SiO2 NPs-PQ/PMMA Photopolymer Material Doped with a High-Concentration Photosensitizer for Holographic Storage
Abstract
:1. Introduction
2. Material Preparation
3. Results
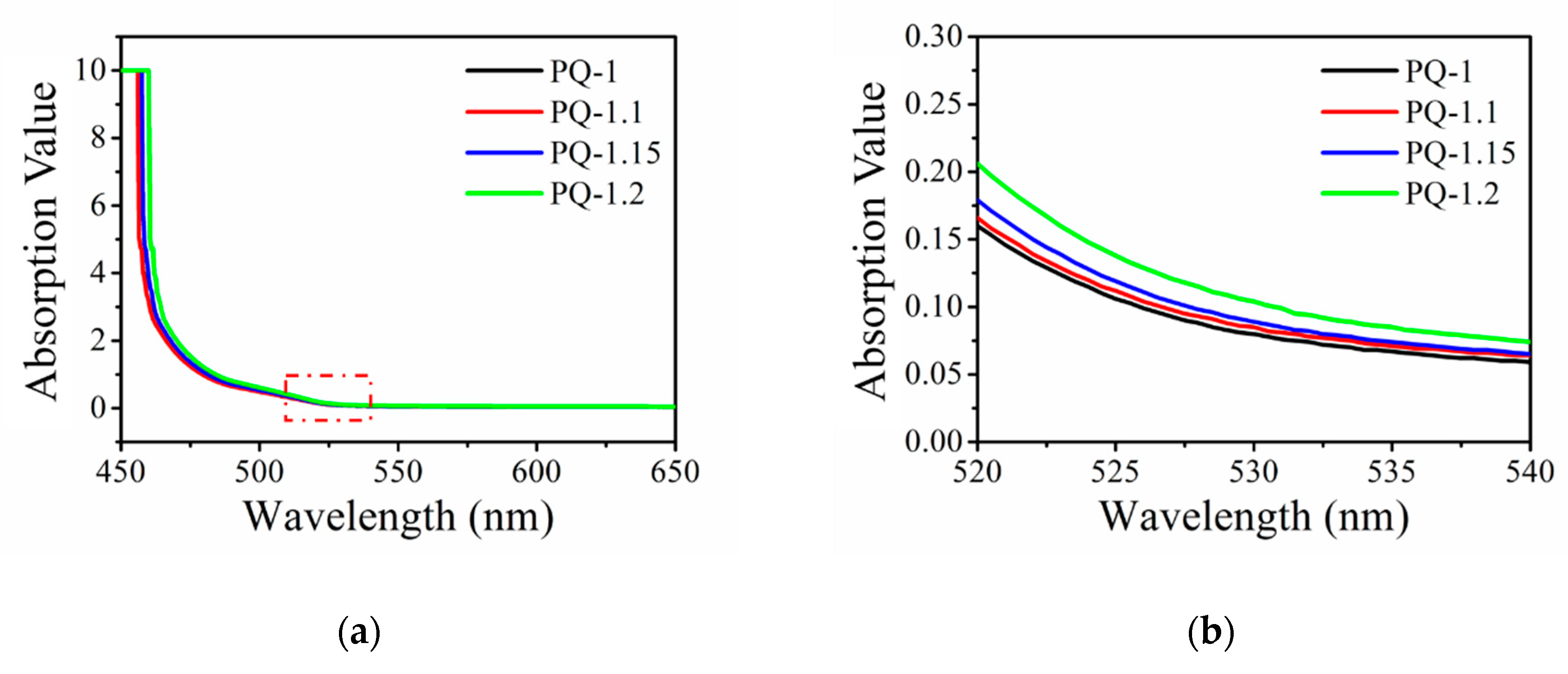
3.1. UV–Vis Spectra Measurements
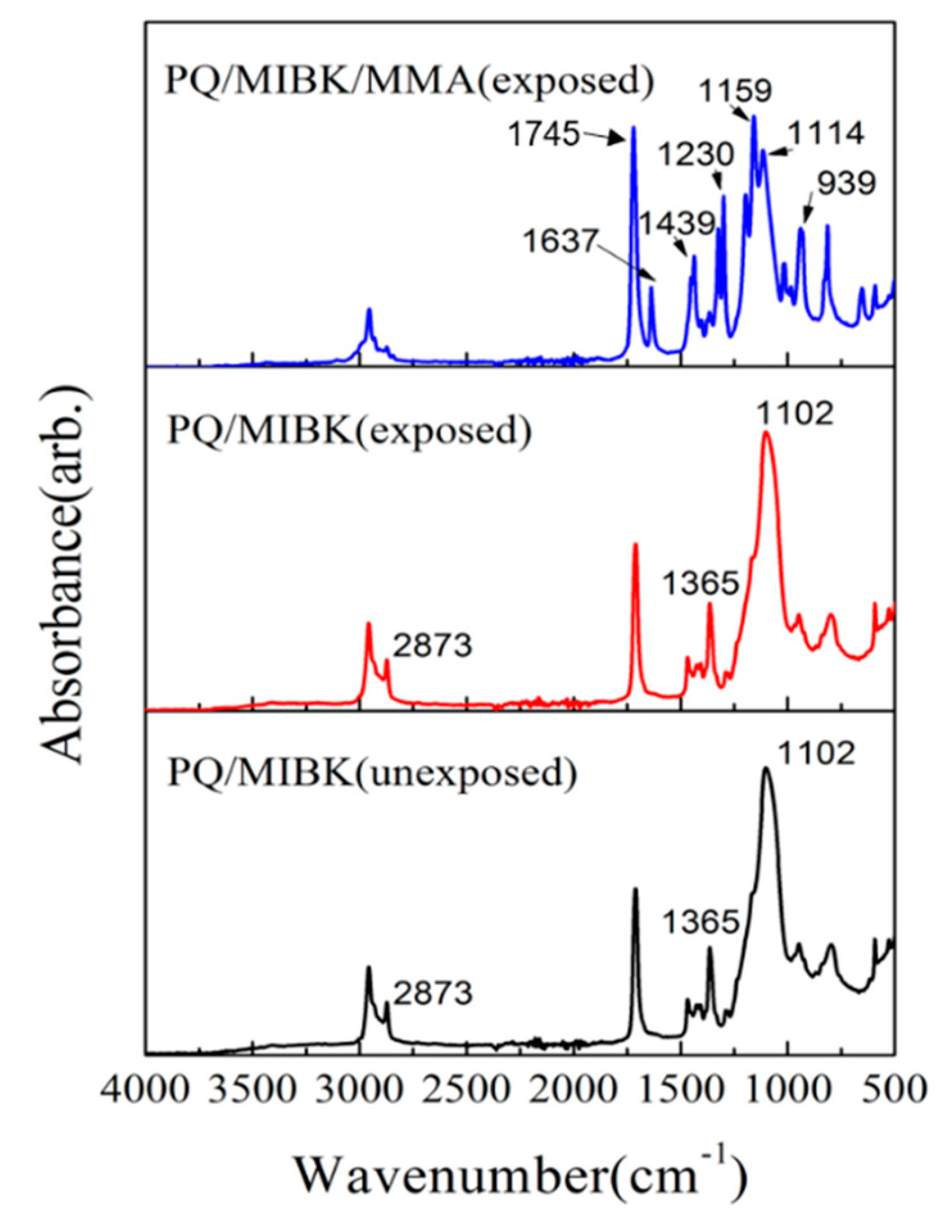
3.2. FT-IR Spectra Measurements
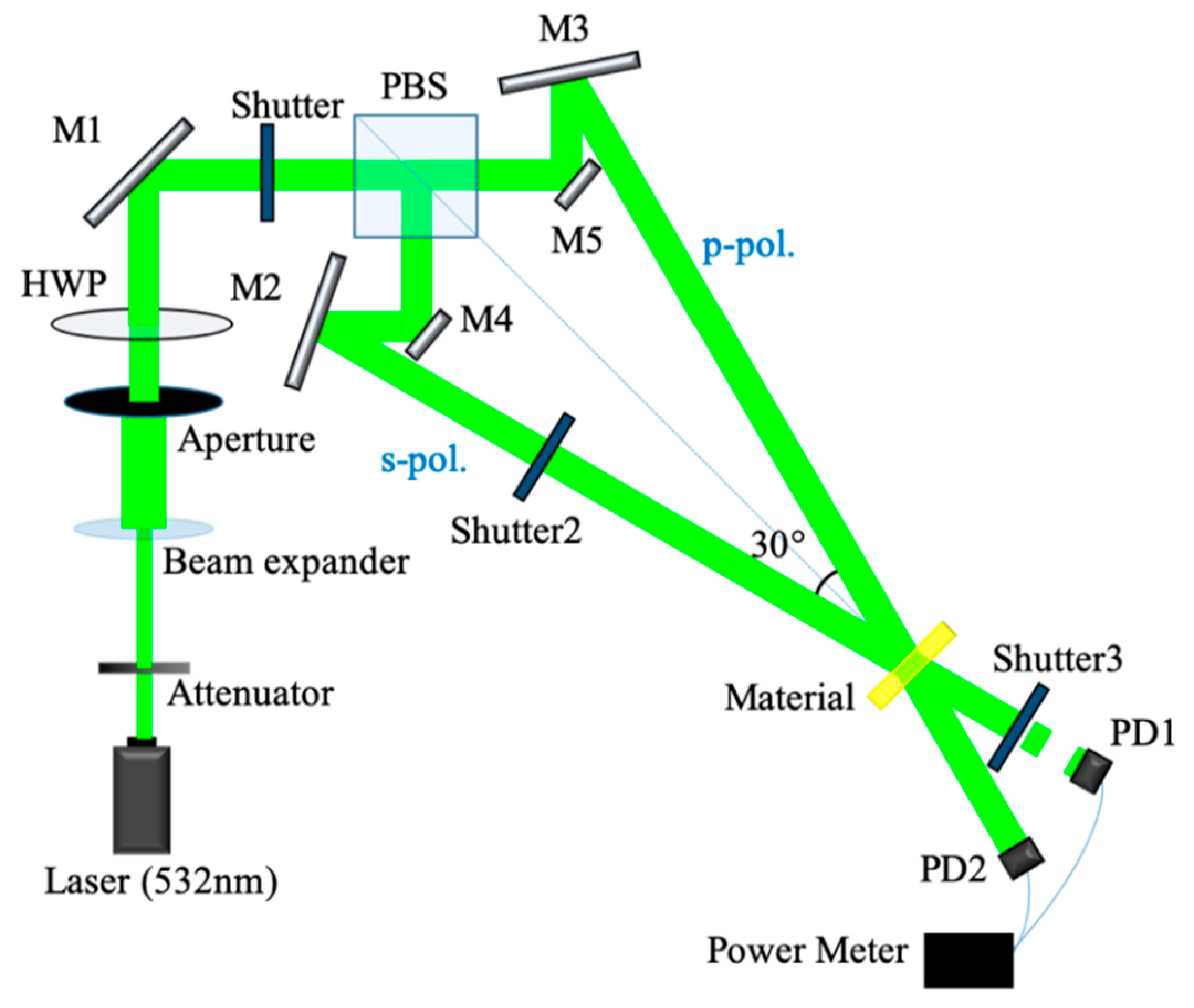
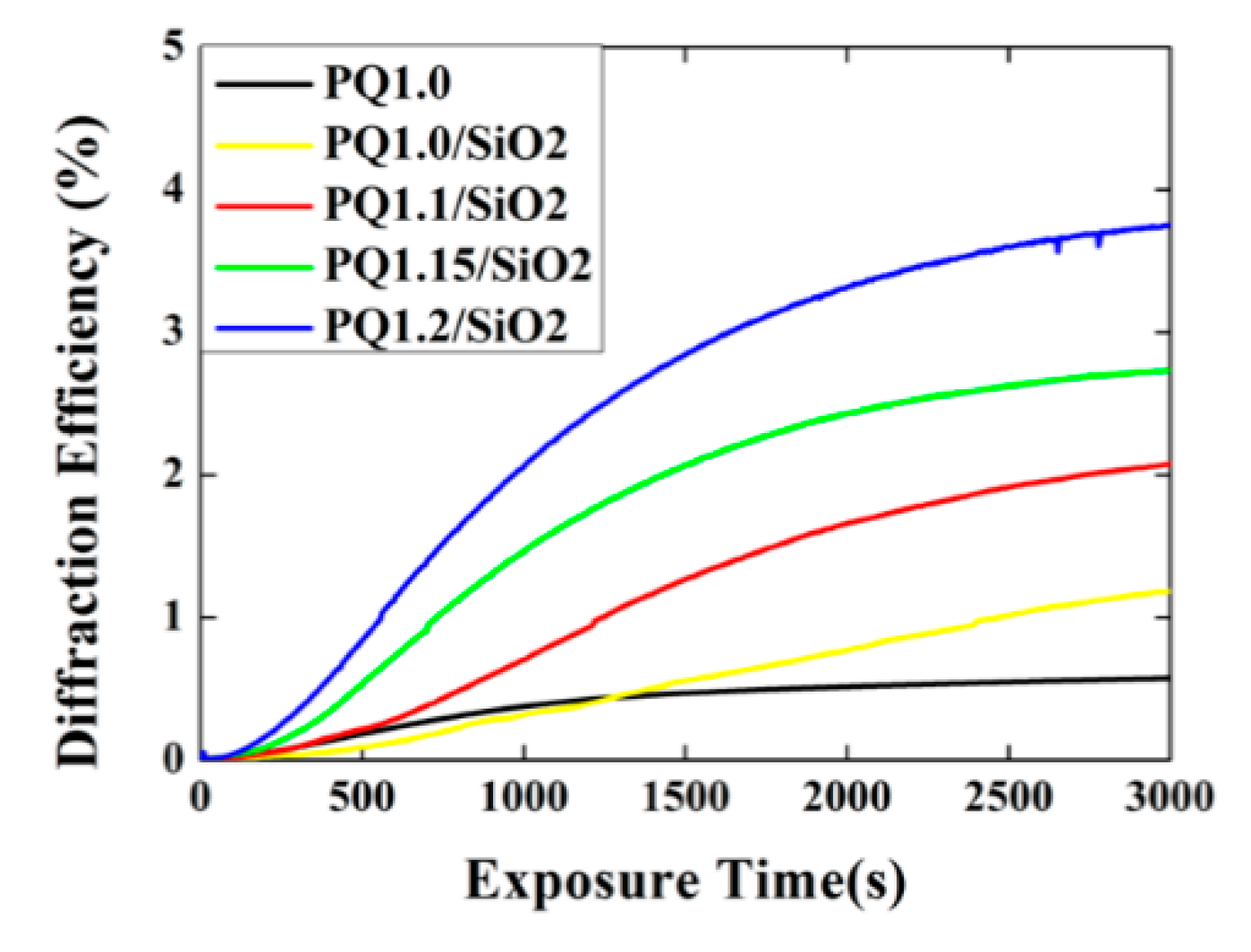
3.3. Holographic Diffraction Characteristics
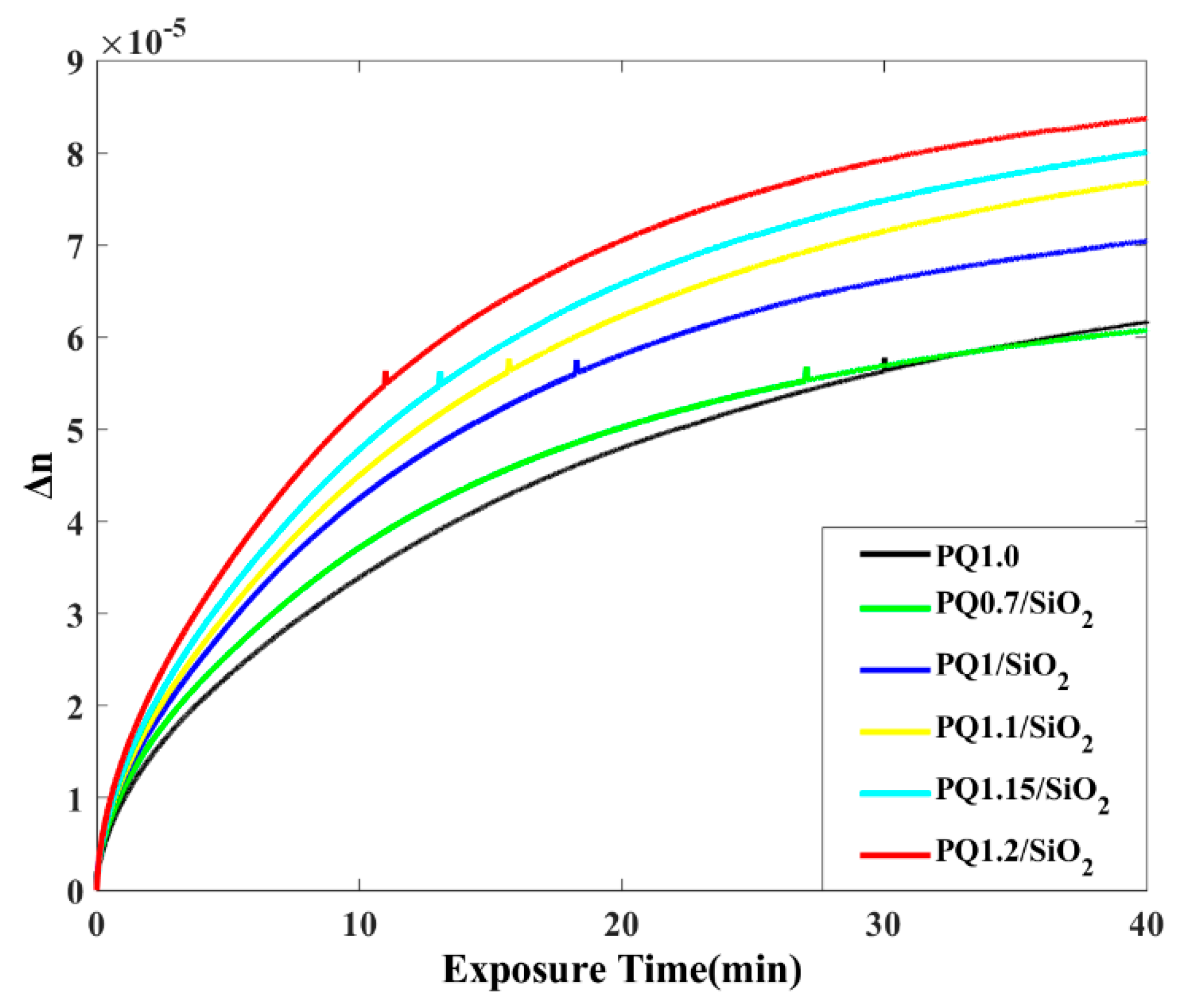
3.4. Photoinduced Birefringence
3.5. Application Experiments
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Murciano, A.; Carretero, L.; Blaya, S.; Madrigal, R.F.; Fimia, A. Experimental Study of Multiplexed Holographic Gratings Recorded in a Photopolymerizable Silica Glass. Appl. Phys. B 2006, 83, 619–622. [Google Scholar] [CrossRef]
