Uncommon Sorption Mechanism of Aromatic Compounds onto Poly(Vinyl Alcohol)/Chitosan/Maleic Anhydride-β-Cyclodextrin Hydrogels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of the Cyclodextrin Functional Monomer (MA-β-CD)
Preparation of PVA/CS/MA-β-CD Hydrogels
2.3. Characterization of the Hydrogels
2.3.1. Swelling Equilibrium Studies
2.3.2. Thermogravimetric Analysis
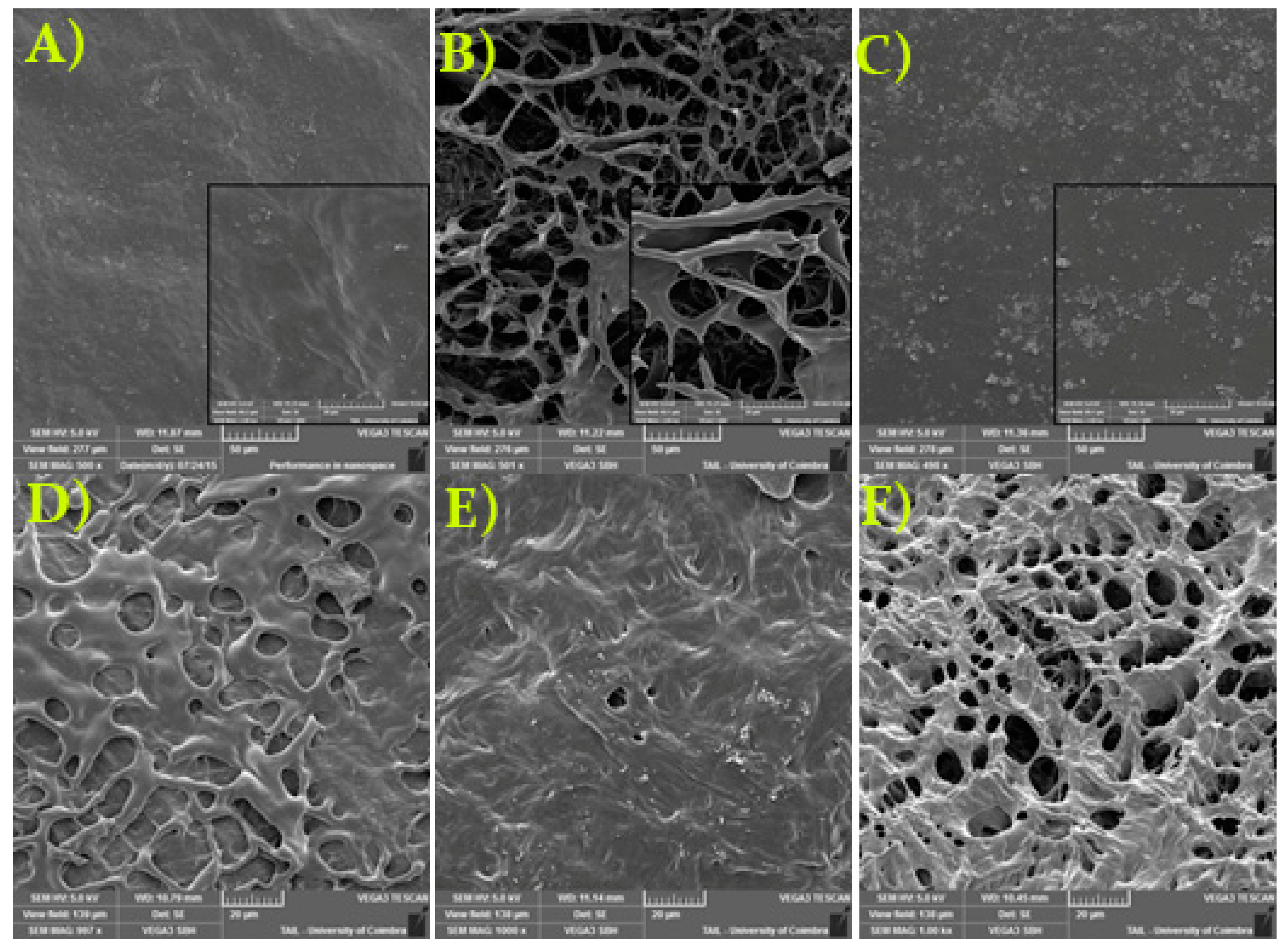
2.3.3. Surface Morphology
2.3.4. Rheological Experiments
2.4. Sorption Studies
2.4.1. Sorption Isotherms
2.4.2. Sorption Kinetics
2.4.3. Reusability of the Composite Hydrogels
2.5. Analytical Procedure
3. Results and Discussion
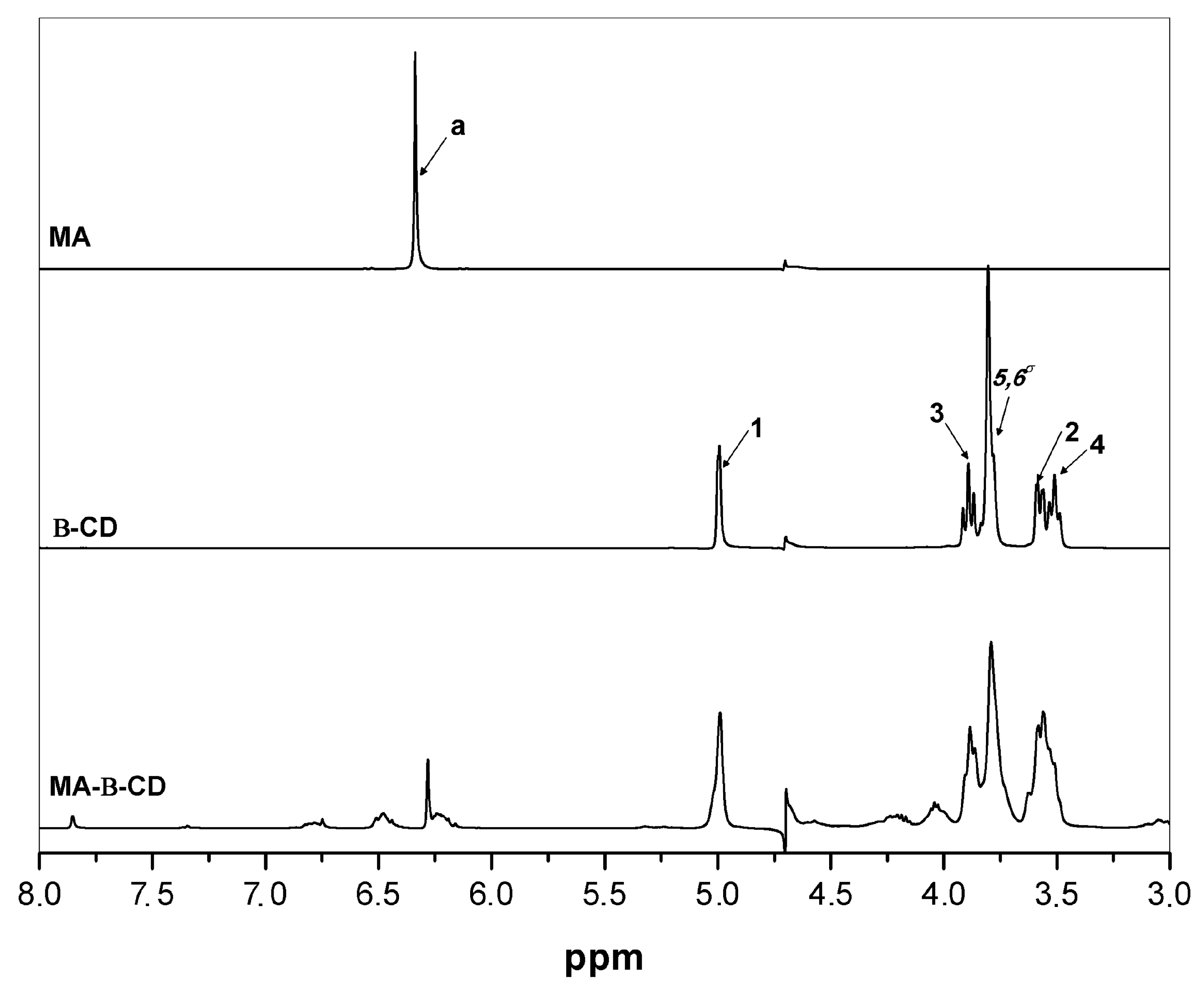
3.1. Synthesis of Maleic Acid-β-Cyclodextrin (MA-β-CD)
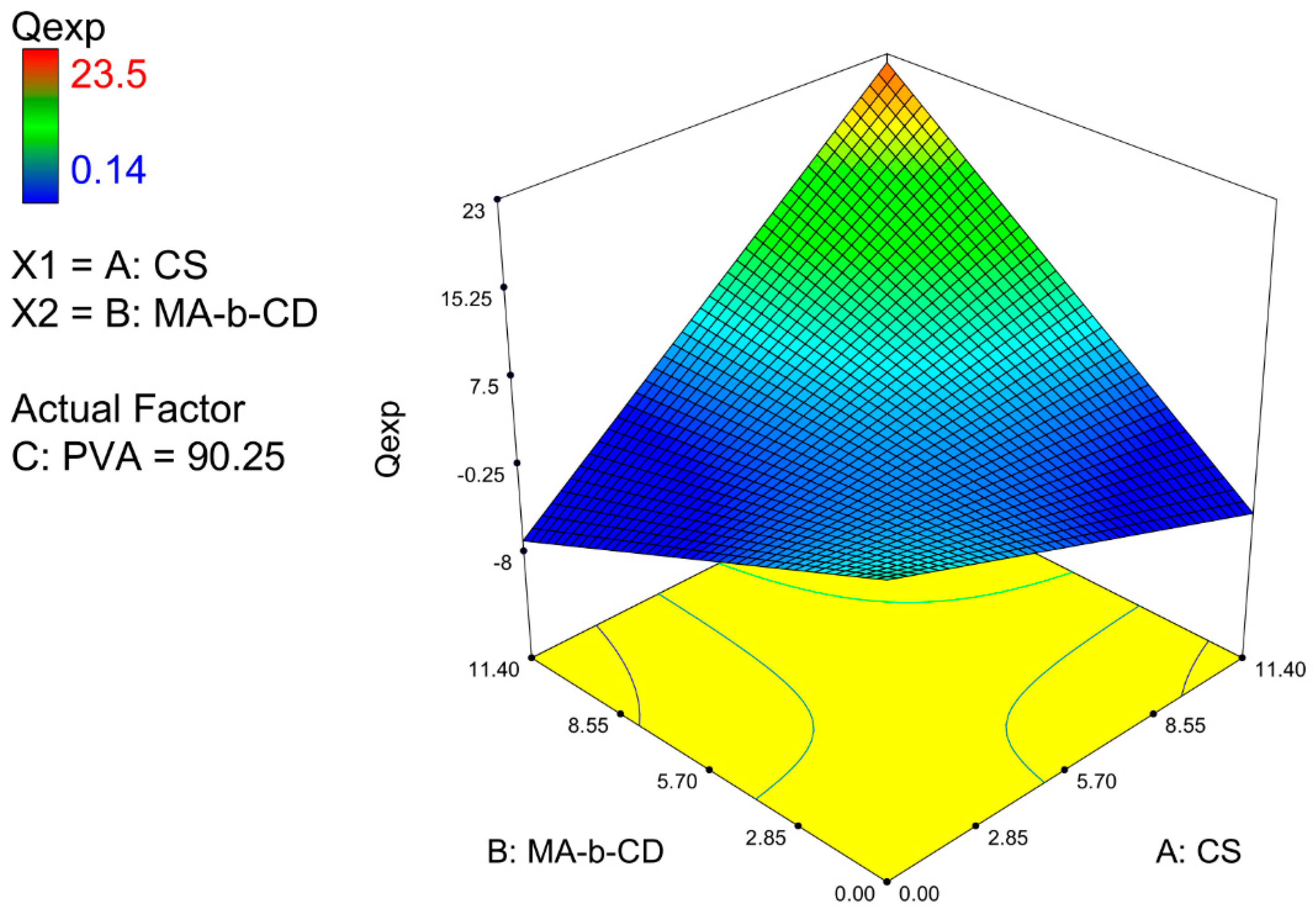
3.2. Experimental Planning and Statistical Analysis
3.3. Characterization of Hydrogels
3.3.1. Swelling Degree
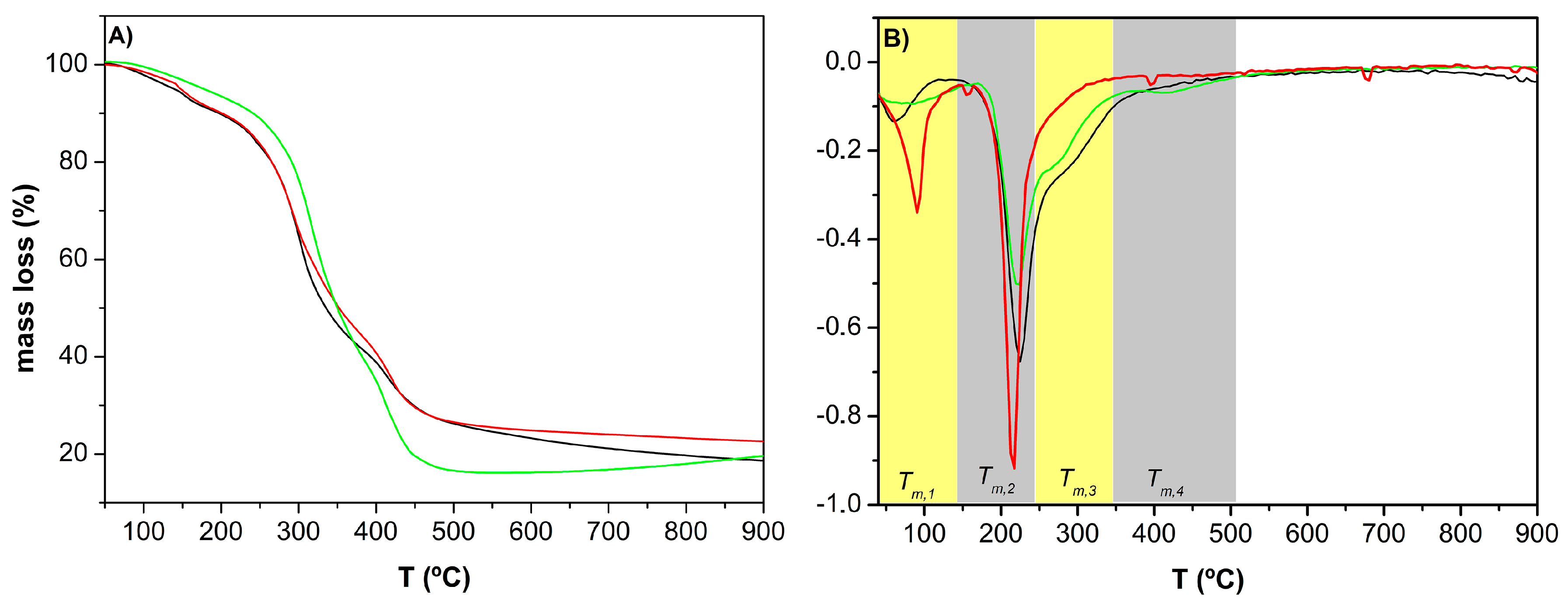
3.3.2. Thermogravimetric Analysis
3.3.3. Surface Morphology Studies
3.3.4. Rheological Properties
3.4. Sorption Studies
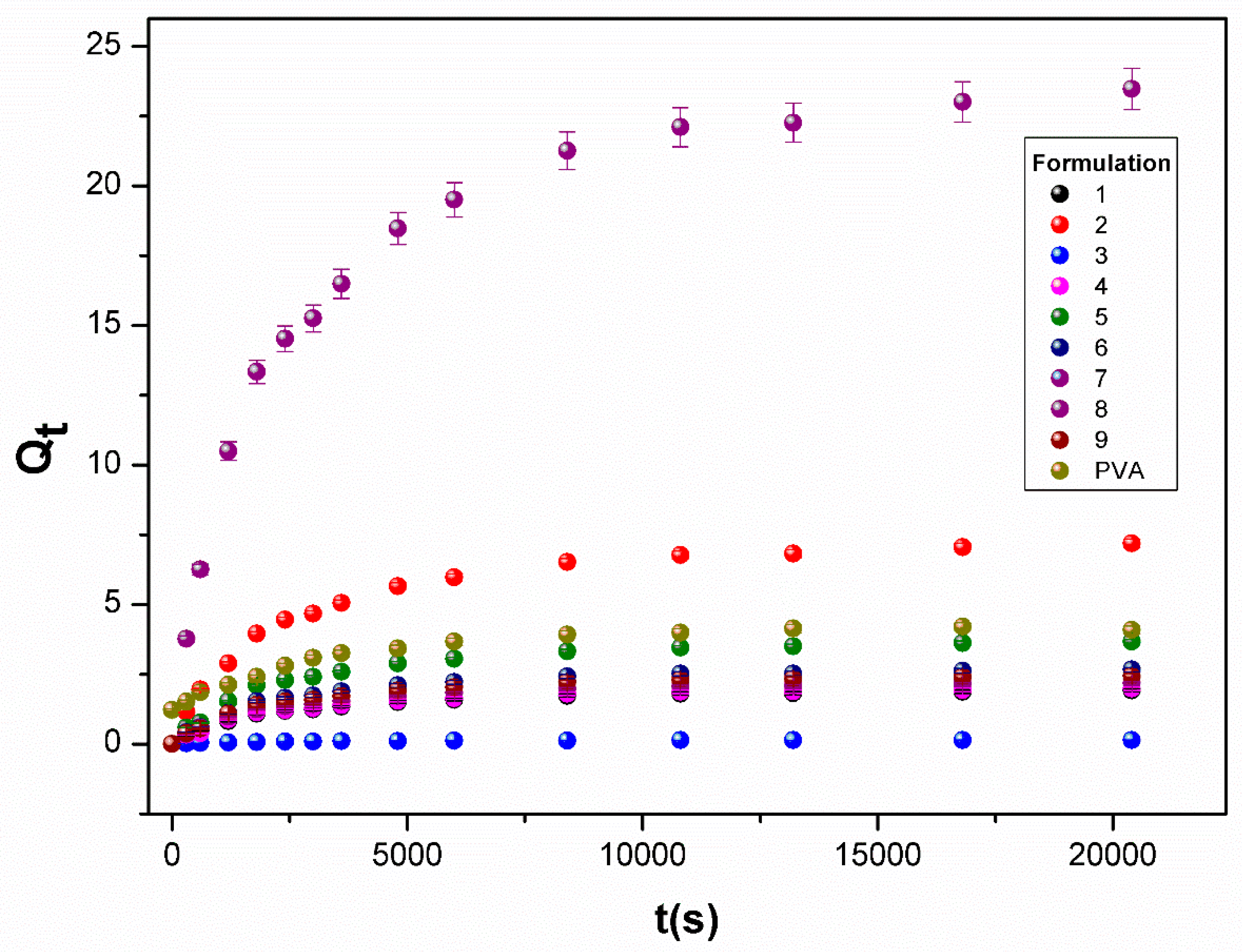
3.4.1. Sorption Kinetics
3.4.2. Sorption Isotherms
3.4.3. Sorption–Desorption Cycles
3.5. Performance of Hydrogels Towards Water Contaminated with A Real Petroleum Sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gu, Y.-G.; Li, H.-B.; Lu, H.-B. Polycyclic aromatic hydrocarbons (PAHs) in surface sediments from the largest deep plateau lake in China: Occurrence, sources and biological risk. Ecol. Eng. 2017, 101, 179–184. [Google Scholar] [CrossRef]
- Szczurek, A.; Maziejuk, M.; Maciejewska, M.; Pietrucha, T.; Sikora, T. BTX compounds recognition in humid air using differential ion mobility spectrometry combined with a classifier. Sens. Actuators B Chem. 2017, 240, 1237–1244. [Google Scholar] [CrossRef]
- Lamichhane, S.; Krishna, K.C.B.; Sarukkalige, R. Polycyclic aromatic hydrocarbons (PAHs) removal by sorption: A review. Chemosphere 2016, 148, 336–353. [Google Scholar] [CrossRef]
- Gou, Y.; Zhao, Q.; Yang, S.; Wang, H.; Qiao, P.; Song, Y.; Cheng, Y.; Li, P. Removal of polycyclic aromatic hydrocarbons (PAHs) and the response of indigenous bacteria in highly contaminated aged soil after persulfate oxidation. Ecotoxicol. Environ. Saf. 2020, 190, 110092. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, C.; Ding, C.; Liu, G.; Zhang, G. Distribution Pattern, Emission Characteristics and Environmental Impact of Polycyclic Aromatic Hydrocarbons (PAHs) in Download Ash and Dust from Iron and Steel Enterprise. Molecules 2019, 24, 3646. [Google Scholar] [CrossRef]
- Akinsanya, B.; Isibor, P.O.; Pentho, K.M.; Mulikat, K.; Saliu, J.K. Parasite prevalence and bioaccumulation of polycyclic aromatic hydrocarbons as stressors in the silver catfish, chrysichthys nigrodigitatus (Siluriformes: Claroteidae). Sci. Afr. 2020, 7, e00225. [Google Scholar] [CrossRef]
- Louis, C.; Liu, Y.; Tassel, P.; Perret, P.; Chaumond, A.; André, M. PAH, BTEX, carbonyl compound, black-carbon, NO2 and ultrafine particle dynamometer bench emissions for Euro 4 and Euro 5 diesel and gasoline passenger cars. Atmos. Environ. 2016, 141, 80–95. [Google Scholar] [CrossRef]
