A Biomimetic Approach to Increasing Soft Actuator Performance by Friction Reduction
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Conductive and Hydrophobic Coatings
2.3. Polymerization of PPy; Actuation of PET-PPy Bilayer
2.4. Electrochemical Measurement Techniques
2.5. Characterization
3. Results and Discussion
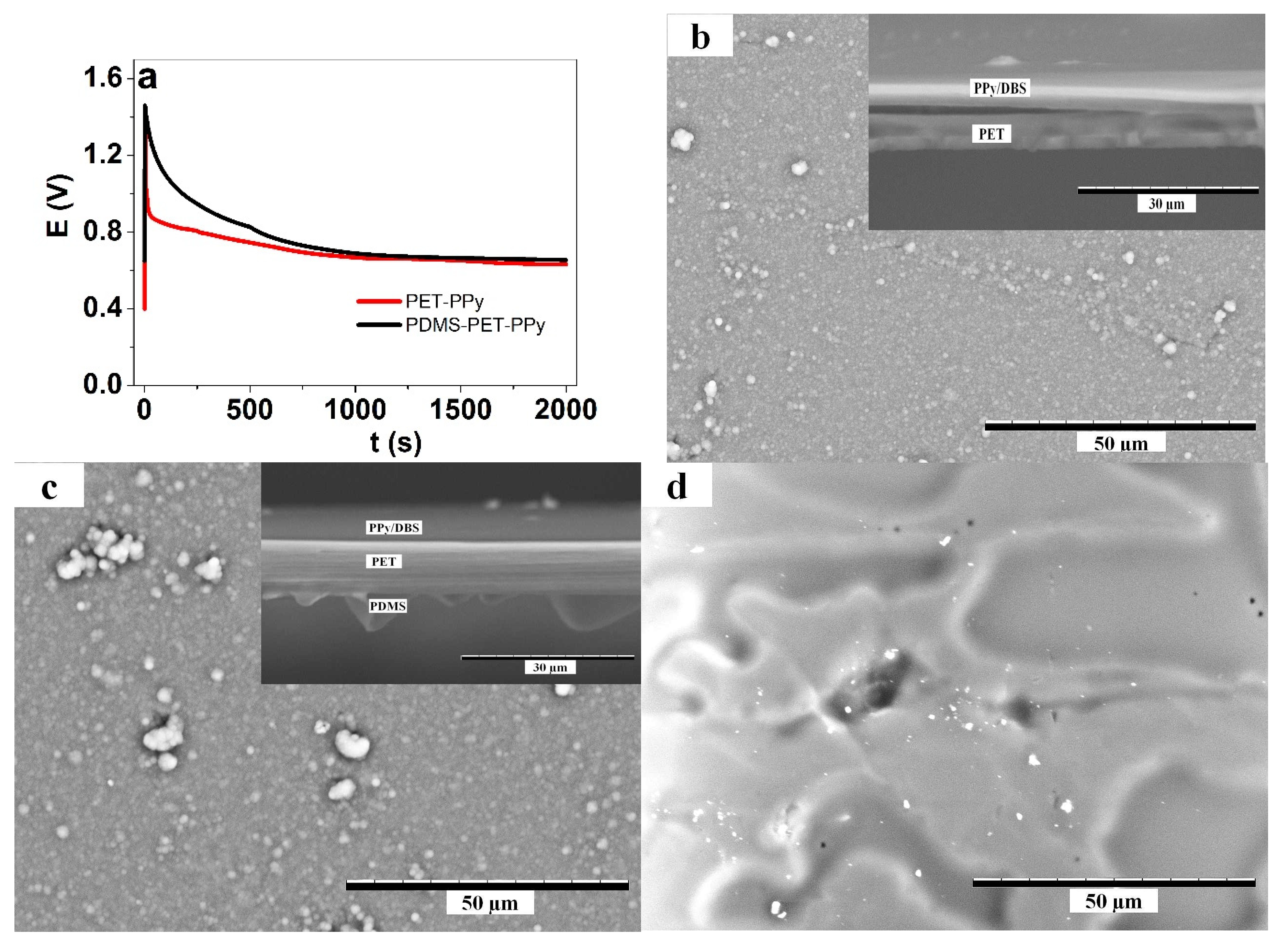
3.1. PPy/DBS Electropolymerization
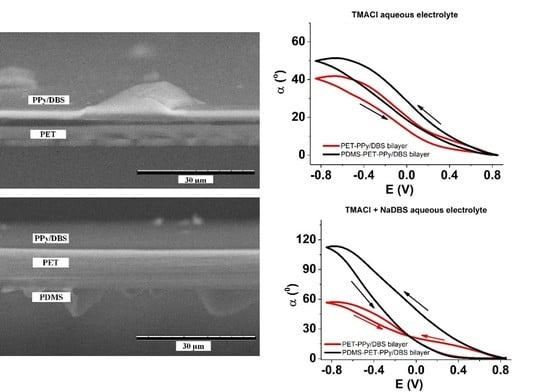
3.2. Friction Reduction
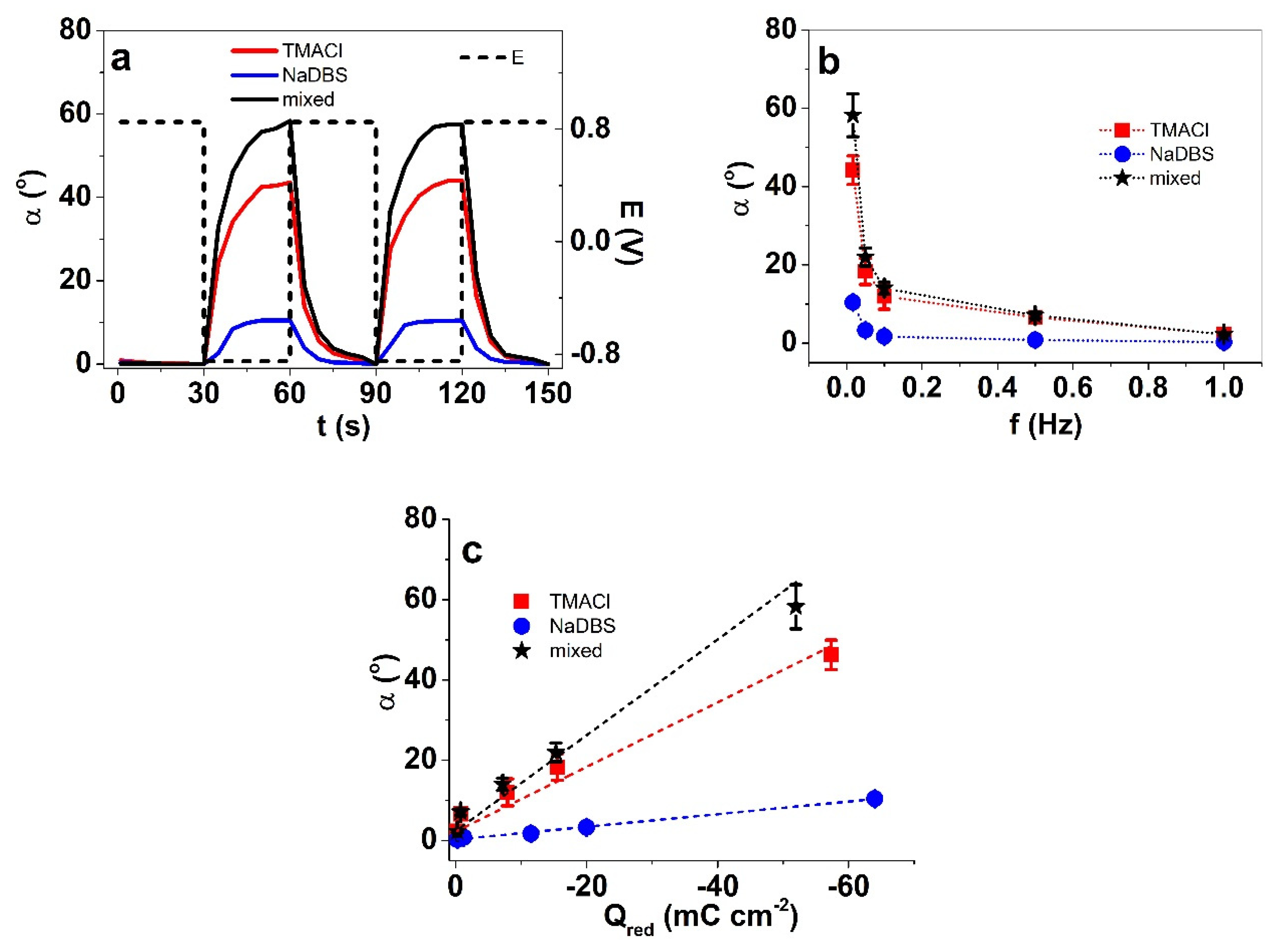
3.2.1. Electrolyte Concentration and Electrolyte Blends
3.2.2. PDMS Coatings on PET Layer
3.2.3. Contact Angle for Combined Approach
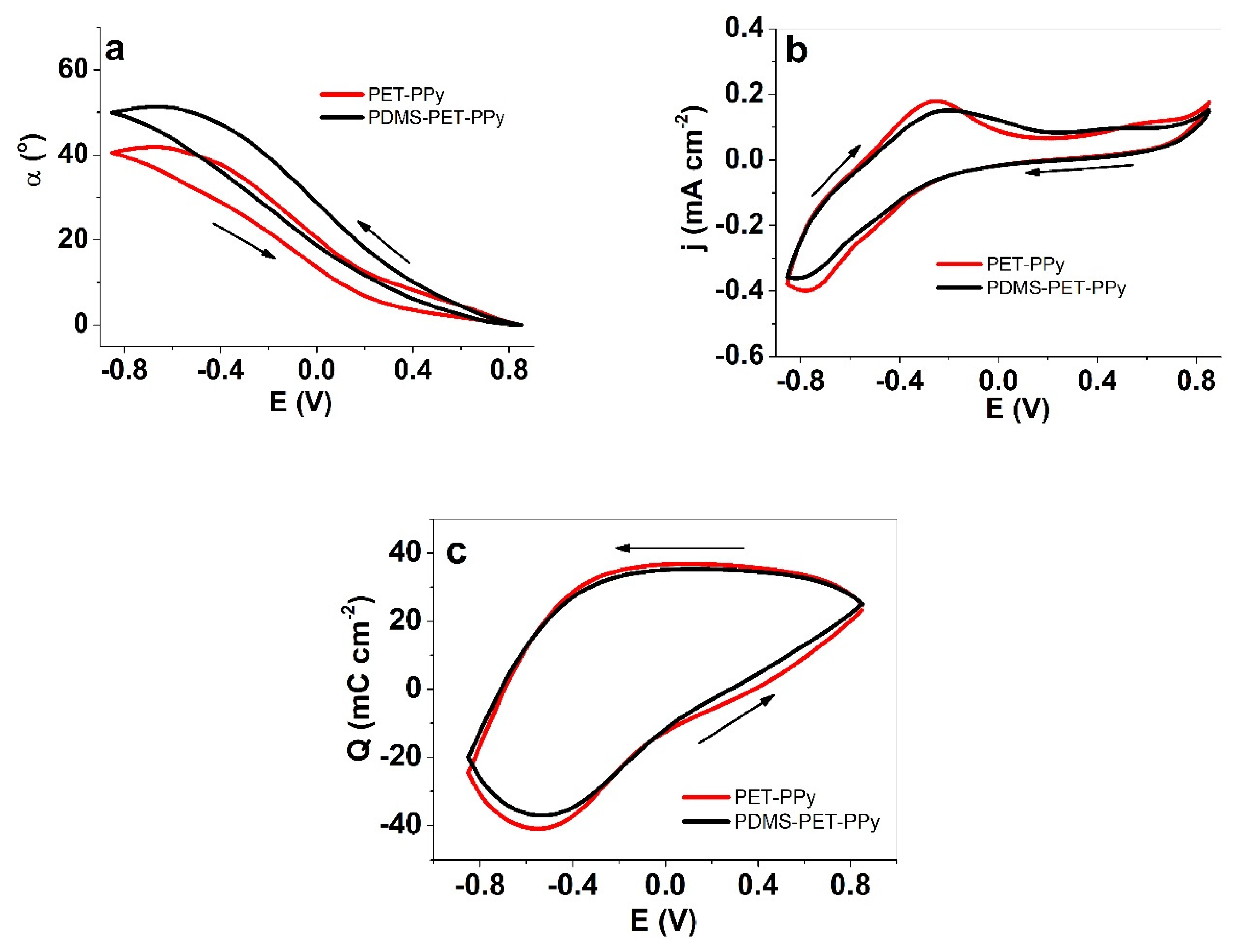
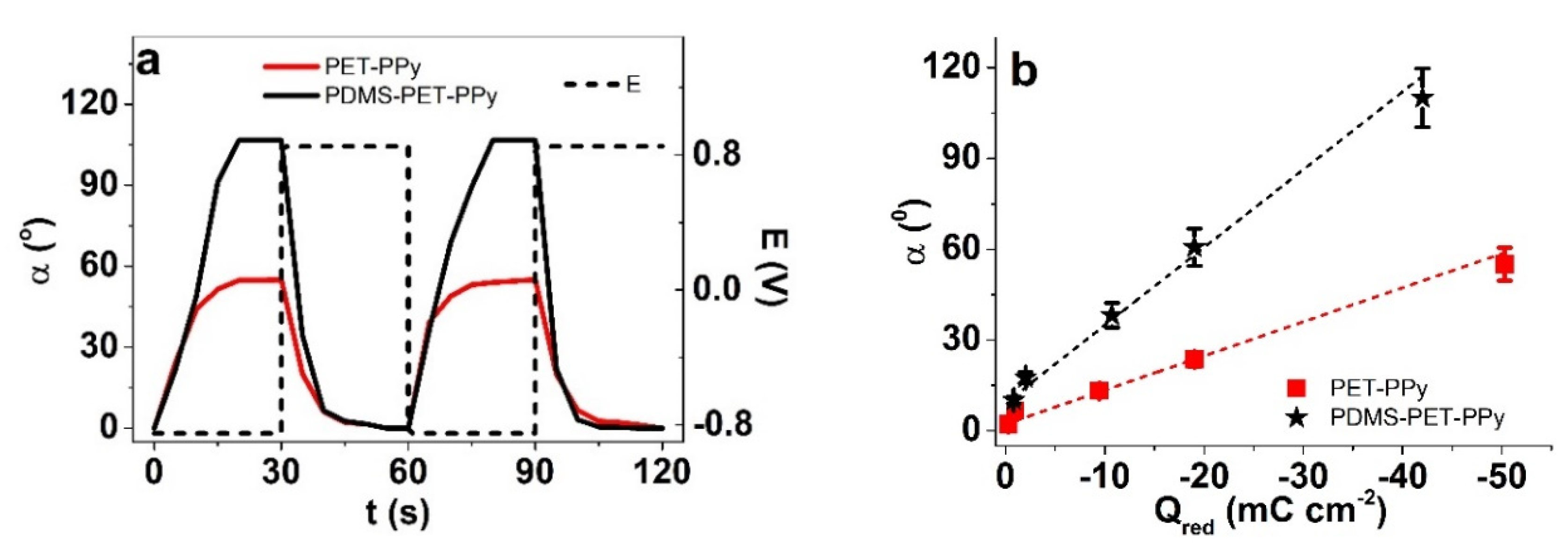
3.3. Effect of Reduced Friction on Actuation
3.3.1. Effect of Electrolyte Composition
3.3.2. Hydrophobic PDMS Coatings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Smela, E.; Inganas, O.; Lundstrom, I. Conducting Polymers as artificial muscles: Challenges and possibilities. J. Micromech. Microeng. 1993, 3, 203–205. [Google Scholar] [CrossRef]
- Jager, E.W.H. Microrobots for Micrometer-Size Objects in Aqueous Media: Potential Tools for Single-Cell Manipulation. Science 2000, 288, 2335–2338. [Google Scholar] [CrossRef]
- Jager, E.W.H.; Smela, E.; Ingana, O.; Inganäs, O. Microfabricating Conjugated Polymer Actuators. Science 2000, 290, 111–114. [Google Scholar] [CrossRef]
- Otero, T.F.; Martinez, J.G. Physical and chemical awareness from sensing polymeric artificial muscles. Experiments and modeling. Prog. Polym. Sci. 2015, 44, 62–78. [Google Scholar] [CrossRef]
