Functional Polymers Structures for (Bio)Sensing Application—A Review
Abstract
1. Introduction
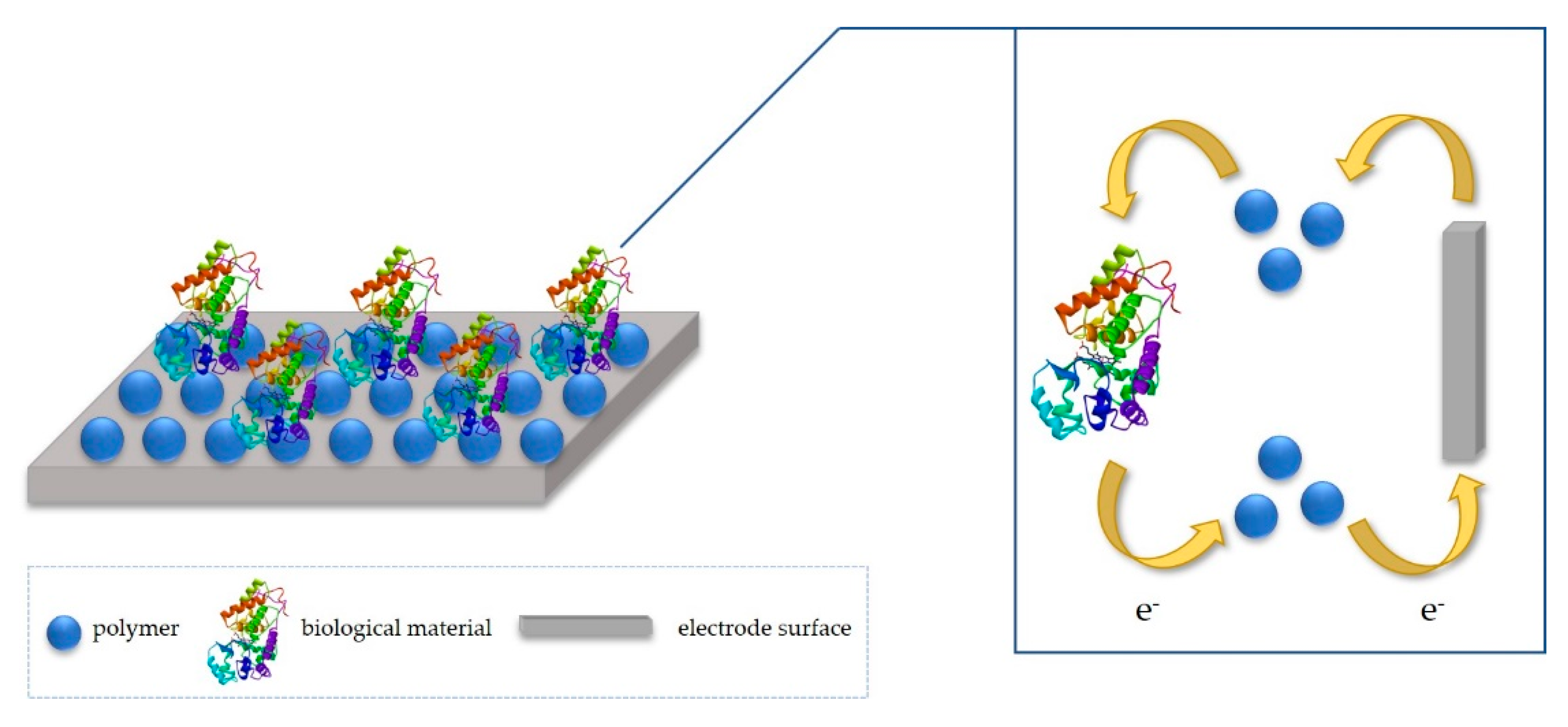
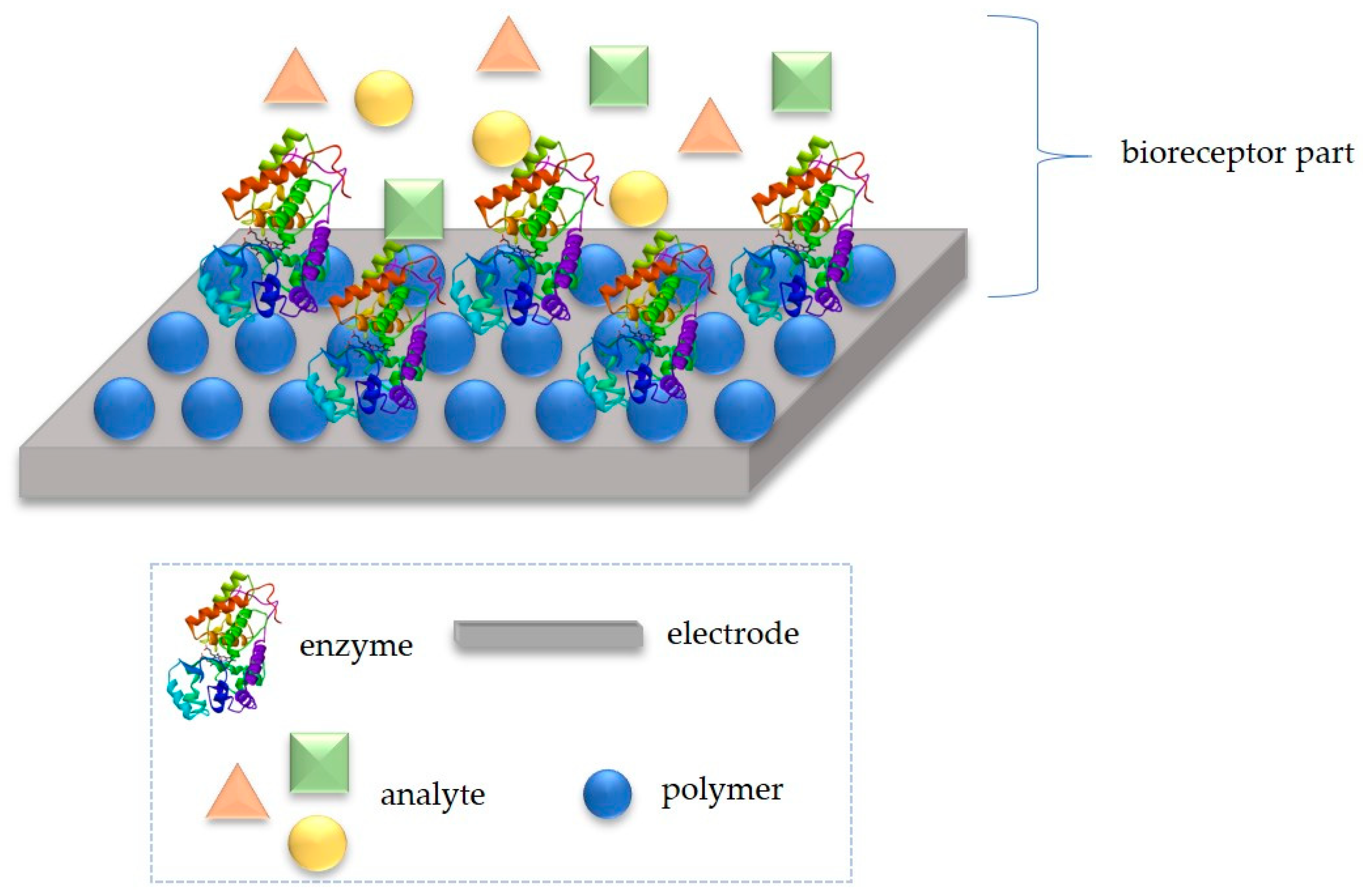
2. Idea of Detection Mechanism
3. Conductive Polymers
3.1. The Thin CP Films
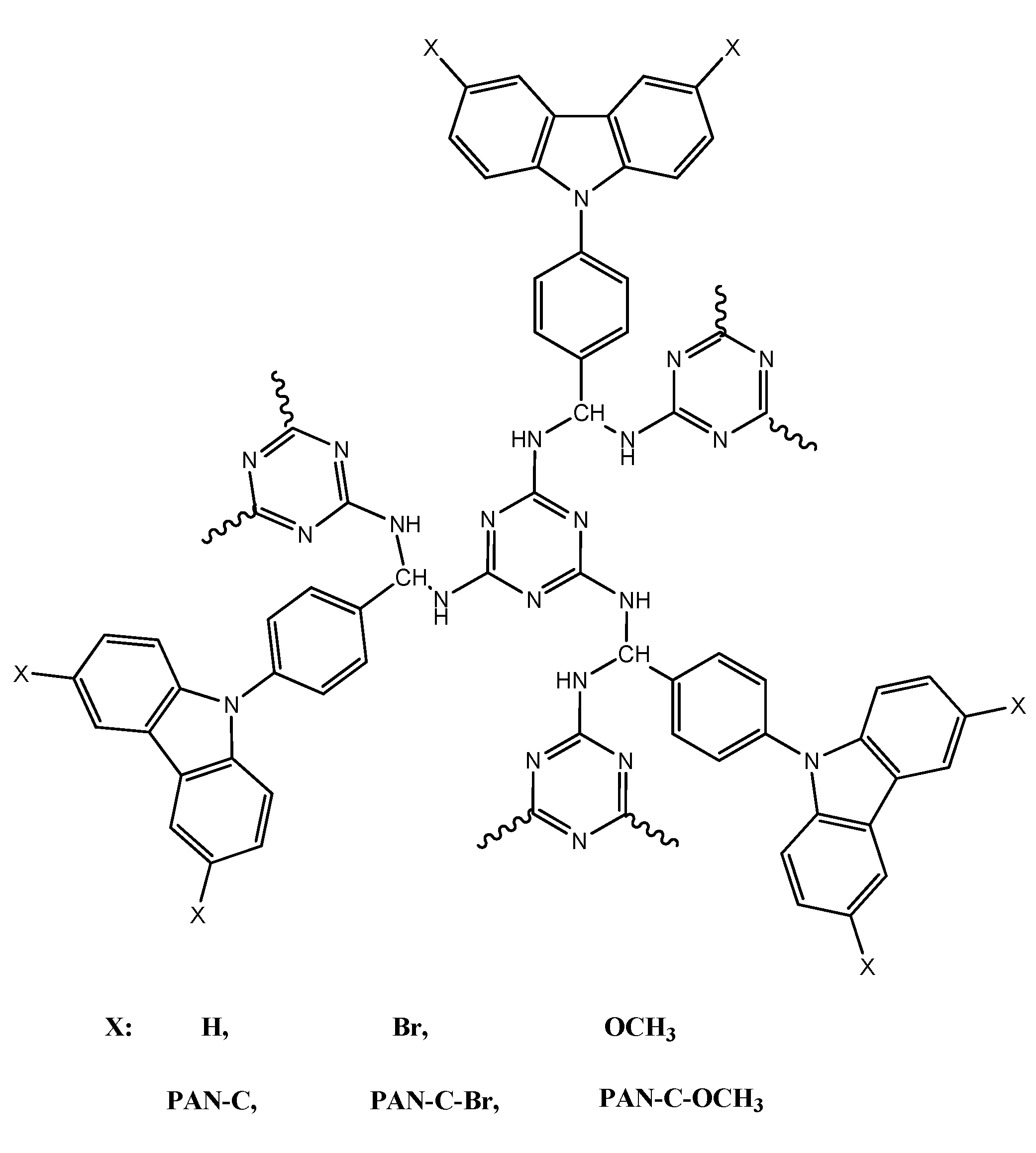
3.2. Conjugated Microporous Polymer (CMP)
3.3. Polymer Gels
4. Composites
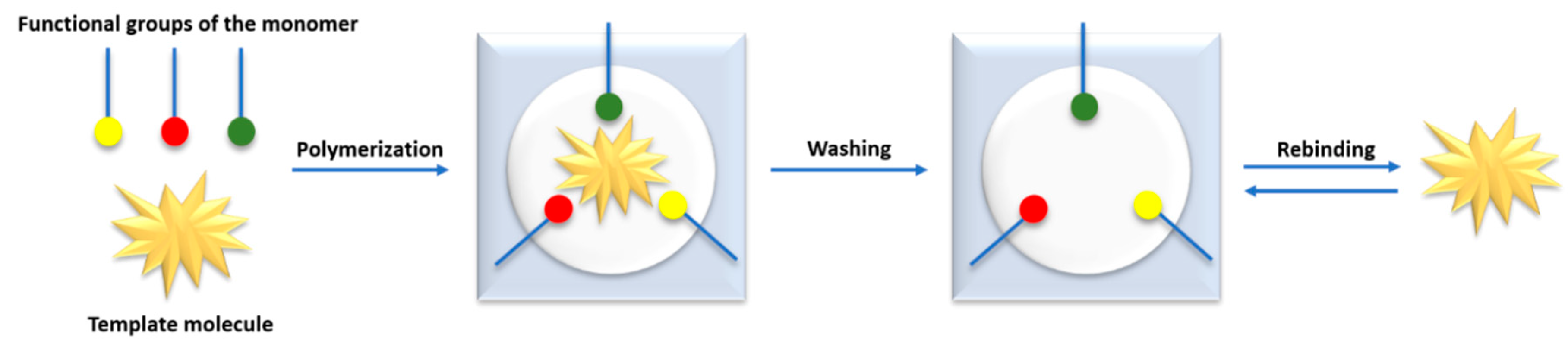
5. Molecularly Imprinted Polymers
5.1. Molecularly Imprinted Polymers (MIPs)
5.2. Selection of the Appropriate Functional Monomer
5.3. MIPs-Based Sensors
5.4. Recent Advances in Molecularly Imprinted Polymer Based Sensors
6. Application of Polymeric Materials in LOC Devices
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ngoepe, M.; Choonara, Y.E.; Tygai, C.; Tomar, L.K.; du Toit, L.C.; Kumar, P.; Ndesendo, V.M.K.; Pillay, V. Integration of Biosensors and Drug Delivery Technologies for Early Detection and Chronic Management of Illness. Sensors 2013, 13, 7680–7713. [Google Scholar] [CrossRef] [PubMed]
- Sabri, N.; Aljunid, S.A.; Salim, M.S.; Ahmad, R.B.; Kamaruddin, R. Toward optical sensors: Review and applications. J. Phys. Conf. Ser. 2013, 423, 012064. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Nabeel, F.; Iqbal, H.M.N.; Li, C.; Zhou, Y. Fluorescent sensor based models for the detection of environmentally-related toxic heavy metals. Sci. Total Environ. 2018, 615, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, T.; Nabeel, F. Luminescent metal-organic frameworks as potential sensory materials for various environmental toxic agents. Coordination Chem. Rev. 2019, 401, 213065. [Google Scholar] [CrossRef]
- Rasheed, T.; Li, C.; Nabeel, F.; Huang, W.; Zhou, Y. Self-assembly of alternating copolymer vesicles for the highly selective, sensitive and visual detection and quantification of aqueous Hg2+. Chem. Eng. J. 2018, 358, 101–109. [Google Scholar] [CrossRef]
- Ren, K.; Zhou, J.; Wu, H. Materials for Microfluidic Chip Fabrication. Acc. Chem. Res. 2013, 46, 2396–2406. [Google Scholar] [CrossRef] [PubMed]
- Klank, J.; Kutter, P.; Geschke, O. CO2-laser micromachining and back-end processing for rapid production of PMMA-based microfluidic systems. Lab Chip 2002, 2, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Sollier, E.; Murray, C.; Maoddi, P.; Di Carlo, D. Rapid prototyping polymers for microfluidic devices and high pressure injections. Lab Chip 2011, 11, 3752–3765. [Google Scholar] [CrossRef]
- Long, J.J.; Benoudjit, A.M.; Arris, F.A.; Ali, F.; Wan Salim, W.W.A. Polymers in biosensors. Multifaced Protocol Biotechnol. 2018, 151–166. [Google Scholar] [CrossRef]
- Hu, J.; Liu, S. Responsive polymers for detection and sensing applications: Current status and future developments. Macromolecules 2010, 43, 8315–8330. [Google Scholar] [CrossRef]
- Rodriguez-Mozas, S.; Lopez de Alda, M.J.; Barcelo, D. Biosensors as useful tools for environmental analysis and monitoring. Anal. Bioanal. Chem. 2006, 386, 1025–1041. [Google Scholar] [CrossRef] [PubMed]
- Yuging, M.; Jiaprong, C.; Xiaohua, W. Using electropolymerized non-conducting polymers to develop enzyme amperometric biosensors. Trends Biotechnol. 2004, 22, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Ess. Biochem. 2016, 60, 1–8. [Google Scholar]
- Bouchta, D.; Izaoumen, N.; Zejli, H.; El Kaoutit, M.; Temsamani, K.R. A novel electrochemical synthesis of poly-3-methylthiophene-gamma-cyclodextrin film Application for the analysis of chlorpromazine and some neurotransmitters. Biosens. Bioelectron. 2005, 20, 2228–2235. [Google Scholar] [CrossRef] [PubMed]
- Cabaj, J.; Sołoducho, J.; Chyla, A.; Jędrychowska, A. Hybrid phenol biosensor based on modified phenoloxidase electrode. Sens. Actuators B 2011, 157, 225–231. [Google Scholar] [CrossRef]
- Nazari, M.; Kashanian, S.; Rafipour, R. Laccase immobilization on the electrode surface to design a biosensor for the detection of phenolic compound such as catechol. Spectrochim. Acta A 2015, 145, 130–138. [Google Scholar] [CrossRef]
- Naveen, M.H.; Gurudatt, N.G.; Shim, Y.-B. Applications of conducting polymer composites to electrochemical sensors: A review. Appl. Mater. Today 2017, 9, 419–433. [Google Scholar] [CrossRef]
- Le, T.-H.; Kim, Y.; Yoon, H. Electrical and electrochemical properties of conducting polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef]