- Filho, C.M.C.; Matias, T.; Durães, L.; Valente, A.J.M. Efficient simultaneous removal of petroleum hydrocarbon pollutants by a hydrophobic silica aerogel-like material. Colloids Surf. A Phys. Eng. Asp. 2017, 520, 550–560. [Google Scholar] [CrossRef]
- Kadri, T.; Rouissi, T.; Kaur Brar, S.; Cledon, M.; Sarma, S.; Verma, M. Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by fungal enzymes: A review. J. Environ. Sci. 2017, 51, 52–74. [Google Scholar] [CrossRef]
- Müller, J.B.; Ramos, D.T.; Larose, C.; Fernandes, M.; Lazzarin, H.S.C.; Vogel, T.M.; Corseuil, H.X. Combined iron and sulfate reduction biostimulation as a novel approach to enhance BTEX and PAH source-zone biodegradation in biodiesel blend-contaminated groundwater. J. Hazard. Mater. 2017, 326, 229–236. [Google Scholar] [CrossRef]
- Rengarajan, T.; Rajendran, P.; Nandakumar, N.; Lokeshkumar, B.; Rajendran, P.; Nishigaki, I. Exposure to polycyclic aromatic hydrocarbons with special focus on cancer. Asian Pac. J. Trop. Biomed. 2015, 5, 182–189. [Google Scholar] [CrossRef]
- Costa, A.S.; Romão, L.P.C.; Araújo, B.R.; Lucas, S.C.O.; Maciel, S.T.A.; Wisniewski, A., Jr.; Alexandre, M.R. Environmental strategies to remove volatile aromatic fractions (BTEX) from petroleum industry wastewater using biomass. Bioresour. Technol. 2012, 105, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Jesus, A.M.D.; Romão, L.P.C.; Araújo, B.R.; Costa, A.S.; Marques, J.J. Use of humin as an alternative material for adsorption/desorption of reactive dyes. Desalination 2011, 274, 13–21. [Google Scholar] [CrossRef]
- Khan, J.A.; He, X.; Shah, N.S.; Khan, H.M.; Hapeshi, E.; Fatta-Kassinos, D.; Dionysiou, D.D. Kinetic and mechanism investigation on the photochemical degradation of atrazine with activated H2O2, S2O82− and HSO5−. Chem. Eng. J. 2014, 252, 393–403. [Google Scholar] [CrossRef]
- Shah, N.S.; Khan, J.A.; Sayed, M.; Khan, Z.U.H.; Rizwan, A.D.; Muhammad, N.; Boczkaj, G.; Murtaza, B.; Imran, M.; Khan, H.M.; et al. Solar light driven degradation of norfloxacin using as-synthesized Bi3+ and Fe2+ co-doped ZnO with the addition of HSO5−: Toxicities and degradation pathways investigation. Chem. Eng. J. 2018, 351, 841–855. [Google Scholar] [CrossRef]
- Mateen, F.; Javed, I.; Rafique, U.; Tabassum, N.; Sarfraz, M.; Safi, S.Z.; Yusoff, I.; Ashraf, M.A. New method for the adsorption of organic pollutants using natural zeolite incinerator ash (ZIA) and its application as an environmentally friendly and cost-effective adsorbent. Desalin. Water Treat. 2016, 57, 6230–6238. [Google Scholar] [CrossRef]
- Mironyuk, I.; Tatarchuk, T.; Naushad, M.; Vasylyeva, H.; Mykytyn, I. Highly efficient adsorption of strontium ions by carbonated mesoporous TiO2. J. Mol. Liq. 2019, 285, 742–753. [Google Scholar] [CrossRef]
- Zhu, Y.; Fan, W.; Zhou, T.; Li, X. Removal of chelated heavy metals from aqueous solution: A review of current methods and mechanisms. Sci. Total Environ. 2019, 678, 253–266. [Google Scholar] [CrossRef]
- Cunha, G.d.C.; Romão, L.P.C.; Santos, M.C.; Araújo, B.R.; Navickiene, S.; de Pádua, V.L. Adsorption of trihalomethanes by humin: Batch and fixed bed column studies. Bioresour. Technol. 2010, 101, 3345–3354. [Google Scholar] [CrossRef]
- Orm, N.B.; Trieu, Q.; Daniele, S. TiO2-Based Hybrid Nanocomposites Modified by Phosphonate Molecules as Selective PAH Adsorbents. Molecules 2018, 23, 3046. [Google Scholar] [CrossRef]