- Hiraoka, M.; Fiorini, P.; O’Callaghan, J.; Yamashita, I.; Van Hoof, C.; Op De Beeck, M. Miniature conductive polymer actuators for high pressure generation in lab on chip systems. Sens. Actuators A Phys. 2012, 177, 23–29. [Google Scholar] [CrossRef]
- Maziz, A.; Concas, A.; Khaldi, A.; Stålhand, J.; Persson, N.-K.; Jager, E.W.H. Knitting and weaving artificial muscles. Sci. Adv. 2017, 3, e1600327. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Moghadam, A.; Kouzani, A.; Torabi, K. Development of a novel soft parallel robot equipped with polymeric artificial muscles. Smart Mater. Struct. 2015, 24, 35017. [Google Scholar]
- Smela, E. Conjugated polymer actuators for biomedical applications. Adv. Mater. 2003, 15, 481–494. [Google Scholar] [CrossRef]
- Hénot, M.; Drockenmuller, E.; Léger, L.; Restagno, F. Friction of Polymers: From PDMS Melts to PDMS Elastomers. ACS Macro Lett. 2018, 7, 112–115. [Google Scholar] [CrossRef]
- Wolf, M.P.; Salieb-beugelaar, G.B.; Hunziker, P. PDMS with designer functionalities—Properties, modifications strategies, and applications. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Bhushan, B.; Burton, Z. Adhesion and friction properties of polymers in microfluidic devices. Nanotechnology 2005, 16, 467–478. [Google Scholar] [CrossRef]
- Maboudian, R.; Carraro, C. Surface chemistry and tribology of MEMS. Annu. Rev. Phys. Chem. 2004, 55, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ueda, E.; Paulssen, D.; Levkin, P.A. Slippery Lubricant-Infused Surfaces: Properties and Emerging Applications. Adv. Funct. Mater. 2019, 29, 1–13. [Google Scholar] [CrossRef]
