Tuning the Wettability and Surface Free Energy of Poly(vinylphenol) Thin Films by Modulating Hydrogen-Bonding Interactions
Abstract
1. Introduction
2. Experimental Procedure
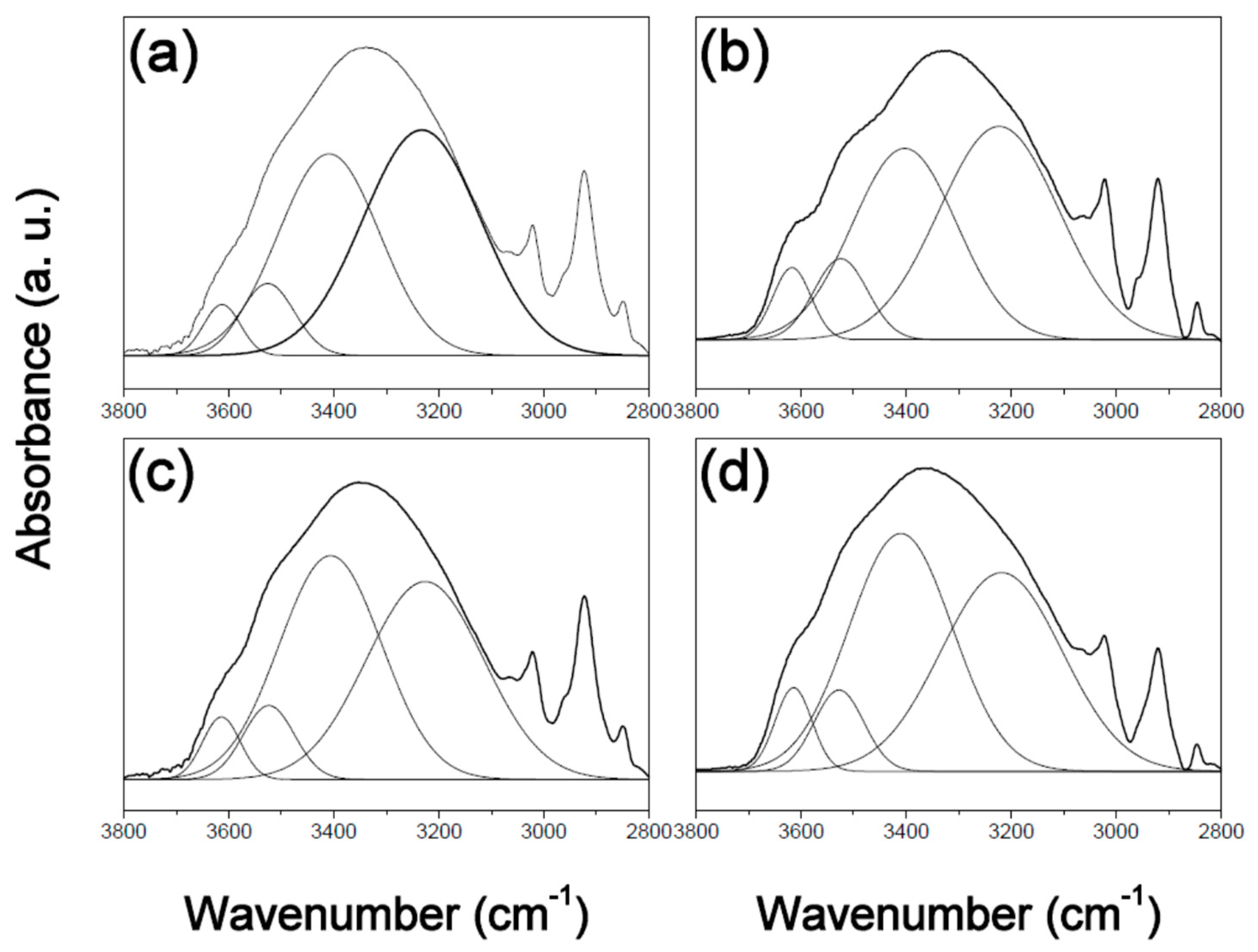
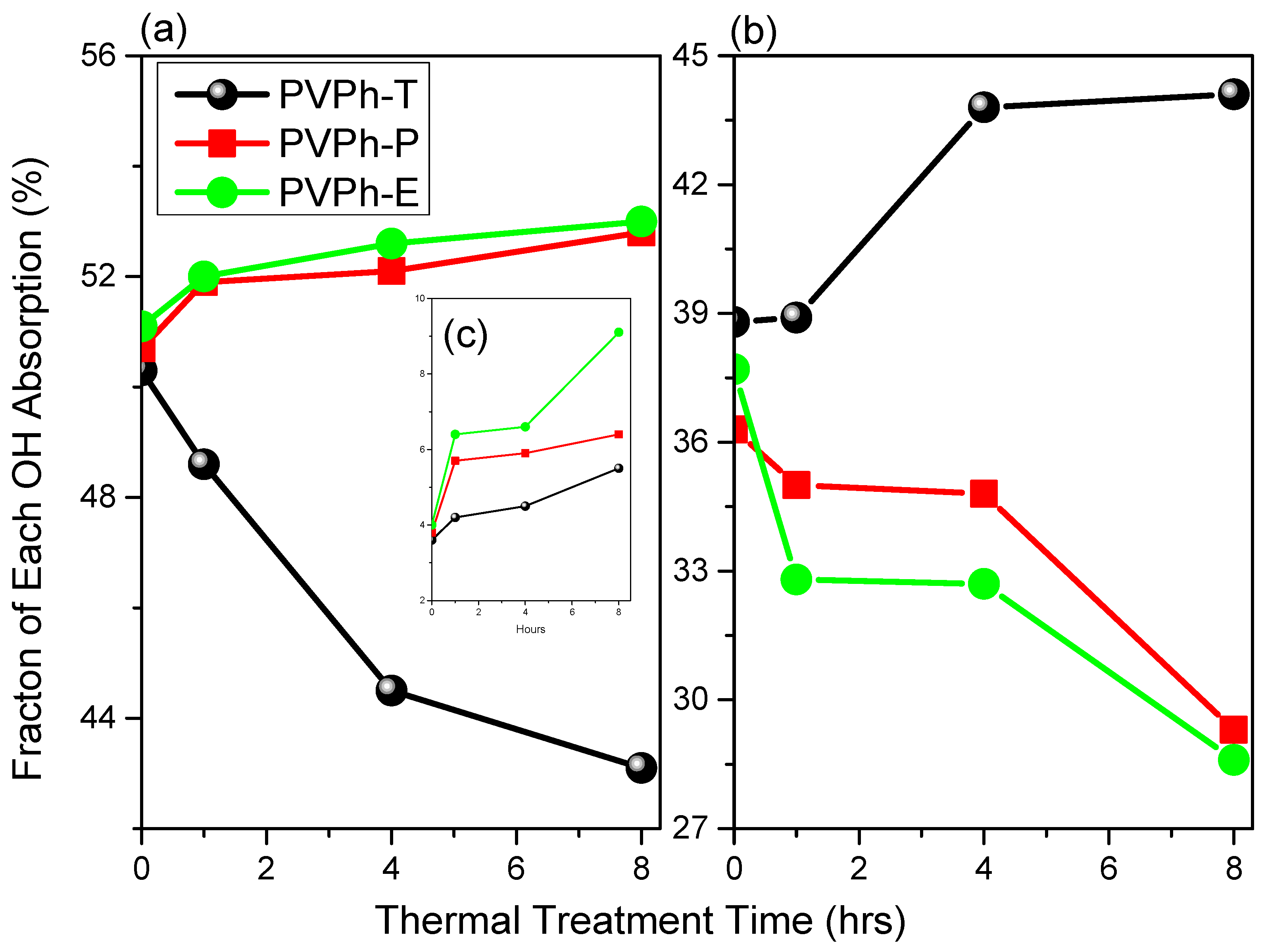
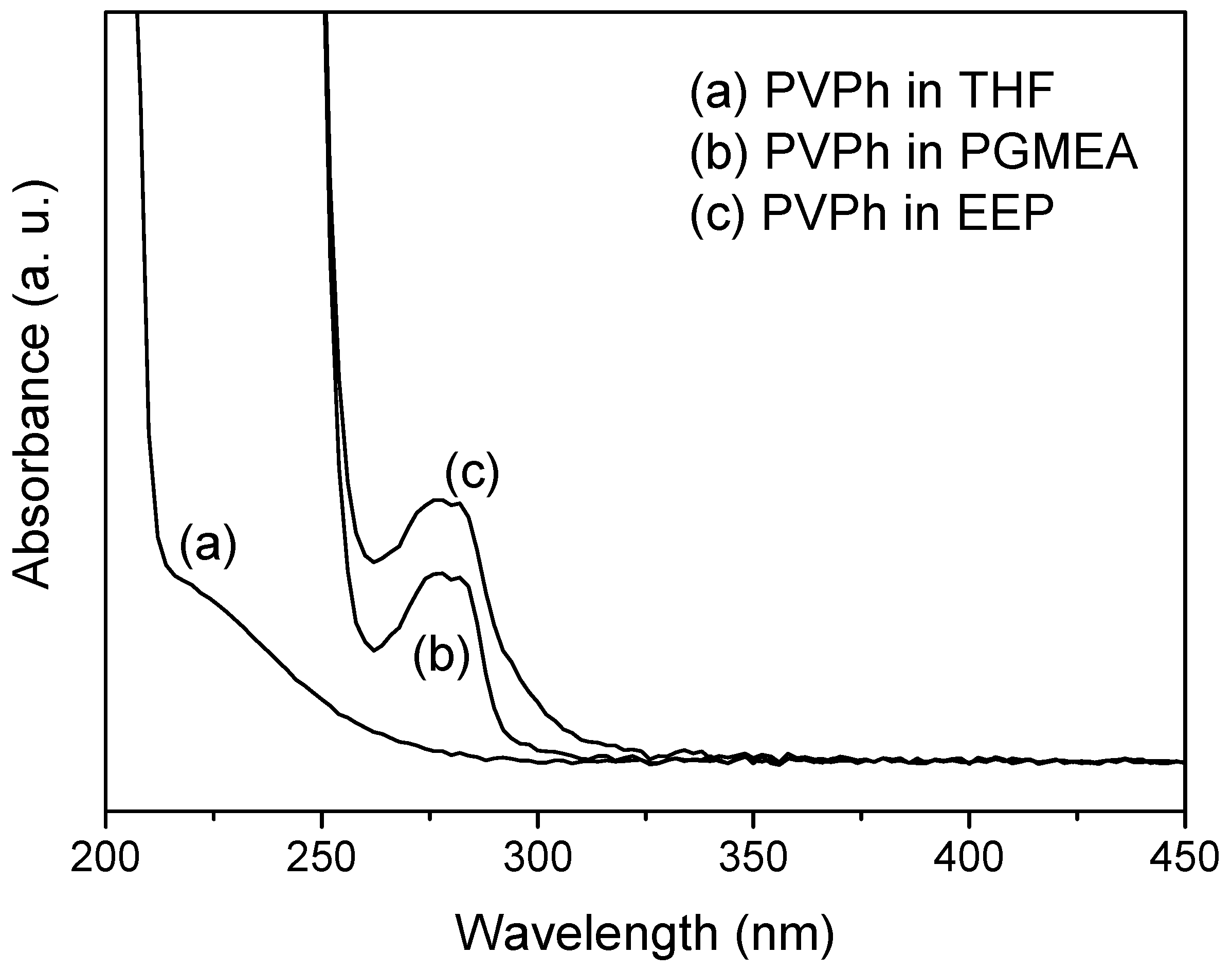
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, C.F.; Chiou, S.F.; Ko, F.H.; Chou, C.T.; Lin, H.C.; Huang, C.F.; Chang, F.C. Fabrication of Biomimetic Super-Amphiphobic Surfaces through Plasma Modification of Benzoxazine Films. Macromol. Rapid Commun. 2006, 27, 333–337. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, G.X.; Zhang, F.X.; Zhang, Y.S. Facile Preparation of Super-Hydrophilic Poly (Ethylene Terephthalate) Fabric Using Dilute Sulfuric Acid under Microwave Irradiation. Appl. Surf. Sci. 2015, 349, 437–444. [Google Scholar] [CrossRef]
- Bui, V.T.; Liu, X.Y.; Ko, S.H.; Choi, H.S. Super-Amphiphilic Surface of Nano Silica/Polyurethane Hybrid Coated Pet Film Via a Plasma Treatment. J. Colloid Interface Sci. 2015, 453, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.P.; Wang, T.M.; Wang, Q.H. Superhydrophilic and Underwater SuperoleophobicPVDF Membranes via Plasma-Induced Surface PEGDA for Effective Separation of Oil-in-Water Emulsions. Colloid Surf. A 2015, 481, 151–157. [Google Scholar] [CrossRef]
- Kuo, S.W. Hydrogen-Bonding in Polymer Blends. J. Polym. Res. 2008, 15, 459–486. [Google Scholar] [CrossRef]
