8 | 51.2 |
| BA/BI = 90/10–0.1 | −54.9 | 23.5 | 19.1 | −52.9 | 41.9, 49.8 | 47.9 |
| BA/BI = 90/10–0.2 | −49.1 | 23.4 | 20.8 | −41.4 | 42.1, 49.6 | 38.2 |
| Sample | Yield Strength (MPa) | Elongation (%) | Young’s Modulus (MPa) |
|---|---|---|---|
| BA/BI = 90/10–0.05 | 7.97 ± 0.04 | 15.03 ± 2.56 | 56.49 ± 3.41 |
| BA/BI = 90/10–0.1 | 10.12 ± 0.36 | 14.79 ± 2.23 | 96.08 ± 7.82 |
| BA/BI = 90/10–0.2 | 11.85 ± 0.14 | 12.19 ± 2.11 | 141.39 ± 7.97 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-W.; Hsu, T.-S.; Huang, K.-W.; Rwei, S.-P. Effect of 1,2,4,5-Benzenetetracarboxylic Acid on Unsaturated Poly(butylene adipate-co-butylene itaconate) Copolyesters: Synthesis, Non-Isothermal Crystallization Kinetics, Thermal and Mechanical Properties. Polymers 2020, 12, 1160. https://doi.org/10.3390/polym12051160
Chen C-W, Hsu T-S, Huang K-W, Rwei S-P. Effect of 1,2,4,5-Benzenetetracarboxylic Acid on Unsaturated Poly(butylene adipate-co-butylene itaconate) Copolyesters: Synthesis, Non-Isothermal Crystallization Kinetics, Thermal and Mechanical Properties. Polymers. 2020; 12(5):1160. https://doi.org/10.3390/polym12051160
Chicago/Turabian StyleChen, Chin-Wen, Te-Sheng Hsu, Kuan-Wei Huang, and Syang-Peng Rwei. 2020. "Effect of 1,2,4,5-Benzenetetracarboxylic Acid on Unsaturated Poly(butylene adipate-co-butylene itaconate) Copolyesters: Synthesis, Non-Isothermal Crystallization Kinetics, Thermal and Mechanical Properties" Polymers 12, no. 5: 1160. https://doi.org/10.3390/polym12051160
APA StyleChen, C.-W., Hsu, T.-S., Huang, K.-W., & Rwei, S.-P. (2020). Effect of 1,2,4,5-Benzenetetracarboxylic Acid on Unsaturated Poly(butylene adipate-co-butylene itaconate) Copolyesters: Synthesis, Non-Isothermal Crystallization Kinetics, Thermal and Mechanical Properties. Polymers, 12(5), 1160. https://doi.org/10.3390/polym12051160