- Caputo, D.; Cesare, G.D.; Vecchio, N.L.; Nascetti, A.; Parisi, E.; Scipinotti, R. Polydimethylsiloxane material as hydrophobic and insulating layer in electrowetting-on-dielectric systems. Microelectron. J. 2014, 45, 1684–1690. [Google Scholar] [CrossRef]
- Yu, J.; Chary, S.; Das, S.; Tamelier, J.; Turner, K.L.; Israelachvili, J.N. Friction and Adhesion of Gecko-Inspired PDMS Flaps on Rough Surfaces. Langmuir 2012, 28, 11527–11534. [Google Scholar] [CrossRef]
- Barthlott, W.; Mail, M.; Neinhuis, C. Superhydrophobic hierarchically structured surfaces in biology: Evolution, structural principles and biomimetic applications. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374. [Google Scholar] [CrossRef]
- Bohn, H.F.; Federle, W. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proc. Natl. Acad. Sci. USA 2004, 101, 14138–14143. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, Z. Recent advances of bioinspired functional materials with specific wettability: From nature and beyond nature. Nanoscale Horiz. 2019, 4, 52–76. [Google Scholar] [CrossRef]
- Urbakh, M.; Klafter, J.; Gourdon, D.; Israelachvili, J. The nonlinear nature of friction. Nature 2004, 430, 525–528. [Google Scholar] [CrossRef]
- Kiefer, R.; Temmer, R.; Tamm, T.; Travas-Sejdic, J.; Kilmartin, P.A.; Aabloo, A. Conducting polymer actuators formed on MWCNT and PEDOT-PSS conductive coatings. Synth. Met. 2013, 171, 69–75. [Google Scholar] [CrossRef]
- De Paoli, M.-A.; Peres, R.D.C.; Panero, S.; Scrosati, B. Properties of electrochemically synthezised polymer electrodes-x. Study of polypyrrole/dodecylbenzene sulfonate. Electrochim. Acta 1992, 37, 1173–1182. [Google Scholar] [CrossRef]