- Kuo, S.W.; Lee, H.F.; Huang, C.F.; Huang, C.J.; Chang, F.C. Synthesis and Self-Assembly of Helical Polypeptide-Random Coil Amphiphilic Diblock Copolymer. J. Polym. Sci. Polym. Chem. 2008, 46, 3108–3119. [Google Scholar] [CrossRef]
- Kuo, S.W.; Tung, P.H.; Chang, F.C. Syntheses and the Study of Strongly Hydrogen-Bonded Poly(Vinylphenol-b-Vinylpyridine) Diblock Copolymer through Anionic Polymerization. Macromolecules 2006, 39, 9388–9395. [Google Scholar] [CrossRef]
- Sun, T.; Wang, G.; Feng, L.; Liu, B.; Ma, Y.; Jiang, L.; Zhu, D. Reversible Switching Between SuperhydrophilicityAndSuperhydrophobicity. Angew. Chem. Int. Ed. 2004, 43, 357–360. [Google Scholar] [CrossRef]
- Ma, K.X.; Chung, T.S. Effect of −C(CF3)2− on the Surface Energy of Main-Chain Liquid Crystalline and Crystalline Polymers. J. Phys. Chem. B 2001, 105, 4145–4150. [Google Scholar] [CrossRef]
- Wang, C.F.; Su, Y.C.; Kuo, S.W.; Huang, C.F.; Sheen, Y.C.; Chang, F.C. Low-Surface-Free-Energy Materials Based on Polybenzoxazines. Angew. Chem. Int. Ed. 2006, 45, 2248–2251. [Google Scholar] [CrossRef]
- Liao, C.S.; Wang, C.F.; Li, H.C.; Chou, H.Y.; Chang, F.C. Tuning the Surface Free Energy of Polybenzoxazine Thin Films. J. Phys. Chem. C 2008, 112, 16189–16191. [Google Scholar] [CrossRef]
