Dentin Bonding and SEM Analysis of a New Experimental Universal Adhesive System Containing a Dendrimer
Abstract
1. Introduction
2. Materials and Methods
2.1. Formulation of the Experimental Adhesives
2.2. Specimen Preparation
2.3. Micro-Tensile Bond Strength (µTBS)
2.4. SEM Analysis
2.5. Statistical Analysis
3. Results
3.1. Dentin µTBS
3.1.1. Part A: GLM Analysis of µTBS Data
- There was no significant interaction between the independent factors (adhesives and protocols) (p = 0.153).
- There were significant differences between the adhesives (p = 0.023), with an observed power of 74.9%.
- Post-hoc tests concluded that AE1 presented a significantly higher µTBS than SBU (p = 0.012), with no differences among the other adhesives.
- The ER protocol showed significantly better results compared with SE protocol (p = 0.015), with an observed power of 71.0%.
3.1.2. Part B: LMM Analysis of µTBS Data
- No significant interaction was observed between the adhesive and protocol factors (p = 0.125).
- The effect of the adhesive type was significant (p = 0.010), as well as of the protocol (p = 0.019), with better results observed for the ER approach.
- Multiple comparisons showed significant differences only between the AE1 and SBU adhesives (p = 0.019).
3.2. Failure Modes and SEM Results
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Munck, J.; Van Landuyt, K.; Peumans, M.; Poitevin, A.; Lambrechts, P.; Braem, M.; Van Meerbeek, B. A critical review of the durability of adhesion to tooth tissue: Methods and results. J. Dent. Res. 2005, 84, 118–132. [Google Scholar] [CrossRef]
- Liu, Y.; Tjäderhane, L.; Breschi, L.; Mazzoni, A.; Li, N.; Mao, J.; Pashley, D.H.; Tay, F.R. Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J. Dent. Res. 2011, 90, 953–968. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.N.; Lee, J.H.; Son, S.A.; Jung, K.H.; Kwon, Y.H.; Park, J.K. Effect of Dentin Wetness on the Bond Strength of Universal Adhesives. Materials 2017, 10, 1224. [Google Scholar] [CrossRef] [PubMed]
- Loguercio, A.D.; Barroso, L.P.; Grande, R.H.; Reis, A. Comparison of intra- and intertooth resin–dentin bond strength variability. J. Adhes. Dent. 2005, 7, 151–158. [Google Scholar] [PubMed]
- Muñoz, M.A.; Sezinando, A.; Luque-Martinez, I.; Szesz, A.L.; Reis, A.; Loguercio, A.D.; Bombarda, N.H.; Perdigão, J. Influence of a hydrophobic resin coating on the bonding efficacy of three universal adhesives. J. Dent. 2014, 42, 595–602. [Google Scholar] [CrossRef]
- Betancourt, D.E.; Baldion, P.A.; Castellanos, J.E. Resin-Dentin Bonding Interface: Mechanisms of Degradation and Strategies for Stabilization of the Hybrid Layer. Int. J. Biomater. 2019, 3, 5268342. [Google Scholar] [CrossRef]
- Sofan, E.; Sofan, A.; Palaia, G.; Tenore, G.; Romeo, U.; Migliau, G. Classification review of dental adhesive systems: From the IV generation to the universal type. Ann. Stomatol 2017, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, B.; De Munk, J.; Yoshida, Y.; Inoue, S.; Vargas, M.; Vijay, P.; Van Landuyt, K.; Lambrechts, P.; Vanherle, G. Adhesion to enamel and dentin: Current status and future challenges. Oper. Dent. 2003, 28, 215–235. [Google Scholar]
- Pashley, D.H.; Tay, F.R.; Breschi, L.; Tjäderhane, L.; Carvalho, R.M.; Carrillo, M.; Tezvergil-Mutluay, A. State of the art etch-and-rinse adhesives. Dent. Mater. 2011, 27, 1–16. [Google Scholar] [CrossRef]
- Moszner, N.; Salz, U.; Zimmermann, J. Chemical aspects of self-etching enamel–dentin adhesives: A systematic review. Dent. Mater. 2005, 21, 895–910. [Google Scholar] [CrossRef]
- Tay, F.R.; Pashley, D.H. Aggressiveness of contemporary self-etching systems. I: Depth of penetration beyond dentin smear layers. Dent. Mater. 2001, 17, 296–308. [Google Scholar] [CrossRef]
- Rosa, W.L.; Piva, E.; Silva, A.F. Bond strength of universal adhesives: A systematic review and meta-analysis. J. Dent. 2005, 43, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Wendler, M.; Petschelt, A.; Belli, R.; Lohbauer, U. Bonding performance of universal adhesives in different etching modes. J. Dent. 2014, 42, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Perdigão, J.; Swift, E.J., Jr. Universal Adhesives. J. Esthet. Restor. Dent. 2015, 27, 331–334. [Google Scholar] [CrossRef]
