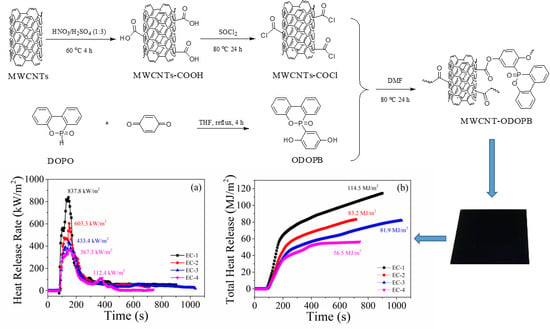
Preparation and Characterization of DOPO-Functionalized MWCNT and Its High Flame-Retardant Performance in Epoxy Nanocomposites
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
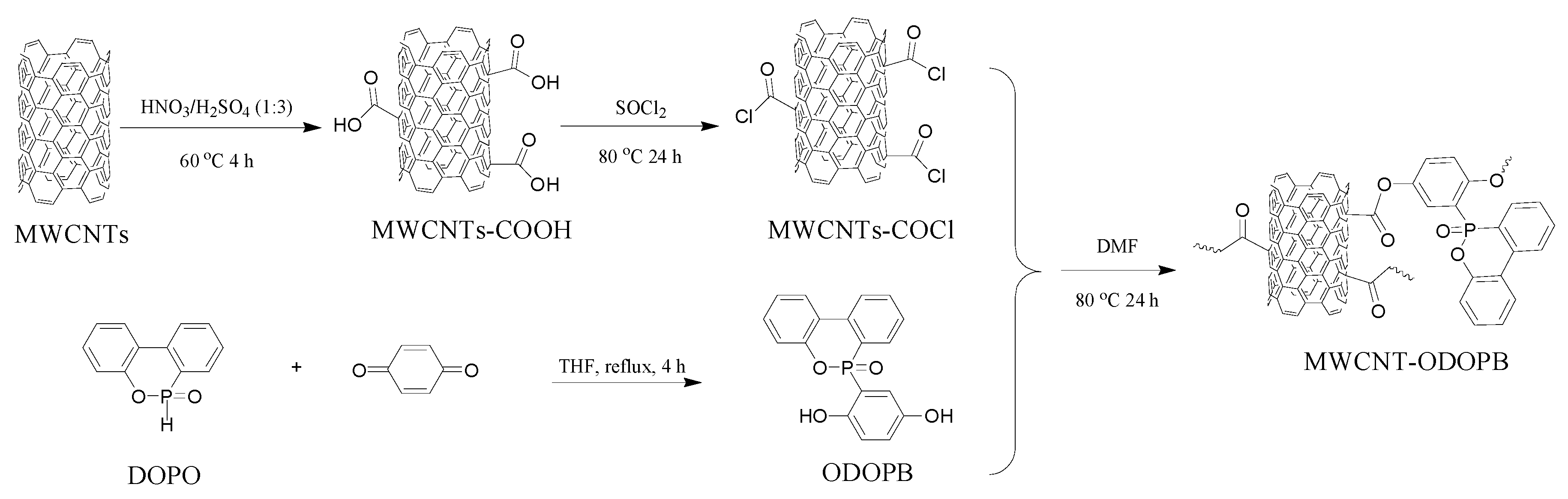
2.2. Preparation of MWCNT-ODOPB
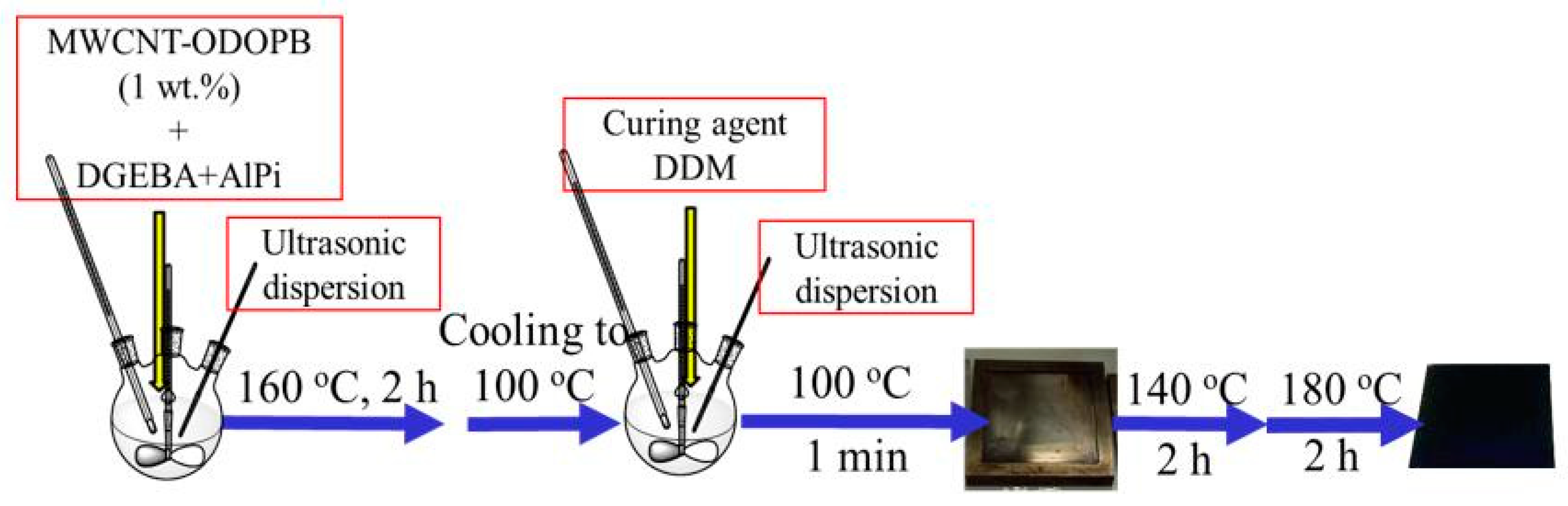
2.3. Preparation of ER/AlPi/MWCNT-ODOPB Nanocomposites
2.4. Instrumental Analysis and Measurements
3. Results and Discussion
3.1. Characterization of MWCNT-ODOPO
3.1.1. FT-IR Analysis
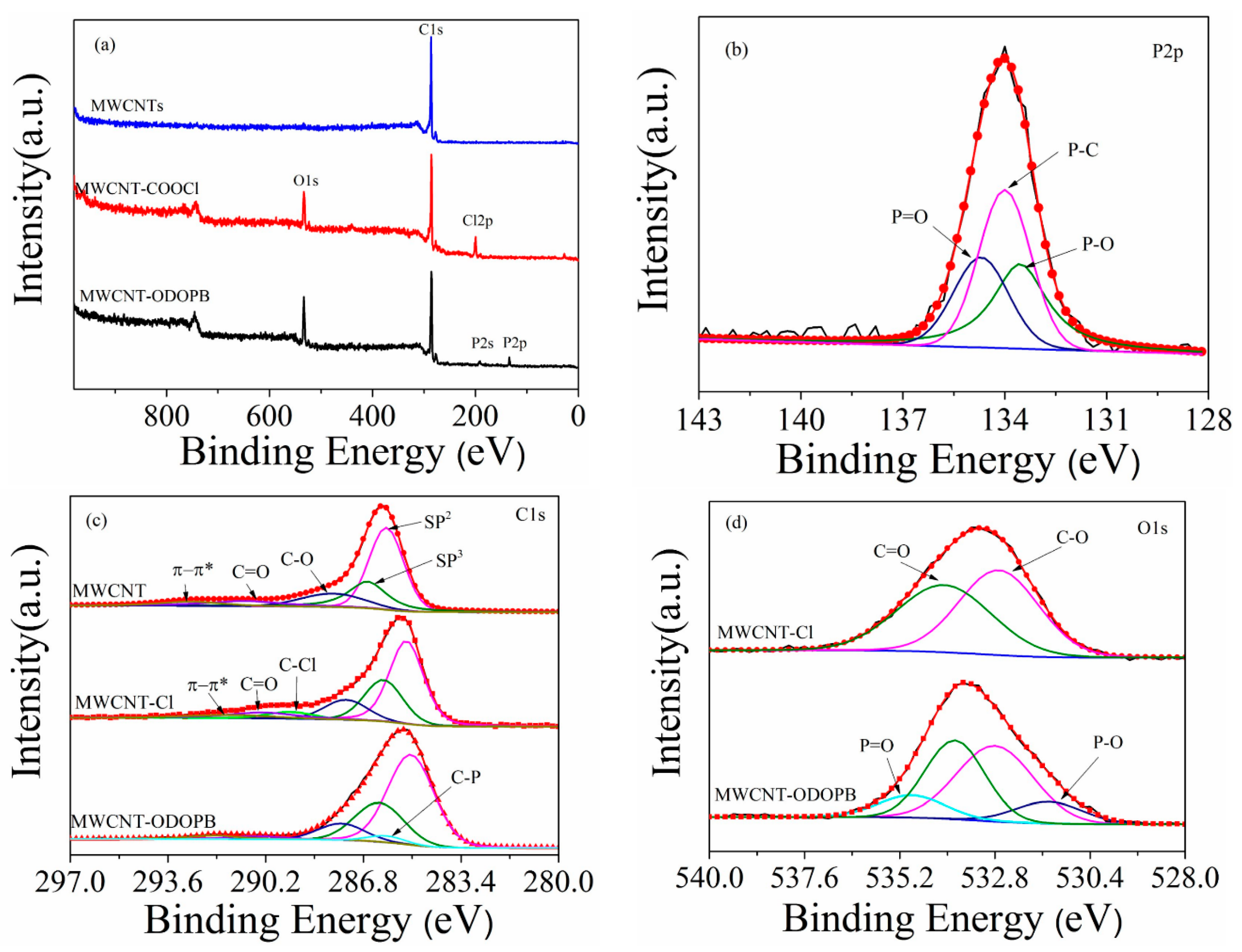
3.1.2. XPS Analysis
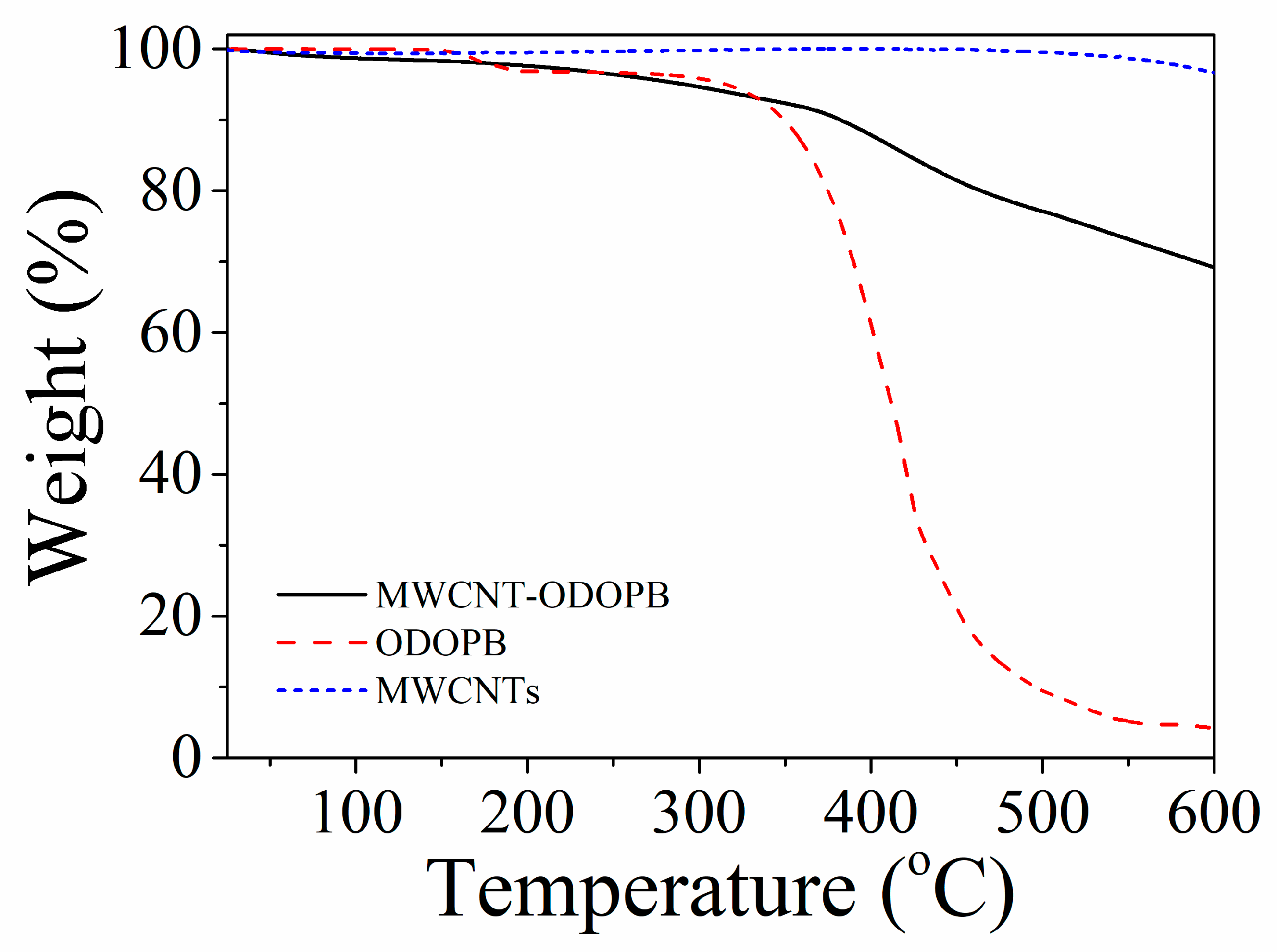
3.1.3. Thermal Analysis
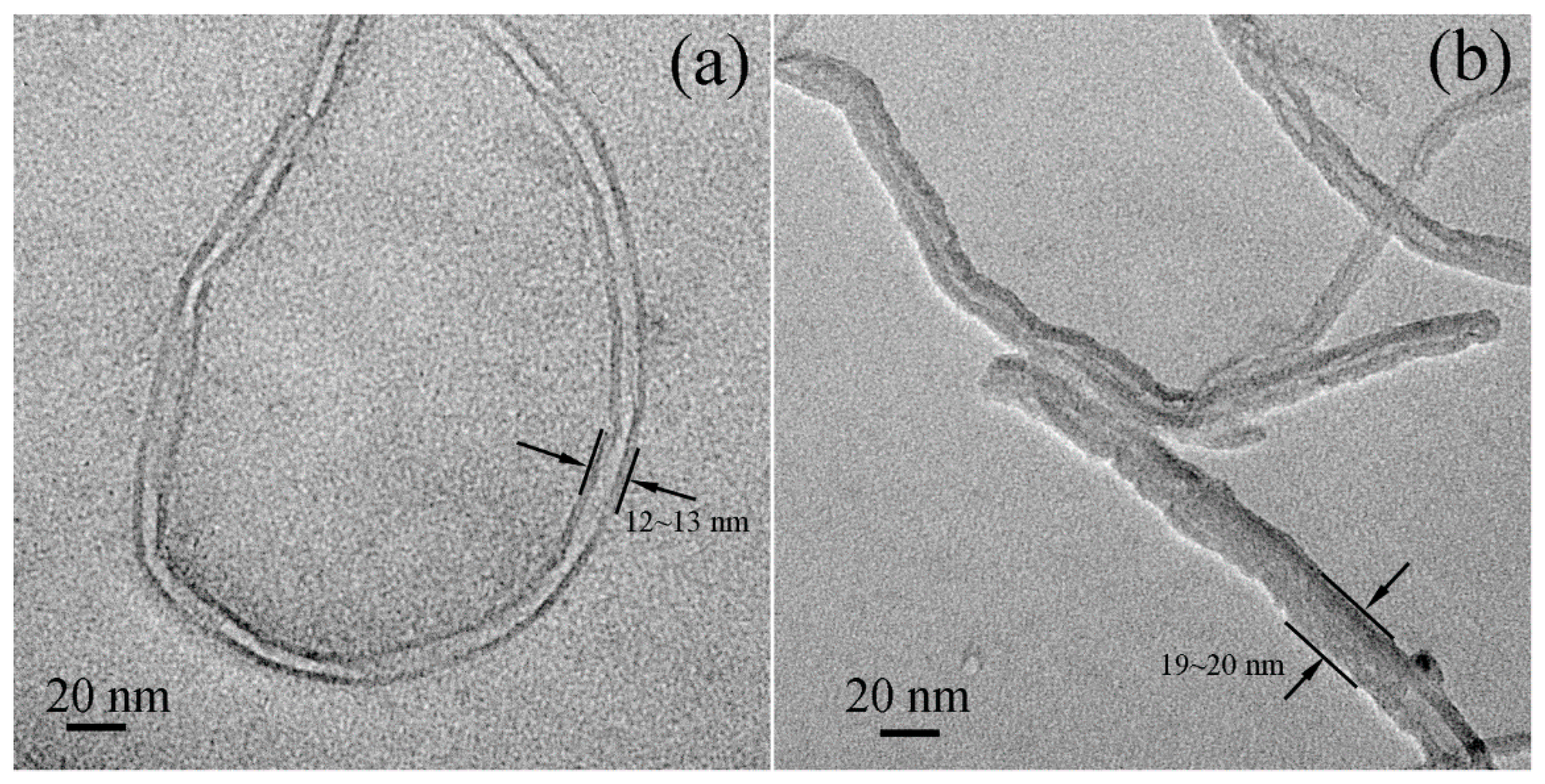
3.1.4. Morphology
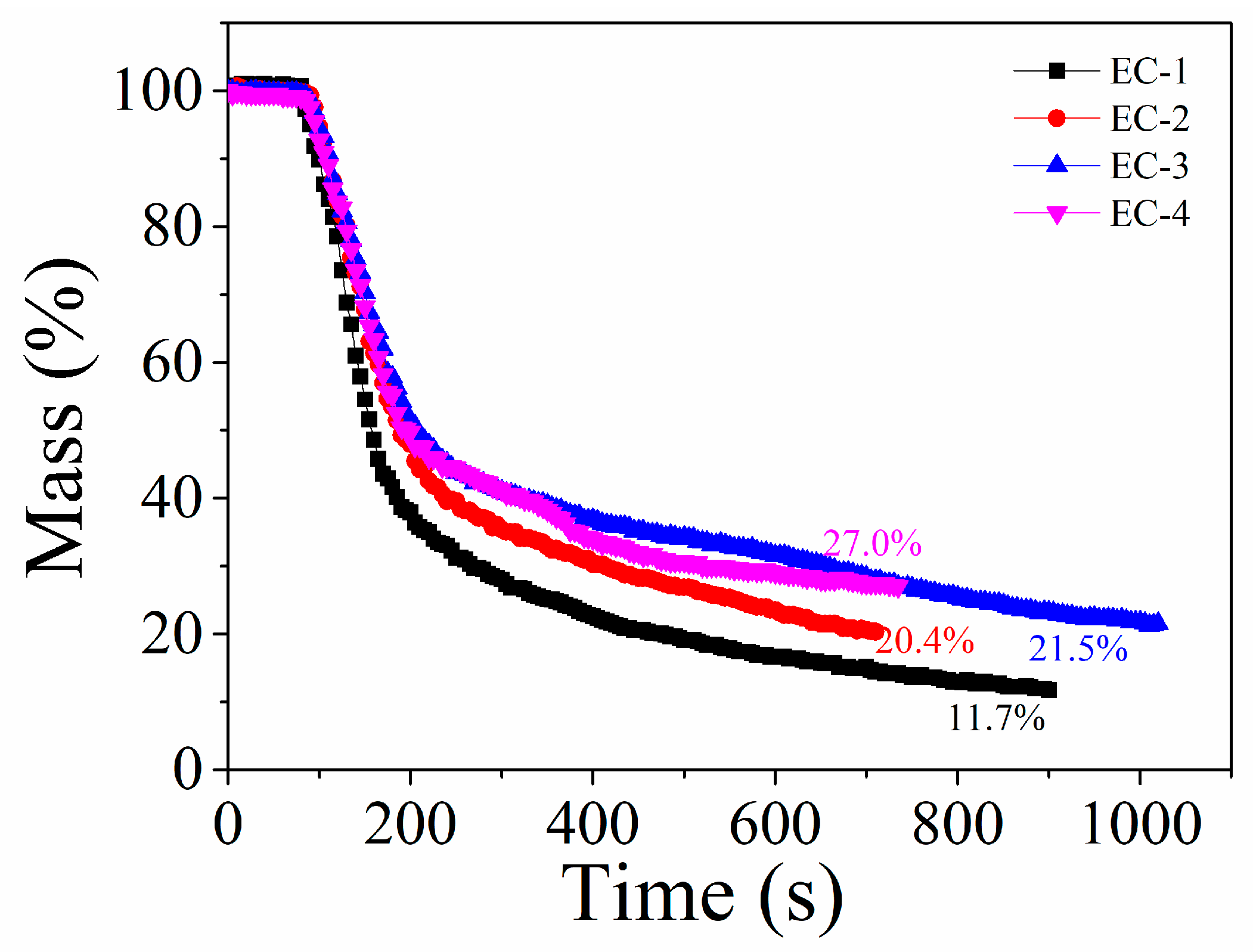
3.2. Flammability and Residual Char Analysis
3.2.1. UL 94 Tests and LOI Measurement
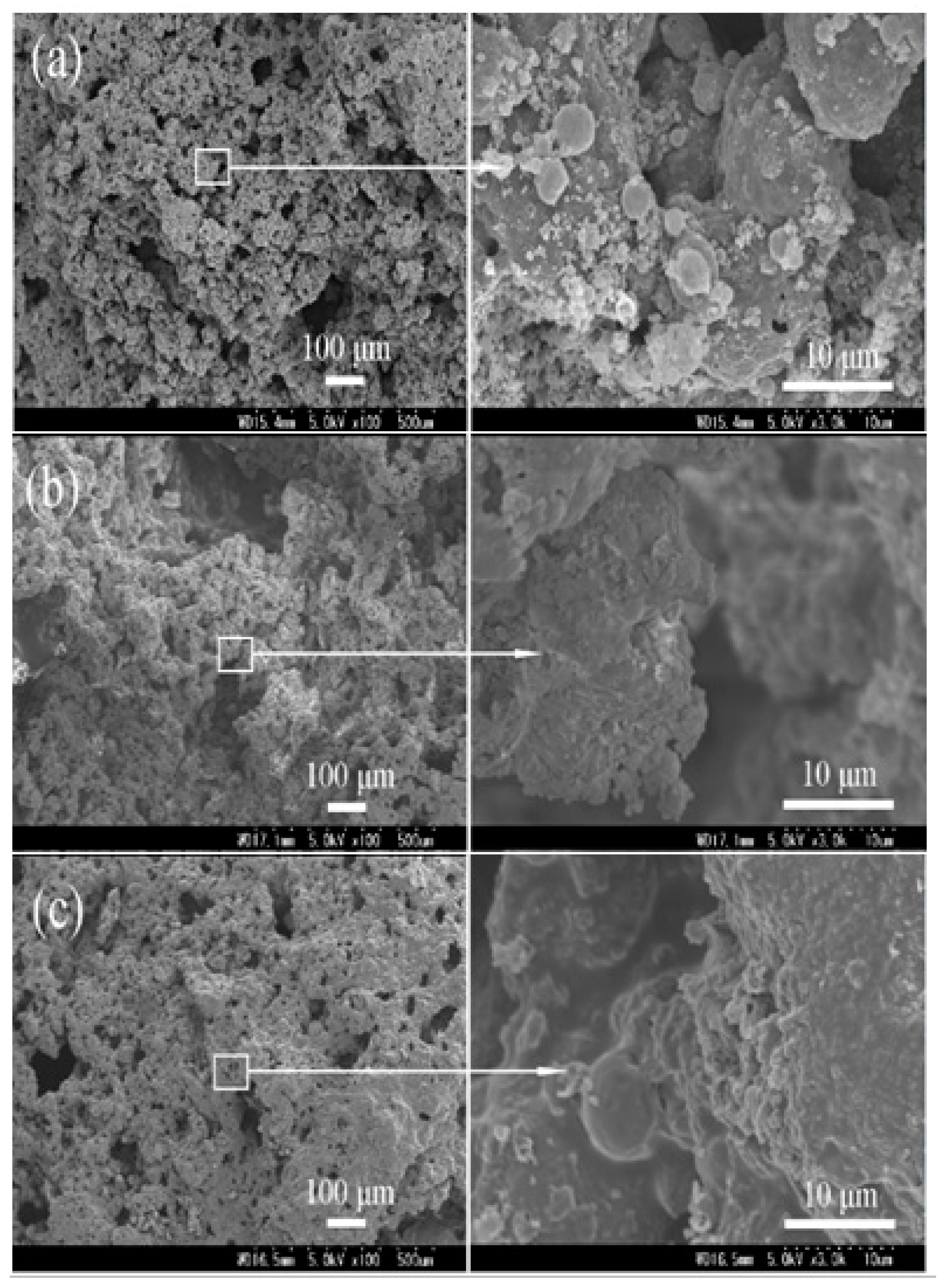
3.2.2. Morphology
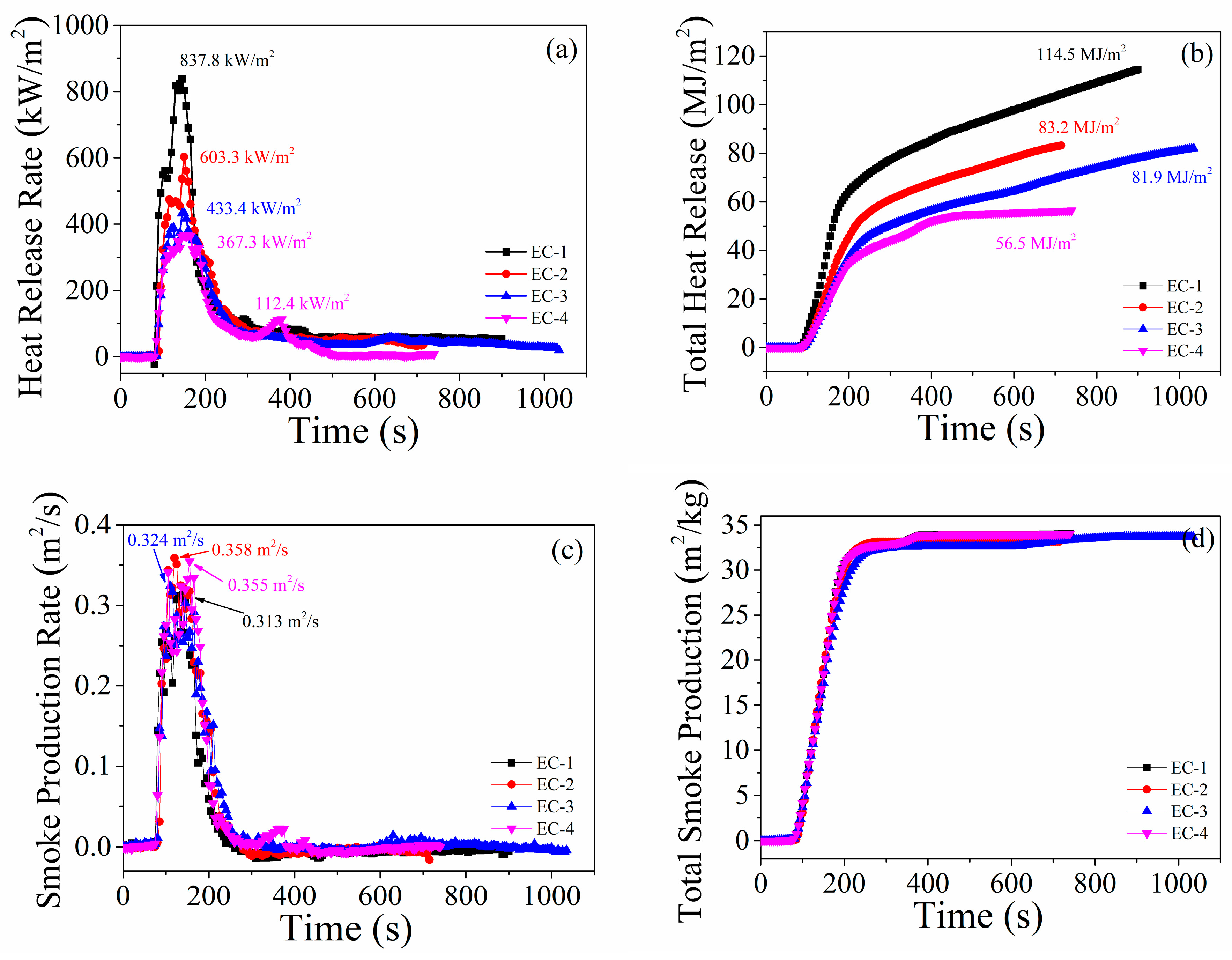
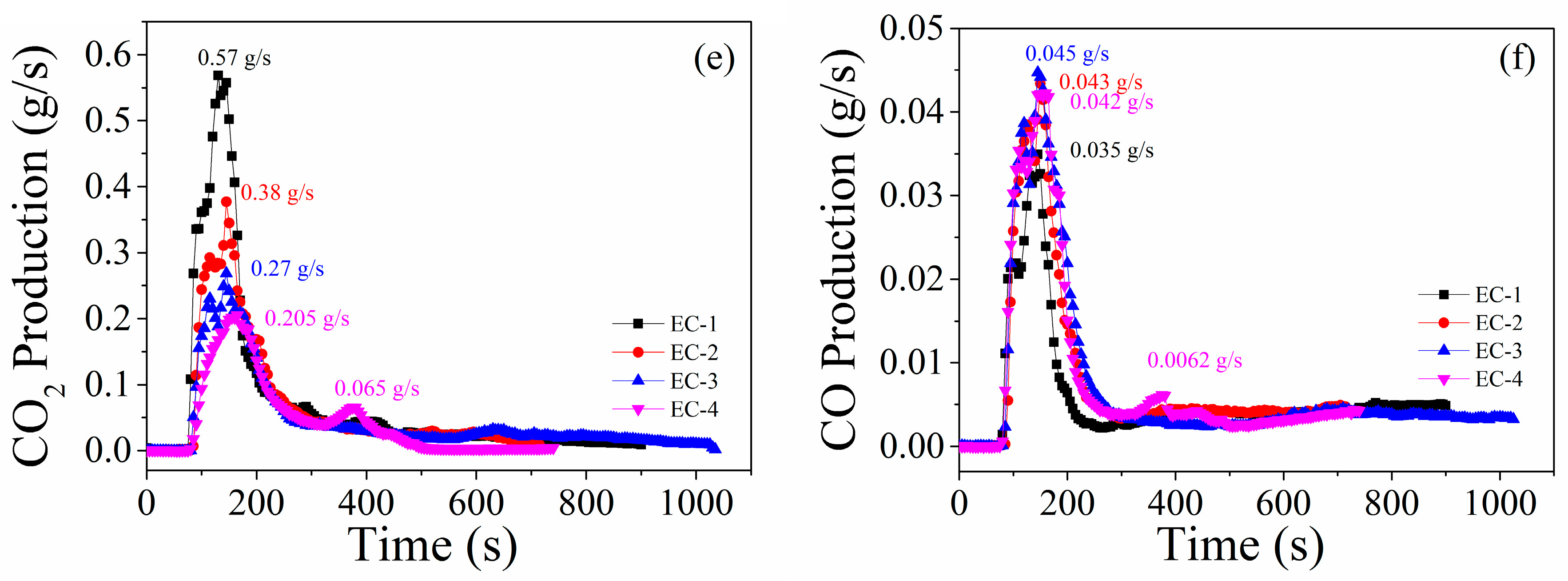
3.2.3. Fire Hazards
3.3. Thermal Behaviors
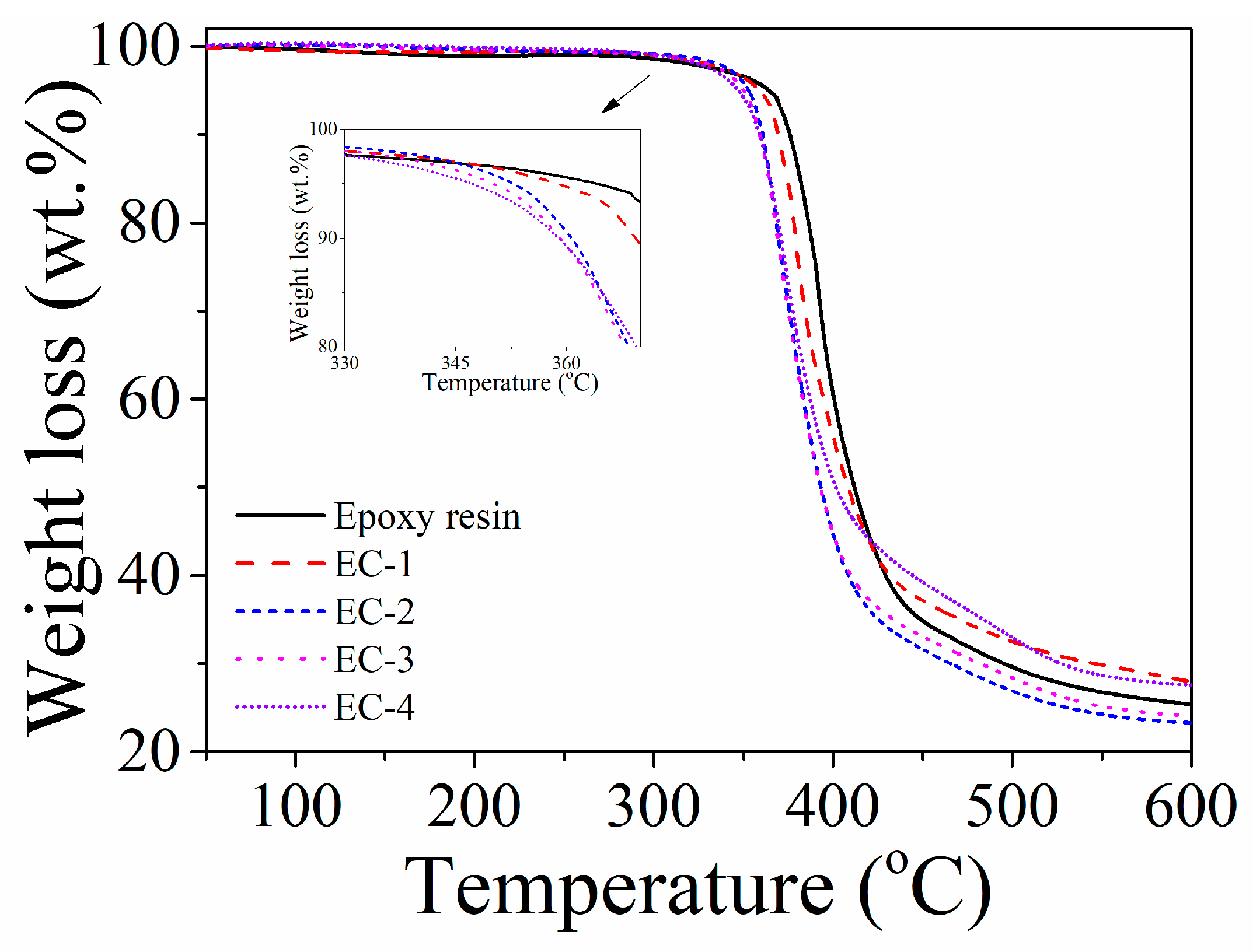
3.3.1. TGA
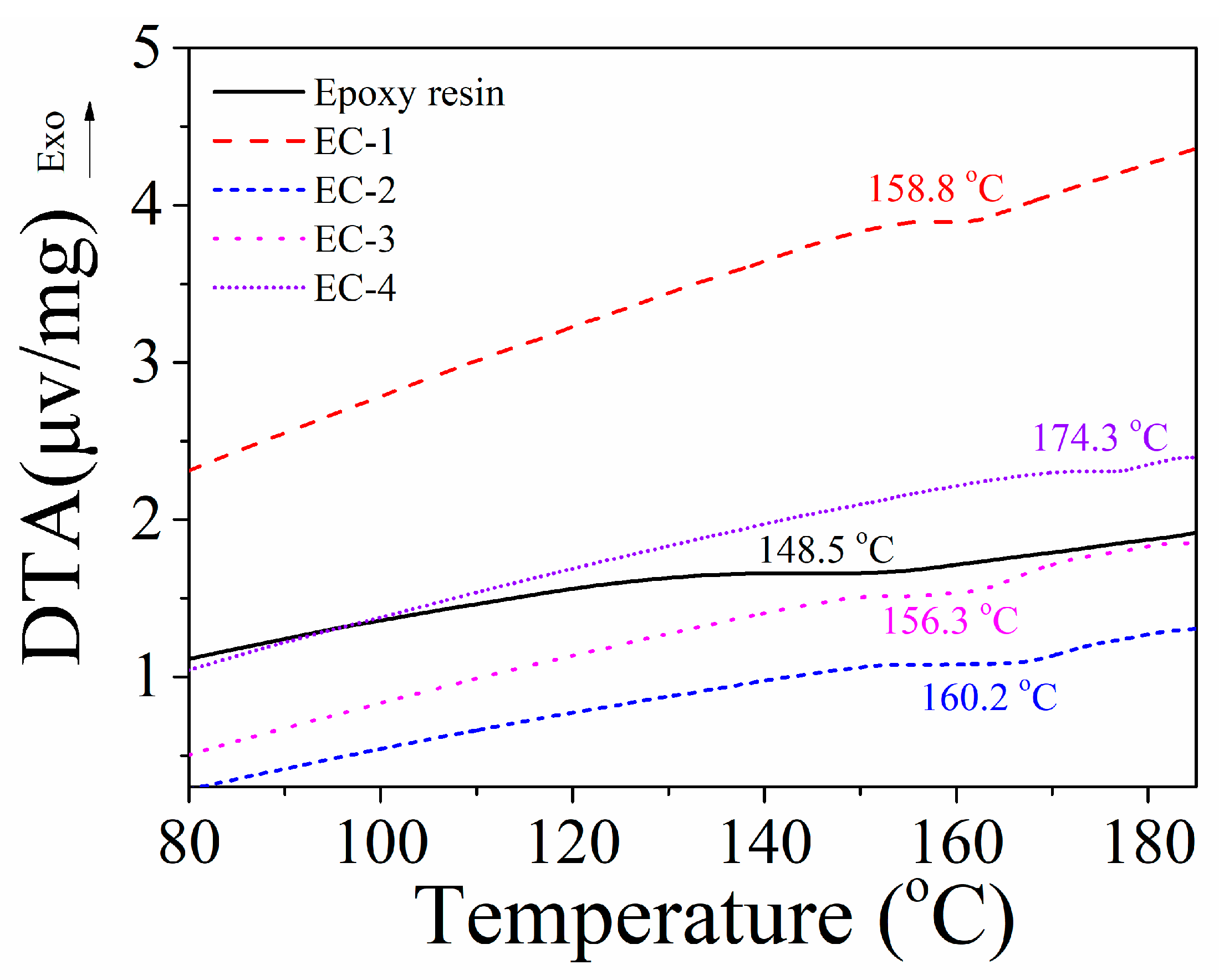
3.3.2. DTA
3.4. DMA Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Spurr, A.R. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 1969, 26, 31–43. [Google Scholar] [CrossRef]
