A Biodegradable Magnetic Nanocomposite as a Superabsorbent for the Simultaneous Removal of Selected Fluoroquinolones from Environmental Water Matrices: Isotherm, Kinetics, Thermodynamic Studies and Cost Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Instrumentation
2.3. Preparation of the Nanocomposite
2.3.1. Synthesis of Mesoporous Carbon (MPC)
2.3.2. Preparation of Magnetic Mesoporous Carbon Coated with Chitosan and β-CD
2.4. Batch Adsorption Studies
2.5. Regeneration and Reusability (Recyclability) of the Nanocomposite
2.6. Application in Real Water Samples
3. Results and Discussion
3.1. Characterization
3.1.1. X-ray Diffraction Spectroscopy
3.1.2. Fourier Transform Infrared Spectroscopy
3.1.3. Nitrogen Adsorption–Desorption
3.1.4. Point of Zero Charge
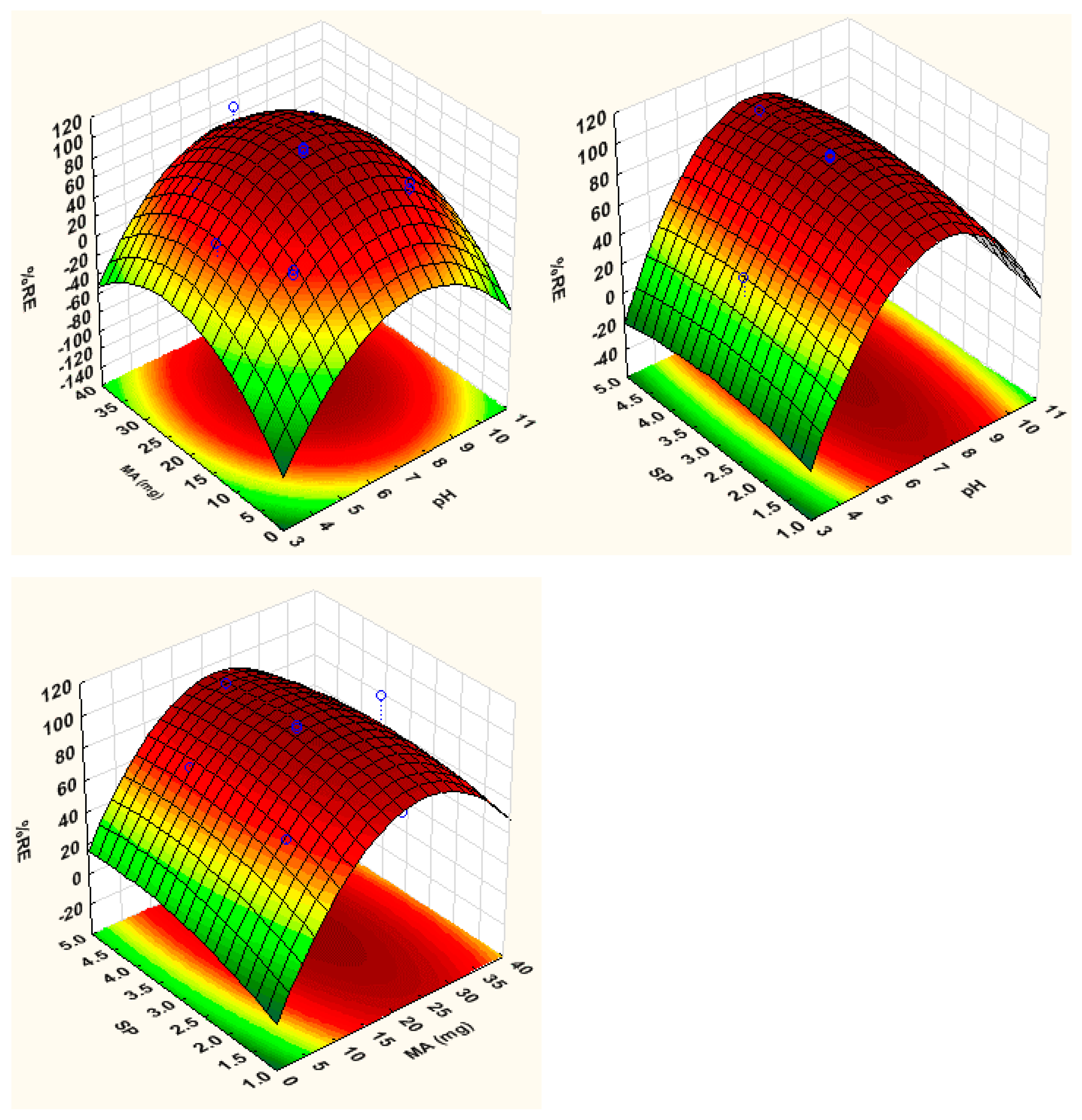
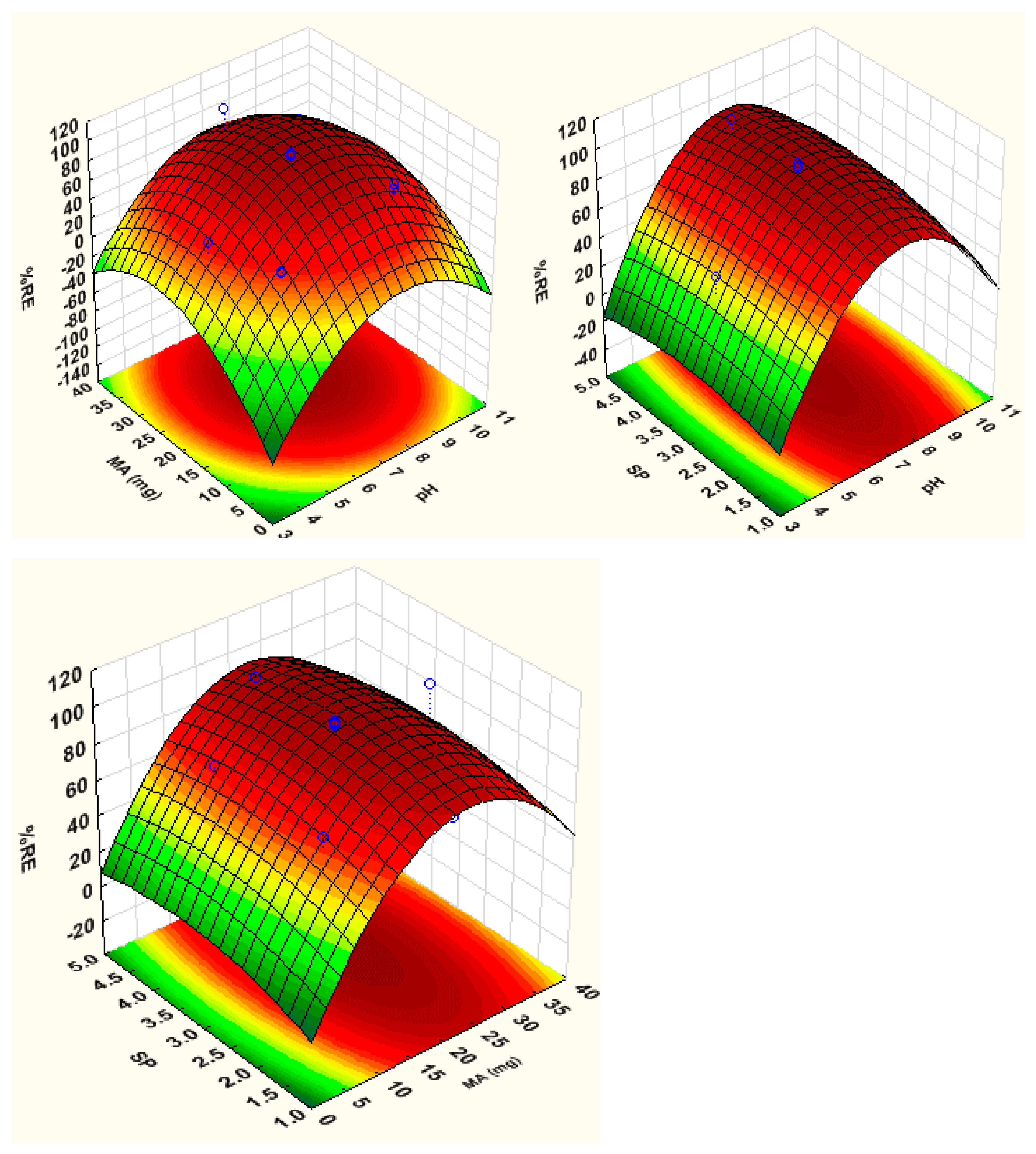
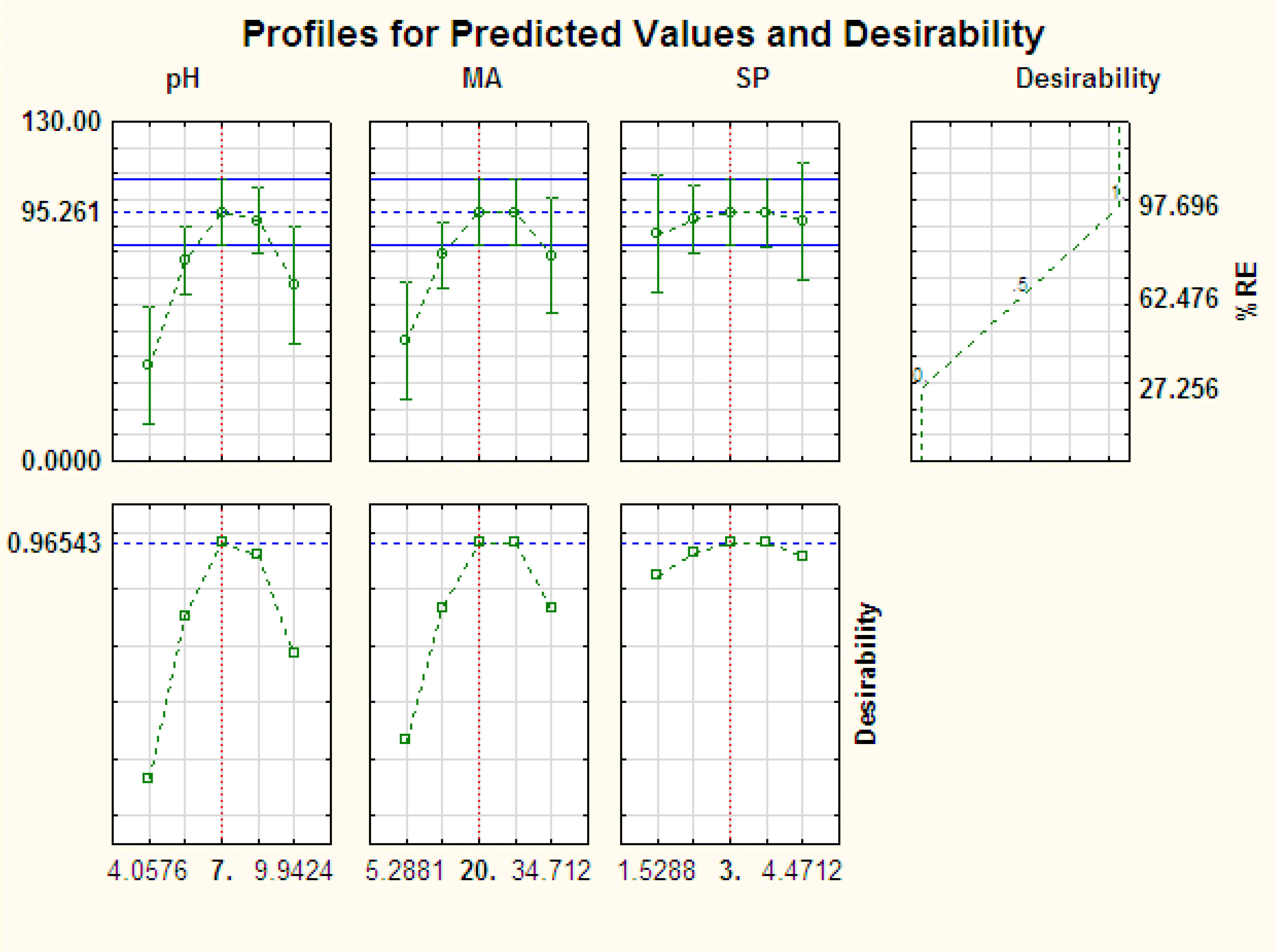
3.2. Optimization
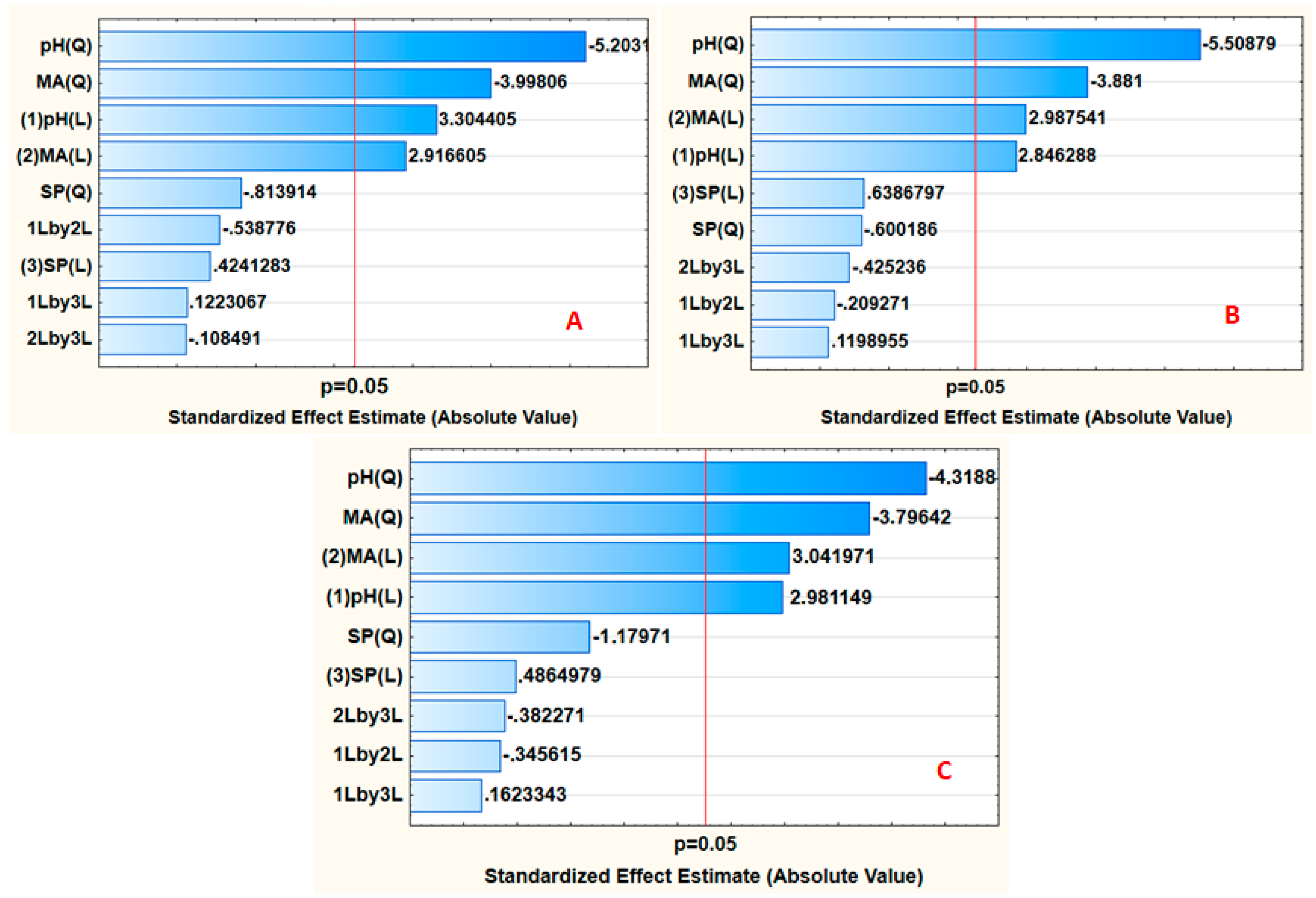
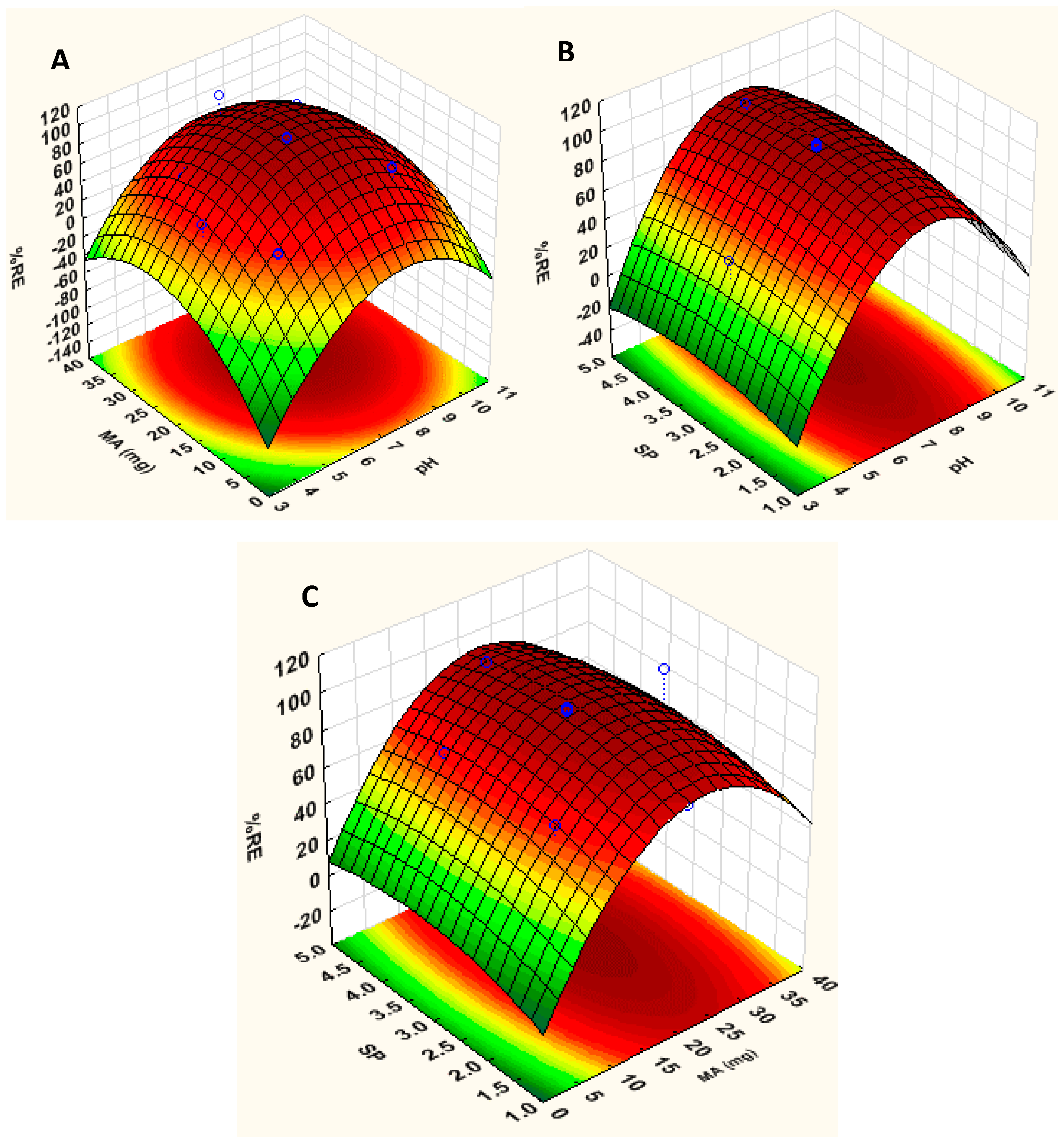
3.2.1. Response Surface Methodology
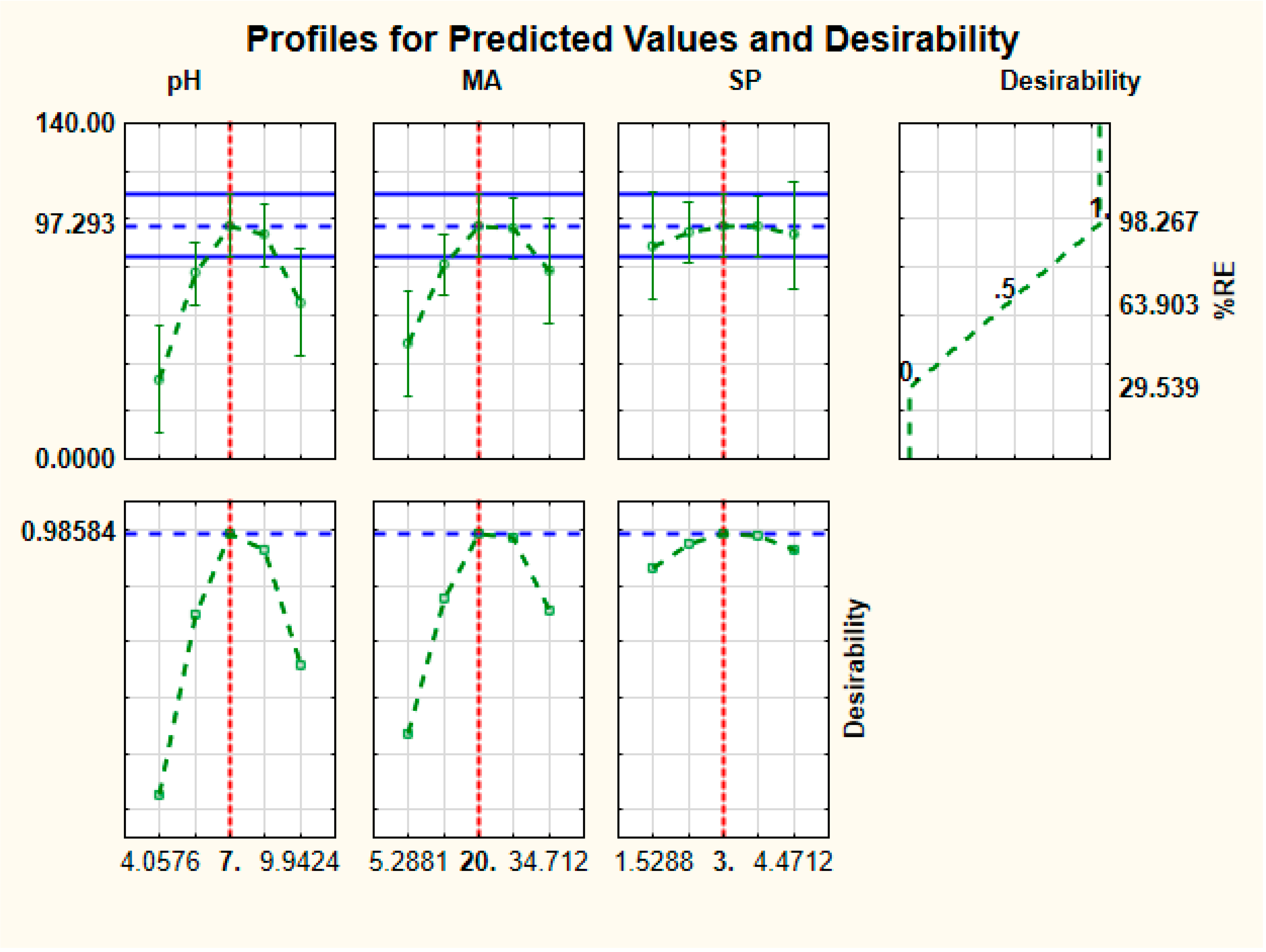
3.2.2. Desirability Function
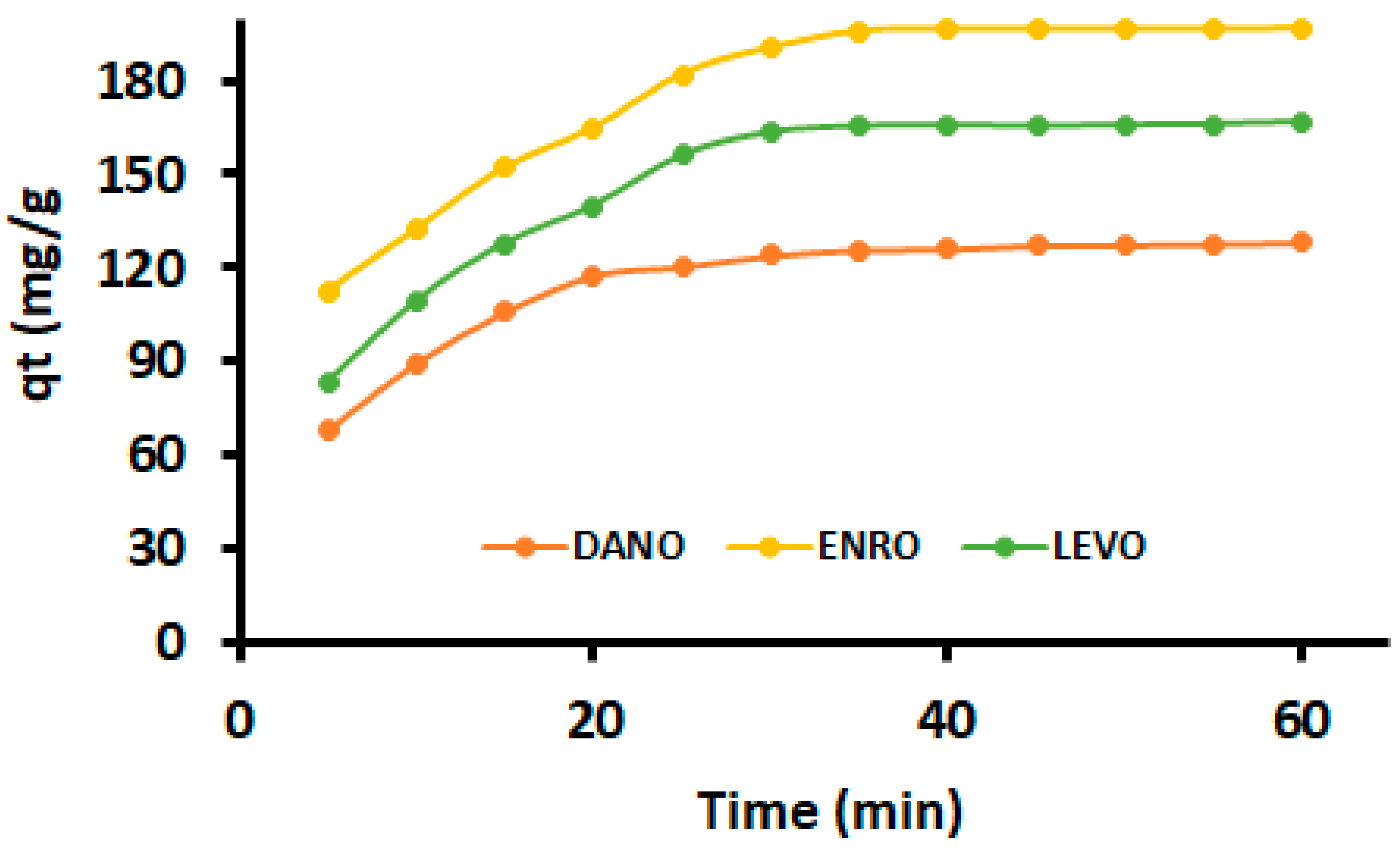
3.3. Adsorption Kinetics
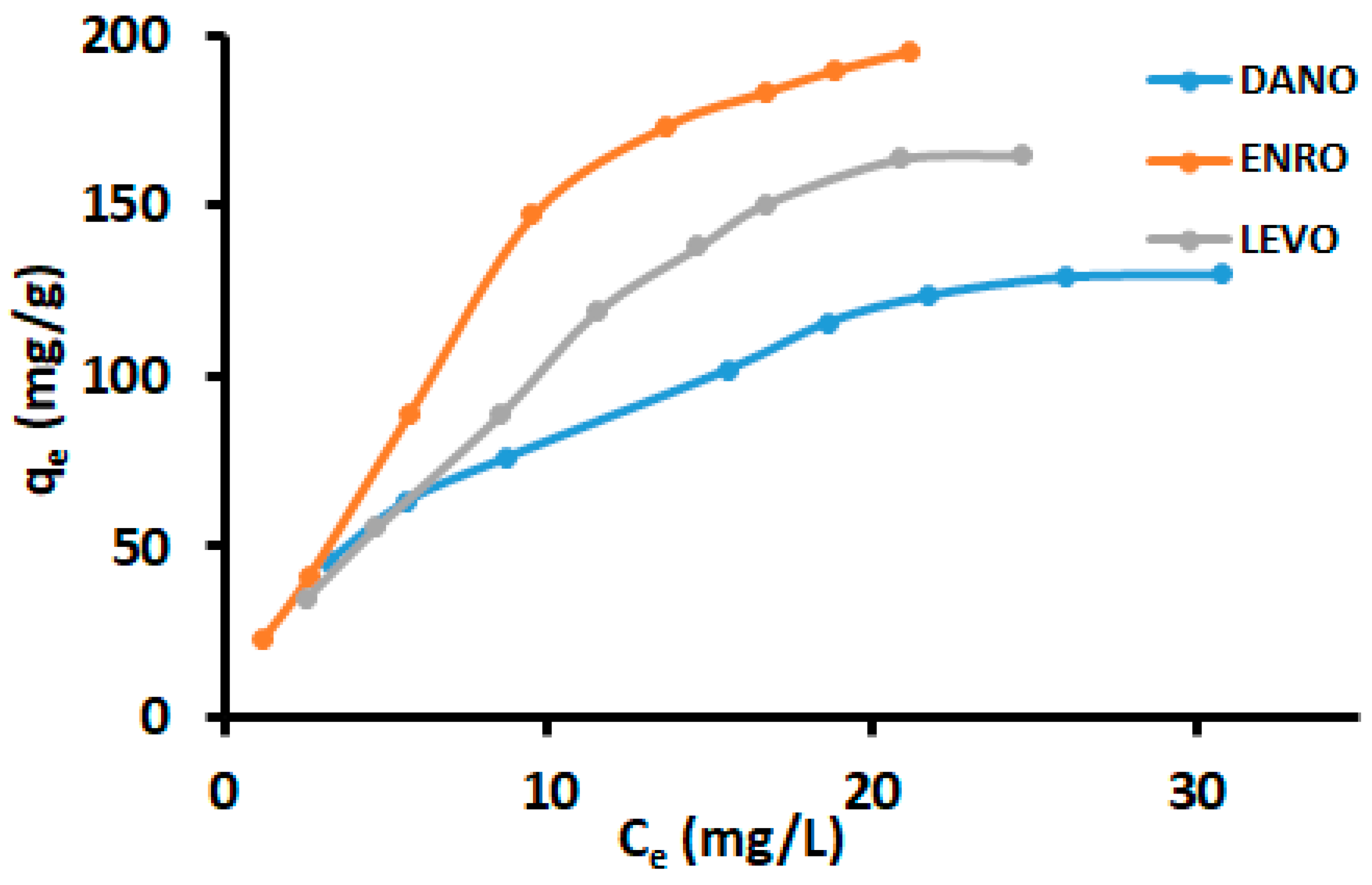
3.4. Adsorption Isotherms
3.5. Adsorption Thermodynamics
3.6. Comparison of Sorption Capacities for Various Adsorbents
3.7. Regeneration and Reusability Studies
3.8. Application to Real Samples
3.9. Cost Analysis for the Preparation of Adsorbent
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Parameters | Tap Water | River Water | Influent | Effluent |
|---|---|---|---|---|
| pH | 7.7 | 7.6 | 6.64 | 7.11 |
| Conductivity (µS/cm) | 150 | 338 | 854 | 513 |
| Total dissolved solid (TDS, mg/L) | 50.6 | 208 | 432 | 235 |
| Dissolved organic carbon (DOC, mg/L) | 1.94 | 15.5 | 20.5 | 12.7 |
| Calcium (mg/L) | 2.46 | 6.65 | 19,652 | 18,509 |
| Mg (mg/L) | 1.33 | 4.30 | 15,231 | 12,861 |
| Fe (mg/L) | 0.98 | 3.20 | 634 | 339 |
| Na (mg/L) | 2.26 | 6.78 | 23,435 | 16,784 |
| K (mg/L) | 1.08 | 1.54 | 9277 | 6745 |

Intraparticle Diffusion Model

References
- Esponda, S.M.; Padrón, M.E.T.; Ferrera, Z.S.; Rodríguez, J.J.S. Solid-phase microextraction with micellar desorption and HPLC-fluorescence detection for the analysis of fluoroquinolones residues in water samples. Anal. Bioanal. Chem. 2009, 394, 927–935. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, H.L.; da Silva Anacleto, S.; da Silva, A.T.M.; Pereira, A.C.; de Souza Borges, W.; Figueiredo, E.C.; Borges, K.B. Molecularly imprinted pipette-tip solid phase extraction for selective determination of fluoroquinolones in human urine using HPLC-DAD. J. Chromatogr. B 2016, 1033, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Baghdadi, M.; Ghaffari, E.; Aminzadeh, B. Removal of carbamazepine from municipal wastewater effluent using optimally synthesized magnetic activated carbon: Adsorption and sedimentation kinetic studies. J. Environ. Chem. Eng. 2016, 4, 3309–3321. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef]
- Denadai, M.; Cass, Q.B. Simultaneous determination of fluoroquinolones in environmental water by liquid chromatography–tandem mass spectrometry with direct injection: A green approach. J. Chromatogr. A 2015, 1418, 177–184. [Google Scholar] [CrossRef]
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef]
- Michael, I.; Rizzo, L.; McArdell, C.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef]
- Espinosa-Mansilla, A.; Girón, A.J.; De La Peña, A.M. Simultaneous determination of the residues of fourteen quinolones and fluoroquinolones in fish samples using liquid chromatography with photometric and fluorescence detection. Czech J. Food Sci. 2012, 30, 74–82. [Google Scholar]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Adsorptive removal of antibiotics from water and wastewater: Progress and challenges. Sci. Total Environ. 2015, 532, 112–126. [Google Scholar] [CrossRef]
