Preparation of Clindamycin Hydrochloride Loaded De-Esterified Low-Methoxyl Mango Peel Pectin Film Used as a Topical Drug Delivery System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Collection of Mango Peel
2.3. Extraction of Pectin from Mango Peels by Microwave-Assisted Method
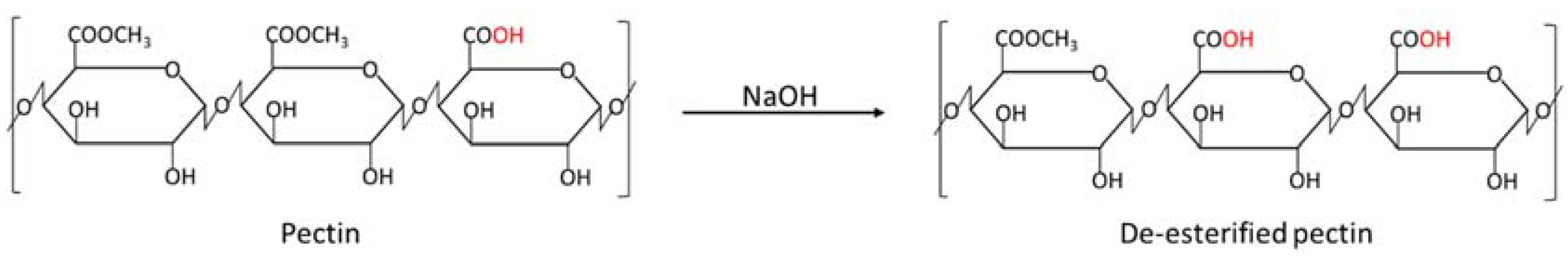
2.4. De-Esterification Using NaOH
2.5. Degree of Esterification
2.6. Galacturonic Acid Content
2.7. Fourier-Transform Infrared Spectrophotometry
2.8. Film Preparation
2.9. Analysis of Morphological Characteristics
2.10. Test of Mechanical Properties
2.11. Preparation of Film Containing Clindamycin HCl
2.12. Clindamycin Content
2.13. In Vitro Drug Release Profile
2.14. Antibacterial Activity Test
2.15. Statistical Analysis
3. Results and Discussion
3.1. Mango Peel Pectin Extraction and Characterization
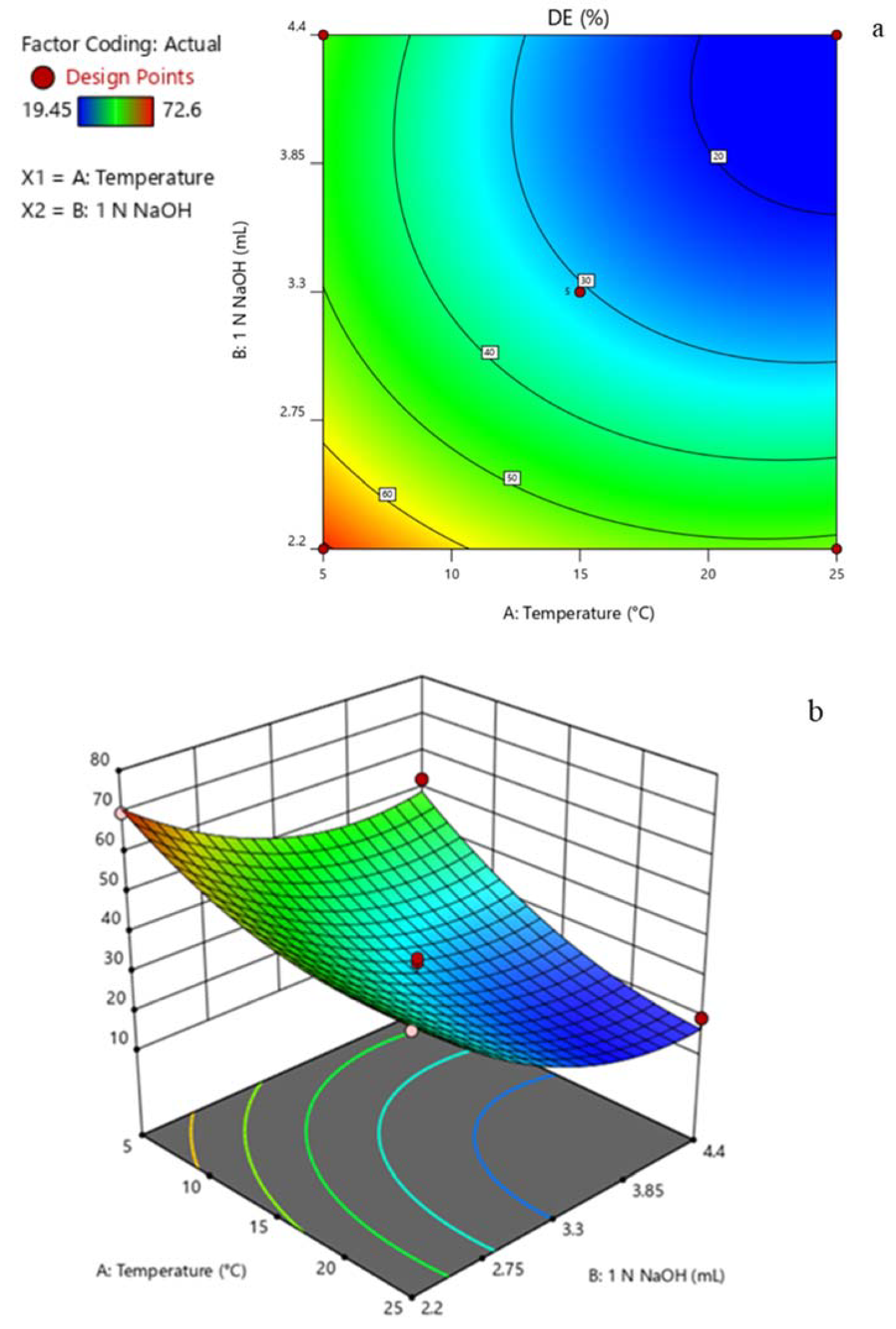
3.2. Experimental Design
3.3. De-Esterification of Pectin
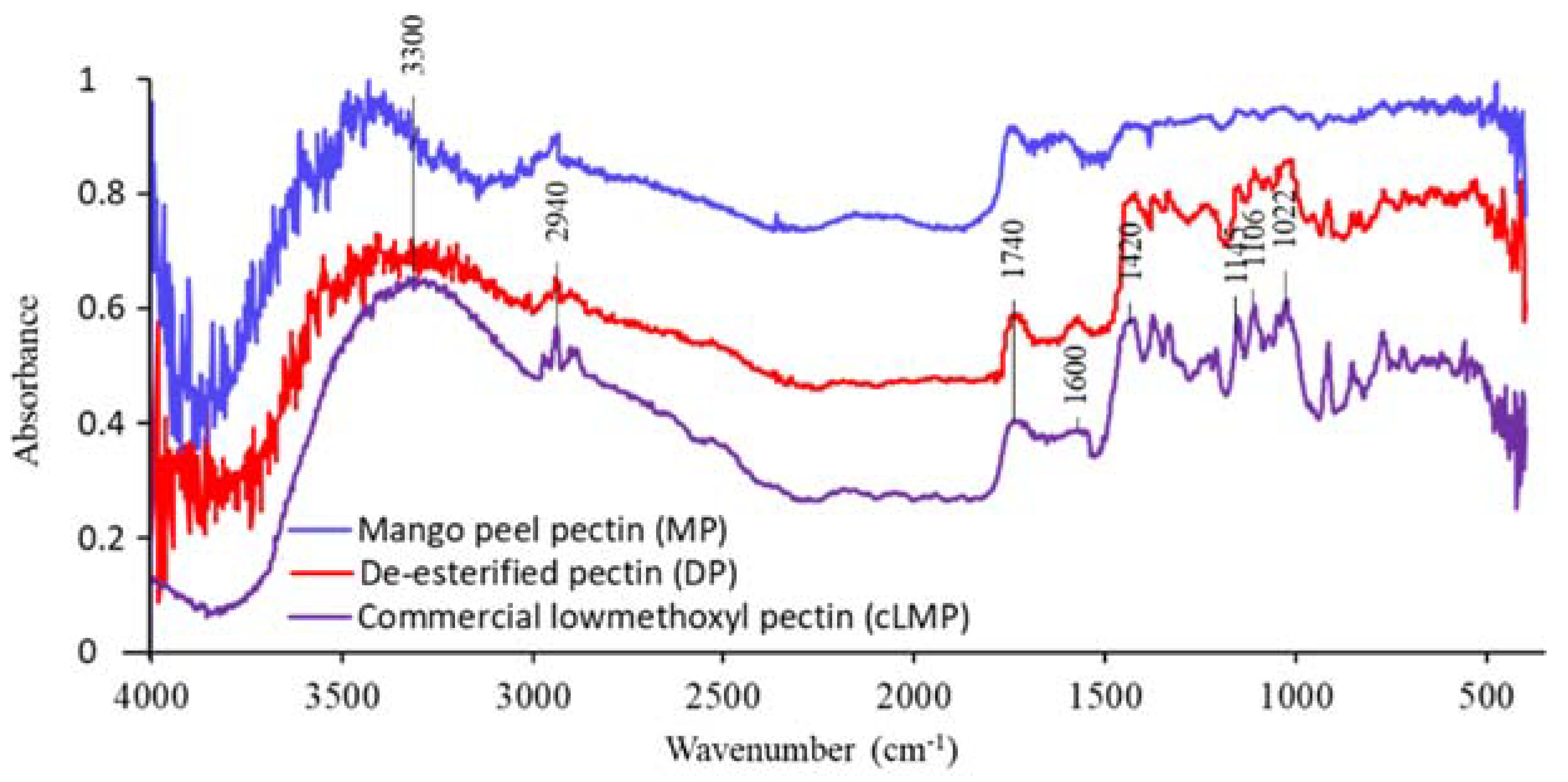
3.4. Fourier-Transform Infrared Spectrophotometry (FTIR)
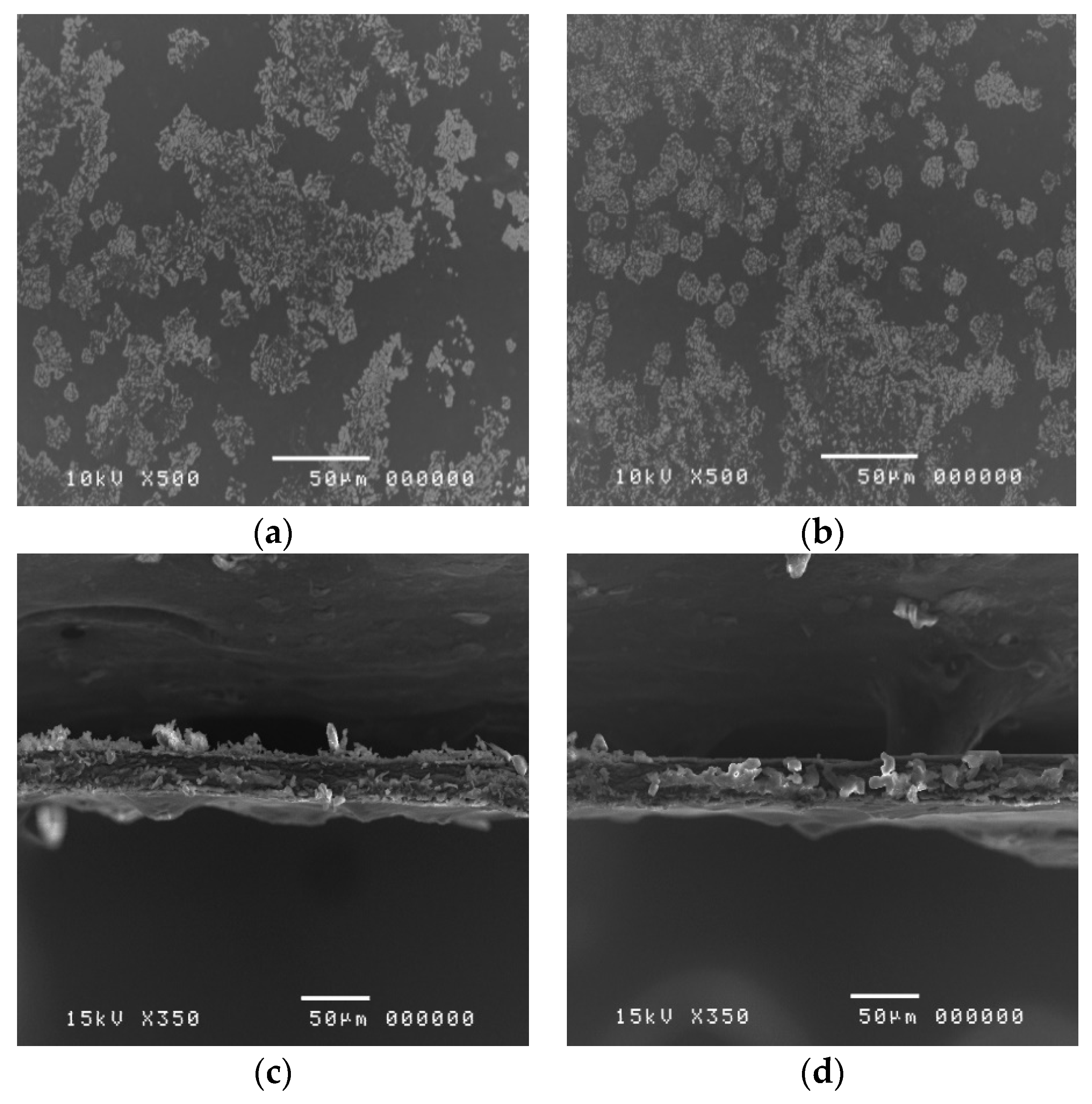
3.5. Morphological Analysis of De-Esterified Pectin Film
3.6. Mechanical Properties of Prepared Pectin Films
3.7. Characteristics of Clindamycin HCl-Loaded Pectin Films
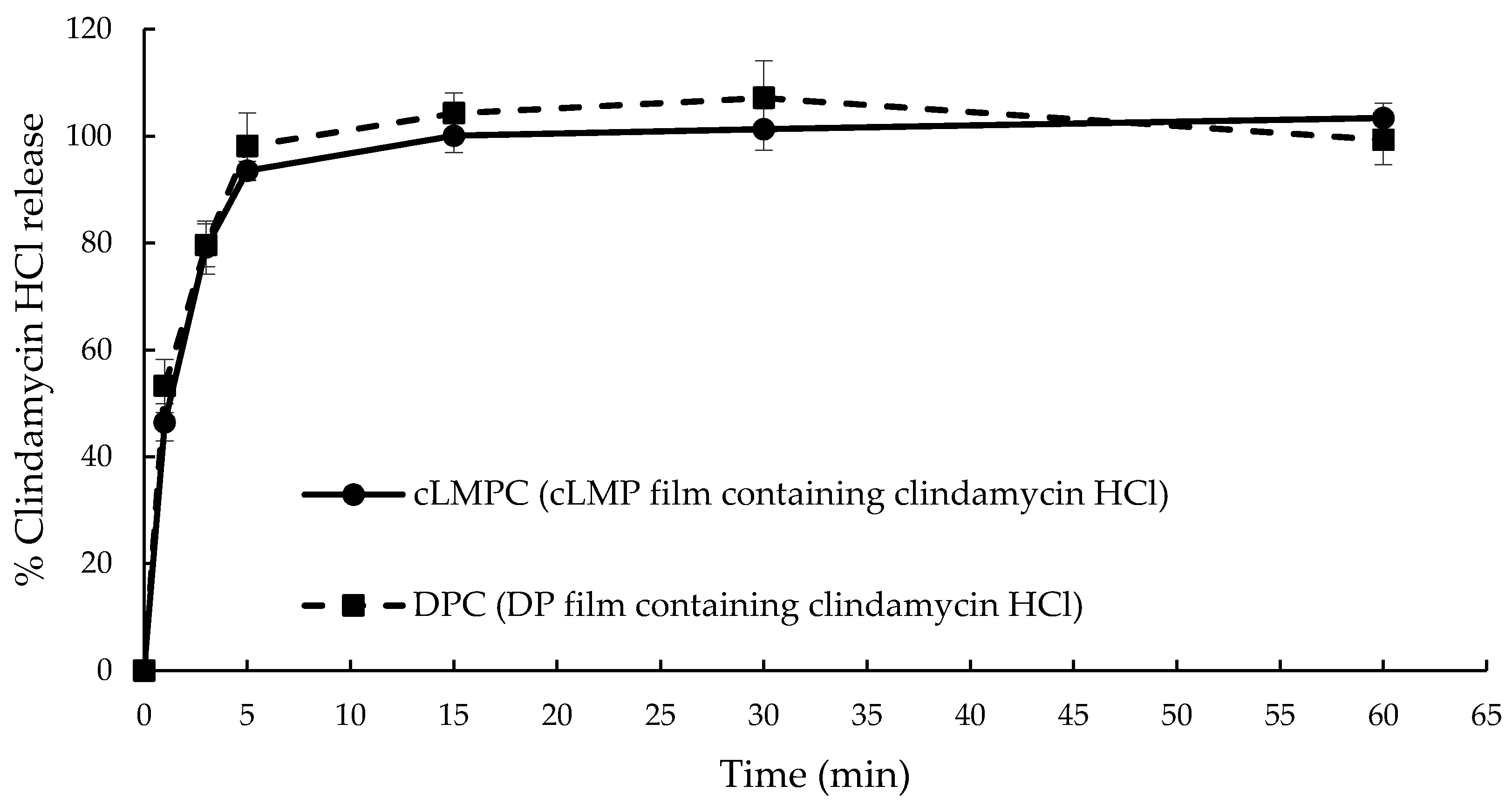
3.8. Drug Release Profile
3.9. Activity against Microbial Growth of Clindamycin HCl-Containing Pectin Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- May, C.D. Industrial pectins: Sources, production and applications. Carbohydr. Polym. 1990, 12, 79–99. [Google Scholar] [CrossRef]
- Department of Agriculture and Fisheris, Queensland Government. Available online: https://www.daf.qld.gov.au/business-priorities/agriculture/plants/fruit-vegetable/fruit-vegetable-crops/mangoes/mango-varieties/nam-doc-mai (accessed on 30 August 2019).