- Rafiee, M.; Nitzsche, F.; Laliberte, J.; Hind, S.; Robitaille, F.; Labrosse, M.R. Thermal properties of doubly reinforced fiberglass/epoxy composites with graphene nanoplatelets, graphene oxide and reduced-graphene oxide. Compos. Part B Eng. 2019, 164, 1–9. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, Z.; Wang, B.; Zhang, C.; Kramer, L. Processing and property investigation of single-walled carbon nanotube (SWNT) buckypaper/epoxy resin matrix nanocomposites. Compos. Part A Appl. Sci. Manuf. 2004, 35, 1225–1232. [Google Scholar] [CrossRef]
- Yang, S.Y.; Lin, W.N.; Huang, Y.L.; Tien, H.W.; Wang, J.Y.; Ma, C.C.M.; Wang, Y.S. Synergetic effects of graphene platelets and carbon nanotubes on the mechanical and thermal properties of epoxy composites. Carbon 2011, 49, 793–803. [Google Scholar] [CrossRef]
- Beheshti, A.; Heris, S.Z. Is MWCNT a good synergistic candidate in APP–PER–MEL intumescent coating for steel structure? Prog. Org. Coat. 2016, 90, 252–257. [Google Scholar] [CrossRef]
- Norouzi, M.; Zare, Y.; Kiany, P. Nanoparticles as effective flame retardants for natural and synthetic textile polymers: Application, mechanism, and optimization. Polym. Rev. 2015, 55, 531–560. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Xing, W.; Lu, H.; Lv, P.; Jie, G. Flame retardancy and thermal degradation mechanism of epoxy resin composites based on a DOPO substituted organophosphorus oligomer. Polymer 2010, 51, 2435–2445. [Google Scholar] [CrossRef]
