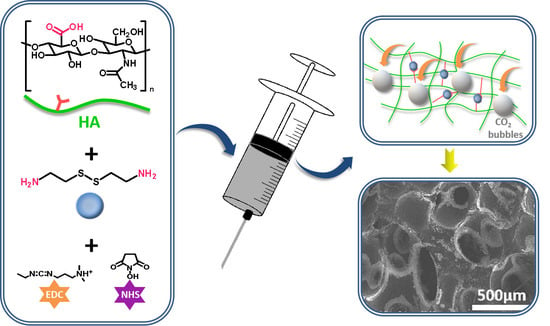
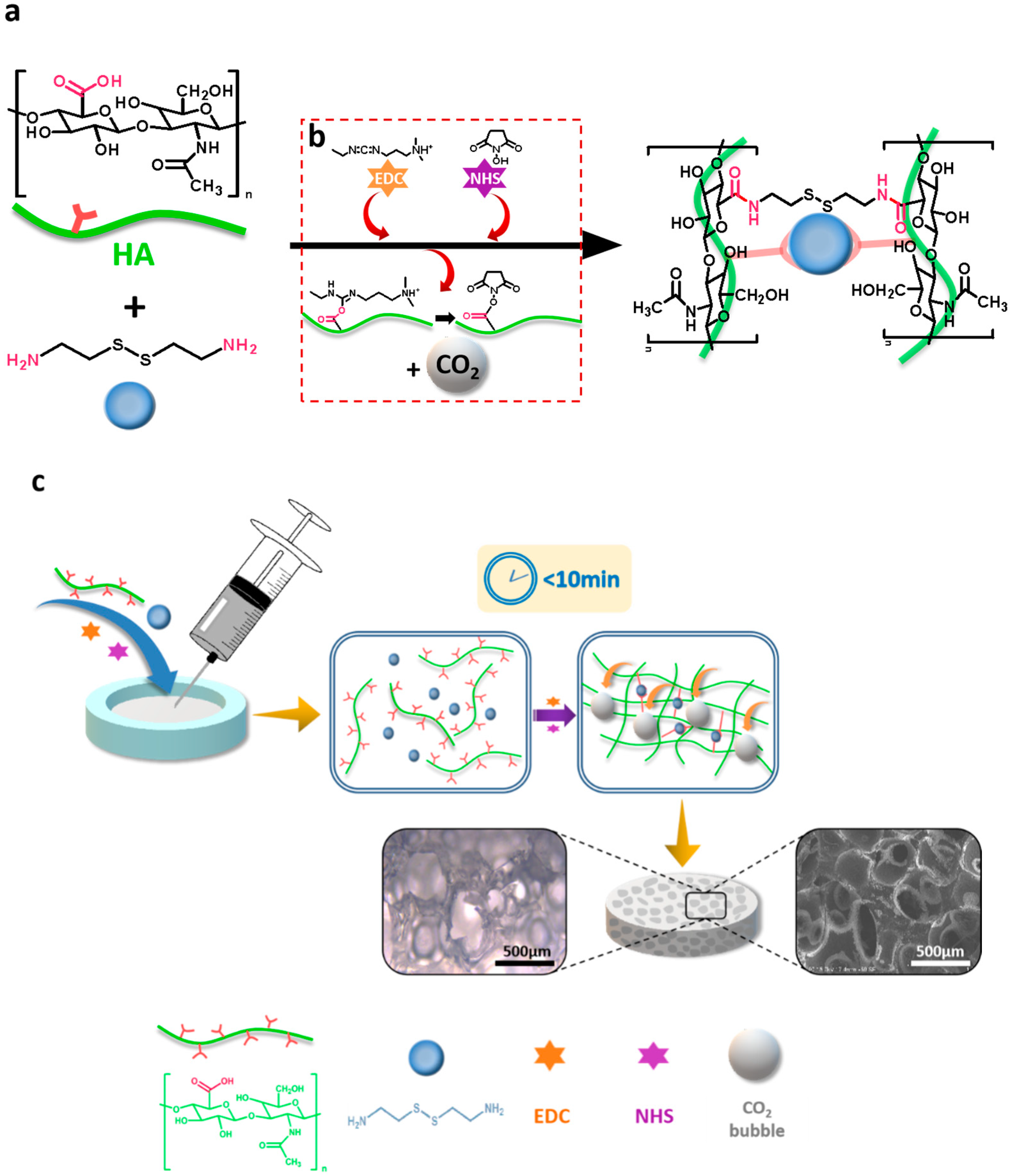
Fabrication of Injectable, Porous Hyaluronic Acid Hydrogel Based on an In-Situ Bubble-Forming Hydrogel Entrapment Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of HA Hydrogel
2.3. Rheological Measurement
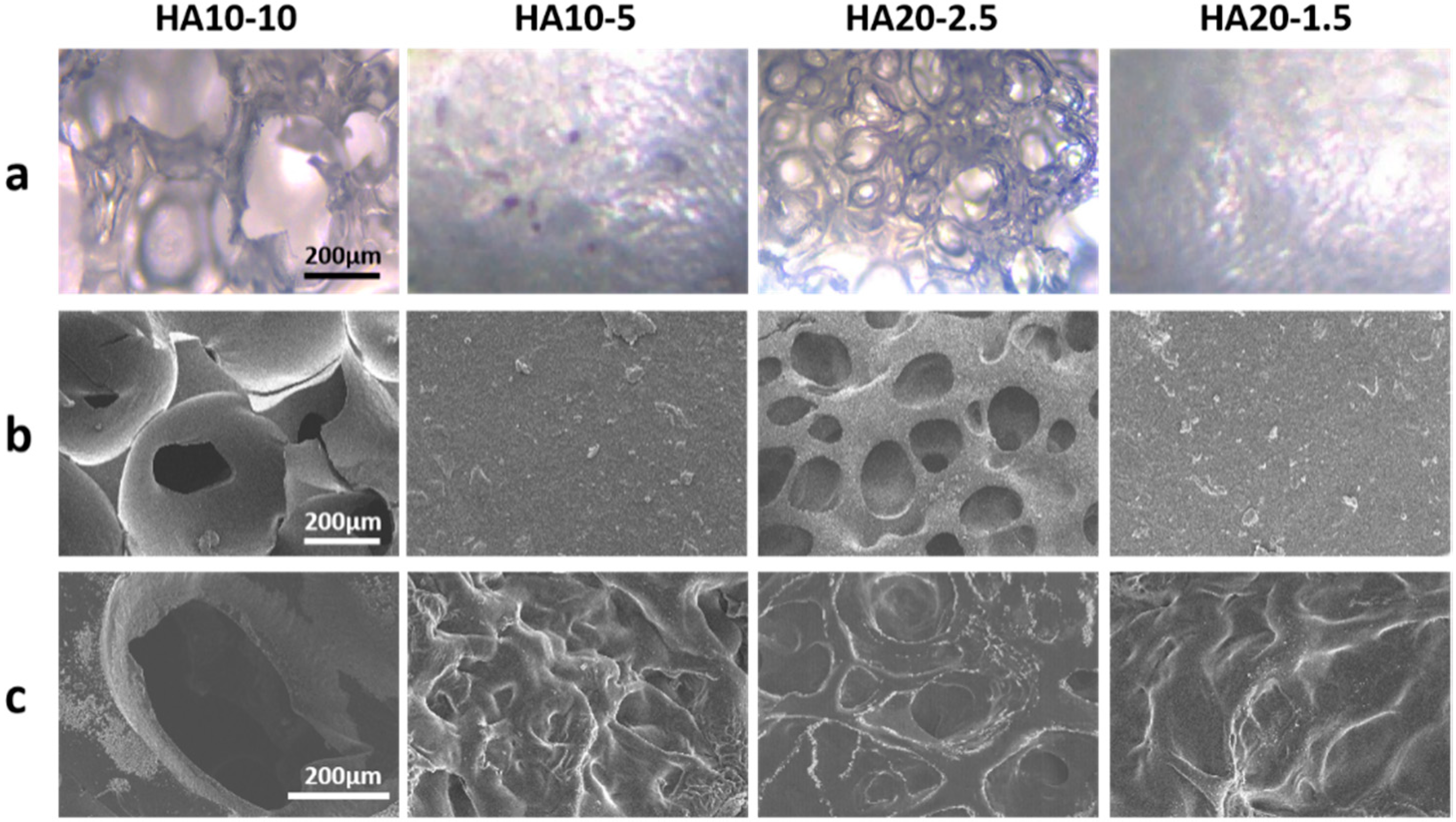
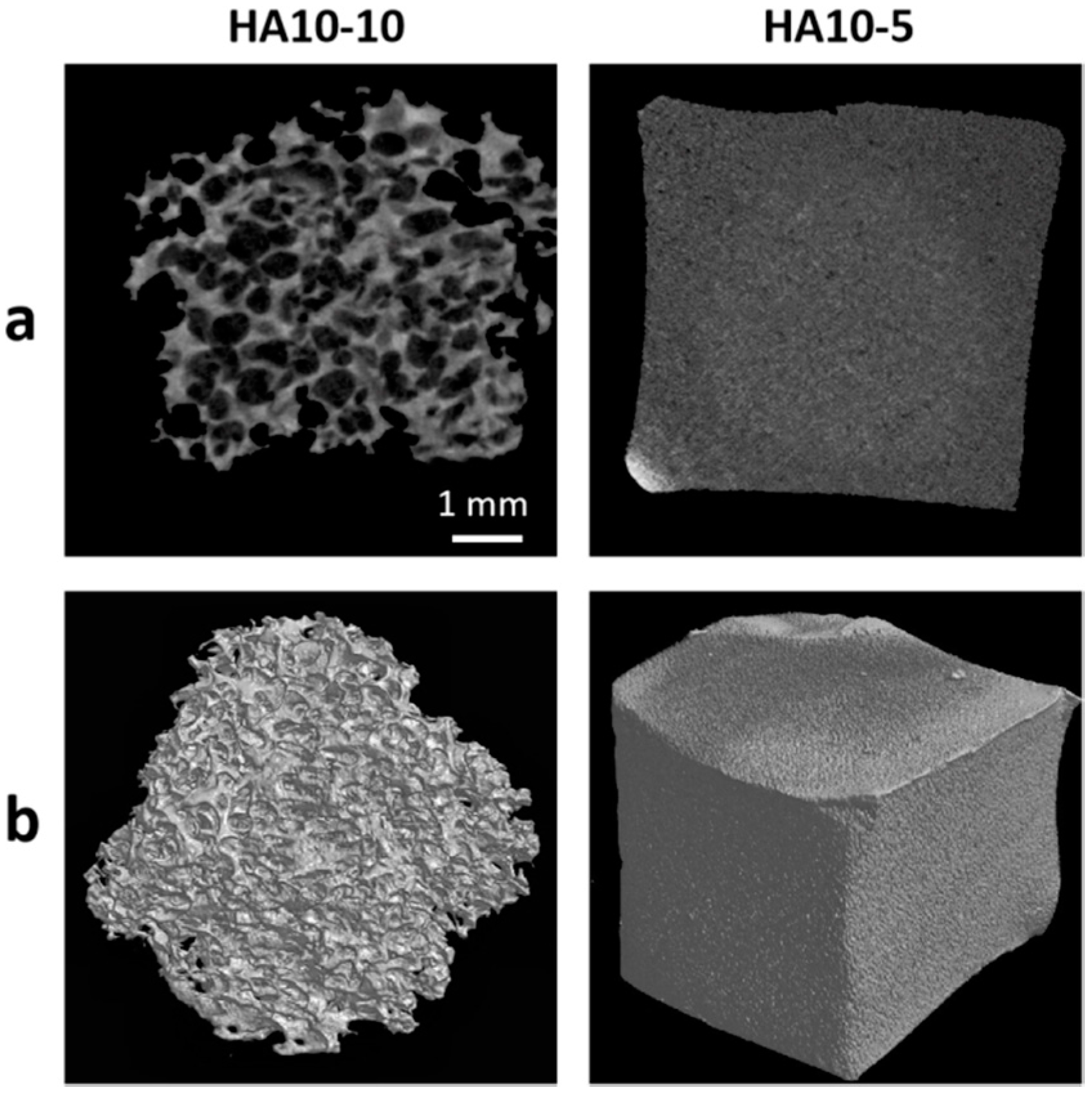
2.4. Micro-Morphologies of the Hydrogels
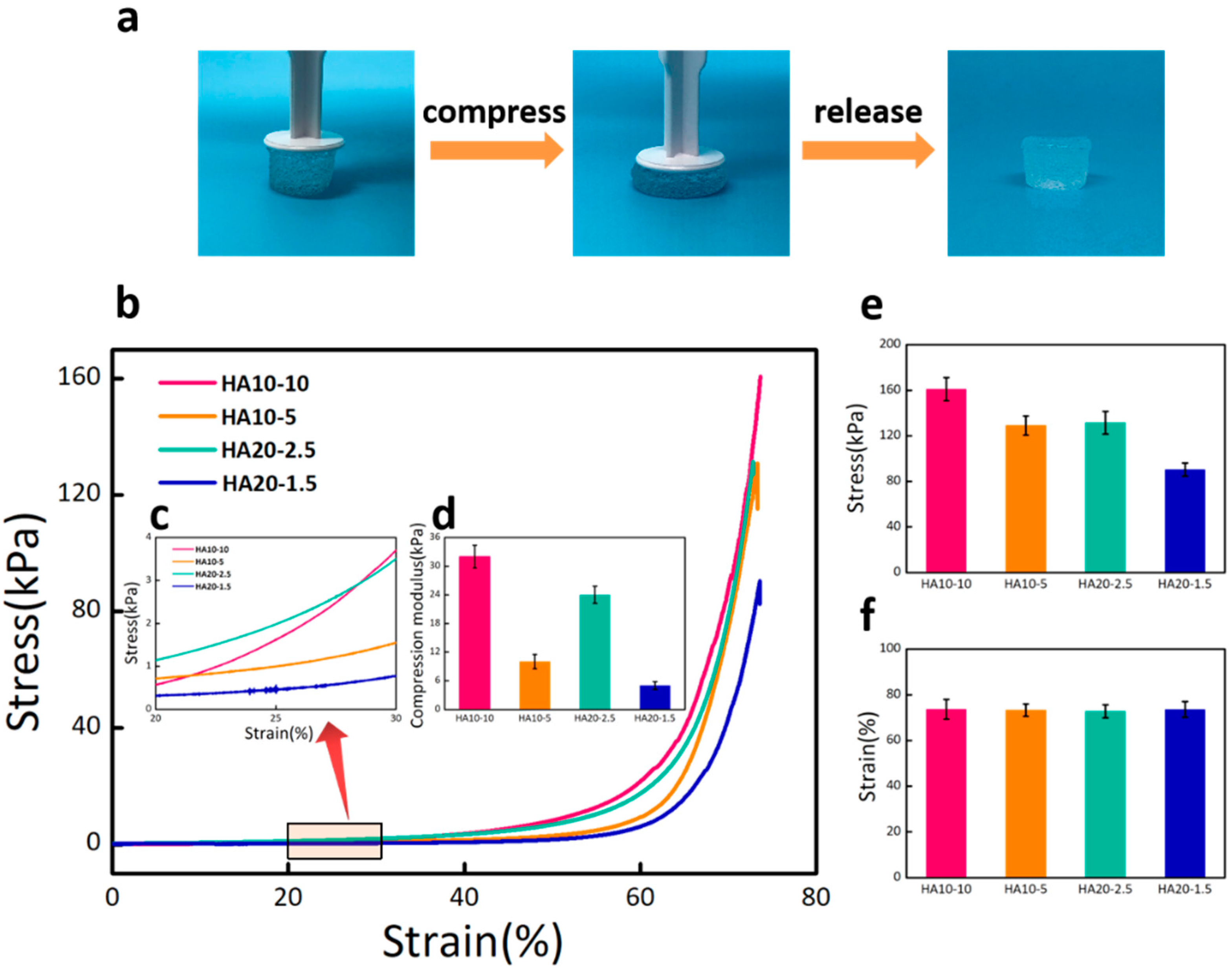
2.5. Compression Test
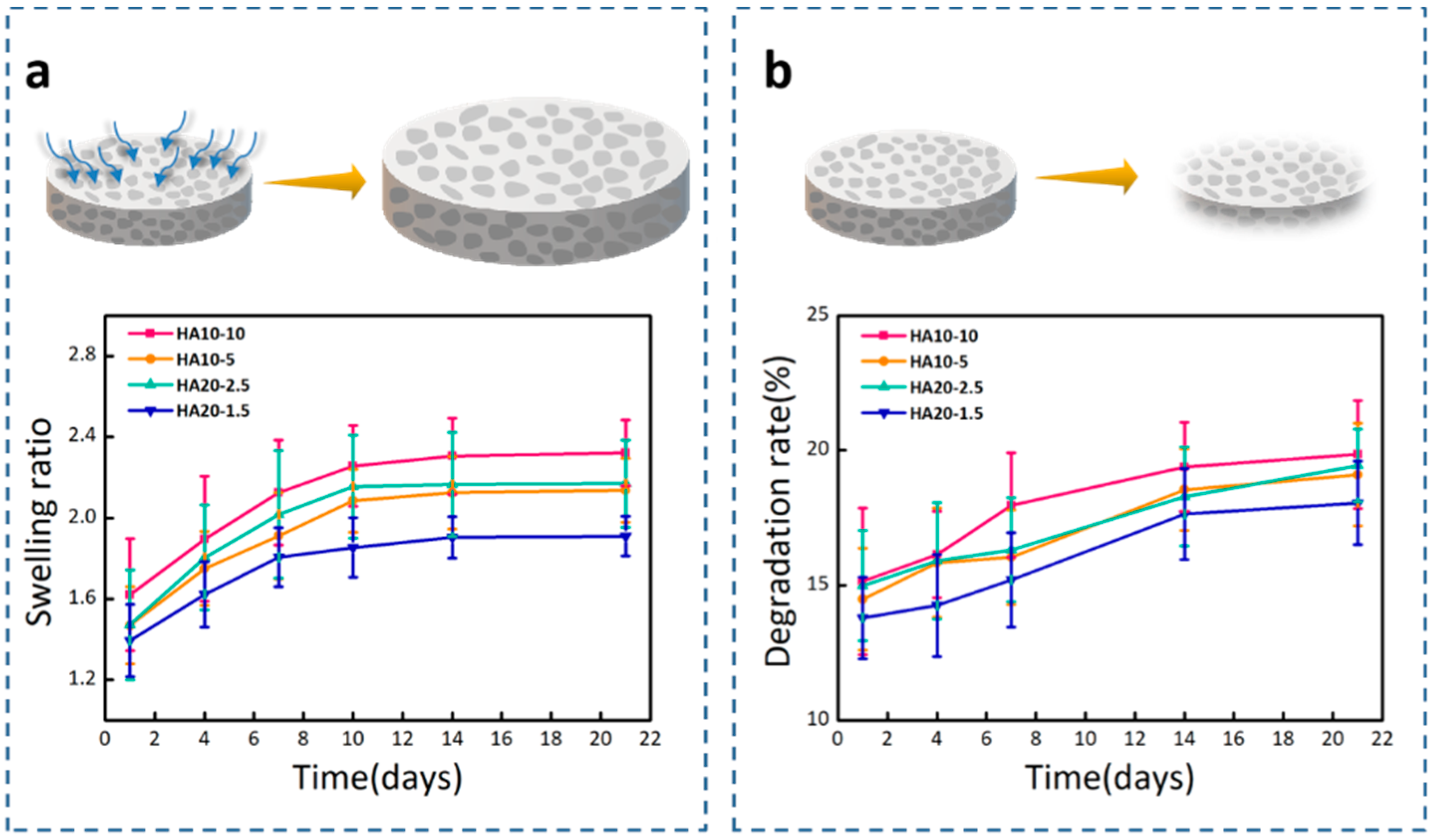
2.6. In Vitro Swelling and Degradation Test
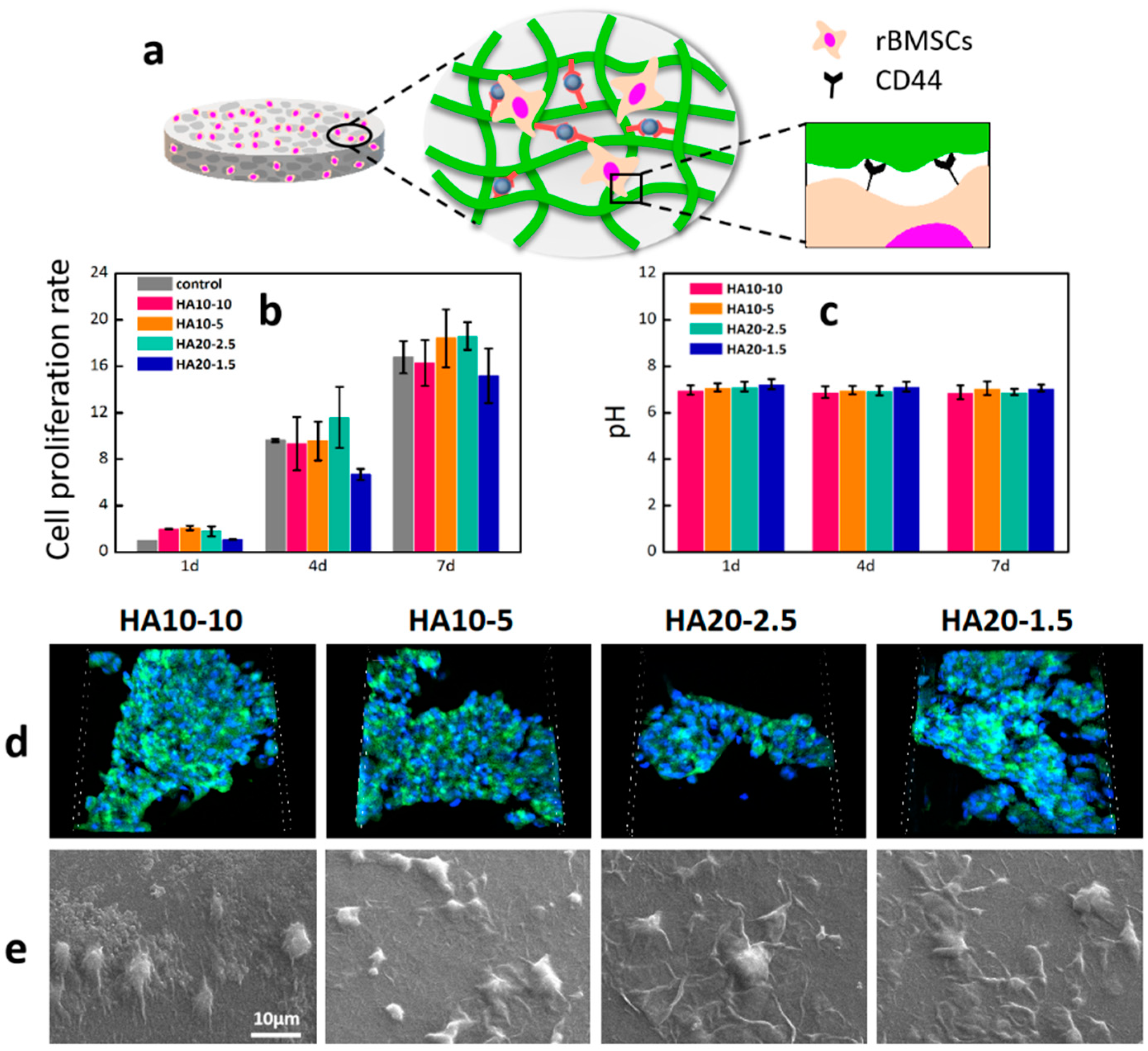
2.7. In Vitro Cellular Adhesion Assay
2.8. In Vitro Cell Proliferation Assay
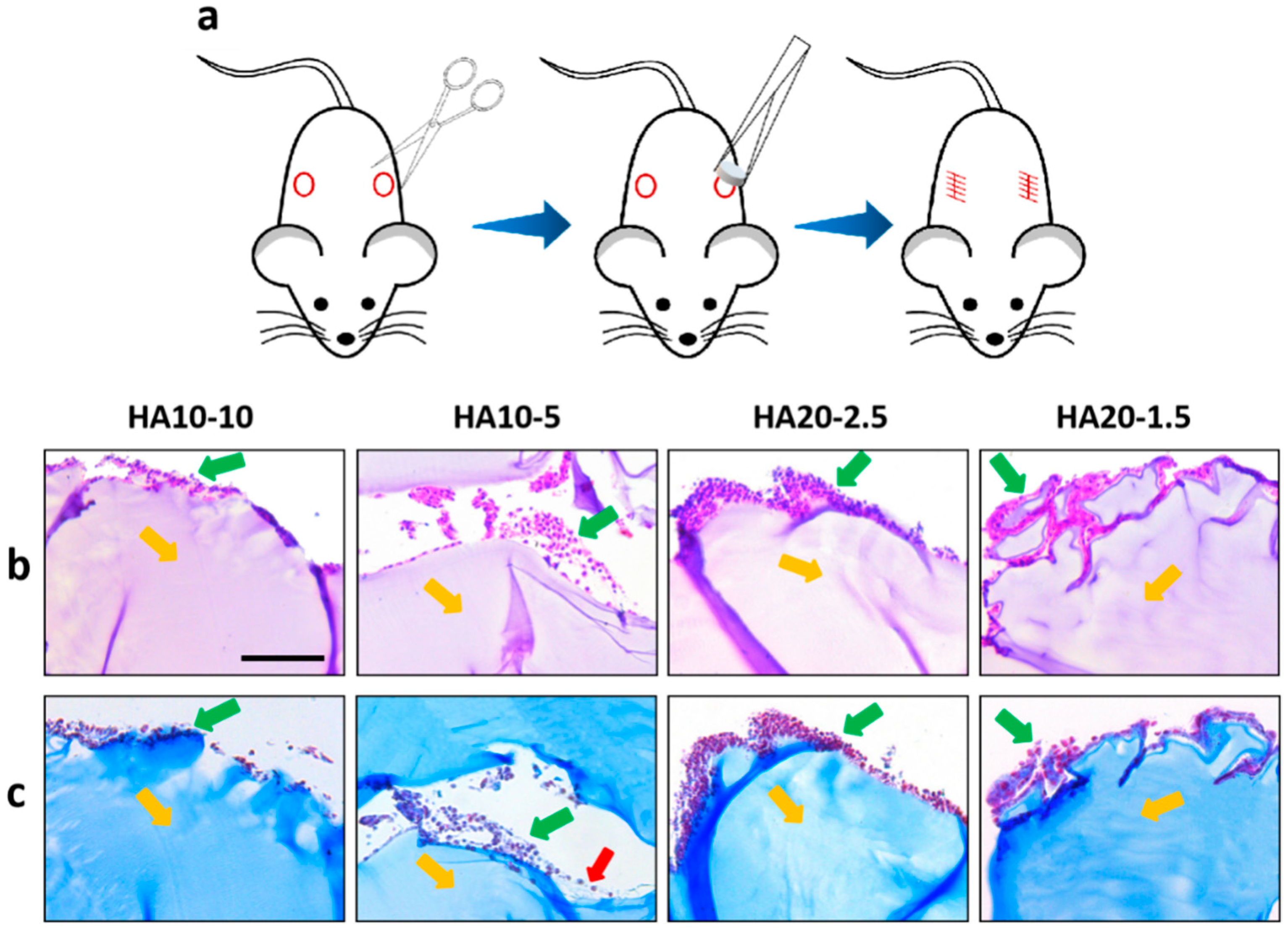
2.9. In Vivo Safety Evaluation
2.10. Statistics Analysis
3. Results
3.1. Preparation of Injectable, Porous HA Hydrogel
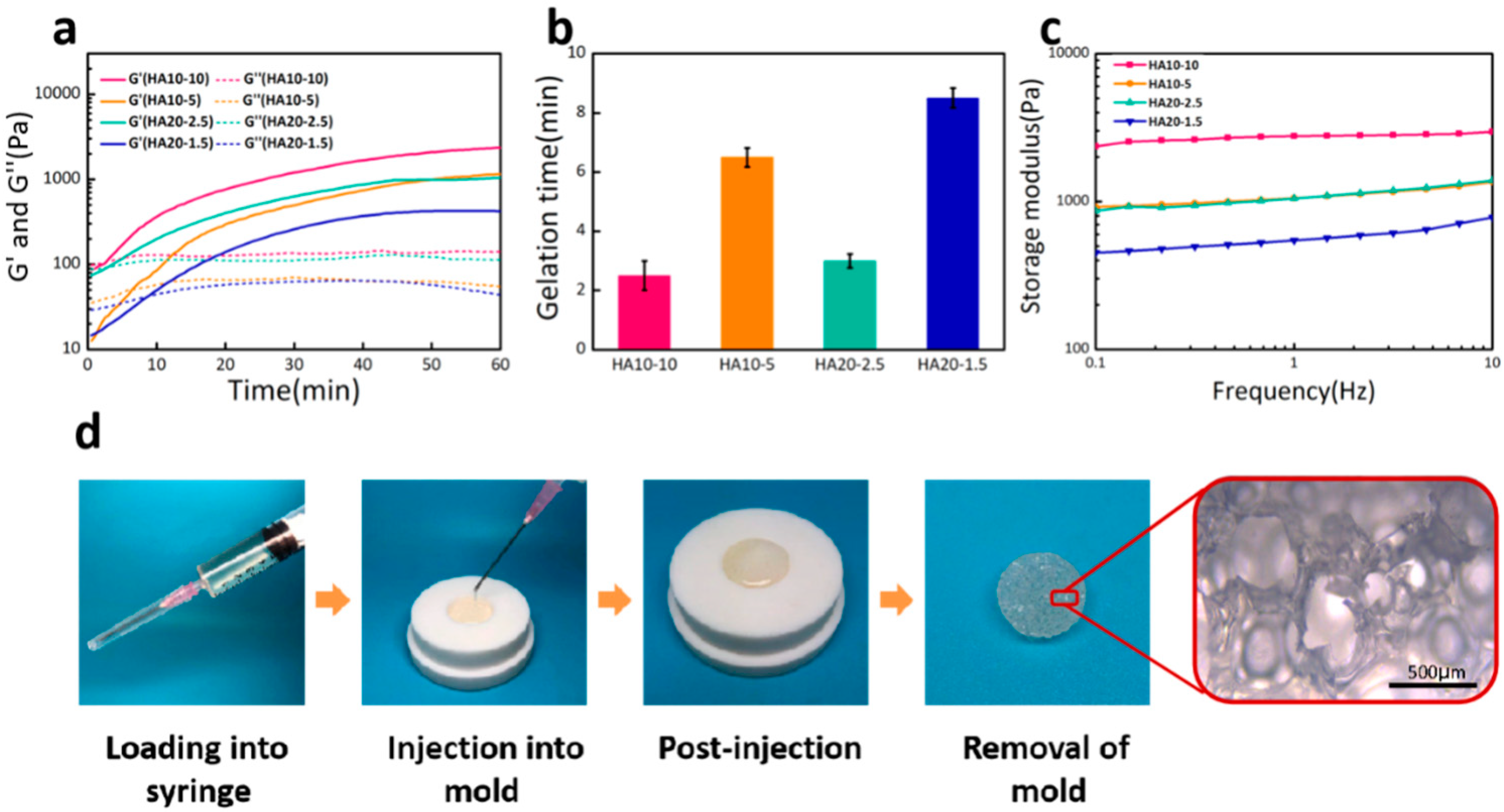
3.2. Characterization of the Injectability and Micro-Morphology of the Hydrogels
3.3. Mechanical Properties, Swelling Ratios, and Degradation Rates of the Hydrogels
3.4. Cell Adhesion, Morphology, and Proliferation of rBMSCs on the Hydrogels
3.5. Biocompatibility of the Hydrogels
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Johnson, T.D.; Christman, K.L. Injectable hydrogel therapies and their delivery strategies for treating myocardial infarction. Expert Opin. Drug Deliv. 2012, 10, 59–72. [Google Scholar] [CrossRef]