- Khanh, T.T.; Lee, R.; Kilmartin, P.A.; Khan, A. Actuation increase in polypyrrole bilayer by photo-activated dopants. Synth. Met. 2018, 246, 57–63. [Google Scholar] [CrossRef]
- Smela, E.; Gadegaard, N. Volume change in polypyrrole studied by atomic force microscopy. J. Phys. Chem. B 2001, 105, 9395–9405. [Google Scholar] [CrossRef]
- Takashima, W.; Hashimoto, H.; Tominaga, K.; Tanaka, A.; Pandey, S.S.; Kaneto, K. Solvation effect on the ion exchange in polypyrrole film doped with sulfonated polyaniline. Thin Solid Film. 2010, 519, 1093–1099. [Google Scholar] [CrossRef]
- Kiefer, R.; Weis, D.G.; Aabloo, A.; Urban, G.; Heinze, J. Dependence of polypyrrole bilayer deflection upon polymerization potential. Synth. Met. 2013, 172, 37–43. [Google Scholar] [CrossRef]
- Khanh, T.T.; Kesküla, A.; Zondaka, Z.; Harjo, M.; Kivilo, A.; Khorram, M.S.; Tamm, T.; Kiefer, R. Role of polymerization temperature on the performance of polypyrrole/dodecylbenzenesulphonate linear actuators. Synth. Met. 2019, 247, 53–58. [Google Scholar] [CrossRef]
- Martinez, J.G.; Otero, T.F.; Jager, E.W.H. Effect of the electrolyte concentration and substrate on conducting polymer actuators. Langmuir 2014, 30, 3894–3904. [Google Scholar] [CrossRef]
- Zondaka, Z.; Valner, R.; Tamm, T.; Aabloo, A.; Kiefer, R. Carbide-derived carbon in polypyrrole changing the elastic modulus with a huge impact on actuation. RSC Adv. 2016, 6, 26380–26385. [Google Scholar] [CrossRef]
- Khadka, R.; Zondaka, Z.; Kesküla, A.; Safaei Khorram, M.; Thien Khanh, T.; Tamm, T.; Travas-Sejdic, J.; Kiefer, R.; Minh City, C. Influence of solvent on linear polypyrrole-polyethylene oxide actuators. J. Appl. Polym. Sci. 2018, 46831, 1–7. [Google Scholar] [CrossRef]