- Wu, N.; Lang, S. Flame retardancy and toughness modification of flame retardant polycarbonate/acrylonitrile-butadiene-styrene/AHP composites. Polym. Degrad. Stab. 2016, 123, 26–35. [Google Scholar] [CrossRef]
- Guan, F.L.; Gui, C.X.; Zhang, H.B.; Jiang, Z.G.; Jiang, Y.; Yu, Z.Z. Enhanced thermal conductivity and satisfactory flame retardancy of epoxy/alumina composites by combination with graphene nanoplatelets and magnesium hydroxide. Compos. Part B Eng. 2016, 98, 134–140. [Google Scholar] [CrossRef]
- Rajaei, M.; Kim, N.K.; Bickerton, S.; Bhattacharyya, D. A comparative study on effects of natural and synthesised nano-clays on the fire and mechanical properties of epoxy composites. Compos. Part B Eng. 2019, 165, 65–74. [Google Scholar] [CrossRef]
- Jian, R.K.; Ai, Y.F.; Xia, L.; Zhang, Z.P.; Wang, D.Y. Organophosphorus heteroaromatic compound towards mechanically reinforced and low-flammability epoxy resin. Compos. Part B Eng. 2019, 168, 458–466. [Google Scholar] [CrossRef]
- Gu, J.; Liang, C.; Zhao, X.; Gan, B.; Qiu, H.; Guo, Y.; Wang, D.Y. Highly thermally conductive flame-retardant epoxy nanocomposites with reduced ignitability and excellent electrical conductivities. Compos. Sci. Technol. 2017, 139, 83–89. [Google Scholar] [CrossRef]
- Atif, R.; Shyha, I.; Inam, F. Mechanical, thermal, and electrical properties of graphene-epoxy nanocomposites—A review. Polymers 2016, 8, 281. [Google Scholar] [CrossRef]