- Roberts, J.; Kumar, A.; Du, J.; Hepplewhite, C.; Ellis, D.J.; Christy, A.G.; Beavis, S.G. Pharmaceuticals and personal care products (PPCPs) in Australia’s largest inland sewage treatment plant, and its contribution to a major Australian river during high and low flow. Sci. Total Environ. 2016, 541, 1625–1637. [Google Scholar] [CrossRef]
- Shi, Y.; Gao, L.; Li, W.; Liu, J.; Cai, Y. Investigation of fluoroquinolones, sulfonamides and macrolides in long-term wastewater irrigation soil in Tianjin, China. Bull. Environ. Contam. Toxicol. 2012, 89, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Archer, E.; Petrie, B.; Kasprzyk-Hordern, B.; Wolfaardt, G.M. The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminants (EDCs), metabolites and illicit drugs in a WWTW and environmental waters. Chemosphere 2017, 174, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, Y.; Candela, L.; Ronen, D.; Teijon, G. Monitoring the occurrence of emerging contaminants in treated wastewater and groundwater between 2008 and 2010. The Baix Llobregat (Barcelona, Spain). J. Hazard. Mater. 2012, 239, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Karnjanapiboonwong, A.; Suski, J.G.; Shah, A.A.; Cai, Q.; Morse, A.N.; Anderson, T.A. Occurrence of PPCPs at a wastewater treatment plant and in soil and groundwater at a land application site. Water Air Soil Pollut. 2011, 216, 257–273. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, T.; Zhang, Y.; Ge, F.; Steel, R.M.; Sun, L. Advances in technologies for pharmaceuticals and personal care products removal. J. Mater. Chem. A 2017, 5, 12001–12014. [Google Scholar] [CrossRef]
- Mehrjouei, M.; Müller, S.; Möller, D. Energy consumption of three different advanced oxidation methods for water treatment: A cost-effectiveness study. J. Clean. Prod. 2014, 65, 178–183. [Google Scholar] [CrossRef]
- Jiang, J.-Q.; Ashekuzzaman, S. Development of novel inorganic adsorbent for water treatment. Curr. Opin. Chem. Eng. 2012, 1, 191–199. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. Adsorption of phenolic compounds on low-cost adsorbents: A review. Adv. Colloid Interface Sci. 2008, 143, 48–67. [Google Scholar] [CrossRef]
- Ramos, S.; Homem, V.; Alves, A.; Santos, L. Advances in analytical methods and occurrence of organic UV-filters in the environment—A review. Sci. Total Environ. 2015, 526, 278–311. [Google Scholar] [CrossRef]
- Aksu, Z. Application of biosorption for the removal of organic pollutants: A review. Process Biochem. 2005, 40, 997–1026. [Google Scholar] [CrossRef]
- Cavazos-Rocha, N.; Carmona-Alvarado, I.; Vera-Cabrera, L.; Waksman-de-Torres, N.; Salazar-Cavazos, M.D.L.L. HPLC method for the simultaneous analysis of fluoroquinolones and oxazolidinones in plasma. J. Chromatogr. Sci. 2014, 52, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Wang, J.; Li, J.; Wang, X.; Chen, Z.; Alsaedi, A.; Hayat, T.; Chen, Y.; Wang, X. The role of graphene oxide and graphene oxide-based nanomaterials in the removal of pharmaceuticals from aqueous media: A review. Environ. Sci. Pollut. Res. 2017, 24, 7938–7958. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Chen, W.; Xu, Z.; Zheng, S.; Zhu, D. Graphene nanosheets and graphite oxide as promising adsorbents for removal of organic contaminants from aqueous solution. J. Environ. Qual. 2013, 42, 191–198. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Carvalho, C.; Rodrigues, D.L.C.; Lima, É.C.; Umpierres, C.S.; Chaguezac, D.F.C.; Machado, F.M. Kinetic, equilibrium, and thermodynamic studies on the adsorption of ciprofloxacin by activated carbon produced from Jerivá (Syagrus romanzoffiana). Environ. Sci. Pollut. Res. 2019, 26, 4690–4702. [Google Scholar] [CrossRef] [PubMed]