- Temmer, R.; Must, I.; Kaasik, F.; Aabloo, A.; Tamm, T. Combined chemical and electrochemical synthesis methods for metal-free polypyrrole actuators. Sens. Actuators B Chem. 2012, 166–167, 411–418. [Google Scholar]
- Alici, G.; Metz, P.; Spinks, G.M. A methodology towards geometry optimization of high performance polypyrrole (PPy) actuators. Smart Mater. Struct. 2006, 15, 243–252. [Google Scholar] [CrossRef]
- Chen, Q.; Natale, D.; Neese, B.; Ren, K.; Lin, M.; Zhang, Q.M.; Pattom, M.; Wang, K.W. Piezoelectric Polymers Actuators for Precise Shape Control of Large Scale Space Antennas. In Proceedings of the SPIE Smart Structures and Materials, Nondestructive Evaluation and Health Monitoring; SPIE: San Diego, CA, USA, 2007; p. 65241P. [Google Scholar]
- Haronian, D. Maximizing microelectromechanical sensor and actuator sensitivity by optimizing geometry. Sens. Actuators A Phys. 1995, 50, 223–236. [Google Scholar] [CrossRef]
- Jin, M.; Feng, X.; Xi, J.; Zhai, J.; Cho, K.; Feng, L.; Jiang, L. Super-Hydrophobic PDMS Surface with Ultra-Low Adhesive Force. Macromol. Rapid Commun. 2005, 26, 1805–1809. [Google Scholar] [CrossRef]
- Unger, M.A.; Chou, H.-P.; Thorsen, T.; Scherer, A.; Quake, S.R. Monolithic Microfabricated Valves and Pumps by Multilayer Soft Lithography. Science 2000, 288, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, M.T.; Mirzadeh, H. In Vitro Blood Compatibility of Modified PDMS Surfaces as Superhydrophobic and Superhydrophilic Materials. J. Appl. Polym. Sci. 2004, 91, 2042–2047. [Google Scholar] [CrossRef]