- Seo, B.B.; Koh, J.T.; Song, S.C. Tuning physical properties and bmp-2 release rates of injectable hydrogel systems for an optimal bone regeneration effect. Biomaterials 2017, 122, 91–104. [Google Scholar] [CrossRef]
- Latifi, N.; Asgari, M.; Vali, H.; Mongeau, L. A tissue-mimetic nano-fibrillar hybrid injectable hydrogel for potential soft tissue engineering applications. Sci. Rep. 2018, 8, 1047. [Google Scholar] [CrossRef]
- Kuang, L.; Ma, X.; Ma, Y.; Yao, Y.; Tariq, M.; Yuan, Y.; Liu, C. Self-assembled injectable nanocomposite hydrogels coordinated by in situ generated cap nanoparticles for bone regeneration. ACS Appl. Mater. Interfaces 2019, 11, 17234–17246. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cao, J.; Han, S.; Liang, Y.; Zhang, T.; Zhao, H.; Wang, L.; Sun, Y. Ecm based injectable thermo-sensitive hydrogel on the recovery of injured cartilage induced by osteoarthritis. Artif. Cells Nanomed. Biotechnol. 2018, 46, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Cao, B.; Lu, C.; Wang, G.; Yu, L.; Ding, J. An injectable hydrogel formed by in situ cross-linking of glycol chitosan and multi-benzaldehyde functionalized peg analogues for cartilage tissue engineering. J. Mater. Chem. B 2015, 3, 1268–1280. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Temenoff, J.S.; Tabata, Y.; Caplan, A.I.; Mikos, A.G. Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials 2007, 28, 3217–3227. [Google Scholar] [CrossRef]