- Tuncaboylu, D.C.; Abdurrahmanoglu, S.; Gazioglu, I. Rheological characterization of starch gels: A biomass based sorbent for removal of polycyclic aromatic hydrocarbons (PAHs). J. Hazard. Mater. 2019, 371, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yao, R.; Guo, J.; He, J.; Meng, G.; Wu, F. Modulation of osteogenic and haemostatic activities by tuning cationicity of genipin-crosslinked chitosan hydrogels. Colloids Surf. B Biointerfaces 2018, 166, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.; Decker, E.A.; McClements, D.J. Influence of Droplet Characteristics on the Formation of Oil-in-Water Emulsions Stabilized by Surfactant−Chitosan Layers. Langmuir 2005, 21, 6228–6234. [Google Scholar] [CrossRef] [PubMed]
- Barros, F.C.D.F.; Vasconcellos, L.C.G.; Carvalho, T.V.; Nascimento, R.F. do Removal of Petroleum Spill in Water by Chitin and Chitosan. Orbital Electron. J. Chem. Orbital 2014, 6, 70–74. [Google Scholar]
- Patachia, S.; Valente, A.J.M.; Papancea, A.; Lobo, V.M.M. Poly(Vinil Alcohol) [PVA]- Based Polymer Membranes; Nova Science Publishers Incorporated: Hauppauge, NY, USA, 2009. [Google Scholar]
- Papancea, A.; Valente, A.J.M.; Patachia, S.; Miguel, M.G.; Lindman, B. PVA-DNA cryogel membranes: Characterization, swelling, and transport studies. Langmuir 2008, 24, 273–279. [Google Scholar] [CrossRef]
- Baptista, J.G.C.; Rodrigues, S.P.J.; Matsushita, A.F.Y.; Vitorino, C.; Maria, T.M.R.; Burrows, H.D.; Pais, A.A.C.C.; Valente, A.J.M. Does poly(vinyl alcohol) act as an amphiphilic polymer? An interaction study with simvastatin. J. Mol. Liq. 2016, 222, 287–294. [Google Scholar] [CrossRef]
- Yan, X.; Li, J.; Yang, R.; Li, Y.; Zhang, X.; Chen, J.; Rhodamine, A.-T.; Photoelectrochemical, B. A new photoelectrochemical aptasensor for prion assay based on cyclodextrin and Rhodamine B. Sens. Actuators B 2018, 255, 2187–2193. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef]
- Rekharsky, M.V.; Inoue, Y. Complexation Thermodynamics of Cyclodextrins. Chem. Rev. 1998, 98, 1875–1918. [Google Scholar] [CrossRef] [PubMed]
- Mauri-Aucejo, A.R.; Ponce-Català, P.; Belenguer-Sapiña, C.; Amorós, P. Determination of phenolic compounds in air by using cyclodextrin-silica hybrid microporous composite samplers. Talanta 2015, 134, 560–567. [Google Scholar] [CrossRef]
- Benvenuta-Tapia, J.J.; Vivaldo-Lima, E.; Tenorio-López, J.A.; de los Ángeles Vargas-Hernández, M.; Vázquez-Torres, H. Kinetic analysis of the RAFT copolymerization of styrene and maleic anhydride by differential scanning calorimetry. Acta 2018, 667, 93–101. [Google Scholar] [CrossRef]
- Filho, C.M.C.; Bueno, P.V.A.; Matsushita, A.F.Y.; Rubira, A.F.; Muniz, E.C.; Durães, L.; Murtinho, D.M.B.; Valente, A.J.M. Synthesis, characterization and sorption studies of aromatic compounds by hydrogels of chitosan blended with β-cyclodextrin- and PVA-functionalized pectin. RSC Adv. 2018, 8, 14609–14622. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and Morphology of Freeze/Thawed PVA Hydrogels. Macromolecules 2000, 33, 2472–2479. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and Applications of Poly ( vinyl alcohol ) Hydrogels Produced by Conventional Crosslinking or by Freezing/Thawing Methods. Adv. Polym. Sci. 2000, 153, 37–65. [Google Scholar]