- Lee, S.H.; Cho, W.; Hwang, D.K.; Lee, T.K.; Kang, Y.S.; Im, S.S. Synthesis of poly(3,4-ethylene dioxythiophene)/ammonium vanadate nanofiber composites for counter electrode of dye-sensitized solar cells. Electrochim. Acta 2017, 245, 607–614. [Google Scholar] [CrossRef]
- Thomas, J.P.; Rahman, M.A.; Srivastava, S.; Kang, J.S.; McGillivray, D.; Abd-Ellah, M.; Heinig, N.F.; Leung, K.T. Highly conducting hybrid silver-nanowire-embedded poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) for high-efficiency planar silicon/organic heterojunction solar cells. ACS Nano 2018, 12, 9495–9503. [Google Scholar] [CrossRef]
- Ghorbani Zamani, F.; Moulahoum, H.; Ak, M.; Demirkol, D.O.; Timur, S. Current trends in the development of conducting polymers-based biosensors. TrAC Trends Anal. Chem. 2019, 118, 264–276. [Google Scholar] [CrossRef]
- Olgac, R.; Soganci, T.; Baygu, Y.; Gok, Y.; Ak, M. Zinc(II) phthalocyanine fused in peripheral positions octa-substituted with alkyl linked carbazole: Synthesis, electropolymerization and its electro-optic and biosensor applications. Biosens. Bioelectron. 2017, 98, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Tekbaşoğlu, T.Y.; Soganci, T.; Ak, M.; Koca, A.; Sener, M.K. Enhancing biosensor properties of conducting polymers via copolymerization: Synthesis of EDOT-substituted bis(2-pyridylimino)isoindolato-palladium complex and electrochemical sensing of glucose by its copolymerized film. Biosens. Bioelectron. 2017, 87, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wen, H.; Zhang, G.; Lei, F.; Feng, Q.; Liu, Y.; Cao, X.; Dong, H. Multifunctional Conductive Hydrogel/Thermochromic Elastomer Hybrid Fibers with a Core–Shell Segmental Configuration for Wearable Strain and Temperature Sensors. ACS Appl. Mater. Interfaces 2020, 12, 7565–7574. [Google Scholar] [CrossRef]
- Tomczykowa, M.; Plonska-Brzezinska, M.E. Conducting polymers, hydrogels and their composites: Preparation, properties and bioapplications. Polymers 2019, 11, 350. [Google Scholar] [CrossRef]
- Stewart, S.; Adams Ivy, M.; Anslyn, E.V. The use of principal component analysis and discriminant analysis in differential sensing routines. Chem. Soc. Rev. 2014, 43, 70–84. [Google Scholar] [CrossRef]
- Mako, T.L.; Racicot, J.M.; Levine, M. Supramolecular Luminescent Sensors. Chem. Rev. 2019, 119, 322–477. [Google Scholar] [CrossRef]
- Makelane, H.R.; John, S.V.; Waryo, T.T.; Baleg, A.; Mayedwa, N.; Rassie, C.; Wilson, L.; Baker, P.; Iwuoha, E.I. AC voltammetric transductions and sensor application of a novel dendritic poly(propylene thiophenoimine)-co-poly(3-hexylthiophene) star co-polymer. Sensor. Actuators B Chem. 2016, 227, 320–327. [Google Scholar] [CrossRef]
- Gurudatt, N.G.; Chung, S.; Kim, J.-M.; Kim, M.-H.; Jung, D.-K.; Han, J.-Y.; Shim, Y.-B. Separation detection of different circulating tumor cells in the blood using an electrochemical microfluidic channel modified with a lipid-bonded conducting polymer. Biosens. Bioelectron. 2019, 146, 111746. [Google Scholar] [CrossRef]
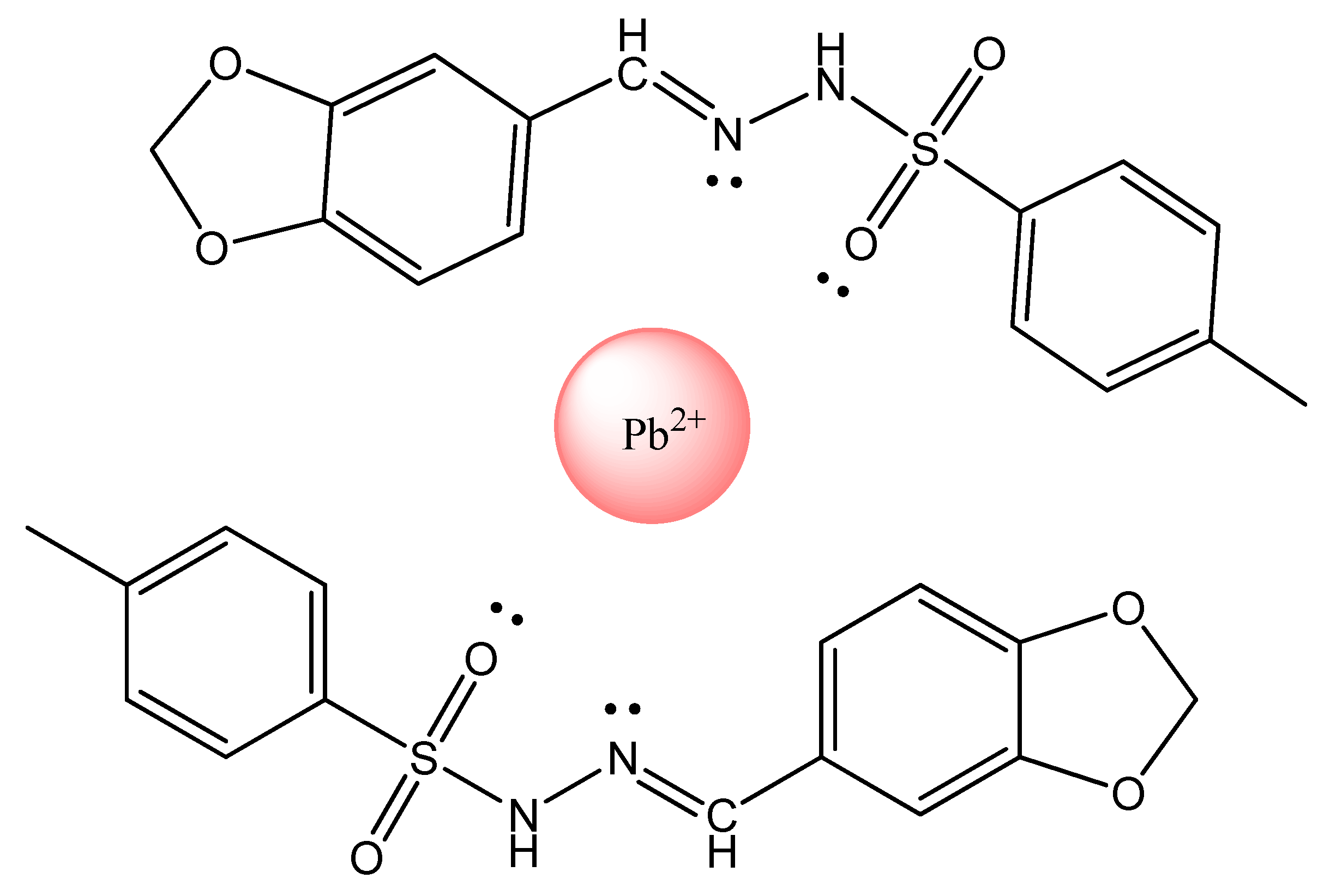
- Rahman, M.M.; Hussain, M.M.; Arshadab, M.N.; Asiri, A.M. The synthesis and application of (E)-N0-(benzo[d]dioxol-5-ylmethylene)-4-methylbenzenesulfonohydrazide for the detection of carcinogenic lead. RSC Adv. 2020, 10, 5316–5327. [Google Scholar] [CrossRef]
- Tan, J.; Chen, W.J.; Guo, J. Conjugated microporous polymers with distinctive π-electronic properties exhibiting enhanced optical applications. Chin. Chem. Lett. 2016, 27, 1405–1411. [Google Scholar] [CrossRef]
- Kou, Y.; Xu, Y.; Guo, Z.; Jiang, D. Supercapacitive energy storage and electric power supply using an aza-fused pi-conjugated microporous framework. Angew. Chem. Int. Ed. Engl. 2011, 50, 8753–8757. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yu, S.; Wang, Q.; Xiao, Q.; Yue, Y.; Ren, S. Fluorene-based conjugated microporous polymers: Preparation and chemical sensing application. Macromol. Rapid Commun. 2017, 38, 1700445. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, S.; Dey, N.; Pradhan, A.; Bhattacharya, S. A conjugated microporous polymer based visual sensing platform for aminoglycoside antibiotic in water. Chem. Commun. 2018, 54, 7495–7498. [Google Scholar] [CrossRef] [PubMed]
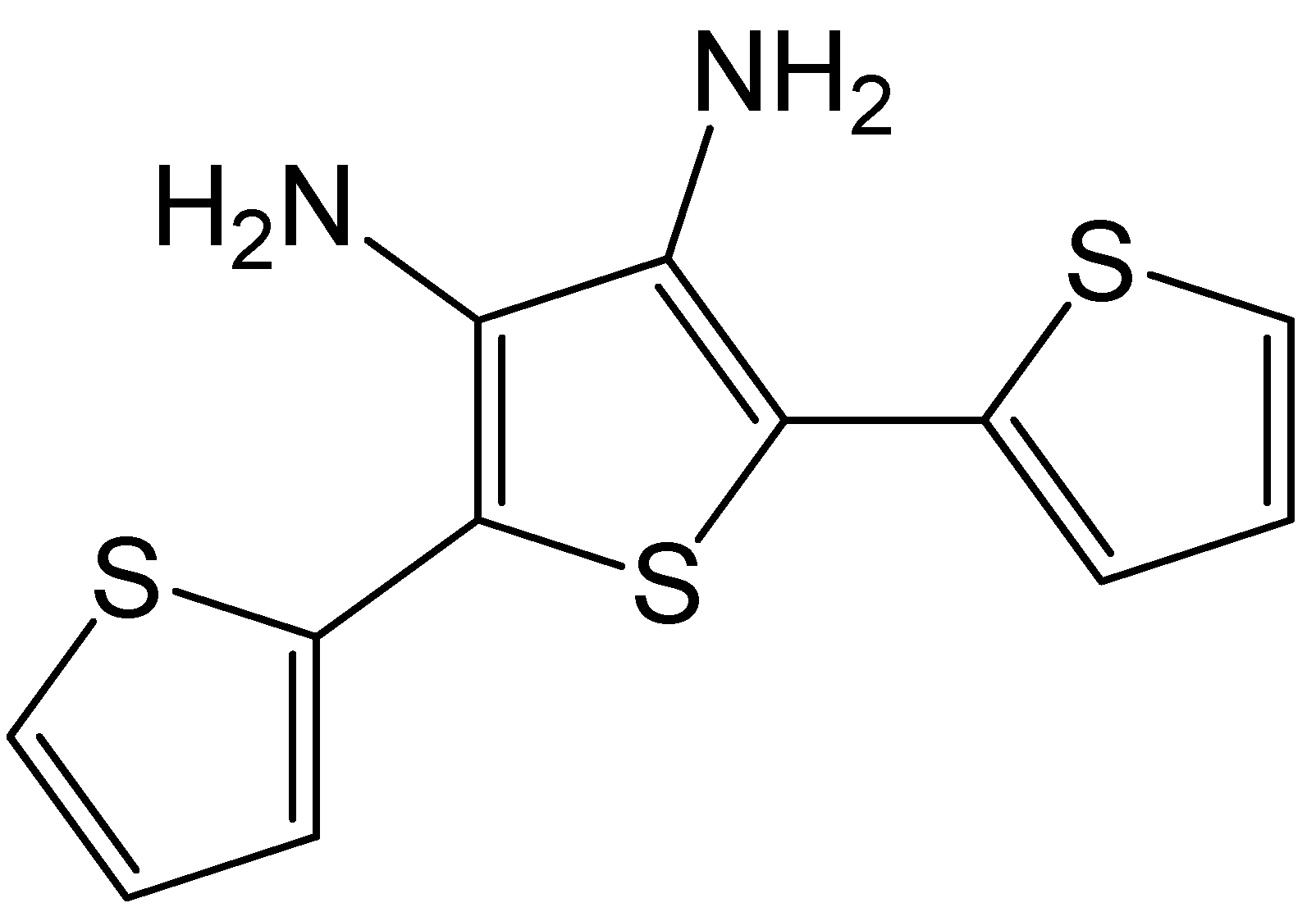