- Dyondi, D.; Webster, T.J.; Banerjee, R. A nanoparticulate injectable hydrogel as a tissue engineering scaffold for multiple growth factor delivery for bone regeneration. Int. J. Nanomed. 2013, 8, 47–59. [Google Scholar] [CrossRef]
- Loebsack, A.; Greene, K.; Wyatt, S.; Culberson, C.; Halberstadt, C. In vivo characterization of a porous hydrogel material for use as a tissue bulking agent. J. Biomed. Mater. Res. Part B Appl. Biomater. 2002, 57, 575–581. [Google Scholar] [CrossRef]
- Betz, M.W.; Yeatts, A.B.; Richbourg, W.J.; Caccamese, J.F.; Coletti, D.P.; Falco, E.E.; Fisher, J.P. Macroporous hydrogels upregulate osteogenic signal expression and promote bone regeneration. Biomacromolecules 2010, 11, 1160–1168. [Google Scholar] [CrossRef]
- Hwang, C.M.; Sant, S.; Masaeli, M.; Kachouie, N.N.; Zamanian, B.; Lee, S.-H.; Khademhosseini, A. Fabrication of three-dimensional porous cell-laden hydrogel for tissue engineering. Biofabrication 2010, 2, 035003. [Google Scholar] [CrossRef] [PubMed]
- Welzel, P.B.; Grimmer, M.; Renneberg, C.; Naujox, L.; Zschoche, S.; Freudenberg, U.; Werner, C. Macroporous starpeg-heparin cryogels. Biomacromolecules 2012, 13, 2349–2358. [Google Scholar] [CrossRef] [PubMed]
- Koshy, S.T.; Ferrante, T.C.; Lewin, S.A.; Mooney, D.J. Injectable, porous, and cell-responsive gelatin cryogels. Biomaterials 2014, 35, 2477–2487. [Google Scholar] [CrossRef] [PubMed]