- Liao, C.S.; Wang, C.F.; Lin, H.C.; Chou, H.Y.; Chang, F.C. Fabrication of Patterned SuperhydrophobicPolybenzoxazine Hybrid Surfaces. Langmuir 2009, 25, 3359–3362. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Chang, S.L.; Shen, T.Y.; Shih, T.Y.; Lin, H.T.; Wang, C.F. Flexible Polybenzoxazine Thermosets with High Glass Transition Temperatures and Low Surface Free Energies. Polym. Chem. 2012, 3, 935–945. [Google Scholar] [CrossRef]
- Lin, C.H.; Shih, Y.S.; Wang, M.W.; Tseng, C.Y.; Juang, T.Y.; Wang, C.F. Emission and surface properties of main-chain type polybenzoxazine with pyridinyl moieties. RSC Adv. 2014, 4, 8692–8698. [Google Scholar] [CrossRef]
- Lin, H.C.; Wang, C.F.; Kuo, S.W.; Tung, P.H.; Huang, C.F.; Lin, C.H.; Chang, F.C. Effect of Intermolecular Hydrogen Bonding on Low-Surface-Energy Material of Poly(vinylphenol). J. Phys. Chem. B 2007, 111, 3404–3410. [Google Scholar] [CrossRef]
- Kuo, S.W.; Wu, Y.C.; Wang, C.F.; Jeong, K.U. Preparing Low-Surface-Energy Polymer Materials by Minimizing Intermolecular Hydrogen-Bonding Interactions. J. Phys. Chem. C 2009, 113, 20666–20673. [Google Scholar] [CrossRef]
- Zha, J.; Lu, X.; Xin, Z. Hydrogen Bonding Effect on Wettability of Polysiloxane Coatings. J. Phys. Chem. C 2015, 119, 420–425. [Google Scholar] [CrossRef]
- Good, R.J.; van Oss, C.J. Modern Approaches to Wettability: Theory and Applications; Schrader, M.E., Loeb, G., Eds.; Plenum Press: New York, NY, USA, 1992; pp. 1–27. [Google Scholar]
- Fowkes, F.W. Adhesion and Adsorption of Polymers, Polymer Science and Technology; Lee, L.H., Ed.; Plenum Press: New York, NY, USA, 1980; Volume 12A, p. 43. [Google Scholar]
