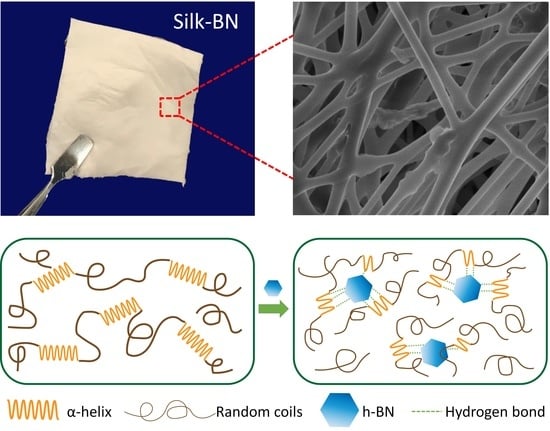
Electrospun Silk-Boron Nitride Nanofibers with Tunable Structure and Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Material Synthesis
2.3. Surface Morphology Analysis
2.4. Structure Analysis
2.5. Thermal Analysis
3. Results and Discussion
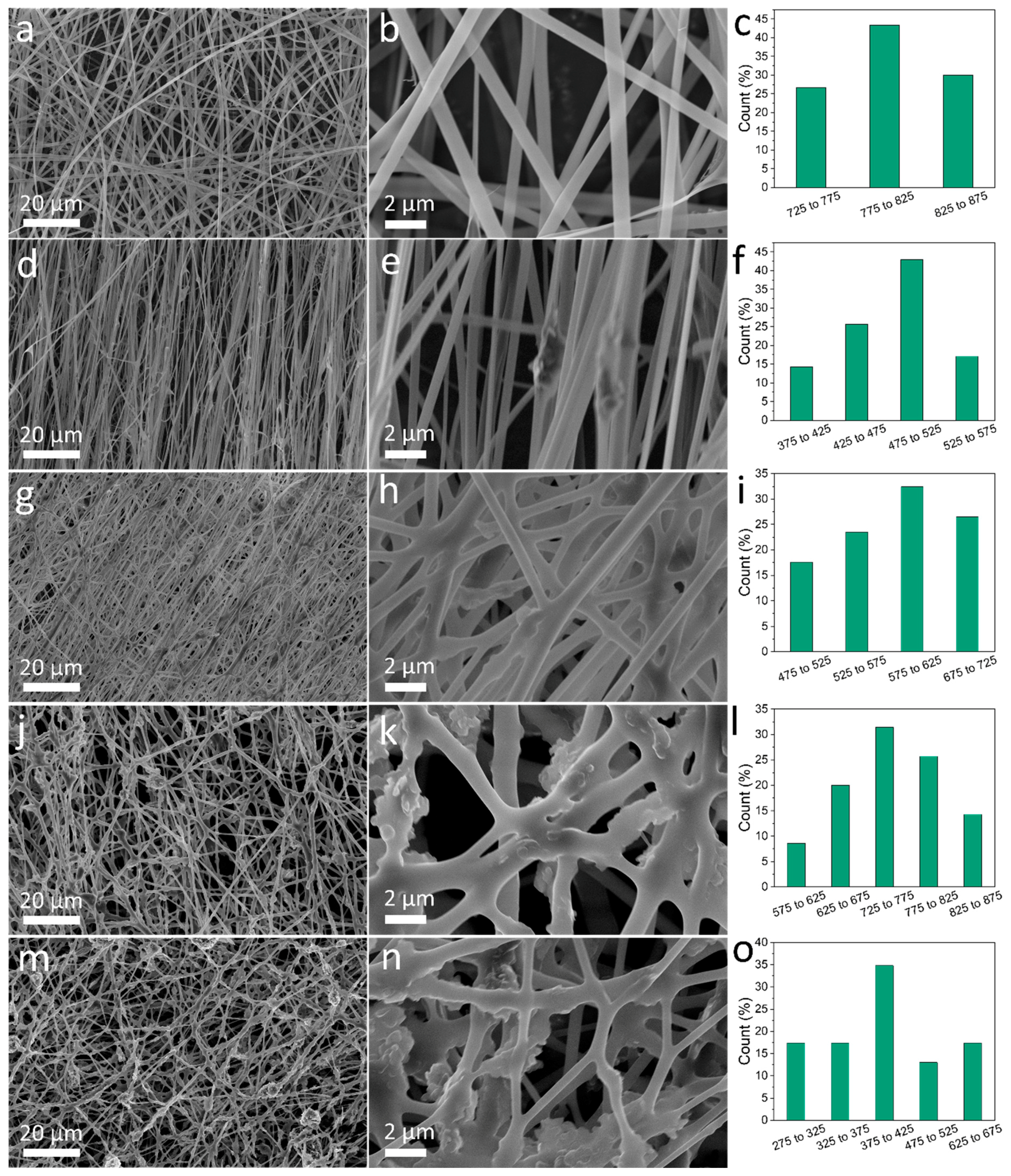
3.1. Morphology Study
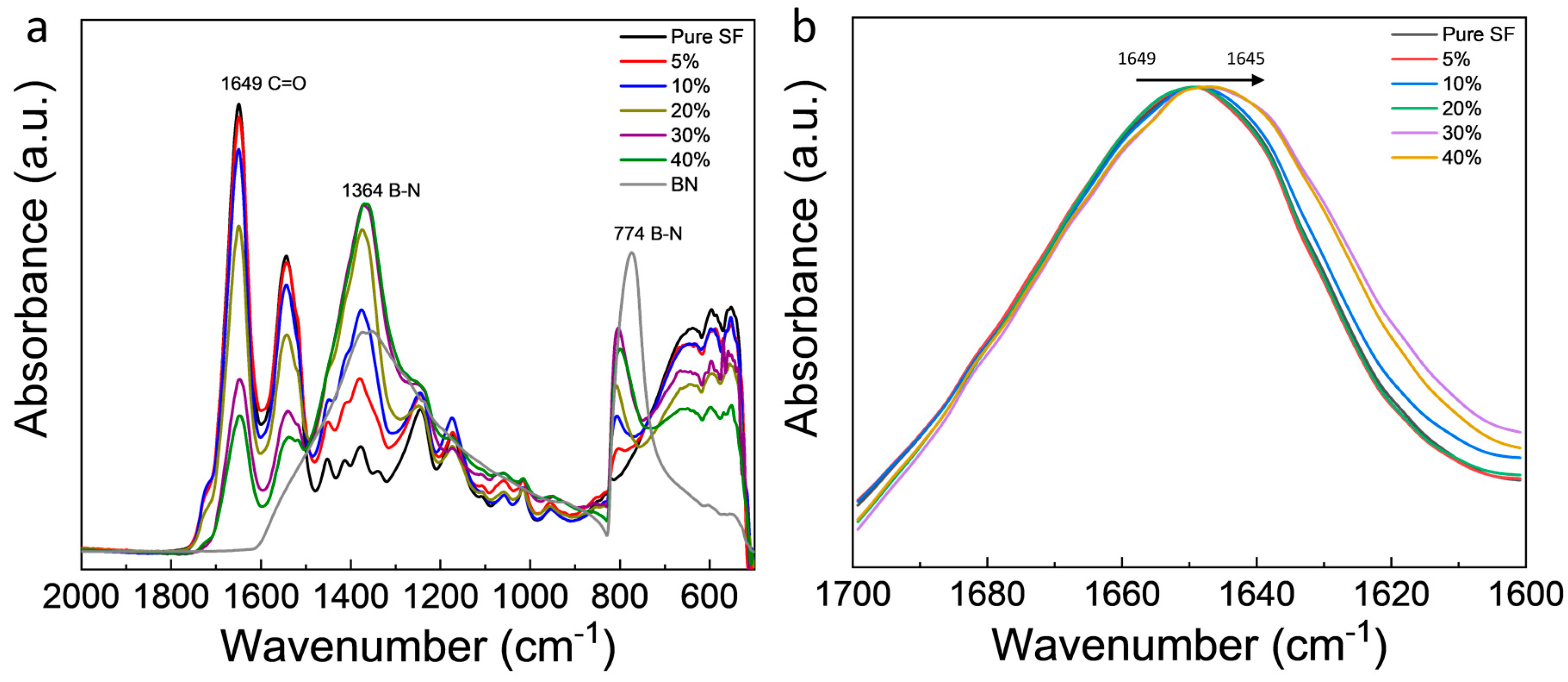
3.2. Structural Study
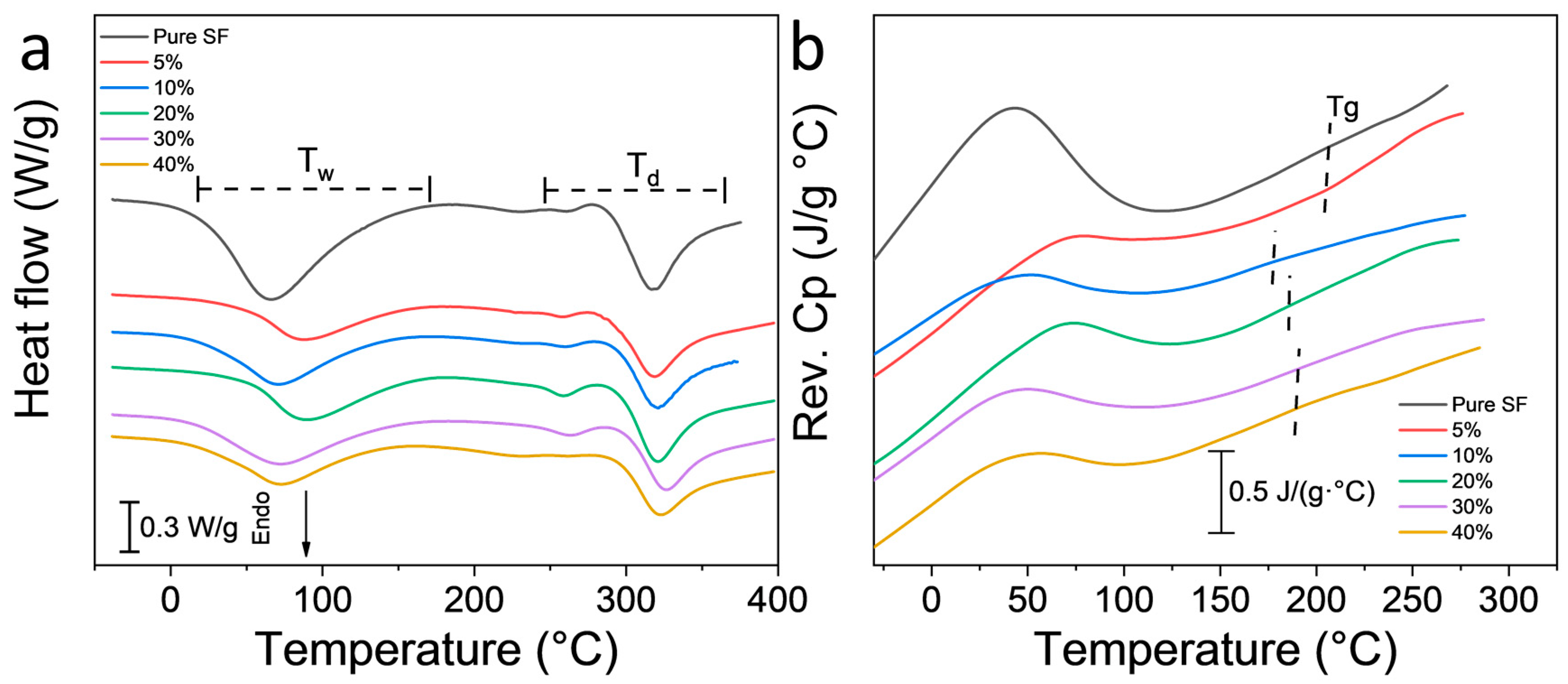
3.3. Thermal Study
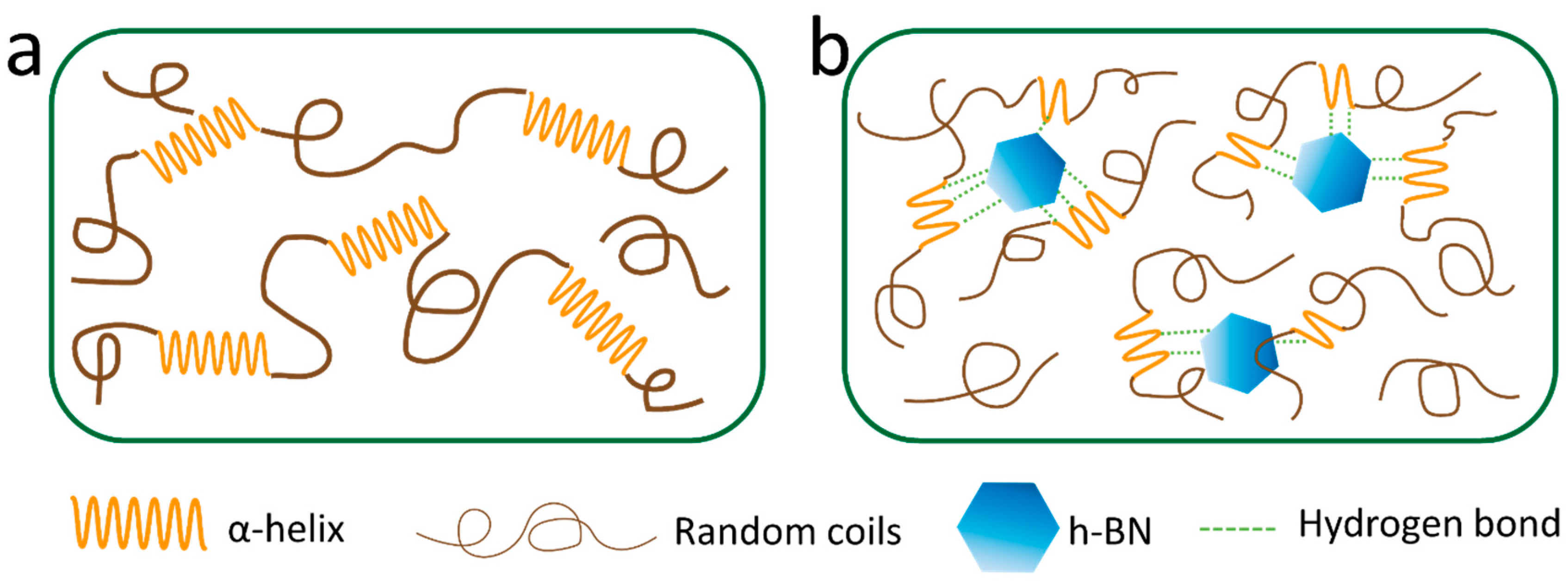
3.4. Mechanism of Self-Assembly
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Conte, A.A.; Shirvani, K.; Hones, H.; Wildgoose, A.; Xue, Y.; Najjar, R.; Hu, X.; Xue, W.; Beachley, V.Z. Effects of post-draw processing on the structure and functional properties of electrospun PVDF-HFP nanofibers. Polymer 2019, 171, 192–200. [Google Scholar] [CrossRef]
- Beachley, V.; Wen, X. Polymer nanofibrous structures: Fabrication, biofunctionalization, and cell interactions. Prog. Polym. Sci. 2010, 35, 868–892. [Google Scholar] [CrossRef]
- Frenot, A.; Chronakis, I.S. Polymer nanofibers assembled by electrospinning. Curr. Opin. Colloid Interface Sci. 2003, 8, 64–75. [Google Scholar] [CrossRef]
- Beachley, V.; Wen, X. Effect of electrospinning parameters on the nanofiber diameter and length. Mater. Sci. Eng. C 2009, 29, 663–668. [Google Scholar] [CrossRef]
- Beachley, V.; Katsanevakis, E.; Zhang, N.; Wen, X. Highly aligned polymer nanofiber structures: Fabrication and applications in tissue engineering. In Biomedical Applications of Polymeric Nanofibers; Springer: Berlin/Heidelberg, Germany, 2011; pp. 171–212. [Google Scholar]