- Sokic, S.; Christenson, M.; Larson, J.; Papavasiliou, G. In situ generation of cell-laden porous mmp-sensitive pegda hydrogels by gelatin leaching. Macromol. Biosci. 2014, 14, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Remya, N.S.; Remya, S.; Nair, P.D. A biodegradable in situ injectable hydrogel based on chitosan and oxidized hyaluronic acid for tissue engineering applications. Carbohydr. Polym. 2011, 85, 838–844. [Google Scholar] [CrossRef]
- Fu, S.Z.; Ni, P.; Wang, B.; Chu, B.; Zheng, L.; Luo, F.; Luo, J.; Qian, Z. Injectable and thermo-sensitive peg-pcl-peg copolymer/collagen/n-ha hydrogel composite for guided bone regeneration. Biomaterials 2012, 33, 4801–4809. [Google Scholar] [CrossRef]
- Zhang, R.; Xue, M.; Yang, J.; Tan, T. A novel injectable and in situ crosslinked hydrogel based on hyaluronic acid and α,β-polyaspartylhydrazide. J. Appl. Polym. Sci. 2012, 125, 1116–1126. [Google Scholar] [CrossRef]
- Yom-Tov, O.; Neufeld, L.; Seliktar, D.; Bianco-Peled, H. A novel design of injectable porous hydrogels with in situ pore formation. Acta Biomater. 2014, 10, 4236–4246. [Google Scholar] [CrossRef]
- Goh, M.; Kim, Y.; Gwon, K.; Min, K.; Hwang, Y.; Tae, G. In situ formation of injectable and porous heparin-based hydrogel. Carbohydr. Polym. 2017, 174, 990–998. [Google Scholar] [CrossRef]
- Li, Y.; Rodrigues, J.o.; Tomás, H. Injectable and biodegradable hydrogels: Gelation, biodegradation and biomedical applications. Chem. Soc. Rev. 2012, 41, 2193–2221. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A.; Greco, F.; Busilacchi, A.; Sollazzo, V.; Gigante, A. Chitosan, hyaluronan and chondroitin sulfate in tissue engineering for cartilage regeneration: A review. Carbohydr. Polym. 2012, 89, 723–739. [Google Scholar] [CrossRef] [PubMed]