- Dimpe, K.M.; Nomngongo, P.N. Application of activated carbon-decorated polyacrylonitrile nanofibers as an adsorbent in dispersive solid-phase extraction of fluoroquinolones from wastewater. J. Pharm. Anal. 2019, 9, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Dil, E.A.; Ghaedi, M.; Asfaram, A.; Bazrafshan, A.A. Ultrasound wave assisted adsorption of congo red using gold-magnetic nanocomposite loaded on activated carbon: Optimization of process parameters. Ultrason. Sonochem. 2018, 46, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Souza, L.S.; Martins, A.C.; Silva, T.L.; Júnior, O.O.S.; Visentainer, J.V.; Almeida, V.C. NaOH-activated carbon of high surface area produced from guava seeds as a high-efficiency adsorbent for amoxicillin removal: Kinetic, isotherm and thermodynamic studies. Chem. Eng. J. 2016, 288, 778–788. [Google Scholar] [CrossRef]
- Jain, A.; Balasubramanian, R.; Srinivasan, M. Production of high surface area mesoporous activated carbons from waste biomass using hydrogen peroxide-mediated hydrothermal treatment for adsorption applications. Chem. Eng. J. 2015, 273, 622–629. [Google Scholar] [CrossRef]
- Khan, N.A.; Hasan, Z.; Jhung, S.H. Adsorptive removal of hazardous materials using metal-organic frameworks (MOFs): A review. J. Hazard. Mater. 2013, 244, 444–456. [Google Scholar] [CrossRef]
- Jung, C.; Son, A.; Her, N.; Zoh, K.-D.; Cho, J.; Yoon, Y. Removal of endocrine disrupting compounds, pharmaceuticals, and personal care products in water using carbon nanotubes: A review. J. Ind. Eng. Chem. 2015, 27, 1–11. [Google Scholar] [CrossRef]
- Sun, K.; Shi, Y.; Chen, H.; Wang, X.; Li, Z. Extending surfactant-modified 2: 1 clay minerals for the uptake and removal of diclofenac from water. J. Hazard. Mater. 2017, 323, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, Y.; Wu, L. Sorption and degradation of pharmaceuticals and personal care products (PPCPs) in soils. Environ. Sci. Pollut. Res. 2013, 20, 4261–4267. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Srinivasan, M.P.; Ni, Y. Preparation of mesoporous high-surface-area activated carbon. Adv. Mater. 2000, 12, 62–65. [Google Scholar] [CrossRef]
- Gao, X.; Du, D.; Li, S.; Yan, X.; Xing, W.; Bai, P.; Xue, Q.; Yan, Z. Outstanding capacitive performance of ordered mesoporous carbon modified by anthraquinone. Electrochim. Acta 2018, 259, 110–121. [Google Scholar] [CrossRef]
- Phan, T.N.; Gong, M.K.; Thangavel, R.; Lee, Y.S.; Ko, C.H. Enhanced electrochemical performance for EDLC using ordered mesoporous carbons (CMK-3 and CMK-8): Role of mesopores and mesopore structures. J. Alloys Compd. 2019, 780, 90–97. [Google Scholar] [CrossRef]
- Sayğılı, H.; Güzel, F. High surface area mesoporous activated carbon from tomato processing solid waste by zinc chloride activation: Process optimization, characterization and dyes adsorption. J. Clean. Prod. 2016, 113, 995–1004. [Google Scholar] [CrossRef]
- Baikousi, M.; Georgiou, Y.; Daikopoulos, C.; Bourlinos, A.B.; Filip, J.; Zbořil, R.; Deligiannakis, Y.; Karakassides, M.A. Synthesis and characterization of robust zero valent iron/mesoporous carbon composites and their applications in arsenic removal. Carbon 2015, 93, 636–647. [Google Scholar] [CrossRef]
- Goel, C.; Bhunia, H.; Bajpai, P.K. Mesoporous carbon adsorbents from melamine–formaldehyde resin using nanocasting technique for CO2 adsorption. J. Environ. Sci. 2015, 32, 238–248. [Google Scholar] [CrossRef]
