Phase Behavior and Phase Diagram of Polystyrene-b-Poly(Perfluorooctylethyl Acrylates)
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
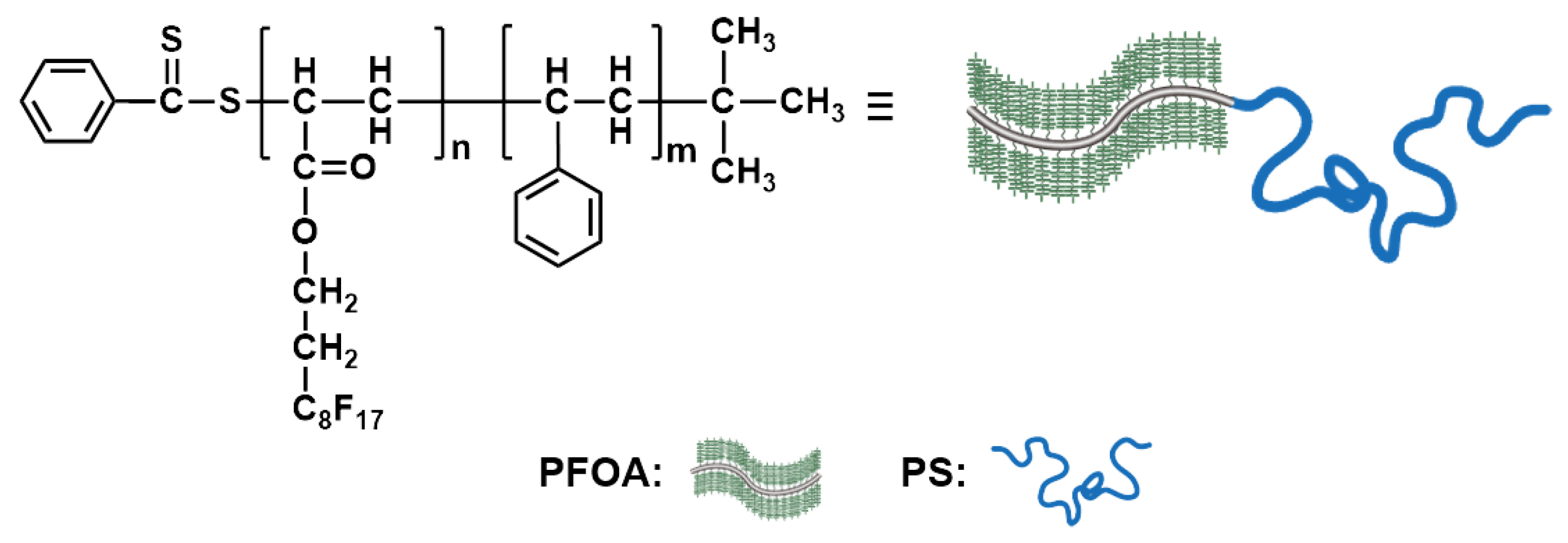
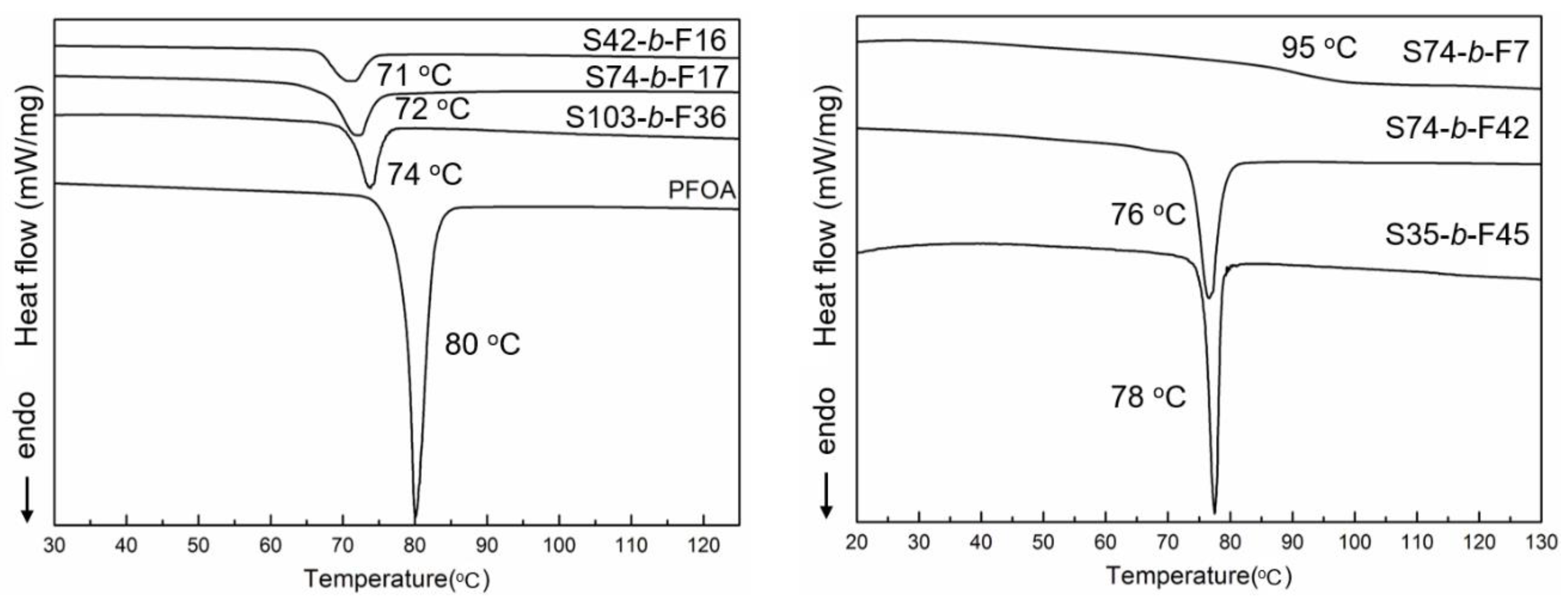
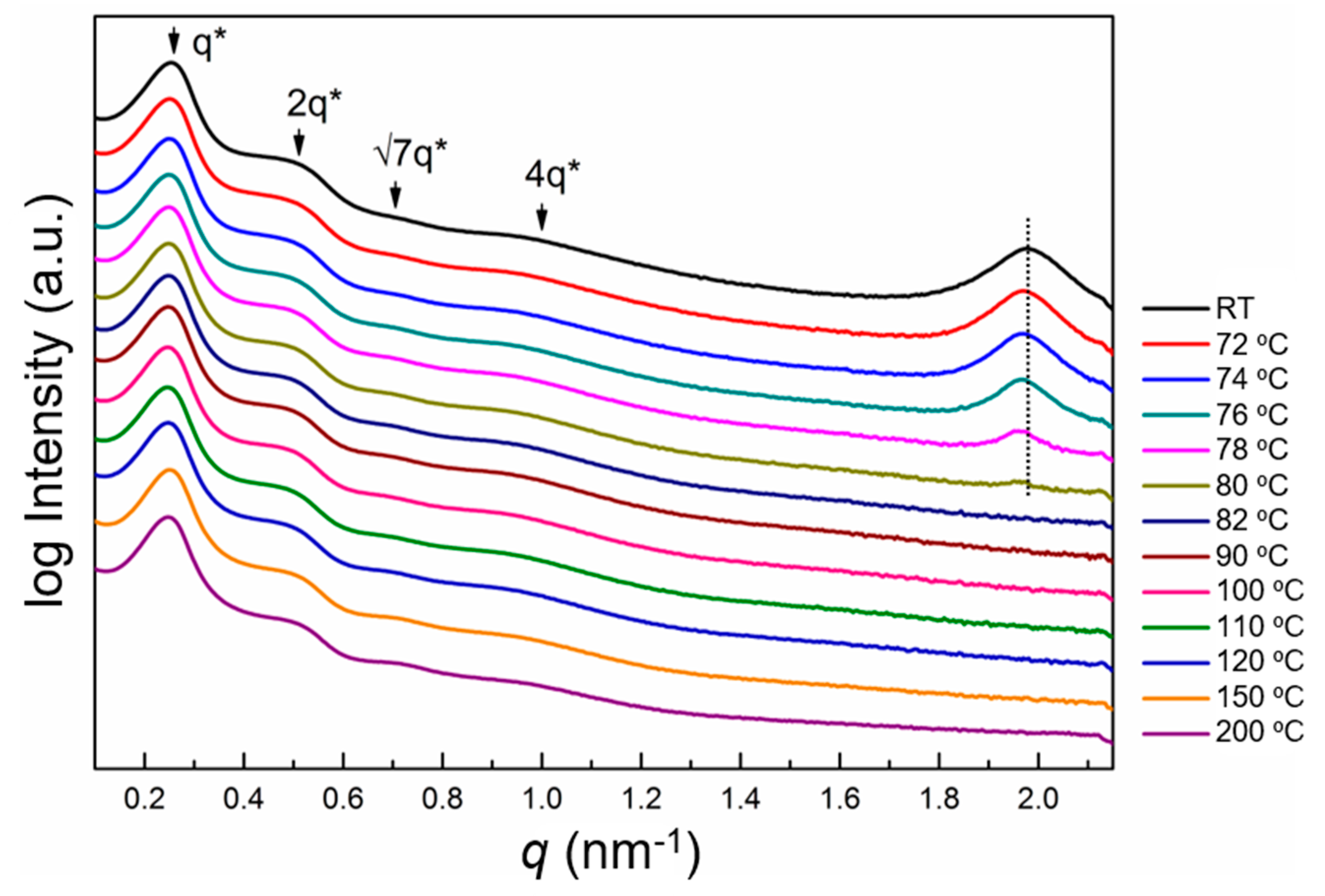
3.1. General Design, Synthesis, and Phase Behavior
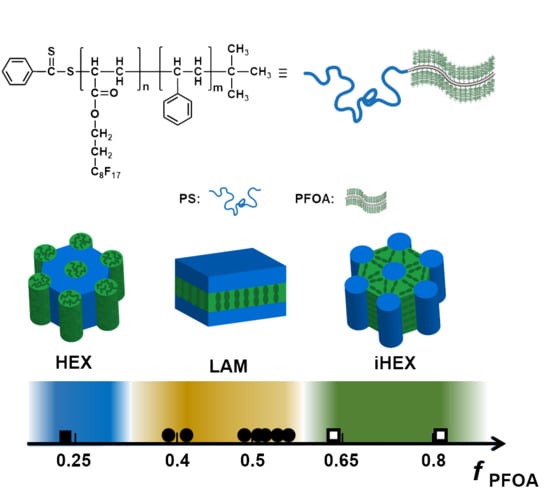
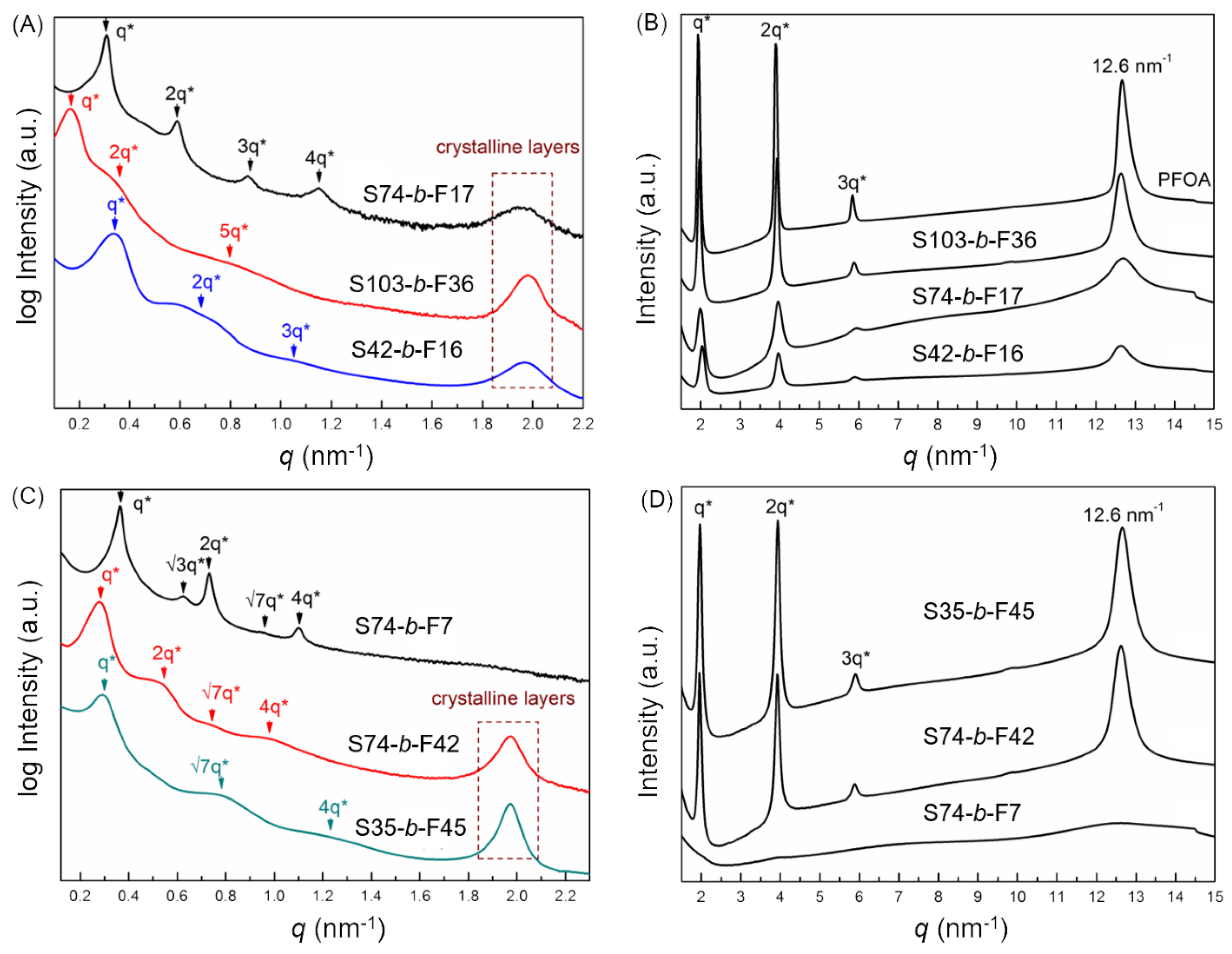
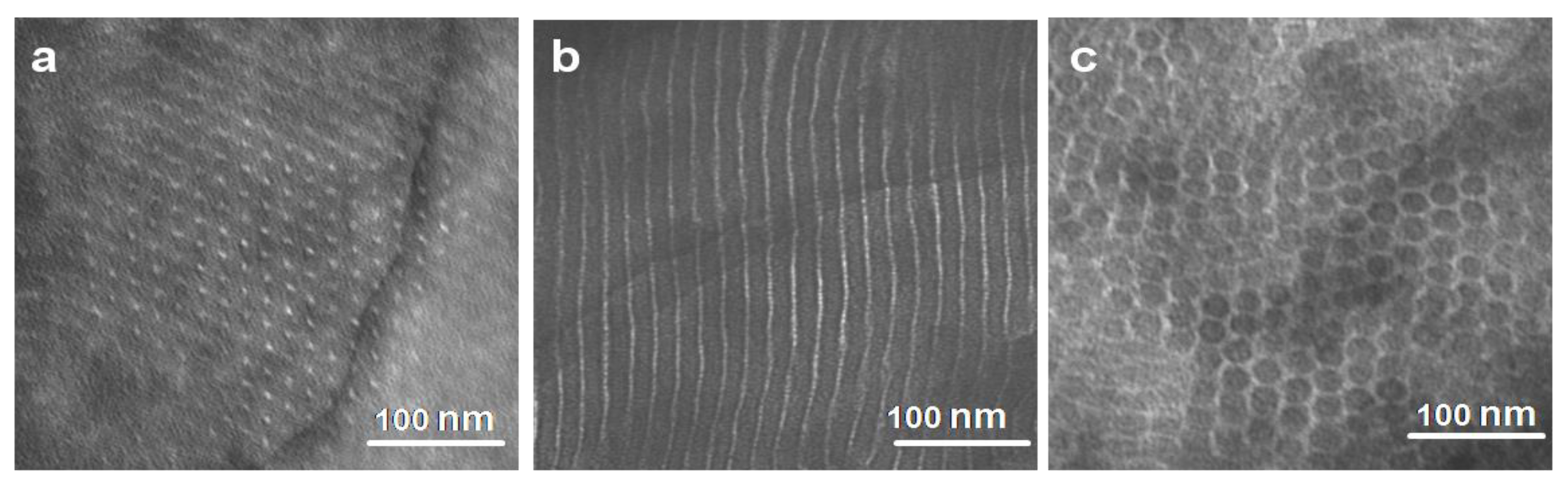
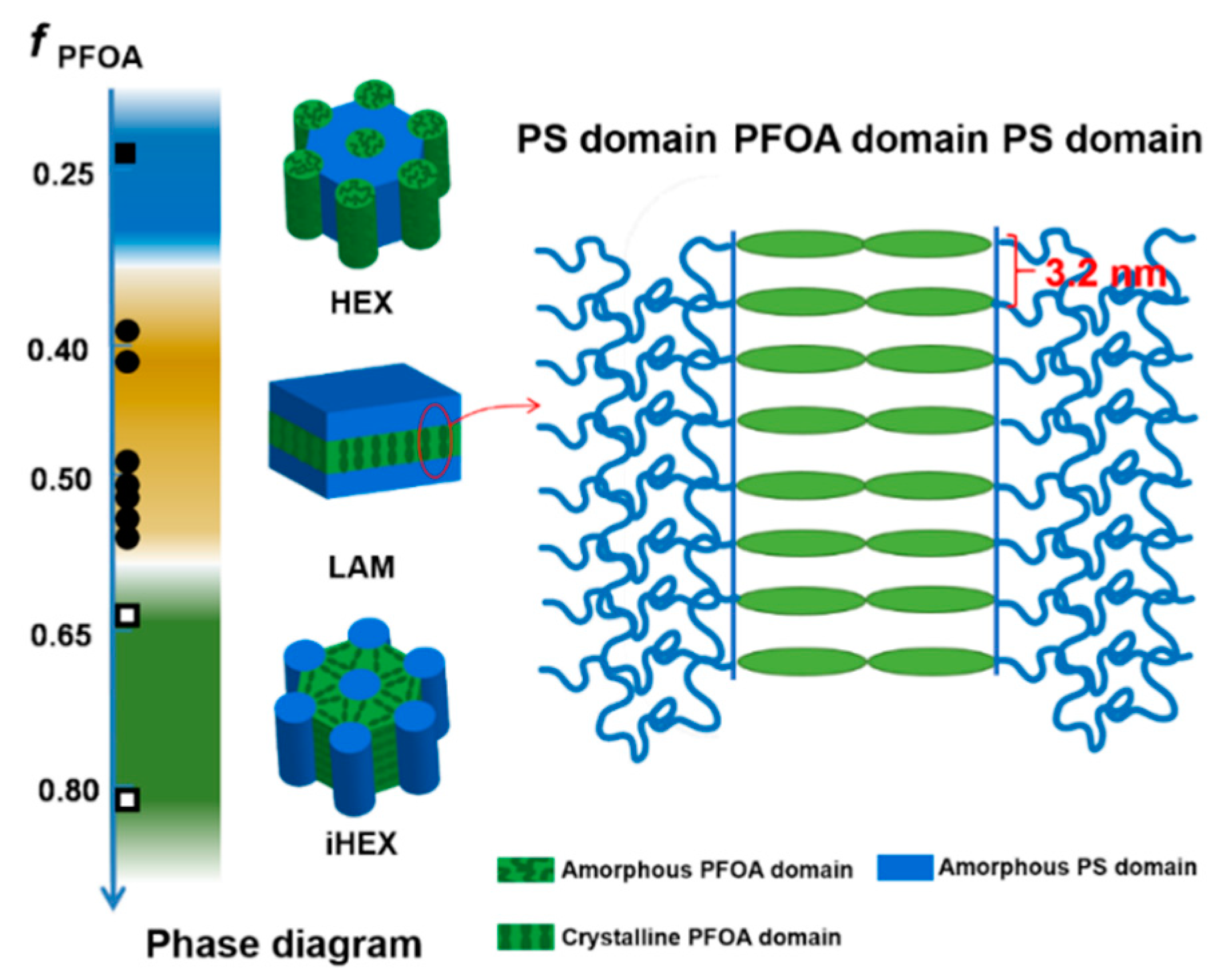
3.2. Self-Assembled Morphologies and Phase Diagram
3.3. Hierarchical Structure and Chain Conformation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cametti, M.; Crousse, B.; Metrangolo, P.; Milani, R.; Resnati, G. The fluorous effect in biomolecular applications. Chem. Soc. Rev. 2012, 41, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, X.; Jin, J.; Wu, Y.; Liang, Y. Difunctionalization of Alkynes: Synthesis of Novel Fluoropolymer Materials. Chin. J. Chem. 2018, 36, 223–226. [Google Scholar] [CrossRef]
- Krukovsky, S.P.; Yarosh, A.A.; Glazkov, A.A.; Batizat, D.V.; Redina, T.N. New fluorine-containing oligomers and polymers. J. Fluor. Chem. 1999, 96, 31–33. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Correia, D.M.; Ribeiro, C.; Fernandes, M.M.; Lanceros-Mendez, S. Fluorinated Polymers as Smart Materials for Advanced Biomedical Applications. Polymers 2018, 10, 161. [Google Scholar] [CrossRef] [PubMed]
