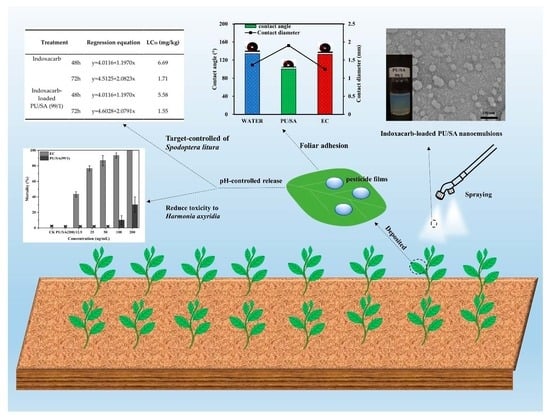
Indoxacarb-Loaded Anionic Polyurethane Blend with Sodium Alginate Improves pH Sensitivity and Ecological Security for Potential Application in Agriculture
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
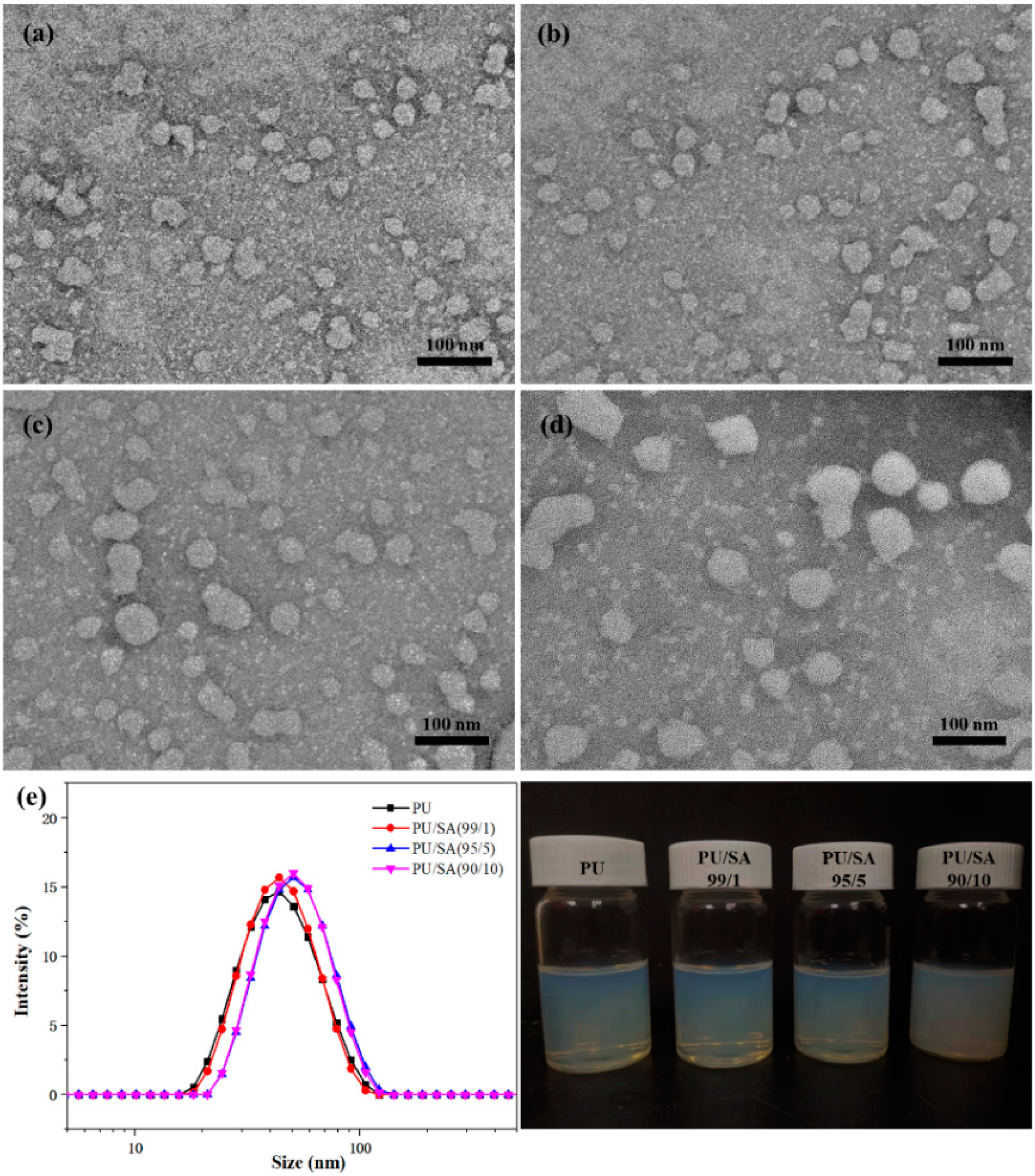
2.2. Preparation of Indoxacarb-Loaded PU/SA Nanoemulsions
2.3. Characterization of the Indoxacarb-Loaded PU/SA Nanoemulsions
2.4. Evaluation of Swelling Properties and Indoxacarb Release In Vitro
2.5. Adhesion and Degradation Dynamics Studies
2.6. Biological Toxicity Determination
3. Results and Discussion
3.1. Preparation of Indoxacarb-Loaded PU/SA Nanoemulsions
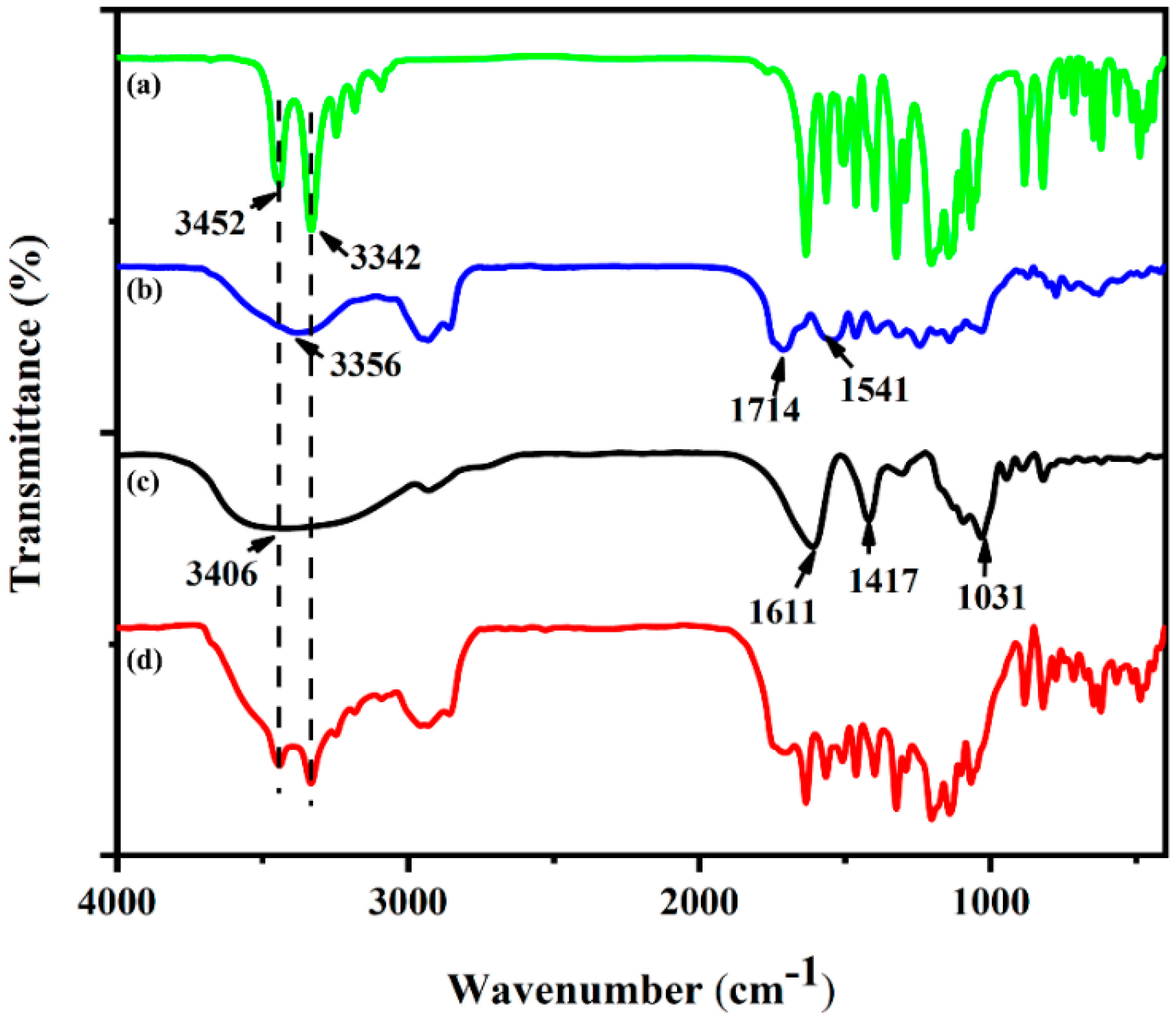
3.2. FTIR Analysis
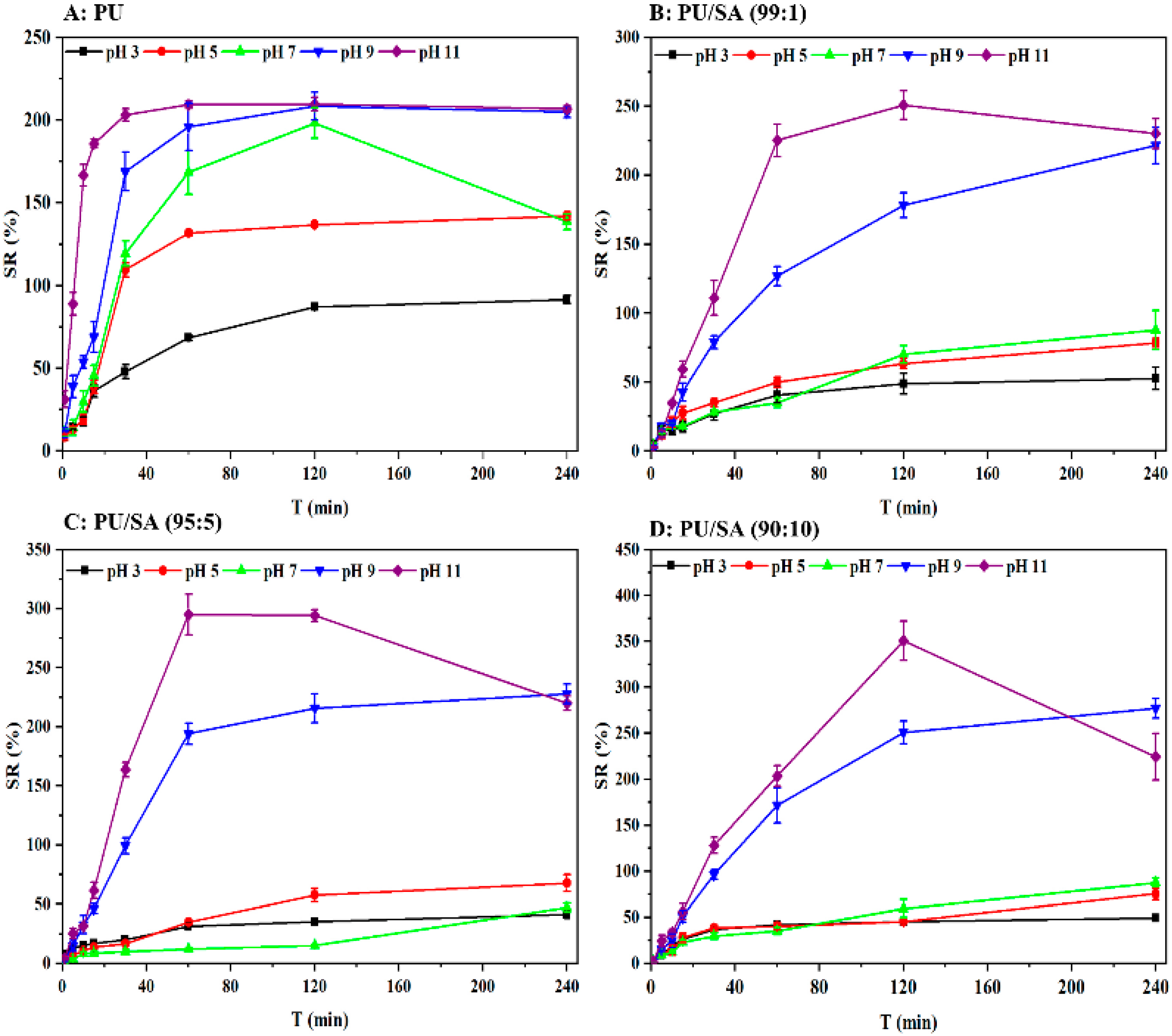
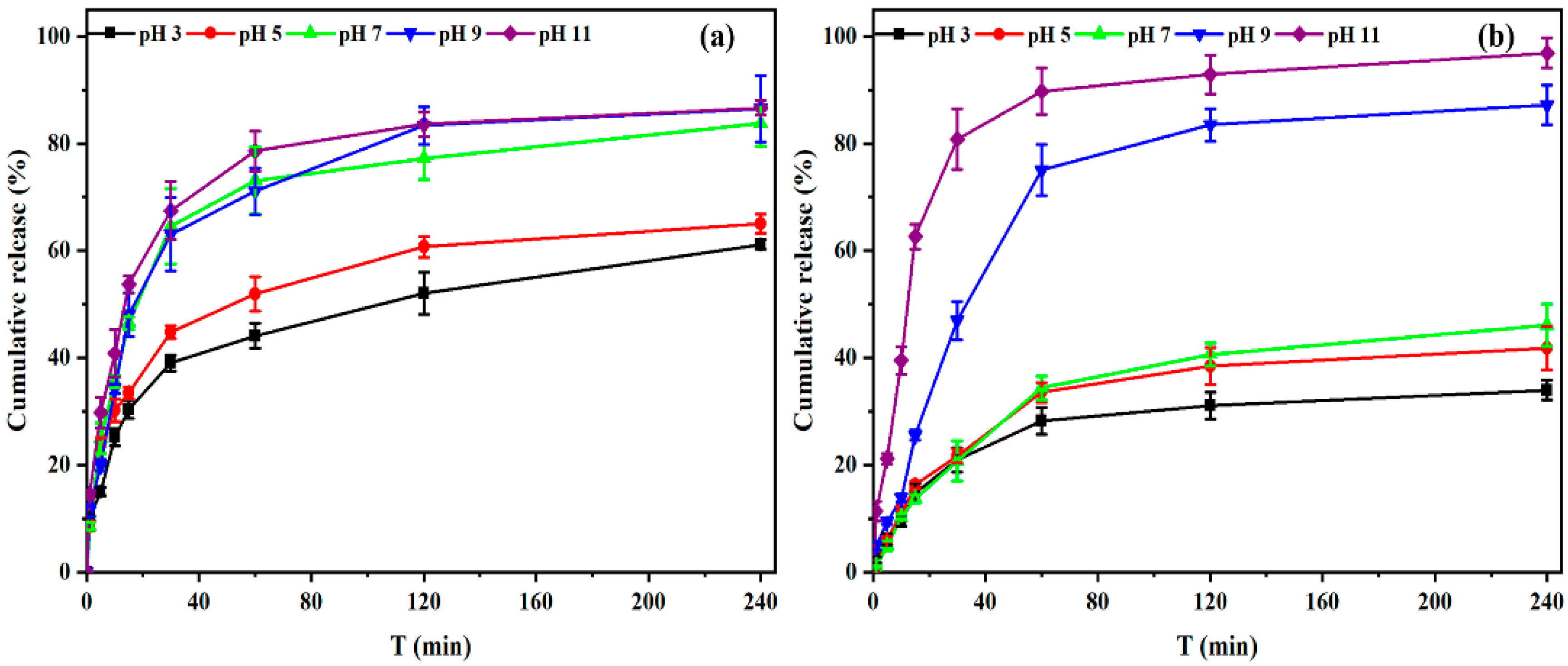
3.3. Responsive Swelling and Release Behaviors
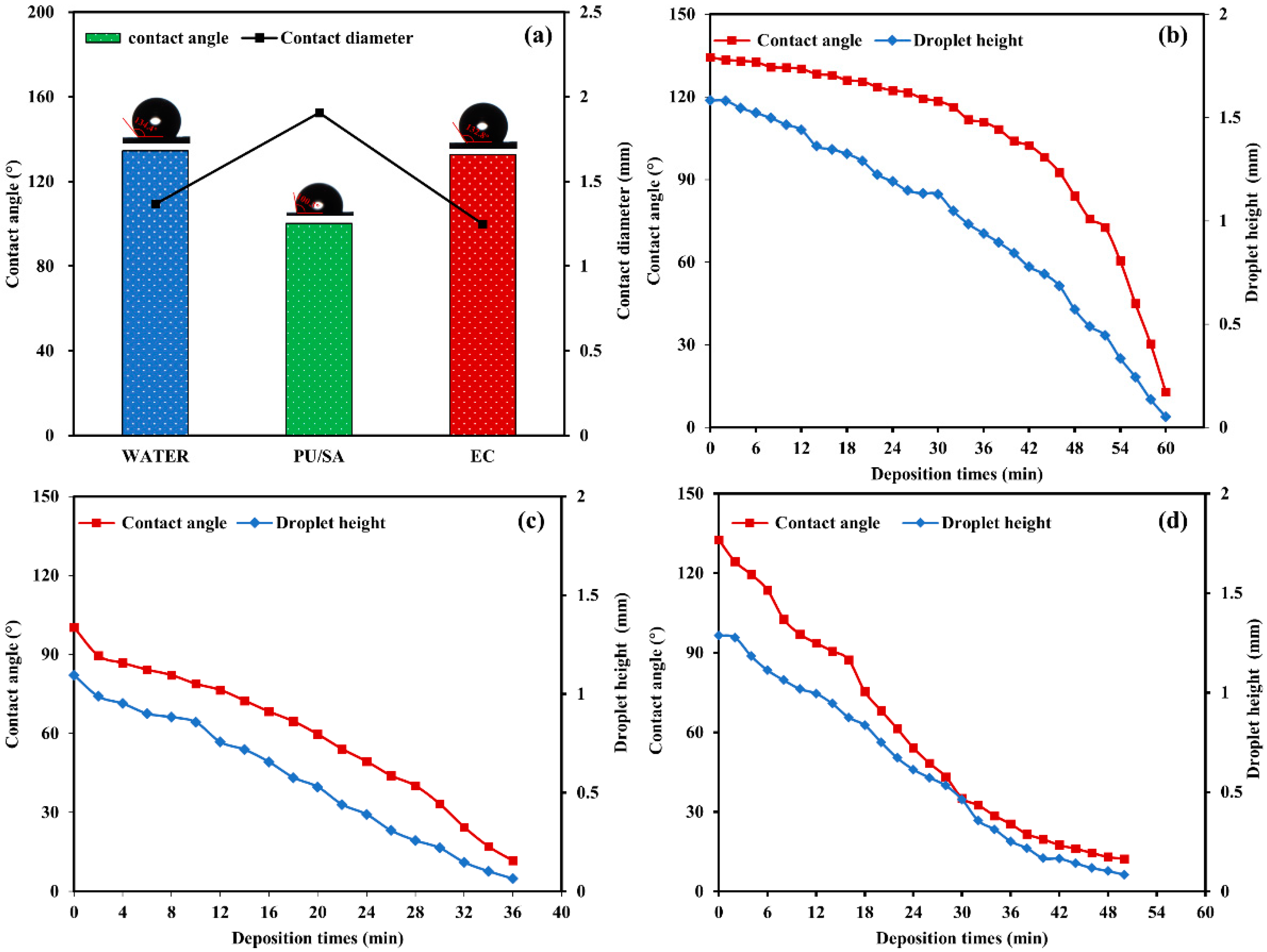
3.4. Foliage Adhesion and Pesticide Deposition
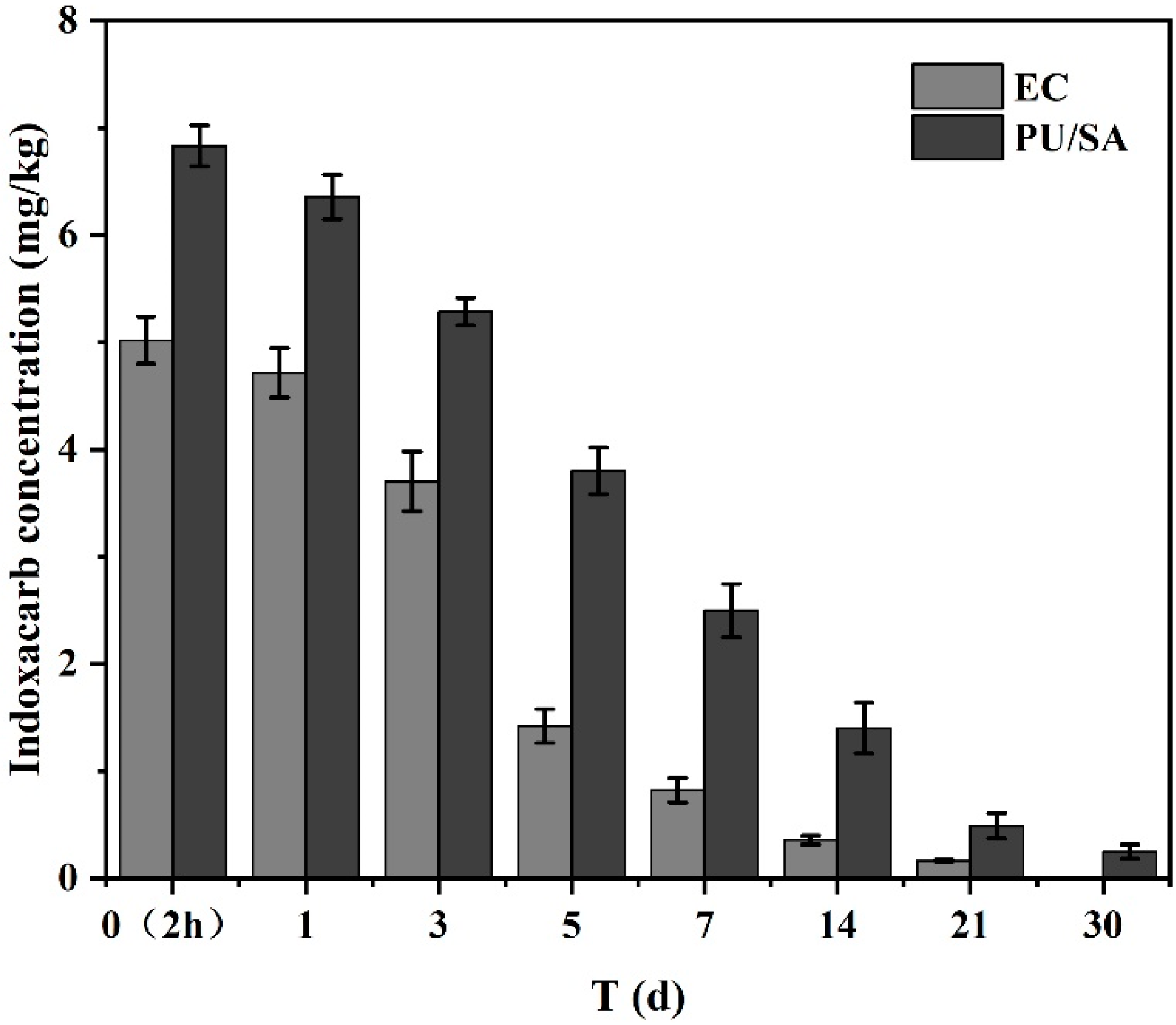
3.5. Digestion Dynamics
3.6. Biological Toxicity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Johnson, K.S.; Felton, G.W. Physiological and dietary influences on midgut redox conditions in generalist lepidopteran larvae. J. Insect Physiol. 1996, 42, 191–198. [Google Scholar] [CrossRef]
- Huang, S.; Xu, J.; Han, Z. Baseline toxicity data of insecticides against the common cutworm Spodoptera litura (Fabricius) and a comparison of resistance monitoring methods. Int. J. Pest Manag. 2006, 52, 209–213. [Google Scholar] [CrossRef]
- Ruttanaphan, T.; Pluempanupat, W.; Aungsirisawat, C.; Boonyarit, P.; Goff, G.L.; Bullangpoti, V. Effect of Plant Essential Oils and Their Major Constituents on Cypermethrin Tolerance Associated Detoxification Enzyme Activities in Spodoptera litura (Lepidoptera: Noctuidae). J. Econ. Entomol. 2019, 112, 2167–2176. [Google Scholar] [CrossRef] [PubMed]