- Shi, S.; Fan, Y.; Huang, Y. Facile low temperature hydrothermal synthesis of magnetic mesoporous carbon nanocomposite for adsorption removal of ciprofloxacin antibiotics. Ind. Eng. Chem. Res. 2013, 52, 2604–2612. [Google Scholar] [CrossRef]
- Chiu, C.-W.; Wu, M.-T.; Lee, J.C.-M.; Cheng, T.-Y. Isothermal adsorption properties for the adsorption and removal of reactive blue 221 dye from aqueous solutions by cross-linked β-chitosan glycan as acid-resistant adsorbent. Polymers 2018, 10, 1328. [Google Scholar] [CrossRef]
- Tzereme, A.; Christodoulou, E.; Kyzas, G.Z.; Kostoglou, M.; Bikiaris, D.N.; Lambropoulou, D.A. Chitosan Grafted Adsorbents for Diclofenac Pharmaceutical Compound Removal from Single-Component Aqueous Solutions and Mixtures. Polymers 2019, 11, 497. [Google Scholar] [CrossRef]
- Fan, L.; Luo, C.; Sun, M.; Qiu, H.; Li, X. Synthesis of magnetic β-cyclodextrin–chitosan/graphene oxide as nanoadsorbent and its application in dye adsorption and removal. Colloids Surf. B Biointerfaces 2013, 103, 601–607. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Kostoglou, M.; Lazaridis, N.K.; Lambropoulou, D.A.; Bikiaris, D.N. Environmental friendly technology for the removal of pharmaceutical contaminants from wastewaters using modified chitosan adsorbents. Chem. Eng. J. 2013, 222, 248–258. [Google Scholar] [CrossRef]
- Fan, L.; Li, M.; Lv, Z.; Sun, M.; Luo, C.; Lu, F.; Qiu, H. Fabrication of magnetic chitosan nanoparticles grafted with β-cyclodextrin as effective adsorbents toward hydroquinol. Colloids Surf. B Biointerfaces 2012, 95, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, B.; Niu, W.; Hong, S.; Saif, B.; Wang, S.; Dong, C.; Shuang, S. β-Cyclodextrin modified graphene oxide–magnetic nanocomposite for targeted delivery and pH-sensitive release of stereoisomeric anti-cancer drugs. RSC Adv. 2015, 5, 89299–89308. [Google Scholar] [CrossRef]
- Lu, D.; Yang, L.; Zhou, T.; Lei, Z. Synthesis, characterization and properties of biodegradable polylactic acid-β-cyclodextrin cross-linked copolymer microgels. Eur. Polym. J. 2008, 44, 2140–2145. [Google Scholar] [CrossRef]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-based nanomaterials: A state-of-the-art review. Int. J. Biol. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Kostoglou, M.; Lazaridis, N.K.; Bikiaris, D.N. N-(2-Carboxybenzyl) grafted chitosan as adsorptive agent for simultaneous removal of positively and negatively charged toxic metal ions. J. Hazard. Mater. 2013, 244, 29–38. [Google Scholar] [CrossRef]
- Lessa, E.F.; Nunes, M.L.; Fajardo, A.R. Chitosan/waste coffee-grounds composite: An efficient and eco-friendly adsorbent for removal of pharmaceutical contaminants from water. Carbohydr. Polym. 2018, 189, 257–266. [Google Scholar] [CrossRef]
- Danalıoğlu, S.T.; Bayazit, Ş.S.; Kuyumcu, Ö.K.; Salam, M.A. Efficient removal of antibiotics by a novel magnetic adsorbent: Magnetic activated carbon/chitosan (MACC) nanocomposite. J. Mol. Liq. 2017, 240, 589–596. [Google Scholar] [CrossRef]
- Hirano, S.; Seino, H.; Akiyama, Y.; Nonaka, I. Chitosan: A biocompatible material for oral and intravenous administrations. In Progress in Biomedical Polymers; Springer: Berlin/Heidelberg, Germany, 1990; pp. 283–290. [Google Scholar]
- Bhanvase, B.; Veer, A.; Shirsath, S.; Sonawane, S. Ultrasound assisted preparation, characterization and adsorption study of ternary chitosan-ZnO-TiO2 nanocomposite: Advantage over conventional method. Ultrason. Sonochem. 2019, 52, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Oveisi, M.; Asli, M.A.; Mahmoodi, N.M. MIL-Ti metal-organic frameworks (MOFs) nanomaterials as superior adsorbents: Synthesis and ultrasound-aided dye adsorption from multicomponent wastewater systems. J. Hazard. Mater. 2018, 347, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Han, Y.; Tao, Y.; Fan, S.; Chu, D.-T.; Ye, X.; Ye, M.; Xie, G. Ultrasound assisted adsorption and desorption of blueberry anthocyanins using macroporous resins. Ultrason. Sonochem. 2018, 48, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Bilal, M.; Khan, R.; Farooq, R.; Siddique, M. Ultrasound-assisted adsorption of phenol from aqueous solution by using spent black tea leaves. Environ. Sci. Pollut. Res. 2018, 25, 22920–22930. [Google Scholar] [CrossRef]