- Gladysz, J.A.; Jurisch, M. Structural, Physical, and Chemical Properties of Fluorous Compounds. In Fluorous Chemistry. Topics in Current Chemistry; Horváth, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 308, pp. 1–23. [Google Scholar]
- Imae, T. Fluorinated polymers. Curr. Opin. Colloid Interface Sci. 2003, 8, 307–314. [Google Scholar] [CrossRef]
- Hansen, N.M.L.; Jankova, K.; Hvilsted, S. Fluoropolymer materials and architectures prepared by controlled radical polymerizations. Eur. Polym. J. 2007, 43, 255–293. [Google Scholar] [CrossRef]
- Hirao, A.; Koide, G.; Sugiyama, K. Synthesis of Novel Well-Defined Chain-End- and In-Chain-Functionalized Polystyrenes with One, Two, Three, and Four Perfluorooctyl Groups and Their Surface Characterization. Macromolecules 2002, 35, 7642–7651. [Google Scholar] [CrossRef]
- Voet, V.S.D.; Tichelaar, M.; Tanase, S.; Mittelmeijer-Hazeleger, M.C.; ten Brinke, G.; Loos, K. Poly(vinylidene fluoride)/nickel nanocomposites from semicrystalline block copolymer precursors. Nanoscale 2013, 5, 184–192. [Google Scholar] [CrossRef]
- Wang, P.; Wang, H.; Dong, Q.; Bai, R. Cobalt-Mediated Radical Copolymerization of Chlorotrifluoroethylene and Vinyl Acetate. Polymers 2019, 11, 101. [Google Scholar] [CrossRef]
- Ge, Z.; Zhang, X.Y.; Dai, J.B.; Li, W.H.; Luo, Y.J. Synthesis and characterization of fluorinated polyurethane with fluorine-containing pendent groups. Chin. Chem. Lett. 2008, 19, 1293–1296. [Google Scholar] [CrossRef]