- Sriamornsak, P. Chemistry of pectin and its pharmaceutical uses: A review. Silpakorn Univ. Int. J. 2003, 3, 206–228. [Google Scholar]
- El-Nawawi, S.A.; Heikal, Y.A. Factors affecting the production of low-ester pectin gels. Carbohydr. Polym. 1995, 26, 189–193. [Google Scholar] [CrossRef]
- Arora, A.; Prausnitz, M.R.; Mitragotri, S. Micro-scale devices for transdermal drug delivery. Int. J. Pharm. 2008, 364, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Smieja, M. Current indications for the use of clindamycin: A critical review. Can. J. Infect. Dis. 1998, 9, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Ishida, N.; Nakaminami, H.; Noguchi, N.; Kurokawa, I.; Nishijima, S.; Sasatsu, M. Antimicrobial susceptibilities of Propionibacterium acnes isolated from patients with acne vulgaris. Microbiol. Immunol. 2008, 52, 621–624. [Google Scholar] [CrossRef]
- Bagherian, H.; Zokaee Ashtiani, F.; Fouladitajar, A.; Mohtashamy, M. Comparisons between conventional, microwave- and ultrasound-assisted methods for extraction of pectin from grapefruit. Chem. Eng. Process. Process Intensif. 2011, 50, 1237–1243. [Google Scholar] [CrossRef]
- Chaiwarit, T.; Ruksiriwanich, W.; Jantanasakulwong, K.; Jantrawut, P. Use of Orange Oil Loaded Pectin Films as Antibacterial Material for Food Packaging. Polymers 2018, 10, 1144. [Google Scholar] [CrossRef]
- Hunter, J.L.; Wicker, L. De-esterification of pectin by alkali, plant and fungal pectinmethylesterases and effect on molecular weight. J. Sci. Food Agric. 2005, 85, 2243–2248. [Google Scholar] [CrossRef]