- Tolstik, E.; Romanov, O.; Matusevich, V.; Tolstik, A.; Kowarschik, R. Formation of self-trapping waveguides in bulk PMMA media doped with Phenanthrenequinone. Opt. Express 2014, 22, 3228–3233. [Google Scholar] [CrossRef] [PubMed]
- Manukhin, B.G.; Chivilikhin, S.A.; Schelkanova, I.J.; Andreeva, N.V.; Materikina, D.A.; Andreeva, O.V. Reversible and irreversible alterations of the optical thickness of PQ/PMMA volume recording media samples. Part I: Experiment. Appl. Opt. 2017, 56, 7351–7357. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chang, F.; Zhao, Y.; Li, Z.; Sun, X. Ultrafast volume holographic storage on PQ/PMMA photopolymers with nanosecond pulsed exposures. Opt. Express 2018, 26, 1072–1082. [Google Scholar] [CrossRef]
- Mahilny, U.; Trofimova, A.; Nazarov, S.; Tolstik, A.; Heintzmann, R.; Tolstik, E. Highly concentrated phenanthrenequinone-polymethylmethacrylate composite for thick reflection holograms recording at 532 nm. Opt. Mater. Express 2016, 6, 3427–3437. [Google Scholar] [CrossRef]
- Vaia, R.A.; Dennis, C.L.; Natarajan, L.V.; Tondiglia, V.P.; Tomlin, D.W.; Bunning, T.J. One step, Micrometer-scale organization of nano- and mesoparticles using holographic photopolymerization: A generic technique. Adv. Mater. 2001, 13, 1570–1574. [Google Scholar] [CrossRef]
- Suzuki, N.; Tomita, Y.; Kojima, T. Holographic recording in TiO2 nanoparticle-dispersed methacrylate photopolymer films. Appl Phys Lett. 2002, 81, 4121–4123. [Google Scholar] [CrossRef]
- Suzuki, N.; Tomita, Y.; Ohmori, K.; Hidaka, M.; Chikama, K. Highly transparent ZrO2 nanoparticle-dispersed acrylate photopolymers for volume holographic recording. Opt. Express 2006, 14, 12712–12719. [Google Scholar] [CrossRef]
- Tomita, Y.; Nishibiraki, H. Improvement of holographic recording sensitivities in the green in SiO2 nanoparticle-dispersed methacrylate photopolymers doped with pyrromethene dyes. Appl. Phys. Lett. 2003, 83, 410–412. [Google Scholar] [CrossRef]