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, F.; Torculas, M.; Lofland, S.; Hu, X. Formic Acid Regenerated Mori, Tussah, Eri, Thai, and Muga Silk Materials: Mechanism of Self-Assembly. ACS Biomater. Sci. Eng. 2019, 5, 6361–6373. [Google Scholar] [CrossRef]
- Wang, F.; Wu, H.; Venkataraman, V.; Hu, X. Silk fibroin-poly (lactic acid) biocomposites: Effect of protein-synthetic polymer interactions and miscibility on material properties and biological responses. Mater. Sci. Eng. C 2019, 104, 109890. [Google Scholar] [CrossRef]
- Lu, Q.; Hu, X.; Wang, X.; Kluge, J.A.; Lu, S.; Cebe, P.; Kaplan, D.L. Water-insoluble silk films with silk I structure. Acta Biomater. 2010, 6, 1380–1387. [Google Scholar] [CrossRef]
- Mandal, B.B.; Grinberg, A.; Gil, E.S.; Panilaitis, B.; Kaplan, D.L. High-strength silk protein scaffolds for bone repair. Proc. Natl. Acad. Sci. USA 2012, 109, 7699–7704. [Google Scholar] [CrossRef]
- Pal, R.K.; Farghaly, A.A.; Wang, C.; Collinson, M.M.; Kundu, S.C.; Yadavalli, V.K. Conducting polymer-silk biocomposites for flexible and biodegradable electrochemical sensors. Biosens. Bioelectron. 2016, 81, 294–302. [Google Scholar] [CrossRef]
- Baoyong, L.; Jian, Z.; Denglong, C.; Min, L. Evaluation of a new type of wound dressing made from recombinant spider silk protein using rat models. Burns 2010, 36, 891–896. [Google Scholar] [CrossRef]
- Tokareva, O.; Jacobsen, M.; Buehler, M.; Wong, J.; Kaplan, D.L. Structure–function–property–design interplay in biopolymers: Spider silk. Acta Biomater. 2014, 10, 1612–1626. [Google Scholar] [CrossRef]