- Rehan, A.; Freed, S. Selection, mechanism, cross resistance and stability of spinosad resistance in Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae). Crop Prot. 2014, 56, 10–15. [Google Scholar] [CrossRef]
- Christeller, J.T.; Amara, S.; Carriere, F. Galactolipase, phospholipase and triacylglycerol lipase activities in the midgut of six species of lepidopteran larvae feeding on different lipid diets. J. Insect Physiol. 2011, 57, 1232–1239. [Google Scholar] [CrossRef]
- Lapied, B.; Grolleau, F.; Sattelle, D.B. Indoxacarb, an oxadiazine insecticide, blocks insect neuronal sodium channels. Br. J. Pharmacol. 2001, 132, 587–595. [Google Scholar] [CrossRef]
- Sayyed, A.H.; Ahmad, M.; Saleem, M.A. Cross-resistance and genetics of resistance to indoxacarb in Spodoptera litura (Lepidoptera: Noctuidae). J. Econ. Entomol. 2008, 101, 472–479. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, D.; Meng, X.; Shi, Q.; Li, P.; Jin, L.; Zhang, K.; Song, B. Enantioselective Degradation of Indoxacarb From Different Commercial Formulations Applied to Tea. Chirality 2015, 27, 262–267. [Google Scholar] [CrossRef]
- Galvan, T.L.; Koch, R.L.; Hutchison, W.D. Effects of spinosad and indoxacarb on survival, development, and reproduction of the multicolored Asian lady beetle (Coleoptera: Coccinellidae). Biol. Control 2005, 34, 108–114. [Google Scholar] [CrossRef]
- Monteiro, H.R.; Pestana, J.L.T.; Novais, S.C.; Soares, A.M.V.M.; Lemos, M.F.L. Toxicity of the insecticides spinosad and indoxacarb to the non-target aquatic midge Chironomus riparius. Sci. Total Environ. 2019, 666, 1283–1291. [Google Scholar] [CrossRef]