- Bai, S.; Hu, Q.; Zeng, Q.; Wang, M.; Wang, L. Variations in surface morphologies, properties, and electrochemical responses to nitro-analyte by controlled electropolymerization of thiophene derivatives. ACS Appl. Mater. Interfaces 2018, 10, 11319–11327. [Google Scholar] [CrossRef]
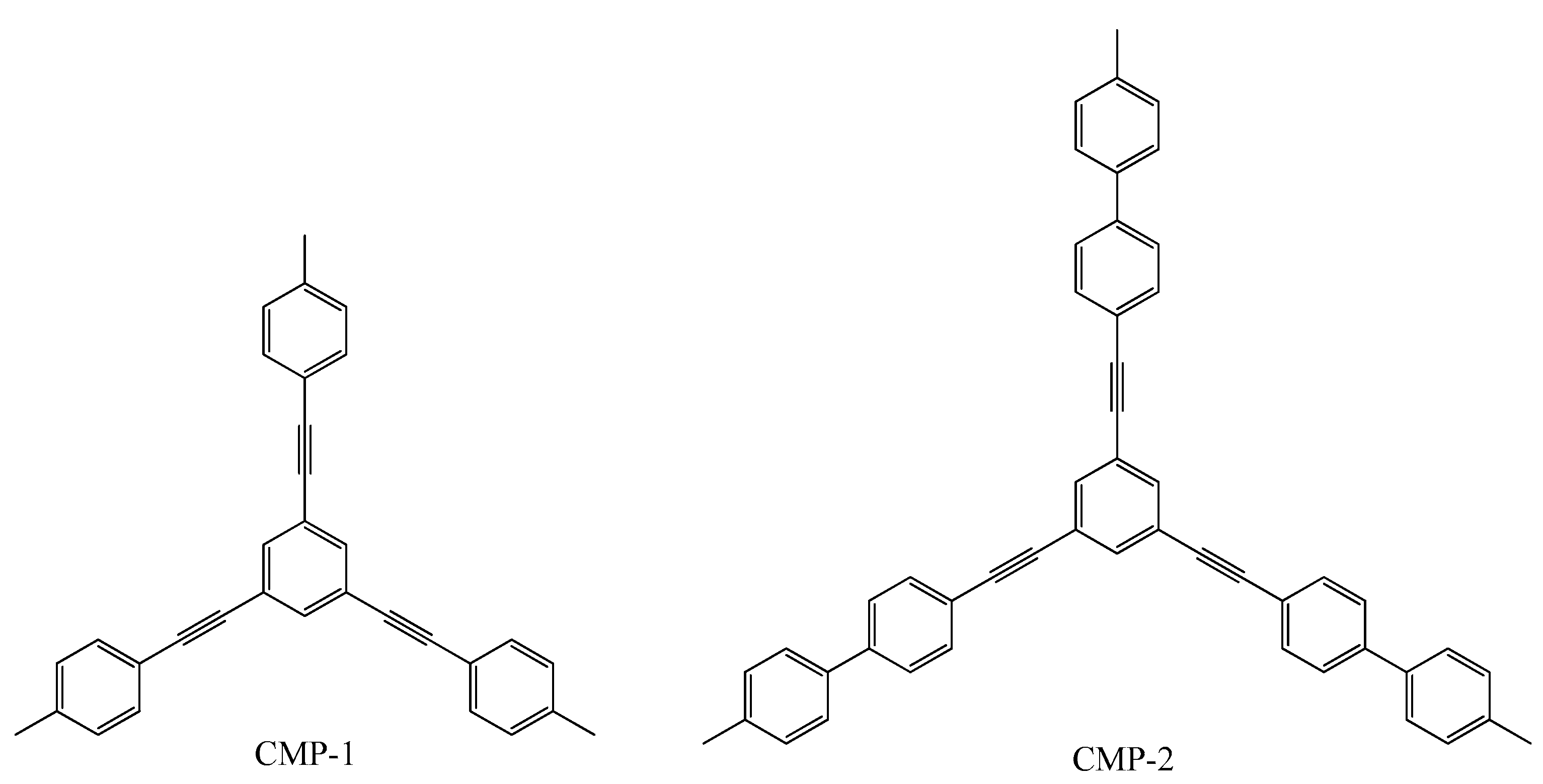
- Jiang, J.-X.; Su, F.; Trewin, A.; Wood, C.D.; Campbell, N.L.; Niu, H.; Dickinson, C.; Ganin, A.Y.; Rosseinsky, M.J.; Khimyak, Y.Z.; et al. Conjugated Microporous Poly(aryleneethynylene)Networks. Angew. Chem. Int. Ed. 2007, 46, 8574–8578. [Google Scholar] [CrossRef]
- Shun, W.; Hu, Q.; Liu, Y.; Meng, X.; Ye, Y.; Liu, X.; Song, X.; Liang, Z. Multifunctional Conjugated Microporous Polymers with Pyridine Unit for Efficient Iodine Sequestration, Exceptional Tetracycline Sensing and Removal. J. Hazard. Mater. 2020, 387, 121049. [Google Scholar]
- Liu, H.; Wang, Y.; Mo, W.; Tang, H.; Cheng, Z.; Chen, Y.; Zhang, S.; Ma, H.; Li, B.; Li, X. Dendrimer-Based, High-Luminescence Conjugated Microporous Polymer Films for Highly Sensitive and Selective Volatile Organic Compound Sensor Arrays. Adv. Funct. Mater. 2020, 1910275. [Google Scholar] [CrossRef]
- Zhang, B.; Li, B.; Wang, Z. Creation of Carbazole-Based Fluorescent Porous Polymers for Recognition and Detection of Various Pesticides in Water. ACS Sens. 2020, 5, 162–170. [Google Scholar] [CrossRef]
- Flory, P.J. Introductory lecture, Faraday Discuss. Chem. Soc. 1974, 57, 7–18. [Google Scholar]
- Cornwell, D.J.; Smith, D.K. Expanding the scope of gels-combining polymers with low- molecular-weight gelators to yield modified self-assembling smart materials with high- tech applications. Mater. Horiz. 2015, 2, 279–293. [Google Scholar] [CrossRef]
- Ross-Murphy, S.B. Physical Gelation of Synthetic and Biological Macromolecules. In Polymer Gels: Fundamentals and Applications; Bohidar, H.B., Dubin, P., Osada, Y., Eds.; Springer: New York, NY, USA, 1991; pp. 21–39. [Google Scholar]
- Li, L.; Shi, Y.; Pan, L.; Shi, Y.; Yu, G. Rational design and applications of conducting polymer hydrogels as electrochemical biosensors. J. Mater. Chem. B Mater. Biol. Med. 2015, 3, 2920–2930. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Y.; Pan, L.; Shi, Y.; Cheng, W.; Shi, Y.; Yu, G. A Nanostructured conductive hydrogels-based biosensor platform for human metabolite detection. Nano Lett. 2015, 15, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, Y.; Pan, L.; Yang, M.; Peng, L.; Zong, S.; Shi, Y.; Yu, G. Multifunctional superhydrophobic surfaces templated from innately microstructured hydrogel matrix. Nano Lett. 2014, 14, 4803–4809. [Google Scholar] [CrossRef]
- Zhao, X.; Li, P.; Guo, B.; Ma, P.X. Antibacterial and conductive injectable hydrogels based on quaternized chitosan-graft-polyaniline/oxidized dextran for tissue engineering. Acta Biomater. 2015, 26, 236–248. [Google Scholar] [CrossRef]
- Xu, Y.; Cui, M.; Patsis, P.A.; Günther, M.; Yang, X.; Eckert, K.; Zhang, Y. Reversibly Assembled Electroconductive Hydrogel via a Host–Guest Interaction for 3D Cell Culture. ACS Appl. Mater. Interfaces 2019, 11, 7715–7724. [Google Scholar] [CrossRef]
- Zhao, F.; Bae, J.; Zhou, X.; Guo, Y.; Yu, G. Nanostructured Functional Hydrogels as an Emerging Platform for Advanced Energy Technologies. Adv. Mater. 2018, 30, 1801796. [Google Scholar] [CrossRef]
- Dong, R.; Zhao, X.; Guo, B.; Ma, P.X. Self-Healing Conductive Injectable Hydrogels with Antibacterial Activity as Cell Delivery Carrier for Cardiac Cell Therapy. ACS Appl. Mater. Interfaces 2016, 8, 17138–17150. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, M.; Ma, C.B.; Wang, Y.Q.; Li, X.P.; Yu, G.H. A Conductive Self-Healing Hybrid Gel Enabled by Metal–Ligand Supramolecule and Nanostructured Conductive Polymer. Nano Lett. 2015, 15, 6276–6281. [Google Scholar] [CrossRef]
- Wang, S.; Guo, G.; Lu, X.; Ji, S.; Tan, G.; Gao, L. Facile Soaking Strategy Toward Simultaneously Enhanced Conductivity and Toughness of Self-Healing Composite Hydrogels Through Constructing Multiple Noncovalent Interactions. ACS Appl. Mater. Interfaces 2018, 10, 19133–19142. [Google Scholar] [CrossRef]
- Mawad, D.; Stewart, E.; Officer, D.L.; Romeo, T.; Wagner, P.; Wagner, K.; Wallace, G.G. A Single Component Conducting Polymer Hydrogel as a Scaffold for Tissue Engineerin. Adv. Funct. Mater. 2012, 22, 2692–2699. [Google Scholar] [CrossRef]