- De Freitas Oliveira, C.; Giordani, D.; Lutckemier, R.; Gurak, P.D.; Cladera-Olivera, F.; Ferreira Marczak, L.D. Extraction of pectin from passion fruit peel assisted by ultrasound. LWT Food Sci. Technol. 2016, 71, 110–115. [Google Scholar] [CrossRef]
- Jantrawut, P.; Chaiwarit, T.; Jantanasakulwong, K.; Brachais, C.H.; Chambin, O. Effect of Plasticizer Type on Tensile Property and In Vitro Indomethacin Release of Thin Films Based on Low-Methoxyl Pectin. Polymers 2017, 9, 289. [Google Scholar] [CrossRef]
- Junmahasathien, T.; Panraksa, P.; Protiarn, P.; Hormdee, D.; Noisombut, R.; Kantrong, N.; Jantrawut, P. Preparation and Evaluation of Metronidazole-Loaded Pectin Films for Potentially Targeting a Microbial Infection Associated with Periodontal Disease. Polymers 2018, 10, 1021. [Google Scholar] [CrossRef] [PubMed]
- Preis, M.; Knop, K.; Breitkreutz, J. Mechanical strength test for orodispersible and buccal films. Int. J. Pharm. 2014, 461, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Felton, L.A.; Ó’Donnell, P.B.; McGinity, J.M. Mechanical properties of polymeric films prepared from aqueous dispersions. In Aqueous Polymeric Coatings for Pharmaceutical Dosage Forms; McGinity, J.M., Felton, L.A., Eds.; CRC Press: Boca Raton, FL, USA, 2017; Volume 215, pp. 105–128. [Google Scholar]
- Otoni, C.G.; de Moura, M.R.; Aouada, F.A.; Camilloto, G.P.; Cruz, R.S.; Lorevice, M.V.; de Soares, N.F.F.; Mattoso, L.H.C. Antimicrobial and physical-mechanical properties of pectin/papaya puree/cinnamaldehyde nanoemulsion edible composite films. Food Hydrocoll. 2014, 41, 188–194. [Google Scholar] [CrossRef]