- Yuan, F.; Wang, W.; Yang, M.; Zhang, X.; Li, J.; Li, H.; He, B.; Minch, B.; Lieser, G.; Wegner, G. Layered Structure and Order-to-Disorder Transition in a Block Codendrimer Caused by Intermolecular Hydrogen Bonds. Macromolecules 2006, 39, 3982–3985. [Google Scholar] [CrossRef]
- Choperena, A.; Painter, P. Hydrogen Bonding in Polymers: Effect of Temperature on the OH Stretching Bands of Poly(vinyl phenol). Macromolecules 2009, 42, 6159–6165. [Google Scholar] [CrossRef]
- Kuo, S.W.; Tung, P.H.; Lai, C.L.; Jeong, K.U.; Chang, F.C. Supramolecularmicellization of diblock copolymer mixtures mediated by hydrogen bonding for the observation of separated coil and chain aggregation in common solvents. Macromol. Rapid Commun. 2008, 29, 229–233. [Google Scholar] [CrossRef]
- Kuo, S.W.; Tung, P.H.; Chang, F.C. Hydrogen bond mediated supramolecular micellization of diblock copolymer mixture in common solvents. Eur. Polym. J. 2009, 45, 1924–1935. [Google Scholar] [CrossRef]
- Lu, Y.S.; Lin, Y.C.; Kuo, S.W. Separated Coil and Chain Aggregation Behaviors on the Miscibility and Helical Peptide Secondary Structure of Poly (tyrosine) with Poly (4-vinylpyridine). Macromolecules 2012, 45, 6547–6556. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Lu, F.H.; Hong, J.L.; Kuo, S.W. Strong emission of 2, 4, 6-triphenylpyridine-functionalized polytyrosine and hydrogen-bonding interactions with poly (4-vinylpyridine). Polym. Chem. 2015, 6, 6340–6350. [Google Scholar] [CrossRef]