- Marzban, R. Midgut pH Profile and Energy Differences in Lipid, Protein and Glycogen Metabolism of Bacillus thuringiensis Cry1Ac Toxin and Cypovirus-infected Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). J. Entomol. Res. Soc. 2012, 14, 45–53. [Google Scholar]
- Kamaraj, C.; Gandhi, P.R.; Elango, G.; Karthi, S.; Chung, I.; Rajakumar, G. Novel and environmental friendly approach; Impact of Neem (Azadirachta indica) gum nano formulation (NGNF) on Helicoverpa armigera (Hub.) and Spodoptera litura (Fab.). Int. J. Biol. Macromol. 2018, 107, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Ramos Campos, E.V.; de Oliveira, J.L.; Fraceto, L.F.; Singh, B. Polysaccharides as safer release systems for agrochemicals. Agron. Sustain. Dev. 2015, 35, 47–66. [Google Scholar] [CrossRef]
- Zare-Akbari, Z.; Farhadnejad, H.; Furughi-Nia, B.; Abedin, S.; Yadollahi, M.; Khorsand-Ghayeni, M. PH-sensitive bionanocomposite hydrogel beads based on carboxymethyl cellulose/ZnO nanoparticle as drug carrier. Int. J. Biol. Macromol. 2016, 93, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.J.; Singh, A. Base triggered release of insecticide from bentonite reinforced citric acid crosslinked carboxymethyl cellulose hydrogel composites. Carbohydr. Polym. 2017, 156, 303–311. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhang, G.; Chi, Y.; Cai, D.; Wu, Z. Fabrication of a controllable nanopesticide system with magnetic collectability. Chem. Eng. J. 2017, 328, 320–330. [Google Scholar] [CrossRef]
- Zeng, L.; Liu, Y.; Pan, J.; Liu, X. Formulation and evaluation of norcanthridin nanoemulsions against the Plutella xylostella (Lepidotera: Plutellidae). BMC Biotechnol. 2019, 19, 16. [Google Scholar] [CrossRef]