- Palma, P.J.; Ramos, J.C.; Martins, J.B.; Diogenes, A.; Figueiredo, M.H.; Ferreira, P.; Viegas, C.; Santos, J.M. Histologic evaluation of regenerative endodontic procedures with the use of chitosan scaffolds in immature dog teeth with apical periodontitis. J. Endodont. 2017, 43, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Leach, J.B.; Bivens, K.A.; Patrick, C.W., Jr.; Schmidt, C.E. Photocrosslinked hyaluronic acid hydrogels: Natural, biodegradable tissue engineering scaffolds. Biotechnol. Bioeng. 2003, 82, 578–589. [Google Scholar] [CrossRef]
- Solchaga, L.A.; Dennis, J.E.; Goldberg, V.M.; Caplan, A.I. Hyaluronic acid-based polymers as cell carriers for tissue-engineered repair of bone and cartilage. J. Orthop. Res. 1999, 17, 205–213. [Google Scholar] [CrossRef]
- Sani, E.S.; Portillo-Lara, R.; Spencer, A.; Yu, W.; Annabi, N. Engineering adhesive and antimicrobial hyaluronic acid/elastin-like polypeptide hybrid hydrogels for tissue engineering applications. ACS Biomater. Sci. Eng. 2018, 4, 2528–2540. [Google Scholar] [CrossRef]
- Zhao, X. Synthesis and characterization of a novel hyaluronic acid hydrogel. J. Biomater. Sci. Polym. Ed. 2006, 17, 419–433. [Google Scholar] [CrossRef]