- Zhang, W.-B.; Yu, X.; Wang, C.-L.; Sun, H.-J.; Hsieh, I.F.; Li, Y.; Dong, X.-H.; Yue, K.; Van Horn, R.; Cheng, S.Z.D. Molecular Nanoparticles Are Unique Elements for Macromolecular Science: From “Nanoatoms” to Giant Molecules. Macromolecules 2014, 47, 1221–1239. [Google Scholar] [CrossRef]
- Yin, G.-Z.; Zhang, W.-B.; Cheng, S.Z.D. Giant molecules: Where chemistry, physics, and bio-science meet. Sci. Chin. Chem. 2017, 60, 338–352. [Google Scholar] [CrossRef]
- Shao, Y.; Xu, X.; Yin, G.-Z.; Han, S.-Y.; Han, D.; Fu, Q.; Yang, S.; Zhang, W.-B. Symmetry-Dictated Mesophase Formation and Phase Diagram of Perfluorinated Polyhedral Oligomeric Silsesquioxanes. Macromolecules 2019, 52, 2361–2370. [Google Scholar] [CrossRef]
- Mabry, J.M.; Vij, A.; Iacono, S.T.; Viers, B.D. Fluorinated polyhedral oligomeric silsesquioxanes (F-POSS). Angew. Chem. Int. Ed. 2008, 47, 4137–4140. [Google Scholar] [CrossRef]
- Fagan, P.J.; Krusic, P.J.; McEwen, C.N.; Lazar, J.; Parkert, D.H.; Herron, N.; Wasserman, E. Production of Perfluoroalkylated Nanospheres from Buckminsterfullerene. Science 1993, 262, 404–407. [Google Scholar] [CrossRef]
- Wang, J.; Mao, G.; Ober, C.K.; Kramer, E.J. Liquid Crystalline, Semifluorinated Side Group Block Copolymers with Stable Low Energy Surfaces: Synthesis, Liquid Crystalline Structure, and Critical Surface Tension. Macromolecules 1997, 30, 1906–1914. [Google Scholar] [CrossRef]
- Li, X.; Andruzzi, L.; Chiellini, E.; Galli, G.; Ober, C.K.; Hexemer, A.; Kramer, E.J.; Fischer, D.A. Semifluorinated Aromatic Side-Group Polystyrene-Based Block Copolymers: Bulk Structure and Surface Orientation Studies. Macromolecules 2002, 35, 8078–8087. [Google Scholar] [CrossRef]