- Ding, M.; Li, J.; He, X.; Song, N.; Tan, H.; Zhang, Y.; Zhou, L.; Gu, Q.; Deng, H.; Fu, Q. Molecular Engineered Super-Nanodevices: Smart and Safe Delivery of Potent Drugs into Tumors. Adv. Mater. 2012, 24, 3639–3645. [Google Scholar] [CrossRef]
- Zhang, Y.; He, W.; Li, J.; Wang, K.; Li, J.; Tan, H.; Fua, Q. Gemini quaternary ammonium salt waterborne biodegradable polyurethanes with antibacterial and biocompatible properties. Mater. Chem. Front. 2017, 1, 361–368. [Google Scholar] [CrossRef]
- Zhang, Y.; He, X.; Ding, M.; He, W.; Li, J.; Li, J.; Tan, H. Antibacterial and Biocompatible Cross-Linked Waterborne Polyurethanes Containing Gemini Quaternary Ammonium Salts. Biomacromolecules 2018, 19, 279–287. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, H.; Li, L.; Zhou, X.; Wang, W.; Kan, C. Preparation and Characterization of Controlled-Release Avermectin/Castor Oil-Based Polyurethane Nanoemulsions. J. Agric. Food Chem. 2018, 66, 6552–6560. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yu, L.; Ding, M.; Li, J.; Tan, H.; Wang, Z.; Fu, Q. Synthesis and Characterization of pH-Sensitive Biodegradable Polyurethane for Potential Drug Delivery Applications. Macromolecules 2011, 44, 857–864. [Google Scholar] [CrossRef]
- Yun, J.; Yoo, H.; Kim, H. Preparation and properties of waterborne polyurethane-urea/sodium alginate blends for high water vapor permeable coating materials. J. Appl. Polym. Sci. 2007, 105, 1168–1176. [Google Scholar] [CrossRef]
- Dai, Y.; Li, P.; Zhang, J.; Wang, A.; Wei, Q. A novel pH sensitive N-succinyl chitosan/alginate hydrogel bead for nifedipine delivery. Biopharm. Drug Dispos. 2008, 29, 173–184. [Google Scholar] [CrossRef]