- Hamza, W.; Dammak, N.; Hadjltaief, H.B.; Eloussaief, M.; Benzina, M. Sono-assisted adsorption of cristal violet dye onto tunisian smectite clay: Characterization, kinetics and adsorption isotherms. Ecotoxicol. Environ. Saf. 2018, 163, 365–371. [Google Scholar] [CrossRef]
- Dehghan, A.; Mohammadi, A.A.; Yousefi, M.; Najafpoor, A.A.; Shams, M.; Rezania, S. Enhanced Kinetic Removal of Ciprofloxacin onto Metal-Organic Frameworks by Sonication, Process Optimization and Metal Leaching Study. Nanomaterials 2019, 9, 1422. [Google Scholar] [CrossRef]
- Hassani, A.; Khataee, A.; Karaca, S.; Karaca, C.; Gholami, P. Sonocatalytic degradation of ciprofloxacin using synthesized TiO2 nanoparticles on montmorillonite. Ultrason. Sonochem. 2017, 35, 251–262. [Google Scholar] [CrossRef]
- Mpupa, A.; Mashile, G.P.; Nomngongo, P.N. Ultrasound-assisted dispersive solid phase nanoextraction of selected personal care products in wastewater followed by their determination using high performance liquid chromatography-diode array detector. J. Hazard. Mater. 2019, 370, 33–41. [Google Scholar] [CrossRef]
- Cortés, M.E.; Sinisterra, R.D.; Avila-Campos, M.J.; Tortamano, N.; Rocha, R.G. The chlorhexidine: Beta;-cyclodextrin inclusion compound: Preparation, characterization and microbiological evaluation. J. Incl. Phenom. Macrocycl. Chem. 2001, 40, 297–302. [Google Scholar] [CrossRef]
- Chaturvedi, G.; Kaur, A.; Umar, A.; Khan, M.A.; Algarni, H.; Kansal, S.K. Removal of fluoroquinolone drug, levofloxacin, from aqueous phase over iron based MOFs, MIL-100 (Fe). J. Solid State Chem. 2019, 121029. [Google Scholar] [CrossRef]
- Singh, G.; Kim, I.Y.; Lakhi, K.S.; Srivastava, P.; Naidu, R.; Vinu, A. Single step synthesis of activated bio-carbons with a high surface area and their excellent CO2 adsorption capacity. Carbon 2017, 116, 448–455. [Google Scholar] [CrossRef]
- Cunha, G.d.C.; Silva, I.A.A.; Alves, J.R.; Oliveira, R.V.M.; Menezes, T.H.S.; Romão, L.P. Magnetic hybrids synthesized from agroindustrial byproducts for highly efficient removal of total chromium from tannery effluent and catalytic reduction of 4-nitrophenol. Cellulose 2018, 25, 7409–7422. [Google Scholar] [CrossRef]
- Cunha, M.R.; Lima, E.C.; Cimirro, N.F.; Thue, P.S.; Dias, S.L.; Gelesky, M.A.; Dotto, G.L.; dos Reis, G.S.; Pavan, F.A. Conversion of Eragrostis plana Nees leaves to activated carbon by microwave-assisted pyrolysis for the removal of organic emerging contaminants from aqueous solutions. Environ. Sci. Pollut. Res. 2018, 25, 23315–23327. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Yuan, W.; Xue, Y.; Wei, H.; Zhang, C.; Li, K. Co-modified MCM-41 as an effective adsorbent for levofloxacin removal from aqueous solution: Optimization of process parameters, isotherm, and thermodynamic studies. Environ. Sci. Pollut. Res. 2017, 24, 5238–5248. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, W.; Liu, Z.; Liu, J.; Zhu, L.; Liu, X.; Huang, K. Renewable Tb/Eu-Loaded Garlic Peels for Enhanced Adsorption of Enrofloxacin: Kinetics, Isotherms, Thermodynamics, and Mechanism. ACS Sustain. Chem. Eng. 2018, 6, 15264–15272. [Google Scholar] [CrossRef]
- Liu, Y.n.; Dong, C.; Wei, H.; Yuan, W.; Li, K. Adsorption of levofloxacin onto an iron-pillared montmorillonite (clay mineral): Kinetics, equilibrium and mechanism. Appl. Clay Sci. 2015, 118, 301–307. [Google Scholar] [CrossRef]