- Berardini, N.; Knödler, M.; Schieber, A.; Carle, R. Utilization of mango peels as a source of pectin and polyphenolics. Innov. Food Sci. Emerg. Technol. 2005, 6, 442–452. [Google Scholar] [CrossRef]
- Sommano, S.; Ounamornmas, P.; Nisoa, M.; Sriwattana, S. Bioactive functionality of pectin from peels of seven Thai mango cultivars. Acta Hortic. 2018, 1213, 423–428. [Google Scholar] [CrossRef]
- Fishman, M.L.; Chau, H.K.; Hoagland, P.D.; Hotchkiss, A.T. Microwave-assisted extraction of lime pectin. Food Hydrocoll. 2006, 20, 1170–1177. [Google Scholar] [CrossRef]
- Sarah, M.; Hanum, F.; Rizky, M.; Hisham, M.F. Microwave-assisted extraction of pectin from cocoa peel. IOP Conf. Ser. Earth Environ. Sci. 2018, 122, 012079. [Google Scholar] [CrossRef]
- Pandit, S.G.; Vijayanand, P.; Kulkarni, S.G. Pectic principles of mango peel from mango processing waste as influenced by microwave energy. LWT Food Sci. Technol. 2015, 64, 1010–1014. [Google Scholar] [CrossRef]
- Rimkeeree, K.; Charoenrein, S. Effect of Cultivar and Ripening Stage on Quality and Microstructure of Frozen Mangoes (Mangifera indica Linn.). Int. J. Food Prop. 2014, 17, 1093–1108. [Google Scholar] [CrossRef]
- Kunnan Singh, J.S.; Ching, Y.C.; Abdullah, L.C.; Ching, K.Y.; Razali, S.; Gan, S.N. Optimization of Mechanical Properties for Polyoxymethylene/Glass Fiber/Polytetrafluoroethylene Composites Using Response Surface Methodology. Polymers 2018, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, E.s.R.; Silva, I.M.D.A.; Gonzaga, L.V.; Amante, E.R.; Teófilo, R.F.; Ferreira, M.M.C.; Amboni, R.D.M.C. Optimization of extraction of high-ester pectin from passion fruit peel (Passiflora edulis flavicarpa) with citric acid by using response surface methodology. Bioresour. Technol. 2008, 99, 5561–5566. [Google Scholar] [CrossRef] [PubMed]