- Luo, C.; Wei, N.; Sun, X.; Luo, F. Fabrication of self-healable, conductive, and ultra-strong hydrogel from polyvinyl alcohol and grape seed–extracted polymer. J. Appl. Polym. Sci. 2020, 49118. [Google Scholar] [CrossRef]
- Bhat, A.; Amanor-Boadu, J.M.; Guiseppi-Elie, A. Toward Impedimetric Measurement of Acidosis with a pH-Responsive Hydrogel Sensor. ACS Sens. 2020, 5, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Ginting, M.; Pasaribu, S.P.; Masmur, I.; Kabana, J.; Hestina. Self-healing composite hydrogel with antibacterial and reversible restorability conductive properties. RSC Adv. 2020, 10, 5050. [Google Scholar] [CrossRef]
- McDonald, J.C.; Whitesides, G.M. Poly (dimethylsiloxane) as a material for fabricating microfluidic devices. Acc. Chem. Res. 2002, 35, 491–499. [Google Scholar] [CrossRef]
- Gong, X.; Wen, W. Polydimethylsiloxane-based conducting composites and their applications in microfluidic chip fabrication. Biomicrofluidics 2009, 3, 012007. [Google Scholar] [CrossRef]
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Kalsoom, U.; Farajikhah, S.; Innis, P.C.; Nesterenko, P.N.; Lewis, T.W.; Breadmore, M.C.; Paull, B. Enhanced physicochemical properties of polydimethylsiloxane based microfluidic devices and thin films by incorporating synthetic micro-diamond. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Chałupniak, A.; Merkoçi, A. Graphene Oxide–Poly (dimethylsiloxane)-Based Lab-on-a-Chip Platform for Heavy-Metals Preconcentration and Electrochemical Detection. ACS Appl. Mater. Interface 2017, 9, 44766–44775. [Google Scholar] [CrossRef]
- Brun, M.; Chateaux, J.F.; Deman, A.L.; Pittet, P.; Ferrigno, R. Nanocomposite Carbon-PDMS Material for Chip-Based Electrochemical Detection. Electroanalysis 2011, 23, 321–324. [Google Scholar] [CrossRef]
- Niu, X.Z.; Peng, S.L.; Liu, L.Y.; Wen, W.J.; Sheng, P. Characterizing and patterning of PDMS-based conducting composites. Adv. Mater. 2007, 19, 2682–2686. [Google Scholar] [CrossRef]
- Liu, L.; Cao, W.; Wu, J.; Wen, W.; Chang, D.C.; Sheng, P. Design and integration of an all-in-one biomicrofluidic chip. Biomicrofluidics 2008, 2, 034103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xu, J.J.; Liu, Y.; Chen, H.Y. In-situ synthesis of poly (dimethylsiloxane)–gold nanoparticles composite films and its application in microfluidic systems. Lab Chip 2008, 8, 352–357. [Google Scholar] [CrossRef] [PubMed]
- SadAbadi, H.; Badilescu, S.; Packirisamy, M.; Wüthrich, R. Integration of gold nanoparticles in PDMS microfluidics for lab-on-a-chip plasmonic biosensing of growth hormones. Biosens. Bioelectron. 2013, 44, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Lu, M.; Wang, W.; Bian, Z.P.; Zhang, J.R.; Zhu, J.J. Electrochemical immunosensor for simultaneous detection of dual cardiac markers based on a poly (dimethylsiloxane)-gold nanoparticles composite microfluidic chip: A proof of principle. Clin. Chem. 2010, 56, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Ozhikandathil, J.; Badilescu, S.; Packirisamy, M. Polymer Composite Optically Integrated Lab on Chip for the Detection of Ammonia. J. Electrochem. Soc. 2018, 165, B3078–B3083. [Google Scholar] [CrossRef]
- Xu, Y.; Lou, B.; Lv, Z.; Zhou, Z.; Zhang, L.; Wang, E. Paper-based solid-state electrochemiluminescence sensor using poly (sodium 4-styrenesulfonate) functionalized graphene/nafion composite film. Anal. Chim. Acta 2013, 763, 20–27. [Google Scholar] [CrossRef]
- Rattanarat, P.; Suea-Ngam, A.; Ruecha, N.; Siangproh, W.; Henry, C.S.; Srisa-Art, M.; Chailapakul, O. Graphene-polyaniline modified electrochemical droplet-based microfluidic sensor for high-throughput determination of 4-aminophenol. Anal. Chim. Acta 2016, 925, 51–60. [Google Scholar] [CrossRef]
- Dou, M.; Sanjay, S.T.; Dominguez, D.C.; Zhan, S.; Li, X. A paper/polymer hybrid CD-like microfluidic SpinChip integrated with DNA-functionalized graphene oxide nanosensors for multiplex qLAMP detection. Chem. Commun. 2017, 53, 10886–10889. [Google Scholar] [CrossRef]
- Peeters, M.; Kobben, S.; Jiménez-Monroy, K.L.; Modesto, L.; Kraus, M.; Vandenryt, T.; Gaulke, A.; van Grinsven, B.; Ingebrandt, S.; Junkers, T. Thermal detection of histamine with a graphene oxide based molecularly imprinted polymer platform prepared by reversible addition–fragmentation chain transfer polymerization. Sens. Actuators B Chem. 2014, 203, 527–535. [Google Scholar] [CrossRef]