- Daemi, H.; Barikani, M.; Barmar, M. Compatible compositions based on aqueous polyurethane dispersions and sodium alginate. Carbohydr. Polym. 2013, 92, 490–496. [Google Scholar] [CrossRef]
- Qin, H.; Zhang, H.; Li, L.; Zhou, X.; Li, J.; Kan, C. Preparation and properties of lambda-cyhalothrin/polyurethane drug-loaded nanoemulsions. Rsc. Adv. 2017, 7, 52684–52693. [Google Scholar] [CrossRef]
- Liang, H.; Wang, S.; He, H.; Wang, M.; Liu, L.; Lu, J.; Zhang, Y.; Zhang, C. Aqueous anionic polyurethane dispersions from castor oil. Ind. Crop. Prod. 2018, 122, 182–189. [Google Scholar] [CrossRef]
- Wang, G.; Xiao, Y.; Xu, H.; Hu, P.; Liang, W.; Xie, L.; Jia, J. Development of Multifunctional Avermectin Poly(succinimide) Nanoparticles to Improve Bioactivity and Transportation in Rice. J. Agric. Food Chem. 2018, 66, 11244–11253. [Google Scholar] [CrossRef]
- Sun, D.; Pang, J.; Qiu, J.; Li, L.; Liu, C.; Jiao, B. Enantioselective Degradation and Enantiomerization of Indoxacarb in Soil. J. Agric. Food Chem. 2013, 61, 11273–11277. [Google Scholar] [CrossRef]
- Zhou, Z.; Cao, C.; Cao, L.; Zheng, L.; Xu, J.; Li, F.; Huang, Q. Effect of surfactant concentration on the evaporation of droplets on cotton (Gossypium hirsutum L.) leaves. Colloid Surface B 2018, 167, 206–212. [Google Scholar] [CrossRef]
- Choi, J.; Zheng, W.; Abd El-Aty, A.M.; Kim, S.; Park, D.; Yoo, K.; Lee, G.; Baranenko, D.A.; Hacimuftuoglu, A.; Jeong, J.H.; et al. Residue analysis of tebufenozide and indoxacarb in chicken muscle, milk, egg and aquatic animal products using liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 2019, 33, e4522. [Google Scholar] [CrossRef] [PubMed]
- Soleymanzade, A.; Khorrami, F.; Forouzan, M. Insecticide Toxicity, Synergism and Resistance in Plutella xylostella (Lepidoptera: Plutellidae). Acta Phytopathol. Entomol. Hung. 2019, 54, 147–154. [Google Scholar] [CrossRef]