- Li, C.; Cao, L.; He, Q.; Jin, G. Holographic Kinetics for Mixed Volume Gratings in Gold Nanoparticles Doped Photopolymer. Opt. Express 2014, 22, 5017–5028. [Google Scholar] [CrossRef]
- Hata, E.; Mitsube, K.; Momose, K.; Tomita, Y. Holographic nanoparticle-polymer composites based on step-growth thiol-ene photopolymerization. Opt. Mater. Express 2011, 1, 207–222. [Google Scholar] [CrossRef]
- Tomita, Y.; Urano, H.; Fukamizu, T.; Kametani, Y.; Nishimura, N.; Odoi, K. Nanoparticle-polymer composite volume holographic gratings dispersed with ultrahigh-refractive-index hyperbranched polymer as organic nanoparticles. Opt. Lett. 2016, 41, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhao, Y.; Li, Z.; Sun, X. Improvement of ultrafast holographic performance in silver nanoprisms dispersed photopolymer. Opt. Express 2018, 26, 6993–7004. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yu, D.; Li, X.; Luo, S.; Jiang, Y.; Sun, X. Diffusional enhancement of volume gratings as an optimized strategy for holographic memory in PQ-PMMA photopolymer. Opt. Express 2010, 18, 6447–6454. [Google Scholar] [CrossRef] [PubMed]
- Mahilny, U.V.; Marmysh, D.N.; Stankevich, A.I.; Tolstik, A.L.; Matusevich, V.; Kowarschik, R. Holographic volume gratings in a glass-like polymer material. Appl. Phys. B 2005, 82, 299–302. [Google Scholar] [CrossRef]
- Fan, F.; Liu, Y.; Hong, Y.; Zang, J.; Kang, G.; Zhao, T.; Tan, X.; Shimura, T. Volume polarization holographic recording in phenanthrenequinone doped poly(MMA-Co-BzMA) photopolymer. Chem. Lett. 2018, 47, 520–523. [Google Scholar] [CrossRef]