- Xue, C.H.; Bai, X.; Jia, S.T. Robust, Self-Healing Superhydrophobic Fabrics Prepared by One-Step Coating of PDMS and Octadecylamine. Sci. Rep. 2016, 6, 27262. [Google Scholar] [CrossRef] [PubMed]
- Otero, T.F.; Martinez, J.G.; Fuchiwaki, M.; Valero, L. Structural Electrochemistry from Freestanding Polypyrrole Films: Full Hydrogen Inhibition from Aqueous Solutions. Adv. Funct. Mater. 2014, 24, 1265–1274. [Google Scholar] [CrossRef]
- Hong, W.; Almomani, A.; Chen, Y.; Jamshidi, R.; Montazami, R. Soft ionic electroactive polymer actuators with tunable non-linear angular deformation. Materials 2017, 10, 664. [Google Scholar] [CrossRef]
- Otero, T.F.; Martinez, J.G.; Arias-Pardilla, J. Biomimetic electrochemistry from conducting polymers. A review. Electrochim. Acta 2012, 84, 112–128. [Google Scholar] [CrossRef]
- Kiefer, R.; Martinez, J.G.; Kesküla, A.; Anbarjafari, G.; Aabloo, A.; Otero, T.F. Polymeric actuators: Solvents tune reaction-driven cation to reaction-driven anion actuation. Sens. Actuators B Chem. 2016, 233, 328–336. [Google Scholar] [CrossRef]
- Von Gioi, R.G.; Jakubowicz, J.; Morel, J.-M.; Randall, G. LSD: A Fast Line Segment Detector with a False Detection Control. IEEE Trans. Pattern Anal. Mach. Intell. 2010, 32, 722–732. [Google Scholar] [CrossRef]
- Vergaftik, N.B.; Volkov, B.N.; Voljak, L.D. International Tables of the Surface Tension of Water. J. Phys. Chem. Ref. Data 1983, 12, 817–820. [Google Scholar] [CrossRef]
- Khuyen, N.Q.; Zondaka, Z.; Harjo, M.; Torop, J.; Tamm, T.; Kiefer, R. Comparative Analysis of Fluorinated Anions for Polypyrrole Linear Actuator Electrolytes. Polymer 2019, 11, 849. [Google Scholar] [CrossRef] [PubMed]