- Dinjaski, N.; Kaplan, D.L. Recombinant protein blends: Silk beyond natural design. Curr. Opin. Biotechnol. 2016, 39, 1–7. [Google Scholar] [CrossRef]
- Frandsen, J.L.; Ghandehari, H. Recombinant protein-based polymers for advanced drug delivery. Chem. Soc. Rev. 2012, 41, 2696–2706. [Google Scholar] [CrossRef]
- Hu, X.; Kaplan, D.; Cebe, P. Determining beta-sheet crystallinity in fibrous proteins by thermal analysis and infrared spectroscopy. Macromolecules 2006, 39, 6161–6170. [Google Scholar] [CrossRef]
- Stanton, J.; Xue, Y.; Pandher, P.; Malek, L.; Brown, T.; Hu, X.; Salas-de la Cruz, D. Impact of ionic liquid type on the structure, morphology and properties of silk-cellulose biocomposite materials. Int. J. Biol. Macromol. 2018, 108, 333–341. [Google Scholar] [CrossRef]
- Wang, X.; Yucel, T.; Lu, Q.; Hu, X.; Kaplan, D.L. Silk nanospheres and microspheres from silk/pva blend films for drug delivery. Biomaterials 2010, 31, 1025–1035. [Google Scholar] [CrossRef]
- Wang, X.; Wenk, E.; Hu, X.; Castro, G.R.; Meinel, L.; Wang, X.; Li, C.; Merkle, H.; Kaplan, D.L. Silk coatings on PLGA and alginate microspheres for protein delivery. Biomaterials 2007, 28, 4161–4169. [Google Scholar] [CrossRef]
- Lammel, A.; Schwab, M.; Hofer, M.; Winter, G.; Scheibel, T. Recombinant spider silk particles as drug delivery vehicles. Biomaterials 2011, 32, 2233–2240. [Google Scholar] [CrossRef]
- Wu, J.; Xie, X.; Zheng, Z.; Li, G.; Wang, X.; Wang, Y. Effect of pH on polyethylene glycol (PEG)-induced silk microsphere formation for drug delivery. Mater. Sci. Eng. C 2017, 80, 549–557. [Google Scholar] [CrossRef]