- Lu, Y.S.; Bastakoti, P.B.; Pramanik, M.; Hossain, M.S.A.; Alshehri, S.M.; Yamauchi, Y.; Kuo, S.W. Nanoarchitectures of self-assembled poly (styrene-b-4-vinyl pyridine) diblock copolymer blended with polypeptide for effective adsorption of mercury (ii) ions. RSC Adv. 2016, 6, 106866–106872. [Google Scholar] [CrossRef]
- Kuo, S.W. Hydrogen Bbonding in Polymeric Materials; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Wang, B.C.; Huang, Y.D.; Liu, L. Solvent-Dependent Adsorption of Phenolic Resin Onto Silica Substrate. J. Adhesion Sci. Technol. 2005, 19, 549–563. [Google Scholar] [CrossRef]


| Temp. (°C) | Time (h) | rms (nm) | Contact Angle (°) | Surface Free Energy (mJ/m2) | |||
|---|---|---|---|---|---|---|---|
| H2O | Diiodomethane (DIM) | ||||||
| 60 | 2 | 10.3 | 76 | 44 | 32.5 | 6.8 | 39.3 |
| 180 | 1 | 4.5 | 90 | 57 | 27.8 | 2.7 | 30.5 |
| 4 | 3.3 | 98 | 74 | 18.5 | 2.5 | 21.1 | |
| 8 | 7.8 | 101 | 78 | 16.7 | 2.1 | 18.8 | |
| Temp. (°C) | Time (h) | rms (nm) | Contact Angle (°) | Surface Free Energy (mJ/m2) | |||
|---|---|---|---|---|---|---|---|
| H2O | DIM | ||||||
| 60 | 2 | 1.1 | 63 | 42 | 30.6 | 14.4 | 45.0 |
| 180 | 1 | 4.2 | 59 | 46 | 27.5 | 18.4 | 45.9 |
| 4 | 3.0 | 49 | 48 | 24.5 | 27.1 | 51.6 | |
| 8 | 5.6 | 45 | 47 | 24.4 | 29.9 | 54.3 | |