- Bay, L.; Mogensen, N.; Skaarup, S.; Jørgensen, M.; Sommer-Larsen, P.; West, K. Polypyrrole Doped with Alkyl Benzenesulfonates. Macromolecules 2002, 35, 9345–9351. [Google Scholar] [CrossRef]
- Otero, T.F. Biomimetic Conducting Polymers: Synthesis, Materials, Properties, Functions, and Devices. Polym. Rev. 2013, 53, 311–351. [Google Scholar] [CrossRef]
- Cortés, M.T.; Moreno, J.C. Artificial muscles based on conducting polymers. e-Polymers 2003, 3, 1–42. [Google Scholar] [CrossRef]
- Hawlicka, E.; Dlugoborski, T. Molecular dynamics simulations of the aqueous solution of tetramethylammonium chloride. Chem. Phys. Lett. 1997, 268, 325–330. [Google Scholar] [CrossRef]
- Ue, M. Mobility and Ionic Association of Lithium and Quaternary Ammonium Salts in Propylene Carbonate and/-Butyrolactone. J. Electrochem. Soc. 1994, 141, 3336–3342. [Google Scholar] [CrossRef]
- Otero, T.F.; Martinez, J.G. Artificial muscles: A tool to quantify exchanged solvent during biomimetic reactions. Chem. Mater. 2012, 24, 4093–4099. [Google Scholar] [CrossRef]
- Onuki, A. Surface tension of electrolytes: Hydrophilic and hydrophobic ions near an interface. J. Chem. Phys. 2008, 128, 1–10. [Google Scholar] [CrossRef]
- Wong, T.-S.; Kang, S.H.; Tang, S.K.Y.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef]

| Medium | Surface Tension, γ [mN m−1] |
|---|---|
| H2O (Milli-Q) | 70.1 ± 2.3 |
| TMACl (0.05 M) | 72.2 ± 5.3 |
| NaDBS (0.05 M) | 30.9 ± 2.2 |
| NaDBS (0.05 M) | |
| +0.05 M TMACl | 31.3 ± 1.3 |
| +0.1 M TMACl | 30.5 ± 2.4 |
| +0.2 M TMACl | 30.3 ± 1.9 |
| +0.5 M TMACl | 29.9 ± 1.1 |