- Pötschke, P.; Mothes, F.; Krause, B.; Voit, B. Melt-mixed PP/MWCNT composites: influence of CNT incorporation strategy and matrix viscosity on filler dispersion and electrical resistivity. Polymers 2019, 11, 189. [Google Scholar]
- Wang, L.; Qiu, J.; Sakai, E.; Wei, X. The Relationship between Microstructure and Mechanical Properties of Carbon Nanotubes/Polylactic acid Nanocomposites Prepared by Twin-screw Extrusion. Compos. Part A Appl. Sci. Manuf. 2016, 89, 18–25. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Grulke, E.; Hilding, J.; Harris, R.; Awad, W.; Douglas, J. Thermal degradation and flammability properties of poly (propylene)/carbon nanotube composites. Macromol. Rapid Commun. 2002, 23, 761–765. [Google Scholar] [CrossRef]
- Deng, X.; Jia, G.; Wang, H.; Sun, H.; Wang, X.; Yang, S.; Wang, T.; Liu, Y. Translocation and fate of multi-walled carbon nanotubes in vivo. Carbon 2007, 45, 1419–1424. [Google Scholar] [CrossRef]
- Lee, S.; min Kim, H.; Seong, D.G.; Lee, D. Synergistic improvement of flame retardant properties of expandable graphite and multi-walled carbon nanotube reinforced intumescent polyketone nanocomposites. Carbon 2019, 143, 650–659. [Google Scholar] [CrossRef]
- Yang, H.; Guan, Y.; Ye, L.; Wang, S.; Li, S.; Wen, X.; Tang, T. Synergistic effect of nanoscale carbon black and ammonium polyphosphate on improving thermal stability and flame retardancy of polypropylene: A reactive network for strengthening carbon layer. Compos. Part B Eng. 2019, 174, 107038. [Google Scholar] [CrossRef]