- Alemzadeh, I. Production of Low Ester (LM) Pectin by De-esterification of High Ester (HM) Apple Pectin. Sci. Iran. 2005, 12, 306–310. [Google Scholar]
- Kurita, O.; Fujiwara, T.; Yamazaki, E. Characterization of the pectin extracted from citrus peel in the presence of citric acid. Carbohydr. Polym. 2008, 74, 725–730. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Jiang, P.; Hu, L.; Zhi, Z.; Chen, J.; Ding, T.; Ye, X.; Liu, D. Characterization of pectin from grapefruit peel: A comparison of ultrasound-assisted and conventional heating extractions. Food Hydrocoll. 2016, 61, 730–739. [Google Scholar] [CrossRef]
- Gurram, R.; Souza Filho, P.F.; Taherzadeh, M.J.; Zamani, A. A Solvent-Free Approach for Production of Films from Pectin and Fungal Biomass. J. Polym. Environ. 2018, 26, 4282–4292. [Google Scholar] [CrossRef]
- Bagliotti Meneguin, A.; Stringhetti Ferreira Cury, B.; Evangelista, R.C. Films from resistant starch-pectin dispersions intended for colonic drug delivery. Carbohydr. Polym. 2014, 99, 140–149. [Google Scholar] [CrossRef]
- Nascimento, T.A.; Calado, V.; Carvalho, C.W.P. Development and characterization of flexible film based on starch and passion fruit mesocarp flour with nanoparticles. Food Res. Int. 2012, 49, 588–595. [Google Scholar] [CrossRef]
- Fishman, M.L.; Coffin, D.R.; Onwulata, C.I.; Konstance, R.P. Extrusion of pectin and glycerol with various combinations of orange albedo and starch. Carbohydr. Polym. 2004, 57, 401–413. [Google Scholar] [CrossRef]
- Dixit, R.P.; Puthli, S.P. Oral strip technology: Overview and future potential. J. Control. Release 2009, 139, 94–107. [Google Scholar] [CrossRef]