- Shao, H.; Xi, N.; Zhang, Y. Microemulsion formulation of a new biopesticide to control the diamondback moth (Lepidoptera: Plutellidae). Sci. Rep. 2018, 8, 10565. [Google Scholar] [CrossRef] [PubMed]
- Memarizadeh, N.; Ghadamyari, M.; Adeli, M.; Talebi, K. Linear-dendritic copolymers/indoxacarb supramolecular systems: Biodegradable and efficient nano-pesticides. Environ. Sci. Process. Impacts 2014, 16, 2380–2389. [Google Scholar] [CrossRef]
- Xi, T.; Lu, Y.; Ai, X.; Tang, L.; Yao, L.; Hao, W.; Cui, P. Ionic liquid copolymerized polyurethane membranes for pervaporation separation of benzene/cyclohexane mixtures. Polymer 2019, 185, 121948. [Google Scholar] [CrossRef]
- Travinskaya, T.V.; Savelyev, Y.V. Aqueous polyurethane-alginate compositions: Peculiarities of behavior and performance. Eur. Polym. J. 2006, 42, 388–394. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; He, S.; Xiao, Y.; Qin, X.; Zhang, Y.; Li, D.; Ma, H.; You, H.; Li, J. Fabrication of a hollow mesoporous silica hybrid to improve the targeting of a pesticide. Chem. Eng. J. 2019, 364, 361–369. [Google Scholar] [CrossRef]
- Shang, Y.; Ding, F.; Xiao, L.; Deng, H.; Du, Y.; Shi, X. Chitin-based fast responsive pH sensitive microspheres for controlled drug release. Carbohydr. Polym. 2014, 102, 413–418. [Google Scholar] [CrossRef]
- Elhariry, H.M. Attachment strength and biofilm forming ability of Bacillus cereus on green-leafy vegetables: Cabbage and lettuce. Food Microbiol. 2011, 28, 1266–1274. [Google Scholar] [CrossRef]
- Sihtmaee, M.; Blinova, I.; Kuennis-Beres, K.; Kanarbik, L.; Heinlaan, M.; Kahru, A. Ecotoxicological effects of different glyphosate formulations. Appl. Soil. Ecol. 2013, 72, 215–224. [Google Scholar] [CrossRef]