- Nambiar, S.; Yeow, J.T. Conductive polymer-based sensors for biomedical applications. Biosens. Bioelectron. 2011, 26, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Chakrabarty, T.; Singh, A.K.; Shahi, V.K. Polymer thin films embedded with metal nanoparticles for electrochemical biosensors applications. Biosens. Bioelectron. 2013, 41, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Lofland, S.; Hu, X. Thermal conductivity of protein-based materials: A review. Polymers 2019, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, G.; Wang, X. New secrets of spider silk: Exceptionally high thermal conductivity and its abnormal change under stretching. Adv. Mater. 2012, 24, 1482–1486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, T.; Ban, H.; Liu, L. Hydrogen bonding-assisted thermal conduction in β-sheet crystals of spider silk protein. Nanoscale 2014, 6, 7786–7791. [Google Scholar] [CrossRef]
- Wu, Y.; Xue, Y.; Qin, S.; Liu, D.; Wang, X.; Hu, X.; Li, J.; Wang, X.; Bando, Y.; Golberg, D. BN nanosheet/polymer films with highly anisotropic thermal conductivity for thermal management applications. ACS Appl. Mater. Interfaces 2017, 9, 43163–43170. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Xue, Y.; Liu, D.; Wang, X.; Hu, X.; Bando, Y.; Lei, W. Super-compatible functional boron nitride nanosheets/polymer films with excellent mechanical properties and ultra-high thermal conductivity for thermal management. J. Mater. Chem. C 2018, 6, 1363–1369. [Google Scholar] [CrossRef]