- Jiang, Y.; Du, L.; Lu, F.; Li, Z.; Yang, J.; Bie, S.; Zhang, J. Synthesis and properties of functionalized β-cyclodextrin copolymer and its metal complexes. Polym. Bull. 2006, 57, 481–489. [Google Scholar] [CrossRef]
- He, Y.; Xu, Z.; Wu, F.; Luo, Z.; Chen, C. Synthesis and characterization of a novel amphiphilic copolymer containing β-cyclodextrin. Colloid Polym. Sci. 2014, 292, 1725–1733. [Google Scholar] [CrossRef]
- Aouada, F.A.; Muniz, E.C.; Vaz, C.M.P.; Mattoso, L.H.C. Correlação entre parâmetros da cinética de intumescimento com características estruturais e hidrofílicas de hidrogéis de poliacrilamida e metilcelulose. Química Nova 2009, 32, 1482–1490. [Google Scholar] [CrossRef]
- Spinks, G.M.; Lee, C.K.; Wallace, G.G.; Kim, S.I.; Kim, S.J. Swelling Behavior of Chitosan Hydrogels in Ionic Liquid−Water Binary Systems. Langmuir 2006, 22, 9375–9379. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.M.M.; Valente, A.J.M.; Polishchuk, A.Y.; Geuskens, G. Transport of non-associated electrolytes in acrylamide hydrogels. J. Mol. Liq. 2001, 94, 179–192. [Google Scholar] [CrossRef]
- Caruso, M.S.F.; Alaburda, J. Hidrocarbonetos policíclicos aromáticos-benzo(a)pireno: Uma revisão. Rev. Inst. Adolfo Lutz 2008, 67, 1–27. [Google Scholar]
- Hirose, S.; Rachman Putra, E.G.; Astrini, N.; Anah, L.; Haryono, A. The International Conference on Innovation in Polymer Science and TechnologyCrosslinking Parameter on the Preparation of Cellulose Based Hydrogel with Divynilsulfone. Procedia Chem. 2012, 4, 275–281. [Google Scholar]
- Zhao, Y.; Su, H.; Fang, L.; Tan, T. Superabsorbent hydrogels from poly(aspartic acid) with salt-, temperature- and pH-responsiveness properties. Polymer 2005, 46, 5368–5376. [Google Scholar] [CrossRef]
- Filho, C.M.C.; Neto, M.N.L.; Teixeira, R.S.; Pais, A.A.C.C.; Valente, A.J.M. Development and optimization of an HPLC–DAD method for quantification of six petroleum hydrocarbon compounds in aqueous samples. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 837–846. [Google Scholar] [CrossRef]
- Carvalho, R.A.; Correia, H.A.; Valente, A.J.M.; Söderman, O.; Nilsson, M. The effect of the head-group spacer length of 12-s-12 gemini surfactants in the host–guest association with β-cyclodextrin. J. Colloid Interface Sci. 2011, 354, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Vicente, A.I.; Pereira, S.G.; Nunes, T.G.; Ribeiro, M.R. 1H-NMR study of maleic anhydride modified ethylene-diene copolymers. J. Polym. Res. 2011, 18, 527–532. [Google Scholar] [CrossRef]
- Izunobi, J.U.; Higginbotham, C.L. Polymer Molecular Weight Analysis by 1 H NMR Spectroscopy. J. Chem. Educ. 2011, 88, 1098–1104. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J.; Bryce, D.L. Spectrometric Identification of Organic Compounds; John Wiley & Sons: Hoboken, NJ, USA, 2014; ISBN 0470616377. [Google Scholar]
- Košťálová, Z.; Hromádková, Z.; Ebringerová, A.; Polovka, M.; Michaelsen, T.E.; Paulsen, B.S. Polysaccharides from the Styrian oil-pumpkin with antioxidant and complement-fixing activity. Ind. Crop. Prod. 2013, 41, 127–133. [Google Scholar] [CrossRef]
- Neufeld, L.; Bianco-Peled, H. Pectin–chitosan physical hydrogels as potential drug delivery vehicles. Int. J. Biol. Macromol. 2017, 101, 852–861. [Google Scholar] [CrossRef]