- Yadav, S.; Goel, N.; Kumar, V.; Tikoo, K.; Singhal, S. Removal of fluoroquinolone from aqueous solution using graphene oxide: Experimental and computational elucidation. Environ. Sci. Pollut. Res. 2018, 25, 2942–2957. [Google Scholar] [CrossRef]
- Al-Jabari, M.H.; Sulaiman, S.; Ali, S.; Barakat, R.; Mubarak, A.; Khan, S.A. Adsorption study of levofloxacin on reusable magnetic nanoparticles: Kinetics and antibacterial activity. J. Mol. Liq. 2019, 291, 111249. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; El-Ghanam, A.M.; Mohamed, R.H.A.; Saad, S.R. Enhanced adsorption of Levofloxacin and Ceftriaxone antibiotics from water by assembled composite of nanotitanium oxide/chitosan/nano-bentonite. Mater. Sci. Eng. C 2019, 110199. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Sun, J.; Chen, J.; Zhang, J.; Wang, Y.; Wang, J.; Zhang, H. Adsorption of enrofloxacin on acid/alkali-modified corn stalk biochar. Spectrosc. Lett. 2019, 52, 367–375. [Google Scholar] [CrossRef]
- Ashiq, A.; Sarkar, B.; Adassooriya, N.; Walpita, J.; Rajapaksha, A.U.; Ok, Y.S.; Vithanage, M. Sorption process of municipal solid waste biochar-montmorillonite composite for ciprofloxacin removal in aqueous media. Chemosphere 2019, 236, 124384. [Google Scholar] [CrossRef]
- Wang, N.; Xiao, W.; Niu, B.; Duan, W.; Zhou, L.; Zheng, Y. Highly efficient adsorption of fluoroquinolone antibiotics using chitosan derived granular hydrogel with 3D structure. J. Mol. Liq. 2019, 281, 307–314. [Google Scholar] [CrossRef]
- Li, R.; Wang, Z.; Zhao, X.; Li, X.; Xie, X. Magnetic biochar-based manganese oxide composite for enhanced fluoroquinolone antibiotic removal from water. Environ. Sci. Pollut. Res. 2018, 25, 31136–31148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-L.; Qiao, G.-L.; Zhao, F.; Wang, Y. Thermodynamic and kinetic parameters of ciprofloxacin adsorption onto modified coal fly ash from aqueous solution. J. Mol. Liq. 2011, 163, 53–56. [Google Scholar] [CrossRef]
- Xiong, W.; Zeng, Z.; Li, X.; Zeng, G.; Xiao, R.; Yang, Z.; Zhou, Y.; Zhang, C.; Cheng, M.; Hu, L. Multi-walled carbon nanotube/amino-functionalized MIL-53 (Fe) composites: Remarkable adsorptive removal of antibiotics from aqueous solutions. Chemosphere 2018, 210, 1061–1069. [Google Scholar] [CrossRef]
- Miraboutalebi, S.M.; Nikouzad, S.K.; Peydayesh, M.; Allahgholi, N.; Vafajoo, L.; McKay, G. Methylene blue adsorption via maize silk powder: Kinetic, equilibrium, thermodynamic studies and residual error analysis. Process Saf. Environ. Prot. 2017, 106, 191–202. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Fekry, N.A. Fabrication of engineered silica-functionalized-polyanilines nanocomposites for water decontamination of cadmium and lead. J. Polym. Environ. 2018, 26, 3858–3876. [Google Scholar] [CrossRef]
- Mohanta, D.; Ahmaruzzaman, M. Bio-inspired adsorption of arsenite and fluoride from aqueous solutions using activated carbon@ SnO2 nanocomposites: Isotherms, kinetics, thermodynamics, cost estimation and regeneration studies. J. Environ. Chem. Eng. 2019, 6, 356–366. [Google Scholar] [CrossRef]
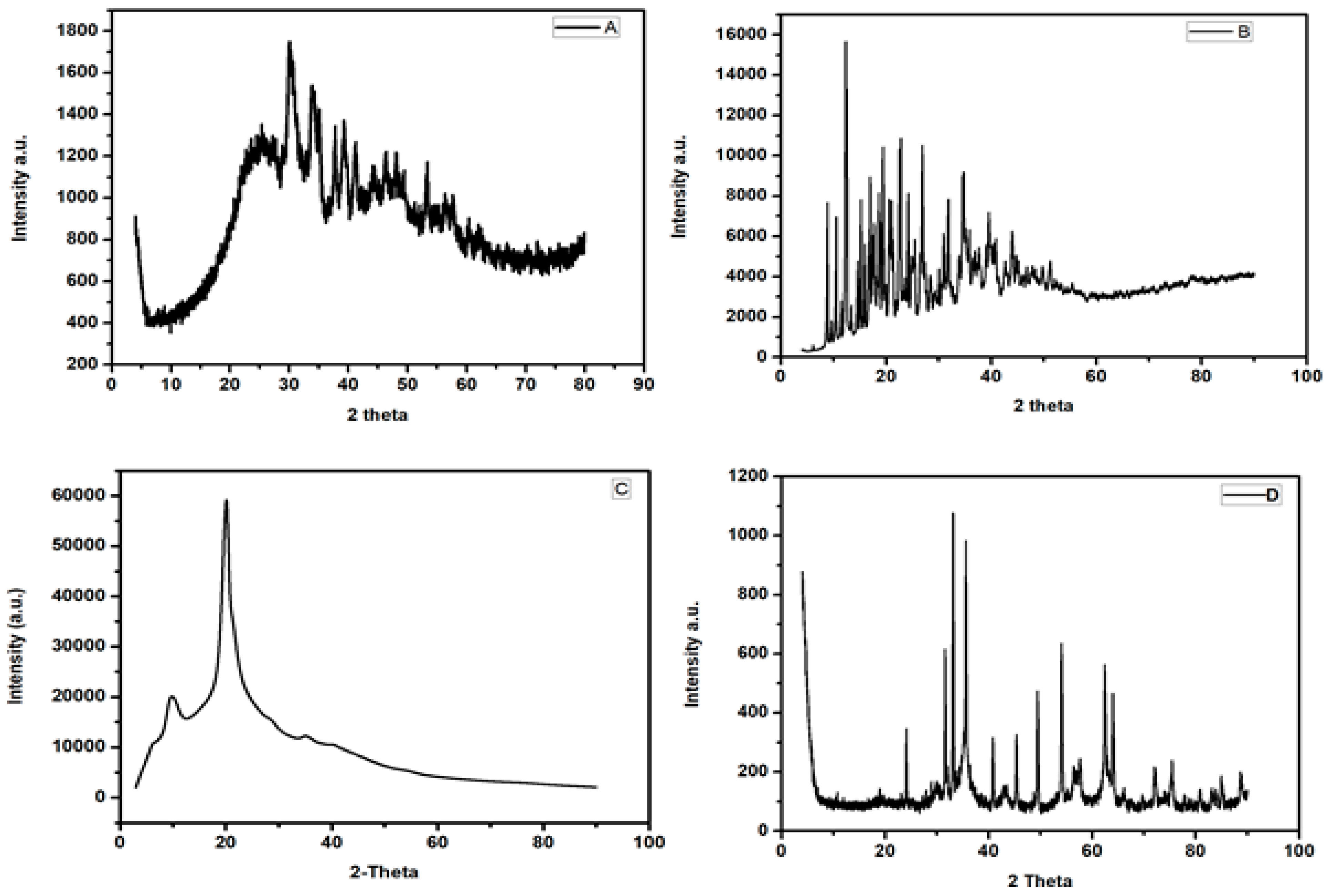



| Isotherm Models | Isotherm Expression | Definition of Terms |
|---|---|---|
| Langmuir | qmax: theoretical monolayer adsorption capacity (mg g−1) Ce: equilibrium concentration (mg L−1), qe: the amount of adsorbate adsorbed per unit weight of adsorbent (mg g−1) C0: initial concentration (mg g−1) KL: Langmuir equilibrium constant (L mg−1) RL: separation factor | |
| Freundlich | KF: Freundlich constant (L g−1) n: is the Freundlich exponent (g L−1) | |
| Hill | nH and KD: Hill isotherm constants qH: maximum equilibrium adsorption capacity (mg/g). | |