- Guerra, V.; Wan, C.; McNally, T. Thermal conductivity of 2D nano-structured boron nitride (BN) and its composites with polymers. Prog. Mater. Sci. 2019, 100, 170–186. [Google Scholar] [CrossRef]
- Zeng, X.; Sun, J.; Yao, Y.; Sun, R.; Xu, J.-B.; Wong, C.-P. A combination of boron nitride nanotubes and cellulose nanofibers for the preparation of a nanocomposite with high thermal conductivity. ACS Nano 2017, 11, 5167–5178. [Google Scholar] [CrossRef]
- Kıvanç, M.; Barutca, B.; Koparal, A.T.; Göncü, Y.; Bostancı, S.H.; Ay, N. Effects of hexagonal boron nitride nanoparticles on antimicrobial and antibiofilm activities, cell viability. Mater. Sci. Eng. C 2018, 91, 115–124. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Zhang, J.; Hanagata, N.; Wang, X.; Weng, Q.; Ito, A.; Bando, Y.; Golberg, D. Hollow boron nitride nanospheres as boron reservoir for prostate cancer treatment. Nat. Commun. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Shi, Y.; Du, P.; Zhang, Z.; Liu, T.; Zhang, R.; Liu, Z. On-Demand Biodegradable Boron Nitride Nanoparticles for Treating Triple Negative Breast Cancer with Boron Neutron Capture Therapy. ACS Nano 2019, 13, 13843–13852. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, H.; Yan, T.; Huang, D.; Zhi, C.; Nakanishi, H.; Gao, X.-D. Folate-conjugated boron nitride nanospheres for targeted delivery of anticancer drugs. Int. J. Nanomed. 2016, 11, 4573. [Google Scholar]
- Cheng, C.-C.; Muhabie, A.A.; Huang, S.-Y.; Wu, C.-Y.; Gebeyehu, B.T.; Lee, A.-W.; Lai, J.-Y.; Lee, D.-J. Dual stimuli-responsive supramolecular boron nitride with tunable physical properties for controlled drug delivery. Nanoscale 2019, 11, 10393–10401. [Google Scholar] [CrossRef]