- Gashti, M.P.; Almasian, A. UV radiation induced flame retardant cellulose fiber by using polyvinylphosphonic acid/carbon nanotube composite coating. Compos. Part B Eng. 2013, 45, 282–289. [Google Scholar] [CrossRef]
- Liang, B.; Cao, J.; Hong, X.; Wang, C. Synthesis and properties of a novel phosphorous-containing flame-retardant hardener for epoxy resin. J. Appl. Polym. Sci. 2013, 128, 2759–2765. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.; Zhan, L.; Ou, S.; Tian, J. High electrocatalytic activity of PtRu nanoparticles supported on starch-functionalized multi-walled carbon nanotubes for ethanol oxidation. J. Mater. Chem. 2011, 21, 4257–4263. [Google Scholar] [CrossRef]
- Yang, X.H.; Guo, J.W.; Yang, S.; Hou, Y.; Zhang, B.; Yang, H.G. A free radical assisted strategy for preparing ultra-small Pt decorated CNTs as a highly efficient counter electrode for dye-sensitized solar cells. J. Mater. Chem. A 2014, 2, 614–619. [Google Scholar] [CrossRef]
- Artner, J.; Ciesielski, M.; Walter, O.; Döring, M.; Perez, R.M.; Sandler, J.K.; Schartel, B. A novel DOPO-based diamine as hardener and flame retardant for epoxy resin systems. Macromol. Mater. Eng. 2008, 293, 503–514. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Zhang, Y.; Wienert, B.; Thomas, A. Nitrogen-and phosphorus-co-doped carbons with tunable enhanced surface areas promoted by the doping additives. Chem. Commun. 2013, 49, 1208–1210. [Google Scholar] [CrossRef] [PubMed]