- Ye, L.; Mosbach, K. Molecularly imprinted microspheres as antibody binding mimics. React. Funct. Polym. 2001, 48, 149–157. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Hudson, A.; Foster, C.W.; Eersels, K.; van Grinsven, B.; Cleij, T.J.; Banks, C.E.; Peeters, M. Recent Advances in Electrosynthesized Molecularly Imprinted Polymer Sensing Platforms for Bioanalyte Detection. Sensors 2019, 19, 1204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Peng, Y.; Bai, J.; Ning, B.; Sun, S.; Hong, X.; Liu, Y.; Liu, Y.; Gao, Z. A novel electrochemical sensor based on electropolymerized molecularly imprinted polymer and gold nanomaterials amplification for estradiol detection. Sens. Actuators B Chem. 2014, 200, 69–75. [Google Scholar] [CrossRef]
- Luppa, P.B.; Sokoll, L.J.; Chan, D.W. Immunosensors—Principles and applications to clinical chemistry. Clin. Chim. Acta 2001, 314, 1–26. [Google Scholar] [CrossRef]
- Rachkov, A.E.; Cheong, S.H.; El’skaya, A.V.; Yano, K.; Karube, I. Molecularly Imprinted Polymers as Artificial Steroid Receptors. Polym. Advan. Technol. 1998, 9, 511–519. [Google Scholar] [CrossRef]
- Des Azevedo, S.; Lakshimi, D.; Chianella, I.; Whitcombe, M.J.; Karim, K.; Ivanova-Mitseva, P.K.; Subrahmanyam, S.; Piletsky, S.A. Molecularly Imprinted Polymer-Hybrid Electrochemical Sensor for the Detection of β-Estradiol. Ind. Eng. Chem. Res. 2013, 52, 13917–13923. [Google Scholar] [CrossRef]
- Lin, Z.T.; Demarr, V.; Bao, J.; Wu, T. Molecularly Imprinted Polymer-Based Biosensors. For the early, rapid detection of pathogens, biomarkers and toxins in clinical, environmental, or food samples. IEEE Nanotechnol. Mag. 2018, 12, 6–13. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Tharpa, K.; Dima, O. Molecularly imprinted membranes: Past, present, and future. Chem. Rev. 2016, 116, 11500–11528. [Google Scholar] [CrossRef]
- Chen, L.; Xu, S.; Li, J. Recent advances in molecular imprinting technology: Current status, challenges, and highlighted applications. Chem. Soc. Rev. 2011, 40, 2922–2942. [Google Scholar] [CrossRef]
- Wackerlig, J.; Lieberzeit, P.A. Molecularly imprinted polymer nanoparticles in chemical sensing—Synthesis, characterisation and application. Sens. Actuators B Chem. 2015, 207, 144–157. [Google Scholar] [CrossRef]
- Guerreiro, J.R.L.; Bochenkov, V.E.; Runager, K.; Aslan, H.; Dong, M.; Enghild, J.J.; De Freitas, V.; Ferreira Sales, M.G.; Sutherland, D.S. Molecular imprinting of complex matrices at localized surface plasmon resonance biosensors for screening of global interactions of polyphenols and proteins. ACS Sens. 2016, 1, 258–264. [Google Scholar] [CrossRef]
- Lahcen, A.A.; Baleg, A.A.; Baker, P.; Iwuoha, E.; Amine, A. Synthesis and electrochemical characterization of nanostructured magnetic molecularly imprinted polymers for 17-β-estradiol determination. Sens. Actuators B Chem. 2017, 241, 698–705. [Google Scholar] [CrossRef]
- Jafari, S.; Dehghani, M.; Nasirizadeh, N.; Azimzadeh, M. An azithromycin electrochemical sensor based on an aniline MIP film electropolymerized on a gold nano urchins/graphene oxide modified glassy carbon electrode. J. Electroanal. 2018, 829, 27–34. [Google Scholar] [CrossRef]
- Lahcen, A.A.; Arduini, F.; Lista, F.; Amine, A. Label-free Electrochemical Sensor based on Spore-Imprinted Polymer for Bacillus cereus Spore Detection. Sens. Actuators B Chem. 2018, 276, 114–120. [Google Scholar] [CrossRef]
- Teng, Y.; Liu, F.; Kan, X. Voltammetric dopamine sensor based on three-dimensional electrosynthesized molecularly imprinted polymers and polypyrrole nanowires. Microchim. Acta. 2017, 184, 2515–2522. [Google Scholar] [CrossRef]
- Chen, S.; Chen, X.; Zhang, L.; Gao, J.; Ma, Q. Electrochemiluminescence Detection of Escherichia coli O157:H7 Based on a Novel Polydopamine Surface Imprinted Polymer Biosensor. ACS Appl. Mater. Interfaces 2017, 9, 5430–5436. [Google Scholar] [CrossRef]
- Zaidi, S.A. Utilization of an environmentally-friendly monomer for an efficient and sustainable adrenaline imprinted electrochemical sensor using graphene. Electrochim. Acta 2018, 274, 370–377. [Google Scholar] [CrossRef]
- Gholivand, M.B.; Karimian, N. Fabrication of a highly selective and sensitive voltammetric ganciclovir sensor based on electropolymerized molecularly imprinted polymer and gold nanoparticles on multiwall carbon nanotubes/glassy carbon electrode. Sens. Actuators B Chem. 2015, 215, 471–479. [Google Scholar] [CrossRef]