- Ren, Y.; Lodge, T.P.; Hillmyer, M.A. Effect of Selective Perfluoroalkylation on the Segregation Strength of Polystyrene−1,2-Polybutadiene Block Copolymers. Macromolecules 2002, 35, 3889–3894. [Google Scholar] [CrossRef]
- Johansson, G.; Percec, V.; Ungar, G.; Smith, K. Fluorophobic Effect Generates a Systematic Approach to the Synthesis of the Simplest Class of Rodlike Liquid Crystals Containing a Single Benzene Unit. Chem. Mater. 1997, 9, 164–175. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Grzybowski, B. Self-assembly at all scales. Science 2002, 295, 2418–2421. [Google Scholar] [CrossRef]
- Matsushita, Y. Creation of Hierarchically Ordered Nanophase Structures in Block Polymers Having Various Competing Interactions. Macromolecules 2007, 40, 771–776. [Google Scholar] [CrossRef]
- Son, J.G.; Hannon, A.F.; Gotrik, K.W.; Alexander-Katz, A.; Ross, C.A. Hierarchical Nanostructures by Sequential Self-Assembly of Styrene-Dimethylsiloxane Block Copolymers of Different Periods. Adv. Mater. 2011, 23, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.-A.; Liu, F.-L.; Han, L.; Zhu, Z.-J.; Zhang, Y.-F.; Wu, Z.-J.; Han, Z.-W.; Zhang, W.-B.; Li, H. Design, synthesis, and optical/electronic properties of a series of sphere-rod shape amphiphiles based on the C60-oligofluorene conjugates. Chin. J. Polym. Sci. 2017, 35, 503–514. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, X.; Zhou, X.; Luo, W.; Zhang, Y.; Cheng, X.; Deng, Y. Amphiphilic block copolymers directed synthesis of mesoporous nickel-based oxides with bimodal mesopores and nanocrystal-assembled walls. Chin. Chem. Lett. 2019, 30, 2003–2008. [Google Scholar] [CrossRef]
- Honda, K.; Morita, M.; Otsuka, H.; Takahara, A. Molecular Aggregation Structure and Surface Properties of Poly(fluoroalkyl acrylate) Thin Films. Macromolecules 2005, 38, 5699–5705. [Google Scholar] [CrossRef]
- Xiang, M.; Li, X.; Ober, C.K.; Char, K.; Genzer, J.; Sivaniah, E.; Kramer, E.J.; Fischer, D.A. Surface Stability in Liquid-Crystalline Block Copolymers with Semifluorinated Monodendron Side Groups. Macromolecules 2000, 33, 6106–6119. [Google Scholar] [CrossRef]
- Al-Hussein, M.; Séréro, Y.; Konovalov, O.; Mourran, A.; Möller, M.; de Jeu, W.H. Nanoordering of Fluorinated Side-Chain Liquid Crystalline/Amorphous Diblock Copolymers. Macromolecules 2005, 38, 9610–9616. [Google Scholar] [CrossRef]
- Dong, X.H.; Ni, B.; Huang, M.; Hsu, C.H.; Bai, R.; Zhang, W.-B.; Shi, A.C.; Cheng, S.Z.D. Molecular-Curvature-Induced Spontaneous Formation of Curved and Concentric Lamellae through Nucleation. Angew. Chem. Int. Ed. 2016, 55, 2459–2463. [Google Scholar] [CrossRef]
- Dong, X.-H.; Ni, B.; Huang, M.; Hsu, C.-H.; Chen, Z.; Lin, Z.; Zhang, W.-B.; Shi, A.-C.; Cheng, S.Z.D. Chain Overcrowding Induced Phase Separation and Hierarchical Structure Formation in Fluorinated Polyhedral Oligomeric Silsesquioxane (FPOSS)-Based Giant Surfactants. Macromolecules 2015, 48, 7172–7179. [Google Scholar] [CrossRef]