- Permyakova, E.S.; Sukhorukova, I.V.; Antipina, L.Y.; Konopatsky, A.S.; Kovalskii, A.M.; Matveev, A.T.; Lebedev, O.I.; Golberg, D.V.; Manakhov, A.M.; Shtansky, D.V. Synthesis and characterization of folate conjugated boron nitride nanocarriers for targeted drug delivery. J. Phys. Chem. C 2017, 121, 28096–28105. [Google Scholar] [CrossRef]
- Saleh, D.A.; Niskanen, J.; Xue, Y.; Golberg, D.; Winnik, F.M.; Sosnik, A. Boron nitride nanotube-based amphiphilic hybrid nanomaterials for superior encapsulation of hydrophobic cargos. Mater. Today Chem. 2017, 6, 45–50. [Google Scholar] [CrossRef]
- Xue, Y.; Jao, D.; Hu, W.; Hu, X. Silk-silk blend materials. J. Therm. Anal. Calorim. 2017, 127, 915–921. [Google Scholar] [CrossRef]
- Callaway, K.A.; Xue, Y.; Altimari, V.; Jiang, G.; Hu, X. Comparative investigation of thermal and structural behavior in renewably sourced composite films of even-even nylons (610 and 1010) with silk fibroin. Polymers 2018, 10, 1029. [Google Scholar] [CrossRef]
- Muratov, D.; Kuznetsov, D.; Il’Inykh, I.; Burmistrov, I.; Mazov, I. Thermal conductivity of polypropylene composites filled with silane-modified hexagonal BN. Compos. Sci. Technol. 2015, 111, 40–43. [Google Scholar] [CrossRef]
- Harrison, H.; Lamb, J.T.; Nowlin, K.S.; Guenthner, A.J.; Ghiassi, K.B.; Kelkar, A.D.; Alston, J.R. Quantification of hexagonal boron nitride impurities in boron nitride nanotubes via FTIR spectroscopy. Nanoscale Adv. 2019, 1, 1693–1701. [Google Scholar] [CrossRef]
- Hu, X.; Cebe, P.; Weiss, A.S.; Omenetto, F.; Kaplan, D.L. Protein-based composite materials. Mater. Today 2012, 15, 208–215. [Google Scholar] [CrossRef]