- Moon, J.M.; Kim, D.M.; Kim, M.H.; Han, J.Y.; Jung, D.K.; Shim, Y.B. A disposable amperometric dual-sensor for the detection of hemoglobin and glycated hemoglobin in a finger prick blood sample. Biosens. Bioelectron. 2017, 91, 128–135. [Google Scholar] [CrossRef]
- Babamiri, B.; Salimi, A.; Hallaj, R. A molecularly imprinted electrochemiluminescence sensor for ultrasensitive HIV-1 gene detection using EuS nanocrystals as luminophore. Biosens. Bioelectron. 2018, 117, 332–339. [Google Scholar] [CrossRef]
- Stojanovic, Z.; Erdossy, J.; Keltai, K.; Scheller, F.W.; Gyurcsanyi, R.E. Electrosynthesized molecularly imprinted polyscopoletin nanofilms for human serum albumin detection. Anal. Chim. Acta 2017, 977, 1–9. [Google Scholar] [CrossRef]
- Shumyantseva, V.V.; Bulko, T.V.; Sigolaeva, L.V.; Kuzikov, A.V.; Archakov, A.I. Electrosynthesis and binding properties of molecularly imprinted poly-o-phenylenediamine for selective recognition and direct electrochemical detection of myoglobin. Biosens. Bioelectron. 2016, 86, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Tamboli, V.K.; Bhalla, N.; Jolly, P.; Bowen, C.R.; Taylor, J.T.; Bowen, J.L.; Allender, C.J.; Estrela, P. Hybrid Synthetic Receptors on MOSFET Devices for Detection of Prostate Specific Antigen in Human Plasma. Anal. Chem. 2016, 88, 11486–11490. [Google Scholar] [CrossRef] [PubMed]
- Yola, M.L.; Atar, N. A Novel Detection Approach for Serotonin by Graphene Quantum Dots/Two-Dimensional (2D) Hexagonal Boron Nitride Nonosheets with Molecularly Imprinted Polymer. Appl. Surf. Sci. 2018, 458, 648–655. [Google Scholar] [CrossRef]
- Zeng, Q.; Huang, X.; Ma, M. A Molecularly Imprinted Electrochemical Sensor Based on Polypyrrole/Carbon Nanotubes Composite for the Detetction of S-ovoalbumin in Egg White. Int. J. Electrochem. Sci. 2017, 12, 3964–3981. [Google Scholar]
- Liu, W.; Ma, Y.; Sun, G.; Wang, S.; Deng, J.; Wei, H. Molecularly imprinted polymers on graphene oxide surface for EIS sensing of testosterone. Biosens. Bioelectron. 2017, 92, 305–312. [Google Scholar] [CrossRef]
- Devkota, L.; Nguyen, L.T.; Vu, T.T.; Piro, B. Electrochemical determination of tetracycline using AuNP-coated molecularly imprinted overoxidized polypyrrole sensing interface. Electrochim. Acta 2018, 270, 535–542. [Google Scholar] [CrossRef]
- Chou, J.; Wong, J.; Christodoulides, N.; Floriano, P.N.; Sanchez, X.; McDevitt, J. Porous Bead-Based Diagnostic Platforms: Bridging the Gaps in Healthcare. Sensors 2012, 12, 15467–15499. [Google Scholar] [CrossRef]
- West, J.; Becker, M.; Tombrink, S.; Manz, A. Micro total analysis systems: Latest achieve-ments. Anal. Chem. 2008, 80, 4403–4419. [Google Scholar] [CrossRef]
- Wang, J.; Chen, G.; Muck, A. Movable contactless-conductivity detector for microchip capillary electrophoresis. Anal. Chem. 2003, 75, 4475–4479. [Google Scholar] [CrossRef]
- Wang, J.; Pumera, M.; Collins, G.E.; Mulchandani, A. Measurements of chemical warfare agent degradation products using an electrophoresis microchip with contactless con-ductivity detector. Anal. Chem. 2002, 74, 6121–6125. [Google Scholar] [CrossRef]
- Crain, M.M.; Keynton, R.S.; Walsh, K.M.; Roussel, T.J., Jr.; Baldwin, R.P.; Naber, J.F.; Jackson, D.J. Fabrication of a glass capillary electrophoresis microchip with integrated electrodes. Methods Mol. Biol. 2006, 339, 13–26. [Google Scholar] [PubMed]
- Lazar, I.M.; Yang, L.L.; Karger, B.L. Microfluidic device for capillary electrochromatography-mass spectrometry. Electrophoresis 2003, 24, 3655–3662. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M. Microchip-based electrochromatography: Designs and applications. Talanta 2005, 66, 1048–1062. [Google Scholar] [CrossRef] [PubMed]
- Végvári, Á.; Hjertén, S. A hybrid microdevice for electrophoresis and electrochromatography using UV detection. Electrophoresis 2002, 23, 3479–3486. [Google Scholar] [CrossRef]
- Kuznetsov, A.E.; Komarova, N.V.; Kuznetsov, E.V.; Andrianova, M.S.; Grudtsov, V.P.; Rybachek, E.N.; Puchnin, K.V.; Ryazantsev, D.V.; Saurov, A.N. Integration of a field effect transistor-based aptasensor under a hydrophobic membrane for bioelectronic nose applications. Biosens. Bioelectron. 2019, 129, 29–35. [Google Scholar] [CrossRef]
- Daprà, E.J.; Lauridsen, L.H.; Nielsen, A.T.; Rozlosnik, N. Comparative study on aptamers as recognition elements for antibiotics in a label-free all-polymer biosensor. Biosens. Bioelectron. 2013, 43, 315–320. [Google Scholar] [CrossRef]