- Hsu, C.H.; Dong, X.H.; Lin, Z.; Ni, B.; Lu, P.; Jiang, Z.; Tian, D.; Shi, A.C.; Thomas, E.L.; Cheng, S.Z. Tunable Affinity and Molecular Architecture Lead to Diverse Self-Assembled Supramolecular Structures in Thin Films. ACS Nano 2016, 10, 919–929. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, W.; Gao, Y.; Zhang, C.; Yang, H. Polysiloxane-Based Side Chain Liquid Crystal Polymers: From Synthesis to Structure–Phase Transition Behavior Relationships. Polymers 2018, 10, 794. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Yin, G.-Z.; Zhao, F.-J.; Bian, Z.-X.; Xu, Y.-J.; Zhang, W.-B.; Miao, X.-R.; Li, H. Facile synthesis and hierarchical assembly of polystyrene-block-poly (perfluorooctylethyl acrylates). Polymer 2017, 113, 46–52. [Google Scholar] [CrossRef]
- Sinturel, C.; Bates, F.S.; Hillmyer, M.A. High χ-Low N Block Polymers: How Far Can We Go? ACS Macro Lett. 2015, 4, 1044–1050. [Google Scholar] [CrossRef]
- De Gennes, P.G. Conformations of Polymers Attached to an Interface. Macromolecules 1980, 13, 1069–1075. [Google Scholar] [CrossRef]
- Kacar, G.; Atilgan, C.; Özen, A.S. Mapping and Reverse-Mapping of the Morphologies for a Molecular Understanding of the Self-Assembly of Fluorinated Block Copolymers. J. Phys. Chem. C 2009, 114, 370–382. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, Y.; Zhang, A.; Calhoun, B.H.; Chun, M.; Quirk, R.P.; Cheng, S.Z.D.; Hsiao, B.S.; Yeh, F.; Hashimoto, T. Phase structures and morphologies determined by competitions among self-organization, crystallization, and vitrification in a disordered poly(ethylene oxide)-b-polystyrene diblock copolymer. Phys. Rev. B 1999, 60, 10022–10031. [Google Scholar] [CrossRef]
- Zheng, J.X.; Xiong, H.; Chen, W.Y.; Lee, K.; Van Horn, R.M.; Quirk, R.P.; Lotz, B.; Thomas, E.L.; Shi, A.-C.; Cheng, S.Z.D. Onsets of Tethered Chain Overcrowding and Highly Stretched Brush Regime via Crystalline-Amorphous Diblock Copolymers. Macromolecules 2006, 39, 641–650. [Google Scholar] [CrossRef]
- Wang, X.-M.; Shao, Y.; Jin, P.-F.; Jiang, W.; Hu, W.; Yang, S.; Li, W.; He, J.; Ni, P.; Zhang, W.-B. Influence of Regio-Configuration on the Phase Diagrams of Double-Chain Giant Surfactants. Macromolecules 2018, 51, 1110–1119. [Google Scholar] [CrossRef]
- Wang, X.-M.; Shao, Y.; Xu, J.; Jin, X.; Shen, R.-H.; Jin, P.-F.; Shen, D.-W.; Wang, J.; Li, W.; He, J.; et al. Precision Synthesis and Distinct Assembly of Double-Chain Giant Surfactant Regioisomers. Macromolecules 2017, 50, 3943–3953. [Google Scholar] [CrossRef]

| Sample a | fPSb | fPFOAb | MPSc | MPFOAd | q1 (nm−1) e | d (nm) f | Structure | Rg (nm) g | Sh |
|---|---|---|---|---|---|---|---|---|---|
| S74-b-F7 | 0.758 | 0.242 | 7700 | 3850 | 0.36 | 17.4 | HEX | 3.58 | 0.96 |
| S74-b-F42 | 0.356 | 0.644 | 7700 | 21,790 | 0.28 | 22.4 | iHEX | 3.58 | 1.17 |
| S35-b-F45 | 0.196 | 0.804 | 3600 | 23,310 | 0.29 | 21.2 | iHEX | 2.44 | 1.13 |