- Okpalugo, T.I.T.; Papakonstantinou, P.; Murphy, H.; McLaughlin, J.; Brown, N.M.D. High resolution XPS characterization of chemical functionalised MWCNTs and SWCNTs. Carbon 2005, 43, 153–161. [Google Scholar] [CrossRef]
- Dubal, D.P.; Gund, G.S.; Lokhande, C.D.; Holze, R. Decoration of spongelike Ni(OH)2 nanoparticles onto MWCNTs using an easily manipulated chemical protocol for supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 2446–2454. [Google Scholar] [CrossRef]
- Xuan, H.; Ren, J.; Wang, X.; Zhang, J.; Ge, L. Flame-retardant, non-irritating and self-healing multilayer films with double-network structure. Compos. Sci. Technol. 2017, 145, 15–23. [Google Scholar] [CrossRef]
- Andreiadis, E.S.; Jacques, P.A.; Tran, P.D.; Leyris, A.; Chavarot-Kerlidou, M.; Jousselme, B.; Matheron, M.; Pécaut, J.; Palacin, S.; Fontecave, M.; et al. Molecular engineering of a cobalt-based electrocatalytic nanomaterial for H2 evolution under fully aqueous conditions. Nat. Chem. 2013, 5, 48–53. [Google Scholar] [CrossRef]
- Song, P.; Shen, Y.; Du, B.; Peng, M.; Shen, L.; Fang, Z. Effects of reactive compatibilization on the morphological, thermal, mechanical, and rheological properties of intumescent flame-retardant polypropylene. ACS Appl. Mater. Interfaces 2009, 1, 452–459. [Google Scholar] [CrossRef]