- Fan, F.; Liu, Y.; Hong, Y.; Zang, J.; Wu, A.; Zhao, T.; Kang, G.; Tan, X.; Shimura, T. Improving the polarization-holography performance of PQ/PMMA photopolymer by doping with THMFA. Opt. Express 2018, 26, 17794–17803. [Google Scholar] [CrossRef]
- Li, C.; Cao, L.; Wang, Z.; Jin, G. Hybrid polarization-angle multiplexing for volume holography in gold nanoparticle-doped photopolymer. Opt. Lett. 2014, 39, 6891. [Google Scholar] [CrossRef]
- Kawatsuki, N. Photoalignment and Photoinduced Molecular Reorientation of Photosensitive Materials. Chem. Lett. 2011, 40, 548. [Google Scholar] [CrossRef]
- Chen, P.L. Phenanthrenequinone-doped copolymers for holographic data storage. Opt. Eng. 2009, 48, 035802. [Google Scholar] [CrossRef]
- Steckman, G.J.; Shelkovnikov, V.; Berezhnaya, V.; Gerasimova, T.; Solomatine, I.; Psaltis, D. Holographic recording in a photopolymer by optically induced detachment of chromophores. Opt. Lett. 2000, 25, 607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomita, Y.; Suzuki, N. Holographic manipulation of nanoparticle distribution morphology in nanoparticle-dispersed photopolymers. Opt. Lett. 2005, 30, 839–841. [Google Scholar] [CrossRef] [PubMed]
- Trofimova, A.V.; Stankevich, A.I.; Mogil’nyi, V.V. Phenanthrenequinone–polymethylmethacrylate composite for polarization phase recording. J. Appl. Spectrosc. 2009, 76, 585. [Google Scholar] [CrossRef]
- Szukalski, A.; Haupa, K.; Miniewicz, A.; Mysliwiec, J. Photoinduced Birefringence in PMMA Polymer Doped with Photoisomerizable Pyrazoline Derivative. J. Phys. Chem. C 2015, 119, 10007–10014. [Google Scholar] [CrossRef]
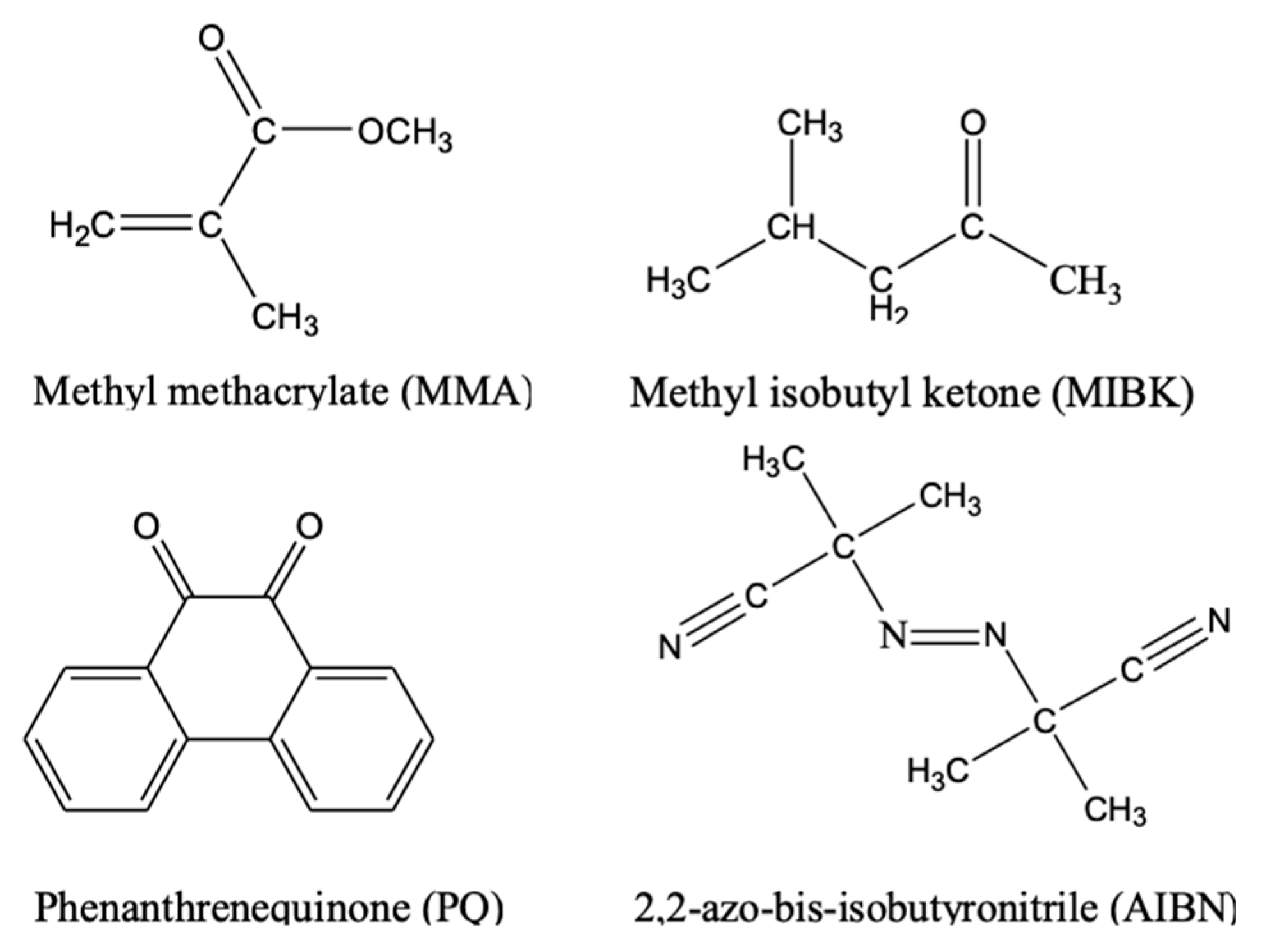


| Sample | MMA (wt %) | SiO2 (wt %) | PQ (wt %) | AIBN (wt %) |
|---|---|---|---|---|
| 1 | 100 | 3 | 0.7 | 1 |
| 2 | 0 | 1 | ||
| 3 | 3 | 1 | ||
| 4 | 3 | 1.1 | ||
| 5 | 3 | 1.15 | ||
| 6 | 3 | 1.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Fan, F.; Tan, X. SiO2 NPs-PQ/PMMA Photopolymer Material Doped with a High-Concentration Photosensitizer for Holographic Storage. Polymers 2020, 12, 816. https://doi.org/10.3390/polym12040816
Liu Y, Fan F, Tan X. SiO2 NPs-PQ/PMMA Photopolymer Material Doped with a High-Concentration Photosensitizer for Holographic Storage. Polymers. 2020; 12(4):816. https://doi.org/10.3390/polym12040816
Chicago/Turabian StyleLiu, Ying, Fenglan Fan, and Xiaodi Tan. 2020. "SiO2 NPs-PQ/PMMA Photopolymer Material Doped with a High-Concentration Photosensitizer for Holographic Storage" Polymers 12, no. 4: 816. https://doi.org/10.3390/polym12040816
APA StyleLiu, Y., Fan, F., & Tan, X. (2020). SiO2 NPs-PQ/PMMA Photopolymer Material Doped with a High-Concentration Photosensitizer for Holographic Storage. Polymers, 12(4), 816. https://doi.org/10.3390/polym12040816