- Varghese, O.P.; Kisiel, M.; Martã Nez-Sanz, E.; Ossipov, D.A.; Hilborn, J.N. Synthesis of guanidinium-modified hyaluronic acid hydrogel. Macromol. Rapid Commun. 2010, 31, 1175–1180. [Google Scholar] [CrossRef]
- Yan, X.M.; Seo, M.S.; Hwang, E.J.; Cho, I.H.; Hahn, S.K.; Sohn, U.D. Improved synthesis of hyaluronic acid hydrogel and its effect on tissue augmentation. J. Biomater. Appl. 2012, 27, 179–186. [Google Scholar] [CrossRef]
- Lévesque, S.G.; Lim, R.M.; Shoichet, M.S. Macroporous interconnected dextran scaffolds of controlled porosity for tissue-engineering applications. Biomaterials 2005, 26, 7436–7446. [Google Scholar]
- Barbetta, A.; Dentini, M.; Zannoni, E.M.; De Stefano, M.E. Tailoring the porosity and morphology of gelatin-methacrylate polyhipe scaffolds for tissue engineering applications. Langmuir ACS J. Surf. Colloids 2005, 21, 12333–12341. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Niu, H.; Zhang, W.; Ma, Y.; Yuan, Y.; Liu, C. Microporous density-mediated response of mscs on 3d trimodal macro/micro/nano-porous scaffolds via fibronectin/integrin and fak/mapk signaling pathways. J. Mater. Chem. B 2017, 5, 3586–3599. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Lin, D.; Tang, W.; Ma, Y.; Duan, B.; Yuan, Y.; Liu, C. Surface topography regulates osteogenic differentiation of mscs via crosstalk between fak/mapk and ilk/β-catenin pathways in a hierarchically porous environment. ACS Biomater. Sci. Eng. 2017, 3, 3161–3175. [Google Scholar] [CrossRef]