- Carosio, F.; Alongi, J. Ultra-Fast Layer-by-Layer Approach for Depositing Flame Retardant Coatings on Flexible PU Foams within Seconds. ACS Appl. Mater. Interfaces 2016, 8, 6315–6319. [Google Scholar] [CrossRef] [PubMed]
- Di, Y.; Iannace, S.; Maio, E.D.; Nicolais, L. Poly (lactic acid)/organoclay nanocomposites: Thermal, rheological properties and foam processing. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 689–698. [Google Scholar] [CrossRef]
- Tang, G.; Wang, X.; Xing, W.; Zhang, P.; Wang, B.; Hong, N.; Yang, W.; Hu, Y.; Song, L. Thermal degradation and flame retardance of biobased polylactide composites based on aluminum hypophosphite. Ind. Eng. Chem. Res. 2012, 51, 12009–12016. [Google Scholar] [CrossRef]
- Wang, L.; Qiu, J.; Sakai, E. Microstructures and mechanical properties of polylactic acid prepared by a cold rolling process. J. Mater. Process. Technol. 2016, 232, 184–194. [Google Scholar] [CrossRef]
- Ma, H.Y.; Tong, L.F.; Xu, Z.B.; Fang, Z.P. Functionalizing carbon nanotubes by grafting on intumescent flame retardant: Nanocomposite synthesis, morphology, rheology, and flammability. Adv. Funct. Mater. 2008, 18, 414–421. [Google Scholar] [CrossRef]
- Li, X.L.; Chen, M.J.; Chen, H.B. Facile fabrication of mechanically-strong and flame retardant alginate/clay aerogels. Compos. Part B Eng. 2019, 164, 18–25. [Google Scholar] [CrossRef]


| ID | AlPi (wt %) a | MWCNT-ODOPB (wt %) b | P (wt %) c | Tg (°C) d | Tg (°C) e | Char Yield f | LOI | UL-94 Grade |