- Zhang, P.W.; Wang, S.Y.; Huang, C.L.; Fu, J.T.; Huang, R.L.; Li, Z.H.; Zhang, Z.X. Dissipation and residue of clothianidin in granules and pesticide fertilizers used in cabbage and soil under field conditions. Environ. Sci. Pollut. R. 2018, 25, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Liang, H.; Zhou, X.; Fang, C.; Zhang, C.; Luo, Y. Synthesis and properties of castor oil-based waterborne polyurethane/sodium alginate composites with tunable properties. Carbohydr. Polym. 2019, 208, 391–397. [Google Scholar] [CrossRef]
- Cao, C.; Song, Y.; Zhou, Z.; Cao, L.; Li, F.; Huang, Q. Effect of adhesion force on the height pesticide droplets bounce on impaction with cabbage leaf surfaces. Soft Matter 2018, 14, 8030–8035. [Google Scholar] [CrossRef] [PubMed]
- Bashir, O.; Claverie, J.P.; Lemoyne, P.; Vincent, C. Controlled-release of Bacillus thurigiensis formulations encapsulated in light-resistant colloidosomal microcapsules for the management of lepidopteran pests of Brassica crops. PeerJ 2016, 4, e2524. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.S.V.K.; Rao, K.M.; Kumar, P.V.N.; Chung, I. Novel Chitosan-based pH Sensitive Micro-networks for the Controlled Release of 5-Fluorouracil. Iran. Polym. J. 2010, 19, 265–276. [Google Scholar]
 ” indicate nontoxic at the corresponding treatment.
” indicate nontoxic at the corresponding treatment.
 ” indicate nontoxic at the corresponding treatment.
” indicate nontoxic at the corresponding treatment.
| Sample | Size (nm) | DPI | ζ (mV) |
|---|---|---|---|
| PU | 57.2 ± 0.6 | 0.113 | −45.9 ± 1.3 |
| PU/SA (99/1) | 58.4 ± 0.3 | 0.114 | −47.3 ± 0.3 |
| PU/SA (95/5) | 60.1 ± 1.9 | 0.133 | −48.8 ± 0.6 |