- Penhasi, A.; Meidan, V.M. Preparation and characterization of in situ ionic cross-linked pectin films: Unique biodegradable polymers. Carbohydr. Polym. 2014, 102, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; McGinity, J.W. Influence of ibuprofen as a solid-state plasticizer in Eudragit RS 30 D on the physicochemical properties of coated beads. AAPS PharmSciTech 2001, 2, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Shim, J.S.; Lee, J.S.; Kim, M.-K.; Chung, M.-S.; Kim, K.H. Pectin-like acidic polysaccharide from Panax ginseng with selective antiadhesive activity against pathogenic bacteria. Carbohydr. Res. 2006, 341, 1154–1163. [Google Scholar] [CrossRef]
- Wright, T.E.; Boyle, K.K.; Duquin, T.R.; Crane, J.K. Propionibacterium acnes Susceptibility and Correlation with Hemolytic Phenotype. Infect. Dis. 2016, 9, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Iinuma, K.; Noguchi, N.; Nakaminami, H.; Sasatsu, M.; Nishijima, S.; Tsuboi, I. Susceptibility of Propionibacterium acnes isolated from patients with acne vulgaris to zinc ascorbate and antibiotics. Clin. Cosmet. Investig. Dermatol. 2011, 4, 161–165. [Google Scholar]



| Variables | Level | ||||
|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | +α | |
| 1 N NaOH (mL) | 1.74 | 2.20 | 3.30 | 4.40 | 4.86 |
| Temperature (°C) | 0.86 | 5.00 | 15.00 | 25.00 | 29.14 |
| Pectin | Chemical Characteristics | ||
|---|---|---|---|
| Yield (% w/w) | Galacturonic Acid (% w/w) | Degree of Esterification (%) | |
| Commercial low methoxyl pectin (cLMP) | - | 84.00 ± 3.11 | 29 |
| Mango peel pectin (MP) | 12.46 ± 0.52 | 75.14 ± 2.09 | 79.02 ± 2.84 |
| De-esterified pectin (DP) | 76.72 ± 4.64 | 69.15 ± 1.19 | 29.40 ± 3.58 |