- De Roover, B.; Sclavons, M.; Carlier, V.; Devaux, J.; Legras, R.; Momtaz, A. Molecular characterization of maleic anhydride-functionalized polypropylene. J. Polym. Sci. Part A Polym. Chem. 1995, 33, 829–842. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, H.; Li, H.; Wu, M.; Zhang, S.; Wang, J. Studies on novel functional β-cyclodextrin and its metal complexes. J. Mol. Struct. 2004, 702, 33–37. [Google Scholar] [CrossRef]
- Meng, Q.; Peng, B.; Shen, C. Synthesis of F127/PAA hydrogels for removal of heavy metal ions from organic wastewater. Colloids Surf. B Biointerfaces 2018, 167, 176–182. [Google Scholar] [CrossRef] [PubMed]
- MacHín, R.; Isasi, J.R.; Vélaz, I. β-Cyclodextrin hydrogels as potential drug delivery systems. Carbohydr. Polym. 2012, 87, 2024–2030. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, N.; Wang, D.; He, Y.; Chen, L.; Zhao, Y. Poly (MAH-β-cyclodextrin-co-NIPAAm) hydrogels with drug hosting and thermo/pH-sensitive for controlled drug release. Polym. Degrad. Stab. 2018, 147, 123–131. [Google Scholar] [CrossRef]
- Knaapila, M.; Stewart, B.; Costa, T.; Rogers, S.E.; Pragana, J.; Fonseca, S.M.; Valente, A.J.M.; Ramos, M.L.; Murtinho, D.; Pereira, J.C.; et al. Incorporation of a Cationic Conjugated Polyelectrolyte CPE within an Aqueous Poly(vinyl alcohol) Sol. Macromolecules 2016, 49, 9119–9131. [Google Scholar] [CrossRef]
- Quintana, J.R.; Valderruten, N.E.; Katime, I. Synthesis and Swelling Kinetics of Poly(Dimethylaminoethyl acrylate methyl chloride quaternary-co-itaconic acid) Hydrogels. Langmuir 1999, 15, 4728–4730. [Google Scholar] [CrossRef]
- Kimura, K.; Waki, H. Minimization of Akaike’s information criterion in linear regression analysis via mixed integer nonlinear program. Optim. Methods Softw. 2018, 33, 633–649. [Google Scholar] [CrossRef]
- Schmidt, J.J.; Jeong, J.H.; Chan, V.; Cha, C.; Baek, K.; Lai, M.-H.; Bashir, R.; Kong, H. Tailoring the Dependency between Rigidity and Water Uptake of a Microfabricated Hydrogel with the Conformational Rigidity of a Polymer Cross-Linker. Biomacromolecules 2013, 14, 1361–1369. [Google Scholar] [CrossRef]
- Corazzari, I.; Nisticò, R.; Turci, F.; Faga, M.G.; Franzoso, F.; Tabasso, S.; Magnacca, G. Advanced physico-chemical characterization of chitosan by means of TGA coupled on-line with FTIR and GCMS: Thermal degradation and water adsorption capacity. Polym. Degrad. Stab. 2015, 112, 1–9. [Google Scholar] [CrossRef]
- Yang, L.; Bi, L.; Lei, Z.; Miao, Y.; Li, B.; Liu, T.; Wu, W. Preparation of Amidoxime-Functionalized β-Cyclodextrin-Graft-(Maleic Anhydride-co-Acrylonitrule) Copolymer and Evaluation of the Adsorption and Regeneration Properties of Uranium. Polymer 2018, 10, 236. [Google Scholar] [CrossRef]
- Gerola, A.P.; Silva, D.C.; Matsushita, A.F.Y.; Borges, O.; Rubira, A.F.; Muniz, E.C.; Valente, A.J.M. The effect of methacrylation on the behavior of Gum Arabic as pH-responsive matrix for colon-specific drug delivery. Eur. Polym. J. 2016, 78, 326–339. [Google Scholar] [CrossRef]