| PU/SA (90/10) | 70.6 ± 1.4 | 0.196 | −49.5 ± 0.4 |
| Treatment | Regression Equation | LC50 (mg/kg) | 95% Fiducial Limit | Correlation Coefficients | |
|---|---|---|---|---|---|
| Indoxacarb | 48 h | y = 4.0116 + 1.1970x | 6.69 | 4.07–11.00 | 0.9923 |
| 72 h | y =4.5125 + 2.0823x | 1.71 | 1.30–2.26 | 0.9855 | |
| Indoxacarb-Loaded PU/SA (99/1) | 48 h | y = 4.0116 + 1.1970x | 5.58 | 3.42–9.08 | 0.9923 |
| 72 h | y = 4.6028 + 2.0791x | 1.55 | 1.17–2.07 | 0.9903 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zhang, Y.; Yang, L.; Zhu, Q.; Ma, Q.; Wang, R.; Zhang, C.; Zhang, Z. Indoxacarb-Loaded Anionic Polyurethane Blend with Sodium Alginate Improves pH Sensitivity and Ecological Security for Potential Application in Agriculture. Polymers 2020, 12, 1135. https://doi.org/10.3390/polym12051135
Wang S, Zhang Y, Yang L, Zhu Q, Ma Q, Wang R, Zhang C, Zhang Z. Indoxacarb-Loaded Anionic Polyurethane Blend with Sodium Alginate Improves pH Sensitivity and Ecological Security for Potential Application in Agriculture. Polymers. 2020; 12(5):1135. https://doi.org/10.3390/polym12051135
Chicago/Turabian StyleWang, Shiying, Yi Zhang, Liupeng Yang, Qizhan Zhu, Qianli Ma, Ruifei Wang, Chaoqun Zhang, and Zhixiang Zhang. 2020. "Indoxacarb-Loaded Anionic Polyurethane Blend with Sodium Alginate Improves pH Sensitivity and Ecological Security for Potential Application in Agriculture" Polymers 12, no. 5: 1135. https://doi.org/10.3390/polym12051135
APA StyleWang, S., Zhang, Y., Yang, L., Zhu, Q., Ma, Q., Wang, R., Zhang, C., & Zhang, Z. (2020). Indoxacarb-Loaded Anionic Polyurethane Blend with Sodium Alginate Improves pH Sensitivity and Ecological Security for Potential Application in Agriculture. Polymers, 12(5), 1135. https://doi.org/10.3390/polym12051135