| Exp. Order | Variables | Response | ||
|---|---|---|---|---|
| DE (%) | ||||
| 1 N NaOH (mL) | Experimental Temperature (°C) | Actual Value | Predicted Value | |
| 1 | 2.20 | 5.0 | 69.82 ± 2.55 | 70.85 |
| 2 | 2.20 | 25.0 | 50.57 ± 0.99 | 52.04 |
| 3 | 4.40 | 5.0 | 52.63 ± 1.02 | 49.33 |
| 4 | 4.40 | 25.0 | 19.45 ± 4.81 | 16.59 |
| 5 | 3.30 | 1.0 | 61.08 ± 7.64 | 62.31 |
| 6 | 3.30 | 29.0 | 25.26 ± 3.18 | 25.86 |
| 7 | 1.74 | 15.0 | 72.60 ± 2.73 | 70.46 |
| 8 | 4.86 | 15.0 | 22.73 ± 3.94 | 30.17 |
| 9 | 3.30 | 15.0 | 33.33 ± 6.73 | 30.59 |
| 10 | 3.30 | 15.0 | 34.45 ± 1.94 | 30.59 |
| 11 | 3.30 | 15.0 | 30.01 ± 4.81 | 30.59 |
| 12 | 3.30 | 15.0 | 23.33 ± 4.89 | 30.59 |
| 13 | 3.30 | 15.0 | 28.97 ± 4.12 | 30.59 |
| DP | 3.05 | 25.0 | 29.40 ± 3.58 | 28.98 |
| Source | Sum of Squares | Mean Square | p-Value |
|---|---|---|---|
| Model | 3887.42 | 777.48 | <0.0001 |
| A-Temperature | 1328.37 | 1328.37 | <0.0001 |
| B-Vol of NaOH | 1622.89 | 1622.89 | <0.0001 |
| AB | 48.51 | 48.51 | 0.0923 |
| A2 | 316.84 | 316.84 | 0.0016 |
| B2 | 676.48 | 676.48 | 0.0002 |
| Residual | 89.37 | 12.77 | |
| Lack of Fit | 44.64 | 14.88 | 0.3819 |
| Pure error | 44.73 | 11.88 |
| Films | Puncture Strength(N/mm2) | Elongation (%) | Young’s Modulus (N/mm2) | Thickness by Thickness Gauge (mm) | Thickness by SEM (µm) | Clindamycin HCl Content (%) |
|---|---|---|---|---|---|---|
| cLMP | 13.72 ± 3.19 a | 2.75 ± 0.68 a | 67.69 ± 12.26 a | 0.048 ± 0.011 a | ~45 | - |
| DP | 11.13 ± 1.92 a | 2.52 ± 0.85 a | 61.79 ± 8.32 a | 0.049 ± 0.010 a | ~45 | - |
| cLMPC | 6.78 ± 1.85 b | 2.46 ± 0.29 a | 47.32 ± 3.35 b | 0.051 ± 0.007 a | ~43 | 93.47 ± 4.71 a |
| DPC | 4.66 ± 0.64 b | 2.77 ± 0.44 a | 44.77 ± 4.45 b | 0.048 ± 0.006 a | ~43 | 98.79 ± 2.69 a |