- Xu, J.; Sun, M.; Tan, Y.; Wang, H.; Wang, H.; Li, P.; Xu, Z.; Xia, Y.; Li, L.; Li, Y. Effect of matrix stiffness on the proliferation and differentiation of umbilical cord mesenchymal stem cells. Differentiation 2017, 96, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, W.; Wang, Z.; Wang, Z.; Xie, Q.; Niu, H.; Guo, H.; Yuan, Y.; Liu, C. Pegylated poly(glycerol sebacate)-modified calcium phosphate scaffolds with desirable mechanical behavior and enhanced osteogenic capacity. Acta Biomater. 2016, 44, 110–124. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Y.; Zhang, J.; Xie, Q.; Wang, Z.; Yu, S.; Yuan, Y.; Liu, C. Mbg-modified beta-tcp scaffold promotes mesenchymal stem cells adhesion and osteogenic differentiation via a fak/mapk signaling pathway. ACS Appl. Mater. Interfaces 2017, 9, 30283–30296. [Google Scholar] [CrossRef]
- Chen, G.; Lv, Y.; Guo, P.; Lin, C.; Zhang, X.; Yang, L.; Xu, Z. Matrix mechanics and fluid shear stress control stem cells fate in three dimensional microenvironment. Curr. Stem. Cell Res. Ther. 2013, 8, 313–323. [Google Scholar] [CrossRef]
- Kabiri, K.; Zohuriaan-Mehr, M.J. Porous superabsorbent hydrogel composites: Synthesis, morphology and swelling rate. Macromol. Mater. Eng. 2004, 289, 653–661. [Google Scholar] [CrossRef]
- Lin, W.J.; Lee, W.-C.; Shieh, M.-J. Hyaluronic acid conjugated micelles possessing cd44 targeting potential for gene delivery. Carbohydr. Polym. 2017, 155, 101–108. [Google Scholar] [CrossRef]
| Sample Codes | MW (Da) | Concentration (wt.%) |
|---|---|---|