- Hawkes, J.J.; Coakley, W.T. Force field particle filter, combining ultrasound standing waves and laminar flow. Sens. Actuators B Chem. 2001, 75, 213–222. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Leung, K.W.; Ting, A.K.; Wong, Z.C.; Ng, W.Y.; Choi, R.C.; Dong, T.T.; Wang, T.; Lau, D.T.; Tsim, K.W. Microfluidic chip based nano liquid chromatography coupled to tandem mass spectrometry for the determination of abused drugs and metabolites in human hair. Anal. Bioanal. Chem. 2012, 402, 2805–2815. [Google Scholar] [CrossRef]
- Chen, C.H.; Tsai, F.; Lien, V.; Justis, N.; Lo, Y.H. Scattering-based cytometric detection using integrated arrayed waveguides with microfluidics. IEEE Photonics Technol. Lett. 2007, 19, 441–443. [Google Scholar] [CrossRef]
- Ozhikandathil, J.; Badilescu, S.; Packirisamy, M. A portable on-chip assay system for absorbance and plasmonic detection of protein hormone in milk. J. Dairy Sci. 2015, 98, 4384–4391. [Google Scholar] [CrossRef]
- Shu, Z.; Kemper, F.; Beckert, E.; Eberhardtaand, R.; Tünnermann, A. Highly sensitive on-chip fluorescence sensor with integrated fully solution processed organic light sources and detectors. RSC Adv. 2017, 7, 26384–26391. [Google Scholar] [CrossRef]
- Giannitsis, A.T. Microfabrication of biomedical lab-on-chip devices. A review. Est. J. Eng. 2011, 17, 109–139. [Google Scholar] [CrossRef]
- Luttge, R. Chemical and biological sensors at component and device level. Nano- Microfabr. Ind. Biomed. Appl. 2016, 161–179. [Google Scholar] [CrossRef]
- Andrianova, M.S.; Gubanova, O.V.; Komarova, N.V.; Kuznetsov, E.V.; Kuznetsov, A.E. Development of a Biosensor Based on Phosphotriesterase and n-Channel ISFET for Detection of Pesticides. Electroanalysis 2016, 28, 1311–1321. [Google Scholar] [CrossRef]
- Lin, Y.H.; Wang, S.H.; Wu, M.H.; Pan, T.M.; Lai, C.S.; Luo, J.D.; Chiou, C.C. Integrating solid-state sensor and microfluidic devices for glucose, urea and creatinine detection based on enzyme-carrying alginate microbeads. Biosens. Bioelectron. 2013, 43, 328–335. [Google Scholar] [CrossRef]
- Li, Y.; Sella, C.; Lemaître, F.; Guille-Collignon, M.; Amatore, C.; Thouin, L. Downstream Simultaneous Electrochemical Detection of Primary Reactive Oxygen and Nitrogen Species Released by Cell Populations in an Integrated Microfluidic Device. Anal. Chem. 2018, 90, 9386–9394. [Google Scholar] [CrossRef]
- Ges, I.A.; Brindley, R.L.; Currie, K.P.M.; Baudenbacher, F.J. A microfluidic platform for chemical stimulation and real time analysis of catecholamine secretion from neuroendocrine cells. Lab. Chip 2013, 13, 4663–4673. [Google Scholar] [CrossRef]
- Lakey, A.; Ali, Z.; Scott, S.M.; Chebil, S.; Korri-Youssoufi, H.; Hunor, S.; Ohlander, A.; Kuphal, M.; Marti, J.S. Impedimetric Array in Polymer Microfluidic Catridge for Low Cost Point-Of-Care Diagnostics. Biosens. Bioelectron. 2019, 129, 147–154. [Google Scholar] [CrossRef]
- Awaja, F.; Wong, T.; Arhatari, B. Lab-on-a-chip device made by autohesion-bonded polymers. Biomed. Microdevices 2018, 20, 1–9. [Google Scholar] [CrossRef]
- Chuang, C.H.; Huang, Y.W.; Wu, Y.T. System-Level Biochip for Impedance Sensing and Programmable Manipulation of Bladder Cancer Cells. Sensors 2011, 11, 11021–11035. [Google Scholar] [CrossRef]
- Onishi, K.; Enomoto, J.; Araki, T.; Takagi, R.; Suzuki, H.; Fukuda, J. Electrochemical microdevices for rapid and on-site determination of the minimum inhibitory concentration of antibiotics. Analyst 2018, 143, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Takagi, R.; Fukuda, J.; Nagata, K.; Yawata, Y.; Nomura, N.; Suzuki, H. A microfluidic microbial culture device for rapid determination of the minimum inhibitory concentration of antibiotics. Analyst 2013, 138, 1000–1003. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yoav, H.; Dykstra, P.H.; Bentley, W.E.; Ghodssi, R. A controlled microfluidic electrochemical lab-on-a-chip for label-free diffusion-restricted DNA hybridization analysis. Biosens. Bioelectron. 2015, 64, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Lisowski, P.; Zarzycki, P.K. Microfluidic Paper-Based Analytical Devices (lPADs) and Micro Total Analysis Systems (lTAS): Development, Applications and Future Trends. Chromatographia 2013, 76, 1201–1214. [Google Scholar] [CrossRef]