- Gačanin, J.; Kovtun, A.; Fischer, S.; Schwager, V.; Quambusch, J.; Kuan, S.L.; Liu, W.; Boldt, F.; Li, C.; Yang, Z.; et al. Spatiotemporally Controlled Release of Rho-Inhibiting C3 Toxin from a Protein–DNA Hybrid Hydrogel for Targeted Inhibition of Osteoclast Formation and Activity. Adv. Healthc. Mater. 2017, 6, 1700392. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Zhao, Y.; Wang, W.; Zhang, T.; Chen, T.; Yi, H.; Rao, F.; Song, S. Removal of methylene blue from water with montmorillonite nanosheets/chitosan hydrogels as adsorbent. Appl. Surf. Sci. 2018, 448, 203–211. [Google Scholar] [CrossRef]
- Ho, Y.-S. Pseudo-Isotherms Using a Second Order Kinetic Expression Constant. Adsorption 2004, 10, 151–158. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Yener, J.; Kopac, T.; Dogu, G.; Dogu, T. Dynamic analysis of sorption of Methylene Blue dye on granular and powdered activated carbon. Chem. Eng. J. 2008, 144, 400–406. [Google Scholar] [CrossRef]
- Do Nascimento, R.F.; de Lima, A.C.A.; Vidal, C.B.; de Quadros Melo, D.; Raulino, G.S.C. Adsorção: Aspectos Teóricos e Aplicações Ambientais; Biblioteca Electrónica de Ciencia y Tecnología: Fortaleza, Brazill, 2014; p. 74. [Google Scholar]
- Sinha, V.; Chakma, S. Advances in the preparation of hydrogel for wastewater treatment: A concise review. J. Environ. Chem. Eng. 2019, 7, 103295. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Arefieva, O.D.; Zemnukhova, L.A.; Morgun, N.P.; Rybin, V.G.; Tsvetnov, M.A.; Kovshun, A.A.; Panasenko, A.E. Removal of (2,4-Dichlorophenoxy)acetic Acid from Aqueous Solutions Using Low-cost Sorbents. Air Soil Water Res. 2015, 8, ASWR.S31623. [Google Scholar] [CrossRef]
- De Naylor, T.V. Permeation Properties; Pergamon Press: Oxford, UK, 1989; Volume 2. [Google Scholar]
- Mohammadi, L.; Bazrafshan, E.; Noroozifar, M.; Ansari-Moghaddam, A.; Barahuie, F.; Balarak, D. Adsorptive Removal of Benzene and Toluene from Aqueous Environments by Cupric Oxide Nanoparticles: Kinetics and Isotherm Studies. J. Chem. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Anjum, H.; Johari, K.; Gnanasundaram, N.; Appusamy, A.; Thanabalan, M. Impact of surface modification on adsorptive removal of BTX onto activated carbon. J. Mol. Liq. 2019, 280, 238–251. [Google Scholar] [CrossRef]
- Bandura, L.; Kołodyńska, D.; Franus, W. Adsorption of BTX from aqueous solutions by Na-P1 zeolite obtained from fly ash. Process Saf. Environ. Prot. 2017, 109, 214–223. [Google Scholar] [CrossRef]
- Alamo-Nole, L.A.; Perales-Perez, O.; Roman-Velazquez, F.R. Sorption study of toluene and xylene in aqueous solutions by recycled tires crumb rubber. J. Hazard. Mater. 2011, 185, 107–111. [Google Scholar] [CrossRef]
- Li, Y.; Chen, B.; Zhu, L. Enhanced sorption of polycyclic aromatic hydrocarbons from aqueous solution by modified pine bark. Bioresour. Technol. 2010, 101, 7307–7313. [Google Scholar] [CrossRef]
- Müller, S.; Totsche, K.U.; Kögel-Knabner, I. Sorption of polycyclic aromatic hydrocarbons to mineral surfaces. Eur. J. Soil Sci. 2007, 58, 918–931. [Google Scholar] [CrossRef]
- Girardello, F.; Rovani, S.; Giovanela, M.; Fernandes, A.N. Removal of pyrene from aqueous solutions by adsorption onto Brazilian peat samples. Adsorpt. Sci. Technol. 2016, 34, 538–551. [Google Scholar] [CrossRef]
- Ray, A.; Selvakumar, A.; Tafuri, A. Removal of selected pollutants from aqueous media by hardwood mulch. J. Hazard. Mater. 2006, 136, 213–218. [Google Scholar] [CrossRef]