- Hu, X.; Shmelev, K.; Sun, L.; Gil, E.-S.; Park, S.-H.; Cebe, P.; Kaplan, D.L. Regulation of silk material structure by temperature-controlled water vapor annealing. Biomacromolecules 2011, 12, 1686–1696. [Google Scholar] [CrossRef] [PubMed]

| Sample | Bound Water (%)-TGA | Tw (°C)-TGA | Tw (°C)-DSC | Tg (°C)-DSC | Td (°C)-TGA | Td (°C)-DSC | Mass Remaining% at 600 °C |
|---|---|---|---|---|---|---|---|
| Pure SF | 13.6 | 50.8 | 66.2 | 204.9 | 332.2 | 319.0 | 43.7 |
| 5% BNSF | 12.2 | 53.2 | 87.7 | 202.7 | 331.3 | 319.4 | 47.4 |
| 10% BNSF | 8.5 | 57.9 | 71.3 | 177.9 | 331.6 | 321.3 | 50.5 |
| 20% BNSF | 10.8 | 49.5 | 89.7 | 185.6 | 328.5 | 321.3 | 55.5 |
| 30% BNSF | 8.4 | 54.2 | 74.4 | 189.5 | 339.1 | 327.2 | 58.2 |
| 40% BNSF | 8.6 | 49.9 | 73.3 | 190.2 | 330.7 | 322.8 | 63.6 |
| Pure BN | <0.1 | N/A | N/A | N/A | N/A | N/A | 98.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Hu, X. Electrospun Silk-Boron Nitride Nanofibers with Tunable Structure and Properties. Polymers 2020, 12, 1093. https://doi.org/10.3390/polym12051093
Xue Y, Hu X. Electrospun Silk-Boron Nitride Nanofibers with Tunable Structure and Properties. Polymers. 2020; 12(5):1093. https://doi.org/10.3390/polym12051093
Chicago/Turabian StyleXue, Ye, and Xiao Hu. 2020. "Electrospun Silk-Boron Nitride Nanofibers with Tunable Structure and Properties" Polymers 12, no. 5: 1093. https://doi.org/10.3390/polym12051093
APA StyleXue, Y., & Hu, X. (2020). Electrospun Silk-Boron Nitride Nanofibers with Tunable Structure and Properties. Polymers, 12(5), 1093. https://doi.org/10.3390/polym12051093