| HA10-10 | 100,000 | 10 |
| HA10-5 | 100,000 | 5 |
| HA20-2.5 | 200,000 | 2.5 |
| HA20-1.5 | 200,000 | 1.5 |
| Sample Code | Closed Porosity (%) | Open Porosity (%) | Total Porosity (%) | Average Pore Size (μm) |
|---|---|---|---|---|
| HA10-10 | 0.00227 ± 0.00081 | 82.93863 ± 3.14905 | 82.93901 ± 3.52803 | 457 ± 34 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Dong, S.; Liu, Y.; Ma, Y.; Zhang, J.; Yang, Z.; Jiang, W.; Yuan, Y. Fabrication of Injectable, Porous Hyaluronic Acid Hydrogel Based on an In-Situ Bubble-Forming Hydrogel Entrapment Process. Polymers 2020, 12, 1138. https://doi.org/10.3390/polym12051138
Wang L, Dong S, Liu Y, Ma Y, Zhang J, Yang Z, Jiang W, Yuan Y. Fabrication of Injectable, Porous Hyaluronic Acid Hydrogel Based on an In-Situ Bubble-Forming Hydrogel Entrapment Process. Polymers. 2020; 12(5):1138. https://doi.org/10.3390/polym12051138
Chicago/Turabian StyleWang, Lixuan, Shiyan Dong, Yutong Liu, Yifan Ma, Jingjing Zhang, Zhaogang Yang, Wen Jiang, and Yuan Yuan. 2020. "Fabrication of Injectable, Porous Hyaluronic Acid Hydrogel Based on an In-Situ Bubble-Forming Hydrogel Entrapment Process" Polymers 12, no. 5: 1138. https://doi.org/10.3390/polym12051138
APA StyleWang, L., Dong, S., Liu, Y., Ma, Y., Zhang, J., Yang, Z., Jiang, W., & Yuan, Y. (2020). Fabrication of Injectable, Porous Hyaluronic Acid Hydrogel Based on an In-Situ Bubble-Forming Hydrogel Entrapment Process. Polymers, 12(5), 1138. https://doi.org/10.3390/polym12051138