| S74-b-F17 | 0.579 | 0.421 | 7700 | 8790 | 0.30 | 20.6 | LAM | 3.58 | 0.83 |
| S42-b-F16 | 0.451 | 0.549 | 4350 | 8380 | 0.34 | 18.5 | LAM | 2.68 | 0.78 |
| S103-b-F36 | 0.478 | 0.522 | 10,750 | 18,620 | 0.18 | 34.8 | LAM | 4.24 | 0.98 |
| S62-b-F19 * | 0.503 | 0.497 | 6400 | 10,000 | 0.33 | 19.0 | LAM | 3.26 | 0.73 |
| S62-b-F21 * | 0.479 | 0.521 | 6400 | 11,000 | 0.32 | 19.7 | LAM | 3.26 | 0.72 |
| S91-b-F18 * | 0.610 | 0.390 | 9450 | 9550 | 0.31 | 20.5 | LAM | 3.97 | 0.79 |
| S91-b-F34 * | 0.462 | 0.538 | 9450 | 17,400 | 0.25 | 25.4 | LAM | 3.97 | 0.74 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, Y.; Dai, H.; Zhao, M.; Li, B.; Yao, J.; Zhang, W.-B.; Li, H. Phase Behavior and Phase Diagram of Polystyrene-b-Poly(Perfluorooctylethyl Acrylates). Polymers 2020, 12, 819. https://doi.org/10.3390/polym12040819
Shao Y, Dai H, Zhao M, Li B, Yao J, Zhang W-B, Li H. Phase Behavior and Phase Diagram of Polystyrene-b-Poly(Perfluorooctylethyl Acrylates). Polymers. 2020; 12(4):819. https://doi.org/10.3390/polym12040819
Chicago/Turabian StyleShao, Yu, Hui Dai, Meng Zhao, Bin Li, Jianan Yao, Wen-Bin Zhang, and Hui Li. 2020. "Phase Behavior and Phase Diagram of Polystyrene-b-Poly(Perfluorooctylethyl Acrylates)" Polymers 12, no. 4: 819. https://doi.org/10.3390/polym12040819
APA StyleShao, Y., Dai, H., Zhao, M., Li, B., Yao, J., Zhang, W.-B., & Li, H. (2020). Phase Behavior and Phase Diagram of Polystyrene-b-Poly(Perfluorooctylethyl Acrylates). Polymers, 12(4), 819. https://doi.org/10.3390/polym12040819