- Sassa, F.; Biswas, G.C.; Suzuki, H. Microfabricated electrochemical sensing devices. Lab. Chip 2020, 20, 1358–1389. [Google Scholar] [CrossRef]
- Seemann, R.; Brinkmann, M.; Pfohl, T.; Herminghaus, S. Droplet based microfluidics. Rep. Prog. Phys. 2012, 75, 016601. [Google Scholar] [CrossRef]
- Yang, F.; Zuo, X.; Li, Z.; Deng, W.; Shi, J.; Zhang, G.; Huang, Q.; Song, S.; Fan, C. A Bubble-Mediated Intelligent Microscale Electrochemical Device for Single-Step Quantitative Bioassays. Adv. Mater. 2014, 26, 4671–4676. [Google Scholar] [CrossRef]
- Manczak, R.; Fouet, M.; Courson, R.; Fabre, P.L.; Montrose, A.; Sudor, J.; Gué, A.M.; Reybier, K. Improved on-chip impedimetric immune-detection of subpopulations of cells toward single-cell resolution. Sens. Actuators B Chem. 2016, 230, 825–831. [Google Scholar] [CrossRef]
- An, L.; Wang, G.; Han, Y.; Li, T.; Jin, P.; Liu, S. Electrochemical biosensor for cancer cell detection based on a surface 3D micro-array. Lab Chip 2018, 18, 335–342. [Google Scholar] [CrossRef]
- Biswas, G.; Watanabe, T.; Carlen, E.T.; Yokokawa, M.; Suzuki, H. Switchable Hydrophobic Valve for Controlled Microfluidic Processing. Chem. Phys. Chem. 2016, 17, 817–821. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Y.; Wang, D.; He, F.; Rotello, V.M.; Carter, K.R.; Watkins, J.J.; Nugen, S.R. UV-nanoimprint lithography as a tool to develop flexible microfluidic devices for electrochemical detection. Lab Chip 2015, 15, 3086–3094. [Google Scholar] [CrossRef] [PubMed]
- Satoh, W.; Takahashi, S.; Sassa, F.; Fukuda, J.; Suzuki, H. On-chip culturing of hepatocytes and monitoring their ammonia metabolism. Lab Chip 2009, 9, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Ainla, A.; Mousavi, M.P.S.; Tsaloglou, M.N.; Redston, J.; Bell, J.G.; Fernández-Abedul, M.T.; Whitesides, G.M. Open-Source Potentiostat for Wireless Electrochemical Detection with Smartphones. Anal. Chem. 2018, 90, 6240–6246. [Google Scholar] [CrossRef]
- Aymerich, J.; Márquez, A.; Terés, L.; Munoz-Berbel, X.; Jiménez, C.; Dominguezm, C.; Serra-Graells, F.; Dei, M. Cost-effective smartphone-based reconfigurable electrochemical instrument for alcohol determination in whole blood sample. Biosens. Bioelectron. 2018, 117, 736–742. [Google Scholar] [CrossRef] [PubMed]



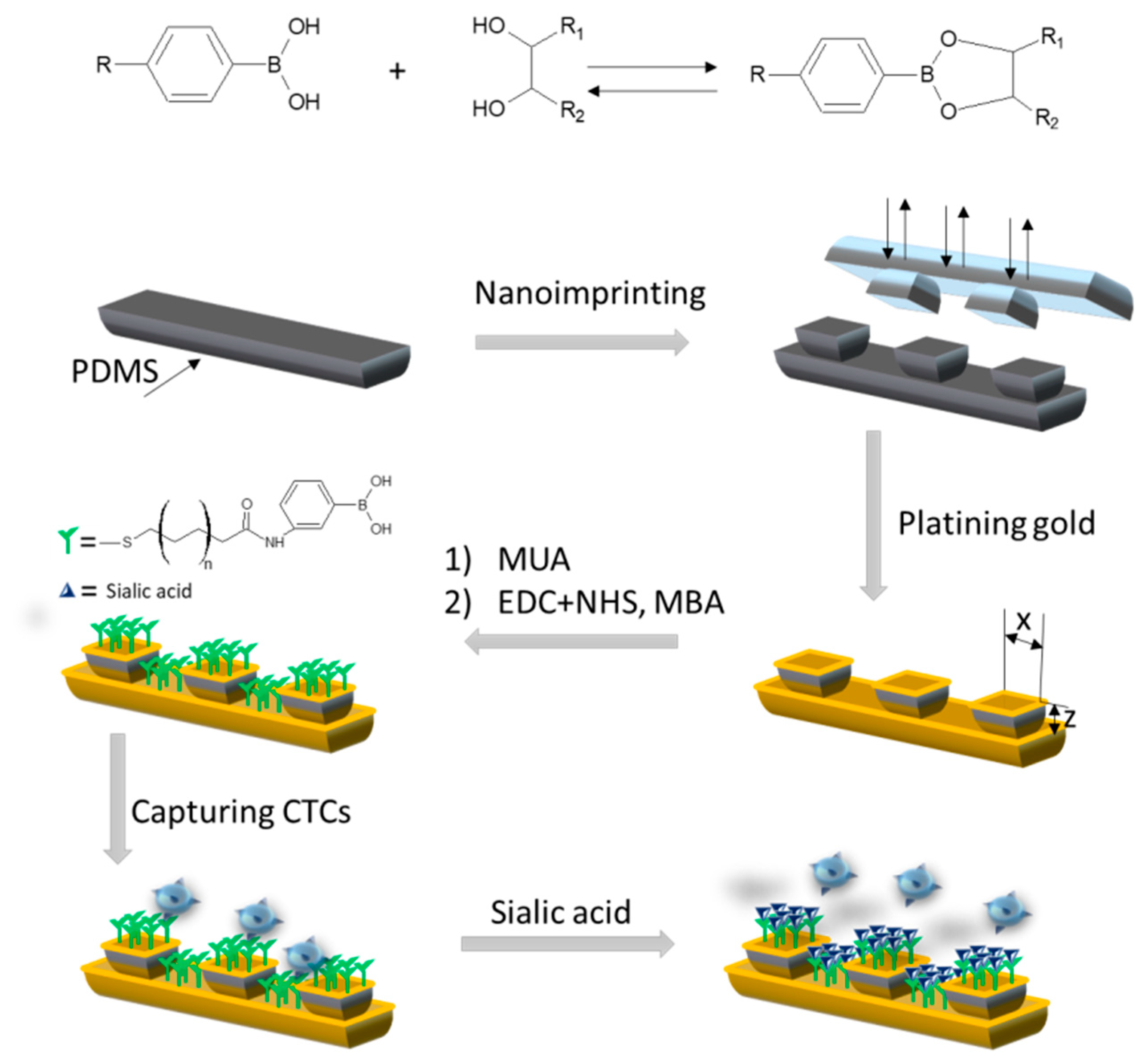
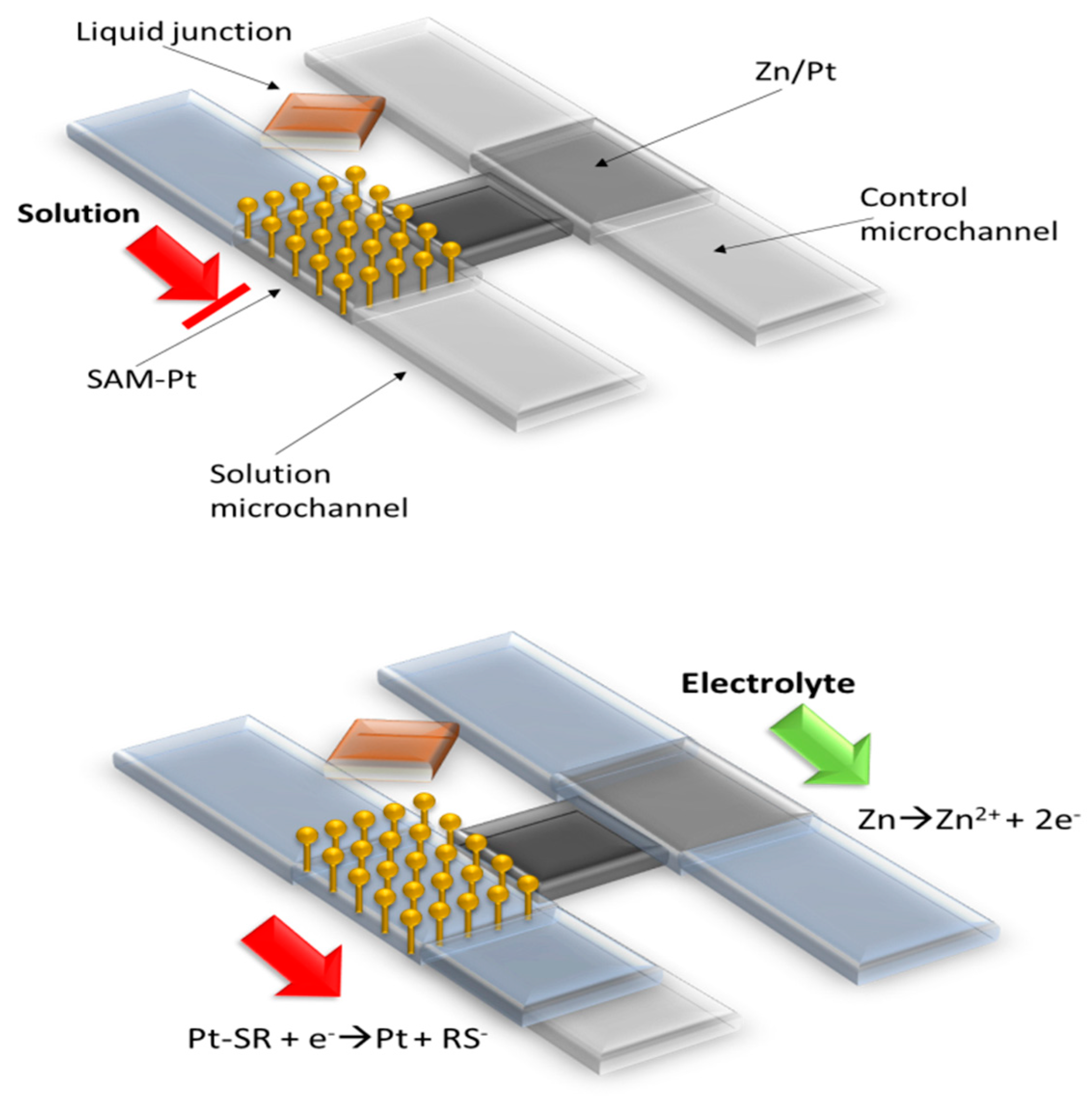
| Template | Functional Monomer | Electrode Material | Detection Method | LOD | Ref. |
|---|---|---|---|---|---|
| 17β-estradiol | aniline | SPCE/MIP-Fe3O4 | SWV | 0.02 μM | [82] |
| azithromycin | aniline | GCE/GO/GNU | DPV | 0.1 nM | [83] |
| B. cereus | pyrrole | CPE | CV | 102 CFU/mL | [84] |
| dopamine | o-phenylenediamine | GCE/PPyNWs | DPV | 33 nM | [85] |
| E.coli | dopamine | GCE | ECL | 8 CFU/mL | [86] |
| epinephrine | nicotinamide | GCE/rGO | DPV | 3 nM | [87] |
| ganciclovir | 2,2′-dithiodianiline | GCE/MWCNT/AuNPs | DPAS | 1.5 nM | [88] |
| hemoglobin | TBA | Au | CA | 82 nM | [89] |
| HIV | o-phenylenediamine | ITO | ECL | 0.3 fM | [90] |
| human serum albumin | bis(2,2-bithien-5-yl)methane | Au | DPV | 0.25 pM | [91] |
| myoglobin | o-phenylenediamine | SPCE | DPV | 0.006 ng/mL | [92] |
| PSA | dopamine | Au | MOSFET | 0.1 pg/mL | [93] |
| serotonin | phenol | GCE/GQDs/2D-hBN | DPV | 0.2 pM | [94] |
| s-ovoalbumin | pyrrole | GCE | DPV | 2.95x10-9 mg/mL | [95] |
| testosterone | o-phenylenediamine | GCE | EIS | 0.4 fM | [96] |
| tetracycline | pyrrole | SPCE/AuNPs | DPV | 0.65 μM | [97] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spychalska, K.; Zając, D.; Baluta, S.; Halicka, K.; Cabaj, J. Functional Polymers Structures for (Bio)Sensing Application—A Review. Polymers 2020, 12, 1154. https://doi.org/10.3390/polym12051154
Spychalska K, Zając D, Baluta S, Halicka K, Cabaj J. Functional Polymers Structures for (Bio)Sensing Application—A Review. Polymers. 2020; 12(5):1154. https://doi.org/10.3390/polym12051154
Chicago/Turabian StyleSpychalska, Kamila, Dorota Zając, Sylwia Baluta, Kinga Halicka, and Joanna Cabaj. 2020. "Functional Polymers Structures for (Bio)Sensing Application—A Review" Polymers 12, no. 5: 1154. https://doi.org/10.3390/polym12051154
APA StyleSpychalska, K., Zając, D., Baluta, S., Halicka, K., & Cabaj, J. (2020). Functional Polymers Structures for (Bio)Sensing Application—A Review. Polymers, 12(5), 1154. https://doi.org/10.3390/polym12051154