| Langmuir–Freundlich | KS: Sips equilibrium constant (1/mg) Qmax: maximum adsorption capacity (mg g−1) n: surface heterogeneity | |
| Pseudo-first order | K1: rate constant (min−1) q–qe: amount of absorbate at equilibrium (mg g−1) | |
| Pseudo-second order | K2: Equilibrium rate constant (g mg−1 min−1) q–qe: amount of adsorbent at equilibrium (mg g−1) | |
| Intra-particle diffusion | Qt: amount of solute on surface of sorbent at time t (mg g−1) Ki: intraparticle diffusion constant (mg g−1 min1/2) |
| Surface Properties | Mesoporous Carbon | Nanocomposite |
|---|---|---|
| SBET (m2 g−1) | 1181 | 1264 |
| Total pore volume (cm3 g−1) | 2.54 | 4.65 |
| Average pore size (nm) | 7.93 | 8.61 |
| t-Plot Smesopore (m2 g−1) | 728 | 755 |
| t-Plot Smicropore (m2 g−1) | 453 | 509 |
| Run | pH | MA | SP | DANO | ENRO | LEVO |
|---|---|---|---|---|---|---|
| 1 | 5 | 10 | 2 | 33.7 | 33.7 | 36.6 |
| 2 | 5 | 10 | 4 | 36.7 | 39.5 | 37.5 |
| 3 | 5 | 30 | 2 | 49.1 | 49.1 | 51.4 |
| 4 | 5 | 30 | 4 | 45.3 | 45.3 | 46.0 |
| 5 | 9 | 10 | 2 | 70.8 | 65.0 | 66.8 |
| 6 | 9 | 10 | 4 | 71.0 | 71.0 | 71.8 |
| 7 | 9 | 30 | 2 | 71.9 | 74.7 | 76.0 |
| 8 | 9 | 30 | 4 | 75.1 | 75.1 | 72.7 |
| 9 | 4.1 | 20 | 3 | 45.4 | 45.4 | 47.3 |
| 10 | 9.9 | 20 | 3 | 59.1 | 59.1 | 66.9 |
| 11 | 7 | 5.3 | 3 | 29.5 | 29.5 | 27.3 |
| 12 | 7 | 35 | 3 | 96.2 | 96.2 | 97.7 |
| 13 | 7 | 20 | 1.5 | 85.4 | 85.4 | 81.5 |
| 14 | 7 | 20 | 4.5 | 96.2 | 99.1 | 97.6 |
| 15 (C) | 7 | 20 | 3 | 97.9 | 97.9 | 94.8 |
| 16 (C) | 7 | 20 | 3 | 97.8 | 97.8 | 95.2 |
| 17 (C) | 7 | 20 | 3 | 96.9 | 99.8 | 96.2 |
| 18 (C) | 7 | 20 | 3 | 98.3 | 98.3 | 94.9 |
| 19 (C) | 7 | 20 | 3 | 96.1 | 96.1 | 95.0 |
| Kinetic Models | Parameters | DANO | ENRO | LEVO |
|---|---|---|---|---|
| qexpt | 130 | 195 | 165 | |
| Pseudo-first order | qe | 93.2 | 161 | 128 |
| k1 | 0.0636 | 0.11 | 0.099 | |
| R2 | 0.9400 | 0.9201 | 0.9372 | |
| Pseudo-second order | qe | 130 | 196 | 167 |
| k2 | 0.0018 | 0.0011 | 0.0011 | |
| R2 | 0.9986 | 0.9971 | 0.9967 | |
| Intraparticle diffusion | kid1 | 17.7 | 24.6 | 24.8 |
| C1 | 32.8 | 56.3 | 29.7 | |
| R21 | 0.9843 | 0.9866 | 0.9754 | |
| kid2 | 1.31 | 0.348 | 0.613 | |
| C2 | 118 | 194 | 162 |
| Two Parameter Models | Three Parameter Models | ||||||
|---|---|---|---|---|---|---|---|
| Langmuir isotherm | Hill isotherm | ||||||
| Parameters | DANO | ENDRO | LEVO | Parameters | DANO | ENRO | LEVO |
| qmax (mg g−1) | 130 | 196 | 165 | qH | 129 | 195 | 164 |
| KL (L g−1) | 0.14 | 0.24 | 0.086 | nH | 2.7 | 2.3 | 2.1 |
| RL | 0.13–0.42 | 0.087–0.22 | 0.11–0.54 | Kd | 115 | 27.6 | 84.9 |
| R2 | 0.9911 | 0.9950 | 0.9934 | R2 | 0.9946 | 0.9859 | 0.9858 |
| Freundlich isotherm | Langmuir-Freundlich (Sips) isotherm | ||||||
| KF | 27.2 | 20.7 | 18.2 | Qmax | 135 | 208 | 170 |
| n | 2.1 | 1.3 | 1.4 | n | 1.1 | 1.1 | 1.3 |
| R2 | 0.9884 | 0.9810 | 0.9806 | Ks | 0.097 | 0.065 | 0.050 |
| R2 | 0.9902 | 0.9884 | 0.9878 | ||||
| Analytes | T (K) | ΔG (kJ mol−1) | ΔH (kJ mol−1) | ΔS (J mol K−1) |
|---|---|---|---|---|
| DANO | 298 | −13.57 | 59.4 | 55.3 |
| 308 | −13.69 | |||
| 313 | −13.74 | |||
| ENRO | 298 | −15.52 | 61.8 | 96.4 |
| 308 | −15.71 | |||
| 313 | −15.81 | |||
| LEVO | 298 | −14.70 | 71.1 | 104 |
| 308 | −14.87 | |||
| 313 | −14.94 |
| Adsorbent | Adsorbate | Adsorption Capacity (mg/g) | Refs |
|---|---|---|---|
| Alkalized biochar | ENRO | 40.91 | [71] |
| Magnetic biochar-based manganese oxide composite | ENRO | 7.19 | [74] |
| iron-pillared montmorillonite | LEVO | 48.61 | [67] |
| MIL-100(Fe) | LEVO | 87.34 | [61] |
| Chitosan derived granular hydrogel with 3D structure | ENRO | 388 | [73] |
| Tb/Eu-Loaded Garlic Peels | ENRO | 769 | [66] |
| Co-modified MCM-41 | LEVO | 108 | [65] |