|---|---|---|---|---|---|---|---|---|
| Epoxy resin | 0 | 0 | 0 | 148.5 | 161.2 | 25.4 | 25.0 | Not rating |
| EC-1 | 0 | 1 | 0.078 | 158.8 | 162.6 | 27.9 | 31.5 | V-1 |
| EC-2 | 2.92 | 1 | 0.75 | 160.2 | 172.7 | 23.3 | 36.5 | V-1 |
| EC-3 | 3.67 | 1 | 1.00 | 156.3 | 168.5 | 24.1 | 39.5 | V-0 |
| EC-4 | 6.18 | 1 | 1.50 | 174.3 | 180.2 | 27.5 | 41.2 | V-0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, L.; Qiu, C.; Qiu, J.; Yao, Y.; Sakai, E.; Yang, L. Preparation and Characterization of DOPO-Functionalized MWCNT and Its High Flame-Retardant Performance in Epoxy Nanocomposites. Polymers 2020, 12, 613. https://doi.org/10.3390/polym12030613
Gu L, Qiu C, Qiu J, Yao Y, Sakai E, Yang L. Preparation and Characterization of DOPO-Functionalized MWCNT and Its High Flame-Retardant Performance in Epoxy Nanocomposites. Polymers. 2020; 12(3):613. https://doi.org/10.3390/polym12030613
Chicago/Turabian StyleGu, Liqiang, Chen Qiu, Jianhui Qiu, Youwei Yao, Eiichi Sakai, and Liting Yang. 2020. "Preparation and Characterization of DOPO-Functionalized MWCNT and Its High Flame-Retardant Performance in Epoxy Nanocomposites" Polymers 12, no. 3: 613. https://doi.org/10.3390/polym12030613
APA StyleGu, L., Qiu, C., Qiu, J., Yao, Y., Sakai, E., & Yang, L. (2020). Preparation and Characterization of DOPO-Functionalized MWCNT and Its High Flame-Retardant Performance in Epoxy Nanocomposites. Polymers, 12(3), 613. https://doi.org/10.3390/polym12030613