| Temp. (°C) | Time (h) | rms (nm) | Contact Angle (°) | Surface Free Energy (mJ/m2) | |||
|---|---|---|---|---|---|---|---|
| H2O | DIM | ||||||
| 60 | 2 | 1.3 | 62 | 42 | 30.4 | 15.1 | 45.5 |
| 180 | 1 | 3.1 | 57 | 43 | 27.7 | 19.6 | 47.3 |
| 4 | 2.4 | 51 | 46 | 26.0 | 24.7 | 50.7 | |
| 8 | 2.3 | 47 | 48 | 24.2 | 28.7 | 52.9 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-F.; Ejeta, D.D.; Wu, J.-Y.; Kuo, S.-W.; Lin, C.-H.; Lai, J.-Y. Tuning the Wettability and Surface Free Energy of Poly(vinylphenol) Thin Films by Modulating Hydrogen-Bonding Interactions. Polymers 2020, 12, 523. https://doi.org/10.3390/polym12030523
Wang C-F, Ejeta DD, Wu J-Y, Kuo S-W, Lin C-H, Lai J-Y. Tuning the Wettability and Surface Free Energy of Poly(vinylphenol) Thin Films by Modulating Hydrogen-Bonding Interactions. Polymers. 2020; 12(3):523. https://doi.org/10.3390/polym12030523
Chicago/Turabian StyleWang, Chih-Feng, Dula Daksa Ejeta, Jian-Yi Wu, Shiao-Wei Kuo, Ching-Hsuan Lin, and Juin-Yih Lai. 2020. "Tuning the Wettability and Surface Free Energy of Poly(vinylphenol) Thin Films by Modulating Hydrogen-Bonding Interactions" Polymers 12, no. 3: 523. https://doi.org/10.3390/polym12030523
APA StyleWang, C.-F., Ejeta, D. D., Wu, J.-Y., Kuo, S.-W., Lin, C.-H., & Lai, J.-Y. (2020). Tuning the Wettability and Surface Free Energy of Poly(vinylphenol) Thin Films by Modulating Hydrogen-Bonding Interactions. Polymers, 12(3), 523. https://doi.org/10.3390/polym12030523