| Film Formulation | Zone of Inhibition (Diameter in mm) | |
|---|---|---|
| S. aureus | C. acnes | |
| Clindamycin solution (containing clindamycin HCl equivalent to clindamycin base 1%) | 42.02 ± 0.52 a | 77.18 ± 1.50 a |
| Blank film | 7.18 ± 0.00 b | 7.63 ± 0.00 b |
| cLMPC (cLMP film containing clindamycin HCl equivalent to clindamycin base 1%) | 41.11 ± 0.33 a | 76.30 ± 0.98 a |
| DPC (DP film containing clindamycin HCl equivalent to clindamycin base 1%) | 40.78 ± 0.37 a | 74.04 ± 1.12 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaiwarit, T.; Rachtanapun, P.; Kantrong, N.; Jantrawut, P. Preparation of Clindamycin Hydrochloride Loaded De-Esterified Low-Methoxyl Mango Peel Pectin Film Used as a Topical Drug Delivery System. Polymers 2020, 12, 1006. https://doi.org/10.3390/polym12051006
Chaiwarit T, Rachtanapun P, Kantrong N, Jantrawut P. Preparation of Clindamycin Hydrochloride Loaded De-Esterified Low-Methoxyl Mango Peel Pectin Film Used as a Topical Drug Delivery System. Polymers. 2020; 12(5):1006. https://doi.org/10.3390/polym12051006
Chicago/Turabian StyleChaiwarit, Tanpong, Pornchai Rachtanapun, Nutthapong Kantrong, and Pensak Jantrawut. 2020. "Preparation of Clindamycin Hydrochloride Loaded De-Esterified Low-Methoxyl Mango Peel Pectin Film Used as a Topical Drug Delivery System" Polymers 12, no. 5: 1006. https://doi.org/10.3390/polym12051006
APA StyleChaiwarit, T., Rachtanapun, P., Kantrong, N., & Jantrawut, P. (2020). Preparation of Clindamycin Hydrochloride Loaded De-Esterified Low-Methoxyl Mango Peel Pectin Film Used as a Topical Drug Delivery System. Polymers, 12(5), 1006. https://doi.org/10.3390/polym12051006