| Surface | Milli-Q [°] | Electrolyte Blend [°] |
|---|---|---|
| PET | 59.7 ± 3.3 | 19 ± 1.2 |
| PDMS | 113 ± 7.2 | 73 ± 5.4 |
| PET-PDMS | 117.6 ± 8.6 | 51.8 ± 3.3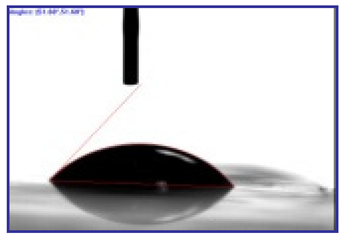 |
| Mixed Electrolyte NaDBS + | Displacement α [°] | Charge Density Qred [mC cm−2] | ||
|---|---|---|---|---|
| 0.017 Hz | 0.1 Hz | 0.017 Hz | 0.1 Hz | |
| 0.05 M TMACl | 58.2 ± 5.5 | 14.1 ± 1.2 | −51.9 ± 4.9 | −7.2 ± 0.6 |
| 0.1 M TMACl | 45.6 ± 4.3 | 8.3 ± 0.7 | −59.9 ± 5.4 | −8.1 ± 0.8 |
| 0.2 M TMACl | 36.3 ± 3.3 | 7.7 ± 0.9 | −56.0 ± 5.2 | −7.4 ± 7.1 |
| 0.5 M TMACl | 24.2 ± 2.5 | 4.1 ± 0.5 | −48.5 ± 4.3 | −5.5 ± 0.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khuyen, N.Q.; Kiefer, R.; Elhi, F.; Anbarjafari, G.; Martinez, J.G.; Tamm, T. A Biomimetic Approach to Increasing Soft Actuator Performance by Friction Reduction. Polymers 2020, 12, 1120. https://doi.org/10.3390/polym12051120
Khuyen NQ, Kiefer R, Elhi F, Anbarjafari G, Martinez JG, Tamm T. A Biomimetic Approach to Increasing Soft Actuator Performance by Friction Reduction. Polymers. 2020; 12(5):1120. https://doi.org/10.3390/polym12051120
Chicago/Turabian StyleKhuyen, Nguyen Quang, Rudolf Kiefer, Fred Elhi, Gholamreza Anbarjafari, Jose G. Martinez, and Tarmo Tamm. 2020. "A Biomimetic Approach to Increasing Soft Actuator Performance by Friction Reduction" Polymers 12, no. 5: 1120. https://doi.org/10.3390/polym12051120
APA StyleKhuyen, N. Q., Kiefer, R., Elhi, F., Anbarjafari, G., Martinez, J. G., & Tamm, T. (2020). A Biomimetic Approach to Increasing Soft Actuator Performance by Friction Reduction. Polymers, 12(5), 1120. https://doi.org/10.3390/polym12051120