| Fe3O4 and Fe3O4@SiO2 | LEVO | 6.85 | [69] |
| NBent-NTiO2-Chit. | LEVO | 90.91 | [70] |
| Activated carbon-decorated polyacrylonitrile nanofibers | DANO, ENRO | 99, 112 | [25] |
| MMPC/Cyc-Chit | DANO, ENRO, LEVO | 130, 196, 165 | This work |
| Samples | Added (mg L−1) | DANO | ENRO | LEVO | |||
|---|---|---|---|---|---|---|---|
| Found (mg L−1) | %RE | Found (mg L−1) | %RE | Found (mg L−1) | %RE | ||
| Tap water | 0 | ND | - | ND | - | ND | - |
| 2.0 | 2.13 ± 0.11 | 99.5 | 1.96 ± 0.08 | 99.7 | 2.03±0.09 | 99.5 | |
| 5.0 | 5.03 ± 0.09 | 98.9 | 4.98 ± 0.05 | 99.0 | 5.11±0.02 | 98.9 | |
| River water | 0 | ND | - | 0.098 ± 0.009 | - | ND | - |
| 2.0 | 1.97 ± 0.12 | 97.1 | 2.11 ± 0.08 | 98.3 | 2.04 ± 0.02 | 97.7 | |
| 5.0 | 5.11 ± 0.09 | 96.8 | 4.95 ± 0.11 | 97.9 | 5.08 ± 0.04 | 95.7 | |
| Influent | 0 | 0.148 ± 0.012 | - | 1.23 ± 0.07 | - | 0.573 ± 0.007 | - |
| 2.0 | 2.15 ± 0.09 | 97.2 | 3.32 ± 0.04 | 96.3 | 2.58 ± 0.06 | 95.4 | |
| 5.0 | 5.21 ± 0.07 | 94.7 | 6.29 ± 0.06 | 90.7 | 5.60 ± 0.06 | 93.6 | |
| Effluent | 0 | 0.058 ± 0.011 | - | 0.443 ± 0.010 | - | 0.078 ± 0.011 | - |
| 2.0 | 2.11 ± 0.05 | 98.7 | 2.40 ± 0.06 | 99.0 | 2.13 ± 0.07 | 98.3 | |
| 5.0 | 5.07 ± 0.07 | 95.7 | 5.51 ± 0.04 | 98.7 | 5.06 ± 0.09 | 97.9 | |
| Processes | Cost Breakdown | Temperature/Time/Mass/Volume | Unit cost (R) | Power Rating (kWh) | Price (R) |
|---|---|---|---|---|---|
| Preparation of MPC | Starch | 15 g | 1917 (2 kg) | 14.37 | |
| Sodium hydroxide | 3 g | 844 (1 Kg) | 2.53 | ||
| Silica | 20 g | 1779 (500 g) | 71.16 | ||
| Heating | 120 °C (power = 205 W), 30 min | 71.65 | 0.06 | 4.30 | |
| Carbonization | 500 °C (power = 520W), 3 h | 71.65 | 1.56 | 111.77 | |
| Cleaning | 70 °C, (power = 120 W), 24 h | 71.65 | 2.9 | 207.79 | |
| Drying | 60 °C (power = 240 W), 1 + 2 h | 71.65 | 0.72 | 51.56 | |
| Subtotal for 10–13 g MPC | 463.48 | ||||
| Preparation of the nanocomposite | Ferrous chloride | 1g | 925 (250 g) | 3.70 | |
| Ferric chloride | 2 g | 936 (1 kg) | 1.87 | ||
| MPC | 3 g | 463.48 (10–13 g) | 106.96 | ||
| Chitosan | 3 g | 1939 (100 g) | 58.17 | ||
| Beta-cyclodextrin | 3 g | 2769.00 (100 g) | 83.07 | ||
| Acetic acid | 1.5 mL | 1006 (2.5 L) | 0.60 | ||
| Net amount of 12 g nanocomposite | 253.77 | ||||
| Overhead cost (10% of net cost) | 25.38 | ||||
| Total cost | 279.15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashile, G.P.; Dimpe, K.M.; Nomngongo, P.N. A Biodegradable Magnetic Nanocomposite as a Superabsorbent for the Simultaneous Removal of Selected Fluoroquinolones from Environmental Water Matrices: Isotherm, Kinetics, Thermodynamic Studies and Cost Analysis. Polymers 2020, 12, 1102. https://doi.org/10.3390/polym12051102
Mashile GP, Dimpe KM, Nomngongo PN. A Biodegradable Magnetic Nanocomposite as a Superabsorbent for the Simultaneous Removal of Selected Fluoroquinolones from Environmental Water Matrices: Isotherm, Kinetics, Thermodynamic Studies and Cost Analysis. Polymers. 2020; 12(5):1102. https://doi.org/10.3390/polym12051102
Chicago/Turabian StyleMashile, Geaneth Pertunia, Kgokgobi Mogolodi Dimpe, and Philiswa Nosizo Nomngongo. 2020. "A Biodegradable Magnetic Nanocomposite as a Superabsorbent for the Simultaneous Removal of Selected Fluoroquinolones from Environmental Water Matrices: Isotherm, Kinetics, Thermodynamic Studies and Cost Analysis" Polymers 12, no. 5: 1102. https://doi.org/10.3390/polym12051102
APA StyleMashile, G. P., Dimpe, K. M., & Nomngongo, P. N. (2020). A Biodegradable Magnetic Nanocomposite as a Superabsorbent for the Simultaneous Removal of Selected Fluoroquinolones from Environmental Water Matrices: Isotherm, Kinetics, Thermodynamic Studies and Cost Analysis. Polymers, 12(5), 1102. https://doi.org/10.3390/polym12051102