- Ake, C.L.; Wiles, M.C.; Huebner, H.J.; McDonald, T.J.; Cosgriff, D.; Richardson, M.B.; Donnelly, K.C.; Phillips, T.D. Porous organoclay composite for the sorption of polycyclic aromatic hydrocarbons and pentachlorophenol from groundwater. Chemosphere 2003, 51, 835–844. [Google Scholar] [CrossRef]
- Hale, S.E.; Elmquist, M.; Brändli, R.; Hartnik, T.; Jakob, L.; Henriksen, T.; Werner, D.; Cornelissen, G. Activated carbon amendment to sequester PAHs in contaminated soil: A lysimeter field trial. Chemosphere 2012, 87, 177–184. [Google Scholar] [CrossRef]
- Pérez-Gregorio, M.R.; García-Falcón, M.S.; Martínez-Carballo, E.; Simal-Gándara, J. Removal of polycyclic aromatic hydrocarbons from organic solvents by ashes wastes. J. Hazard. Mater. 2010, 178, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.A.S.; Garcia, A.C.F.S.; Santos, D.O.; Sarmento, V.H.V.; de Mesquita, M.E.; Romão, L.P.C. Applications of inorganic–organic mesoporous materials constructed by self-assembly processes for removal of benzo[k]fluoranthene and benzo[b]fluoranthene. J. Sol-Gel Sci. Technol. 2015, 75, 495–507. [Google Scholar] [CrossRef]
- TPHCWG Total Petroleum Hydrocarbon Criteria Working Group Series-Volume 2. Composition of Petroleum Mixtures; Potter, T.L., Simmons, K., Eds.; Amherst Scientific Publishers: Amherst, MA, USA, 1998. [Google Scholar]


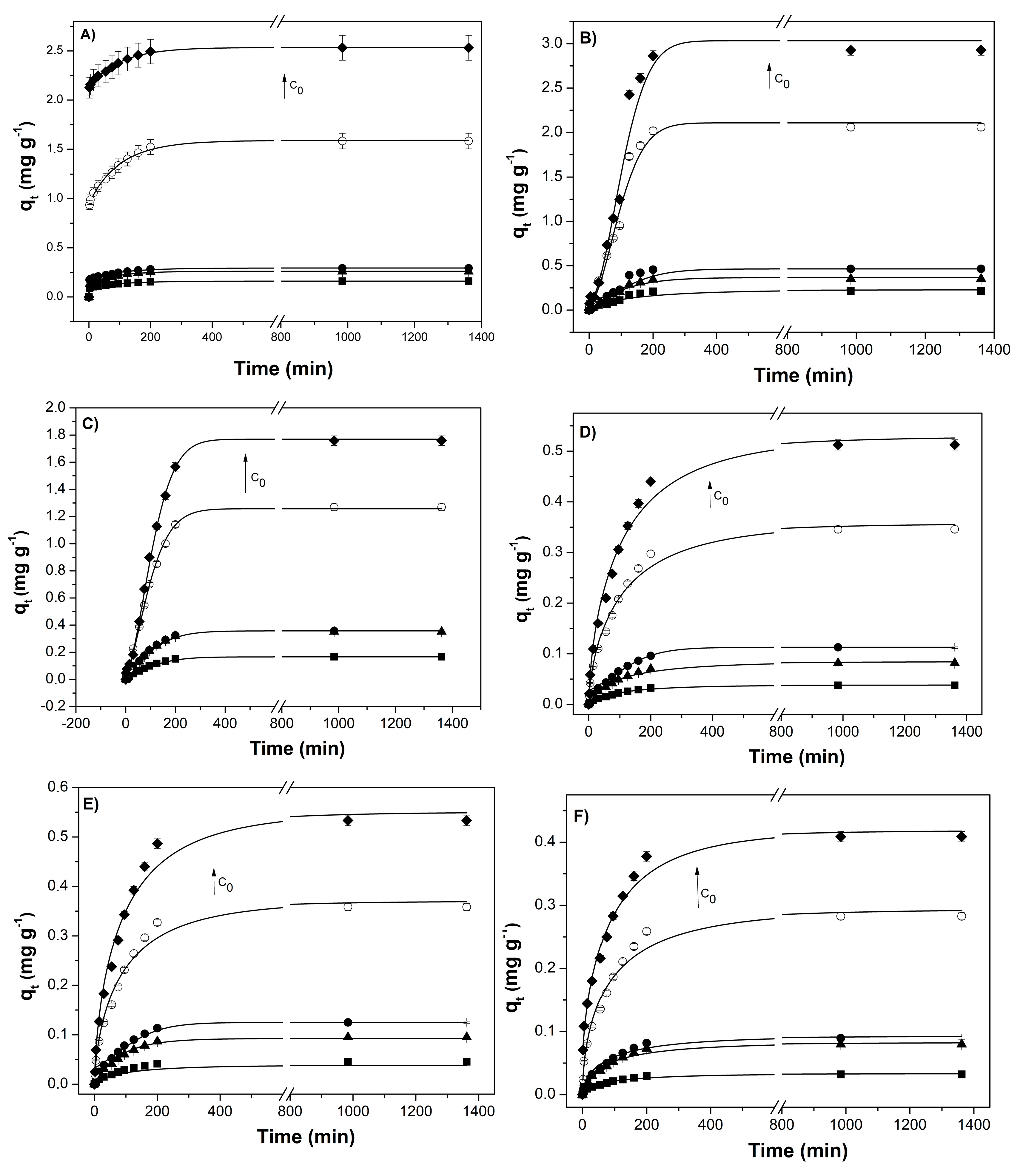

| C0 (mg L−1) | ||||||
|---|---|---|---|---|---|---|
| Benzene | Xylenes | Toluene | Pyrene | B(b)F | B(a)P | |
| IS * | ||||||
| I1K | 6.9 (±0.2) * | 8.1 (±0.4) | 8.4 (±0.3) | 2.2 (±0.1) | 2.4 (±0.1) | 2.2 (±0.1) |
| I2K | 9.9 (±0.3) | 14.4 (±0.4) | 10.2 (±0.3) | 4.0 (±0.1) | 4.3 (±0.1) | 4.7 (±0.1) |
| I3K | 12.7 (±0.4) | 15.9 (±0.5) | 12.5 (±0.4) | 6.3 (±0.1) | 6.2 (±0.1) | 5.9 (±0.1) |
| I4K | 32 (±1) | 33.4 (±0.9) | 32 (±1) | 10.1 (±0.3) | 9.31 (±0.36) | 9.9 (±0.2) |
| I5K | 48.1 (±1.4) | 50.1 (±1.5) | 48.0 (±1.2) | 15.1 (±0.5) | 13.9 (±0.4) | 13.0 (±0.3) |
| Equation (6) | Equation (7) | ||||||
|---|---|---|---|---|---|---|---|
| Form | Qe,exp | Qe,1 | k1,w (10−4s−1) | AIC | Qe,2 | k2,w (10−5 s−1) | AIC |
| 1 | 1.91 (±0.04) | 1.3 (±0.1) | 2.1 (±0.1) | 4.46 | 2.10 (±0.04) | 0.02 (±0.01) | 9.48 |
| 2 | 7.2 (±0.1) | 4.9 (±0.1) | 2.2 (±0.1) | 4.44 | 7.92 (±0.01) | 0.003 (±0.002) | 8.30 |
| 3 | 0.14 (±0.04) | 0.1 (±0.1) | 2.2 (±0.1) | 4.51 | 0.16 (±0.04) | 1.23 (±0.02) | 11.79 |
| 4 | 1.98 (±0.03) | 1.4 (±0.1) | 2.2 (±0.1) | 4.46 | 2.2 (±0.1) | 0.02 (±0.06) | 10.12 |
| 5 | 3.7 (±0.1) | 2.6 (±0.1) | 2.3 (±0.1) | 4.38 | 4.07 (±0.02) | 0.08 (±0.03) | 9.41 |
| 6 | 2.7 (±0.1) | 2.0 (±0.1) | 2.3 (±0.1) | 4.46 | 2.94 (±0.03) | 0.04 (±0.01) | 9.45 |
| 7 | 2.2 (±0.1) | 1.6 (±0.1) | 2.3 (±0.1) | 4.36 | 2.41 (±0.03) | 0.029 (±0.003) | 9.32 |
| 8 | 23.5 (±0.4) | 16.8 (±0.1) | 2.2 (±0.1) | 4.44 | 25.73 (±0.03) | 0.0003 (±0.0001) | 7.26 |
| 9 | 2.4 (±0.1) | 1.8 (±0.1) | 2.3 (±0.1) | 4.37 | 2.69 (±0.03) | 0.04 (±0.03) | 9.57 |
| PVA | 4.08 (±0.12) | 2.7 (±0.1) | 3.09 (±0.04) | 3.66 | 4.37 (±0.04) | 0.002 (± 0.002) | 9.91 |
| Freundlich | Henry | |||||||
|---|---|---|---|---|---|---|---|---|
| IS ** | C0 (mg L−1) | 1/nF | KF (mg (n−1)/n L1/n g−1) | R2 | AIC | KH (L g−1) | R2 | AIC |
| Benzene | 6.9–52.8 | 1.04 (±0.09) * | 0.07 (±0.02) | 0.9308 | 4.04 | 0.09 (±0.003) | 0.9924 | 3.39 |
| Xylenes | 13.1–55.1 | 1.006 (±0.004) | 0.026 (±0.004) | 0.9830 | 5.16 | 0.027 (±0.001) | 0.9858 | 3.76 |
| Toluene | 8.4–52.4 | 0.97 (±0.05) | 0.032 (±0.005) | 0.9731 | 3.63 | 0.028 (±0.001) | 0.9862 | 2.77 |
| B(b)F | 2.4–15.4 | 1.21 (±0.07) | 0.027 (±0.004) | 0.9594 | 3.56 | 0.038 (±0.002) | 0.9890 | 2.98 |
| Pyrene | 3.4–16.6 | 0.98 (±0.06) | 0.022 (±0.003) | 0.9638 | 3.47 | 0.021 (±0.001) | 0.9696 | 2.38 |
| B(a)P | 1.9–7.3 | 1.12 (±0.02) | 6.94 (±0.2) × 10−3 | 0.9984 | 3.49 | 0.023 (±0.001) | 0.9784 | 2.46 |
| Hydrocarbon | Adsorbent | Removal Efficiency (%) | |
|---|---|---|---|
| Benzene | cupric oxide nanoparticles | 98.7 | [75] |
| activated carbon | 98.89 | [76] | |
| zeolite | 35 | [77] | |
| Toluene | cupric oxide nanoparticles | 92.5 | [75] |
| activated carbon | 99.86 | [76] | |
| zeolite | 55 | [77] | |
| Xylenes | activated carbon | 99.99 | [76] |
| zeolite | 77 */99 ** | [77] | |
| crumb rubber | 81 | [78] | |
| Pyrene | modified pine bark | 55.28–93.53 | [79] |
| mineral surfaces | 25 | [80] | |
| Brazilian peat | 75.5–92.1 | [81] | |
| B(a)P | hardwood mulch | 92 | [82] |
| mineral surfaces | 82 | [80] | |
| Porous organoclay composite | 99 | [83] | |
| B(b)F | activated carbon | 72 | [84] |
| wood ashes | 98.6 | [85] | |
| mesoporous materials | 32 | [86] |
| C0 in the Used Fossil Fuel Before Dilution (wt-%)43 | C0 in the Spiked Solution (mg L−1) | Removal Efficiency (%) | Qe,exp (10−2 mg g−1) | |
|---|---|---|---|---|
| Benzene | 0.08 (±0.01) * | 4.1 (±0.1) | 0.37 (±0.01) | 0.4 (±0.1) |
| Xylenes | 1.32 (±0.04) | 11.2 (±0.4) | 0.28 (±0.01) | 0.95 (±0.02) |
| Toluene | 0.78 (±0.01) | 7.4 (±0.2) | 0.83 (±0.02) | 1.71(±0.03) |
| Pyrene | 0.012 (±0.001) | 1.3 (±0.1) | 4.3 (±0.1) | 0.48 (±0.01) |
| B(a)P | 5.0 (±0.2) × 10−3 | 0.49 (±0.02) | 9.8 (±0.2) | 5.8 (±0.1) |
| B(b)F | 4.0 (±0.1) × 10−4 | 0.31 (±0.03) | 11.02 (±0.22) | 0.3 (±0.1) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filho, C.M.C.; Bueno, P.V.A.; Matsushita, A.F.Y.; Vilsinski, B.H.; Rubira, A.F.; Muniz, E.C.; Murtinho, D.M.B.; Valente, A.J.M. Uncommon Sorption Mechanism of Aromatic Compounds onto Poly(Vinyl Alcohol)/Chitosan/Maleic Anhydride-β-Cyclodextrin Hydrogels. Polymers 2020, 12, 877. https://doi.org/10.3390/polym12040877
Filho CMC, Bueno PVA, Matsushita AFY, Vilsinski BH, Rubira AF, Muniz EC, Murtinho DMB, Valente AJM. Uncommon Sorption Mechanism of Aromatic Compounds onto Poly(Vinyl Alcohol)/Chitosan/Maleic Anhydride-β-Cyclodextrin Hydrogels. Polymers. 2020; 12(4):877. https://doi.org/10.3390/polym12040877
Chicago/Turabian StyleFilho, Cesar M. C., Pedro V. A. Bueno, Alan F. Y. Matsushita, Bruno H. Vilsinski, Adley F. Rubira, Edvani C. Muniz, Dina M. B. Murtinho, and Artur J. M. Valente. 2020. "Uncommon Sorption Mechanism of Aromatic Compounds onto Poly(Vinyl Alcohol)/Chitosan/Maleic Anhydride-β-Cyclodextrin Hydrogels" Polymers 12, no. 4: 877. https://doi.org/10.3390/polym12040877
APA StyleFilho, C. M. C., Bueno, P. V. A., Matsushita, A. F. Y., Vilsinski, B. H., Rubira, A. F., Muniz, E. C., Murtinho, D. M. B., & Valente, A. J. M. (2020). Uncommon Sorption Mechanism of Aromatic Compounds onto Poly(Vinyl Alcohol)/Chitosan/Maleic Anhydride-β-Cyclodextrin Hydrogels. Polymers, 12(4), 877. https://doi.org/10.3390/polym12040877