- Nunes, T.G.; Polido, M.; Amorim, A.; Nunes, S.G.; Toledano, M. Multinuclear magnetic resonance studies on the chemical interaction of a self-etching adhesive with radicular and coronal human dentin. J. Mater. Sci. Mater. Med. 2007, 18, 2093–2099. [Google Scholar] [CrossRef]
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resins. Biomaterials 2002, 23, 1819–1829. [Google Scholar] [CrossRef]
- Landuyt, K.L.; Snauwaert, J.; De Munck, J.; Peumans, M.; Yoshida, Y.; Poitevin, A.; Coutinho, E.; Suzuki, K.; Lambrechts, P.; Van Meerbeek, B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007, 28, 3757–3785. [Google Scholar] [CrossRef]
- Fleisch, A.F.; Sheffield, P.E.; Chinn, C.; Edelstein, B.L.; Landrigan, P.J. Bisphenol A and related compounds in dental materials. Pediatrics 2010, 126, 760–768. [Google Scholar] [CrossRef]
- Soderholm, K.J.; Mariotti, A. BIS-GMA-based resins in dentistry: Are they safe. J. Am. Dent. Assoc. 1999, 130, 201–209. [Google Scholar] [CrossRef]
- Yu, B.; Liu, F.; He, J. Preparation of low shrinkage methacrylate-based resin system without Bisphenol A structure by using a synthesized dendritic macromere (G-IEMA). J. Mech. Behav. Biomed. Mater. 2014, 35, 1–8. [Google Scholar] [CrossRef]
- Silva e Souza, M.H., Jr.; Carneiro, K.G.K.; Lobato, M.F.; e Souza, S.; de Almeida Rodrigues, P.; Góes, M.F.D. Adhesive systems: Important aspects related to their composition and clinical use. J. Appl. Oral Sci. 2010, 18, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos e Cruz, J.; Brito, J.; Polido, M.; Gonçalves, L. A new experimental adhesive system containing G-IEMA—Physicochemical properties. J. Adhes. Sci. Technol. 2019, 33, 418–432. [Google Scholar] [CrossRef]
- Shono, Y.; Ogawa, T.; Terashita, M. Regional measurement of resin-dentin bonding as an array. J. Dent. Res. 1999, 78, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Perdigao, J.; Lambrechts, P.; Van Meerbeek, B.; Vanherle, G.; Lopes, A.L. Field emission SEM comparison of four postfixation drying techniques for human dentin. J. Biomed. Mater. Res. 1995, 29, 1111–1120. [Google Scholar] [CrossRef]
- Schneider, H.; Fröhlich, M.; Erler, G.; Engelke, C.; Merte, K. Interaction patterns between dentin and adhesive on prepared class V cavities in vitro and in vivo. J. Biomed. Mater. Res. 2000, 53, 86–92. [Google Scholar] [CrossRef]
- Gallusi, G.; Galeano, P.; Libonati, A.; Giuca, M.R.; Campanella, V. Evaluation of bond strength of different adhesive systems: Shear and Microtensile Bond Strength Test. Oral Implantol. 2009, 2, 19–25. [Google Scholar]
- Pashley, D.H.; Sano, H.; Ciucchi, B.; Yoshiyama, M.; Carvalho, R.M. Adhesion testing of dentin bonding agents: A review. Dent. Mater. 1995, 11, 117–125. [Google Scholar] [CrossRef]
- Sezinando, A. Looking for the ideal adhesive—A review. Rev. Port. Estomatol. Med. Dentária Cir. Maxilofac. 2014, 55, 194–206. [Google Scholar] [CrossRef]
- Hanabusa, M.; Mine, A.; Kuboki, T.; Momoi, Y.; Van Ende, A.; Van Meerbeek, B.; De Munck, J. Bonding effectiveness of a new ‘multi-mode’ adhesive to enamel and dentine. J. Dent. 2012, 40, 475–484. [Google Scholar] [CrossRef]
- Nicoloso, G.F.; Antoniazzi, B.F.; Lenzi, T.L.; Soares, F.Z.M.; Rocha, R.O. The Bonding Performance of a Universal Adhesive to Artificially-created Caries-affected Dentin. J. Adhes. Dent. 2017, 19, 317–321. [Google Scholar] [CrossRef]
- Yoshida, Y.; Yoshihara, K.; Nagaoka, N.; Hayakawa, S.; Torii, Y.; Ogawa, T.; Osaka, A.; Meerbeek, B.V. Self-assembled Nano-layering at the Adhesive interface. J. Dent. Res. 2012, 91, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Perdigao, J.; Sezinando, A.; Monteiro, P.C. Laboratory bonding ability of a multi-purpose dentin adhesive. Am. J. Dent. 2012, 25, 153–158. [Google Scholar] [PubMed]
- Munoz, M.A.; Luque, I.; Hass, V.; Reis, A.; Loguercio, A.D.; Bombarda, N.H. Immediate bonding properties of universal adhesives to dentine. J. Dent. 2013, 41, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Luque-Martinez, I.V.; Perdigão, J.; Muñoz, M.A.; Sezinando, A.; Reis, A.; Loguercio, A.D. Effects of solvent evaporation time on immediate adhesive properties of universal adhesives to dentin. Dent. Mater. 2014, 30, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, B.; Yoshihara, K.; Yoshida, Y.; Mine, A.; De Munck, J.; Van Landuyt, K.L. State of the art of self-etch adhesives. Dent. Mater. 2011, 27, 17–28. [Google Scholar] [CrossRef]
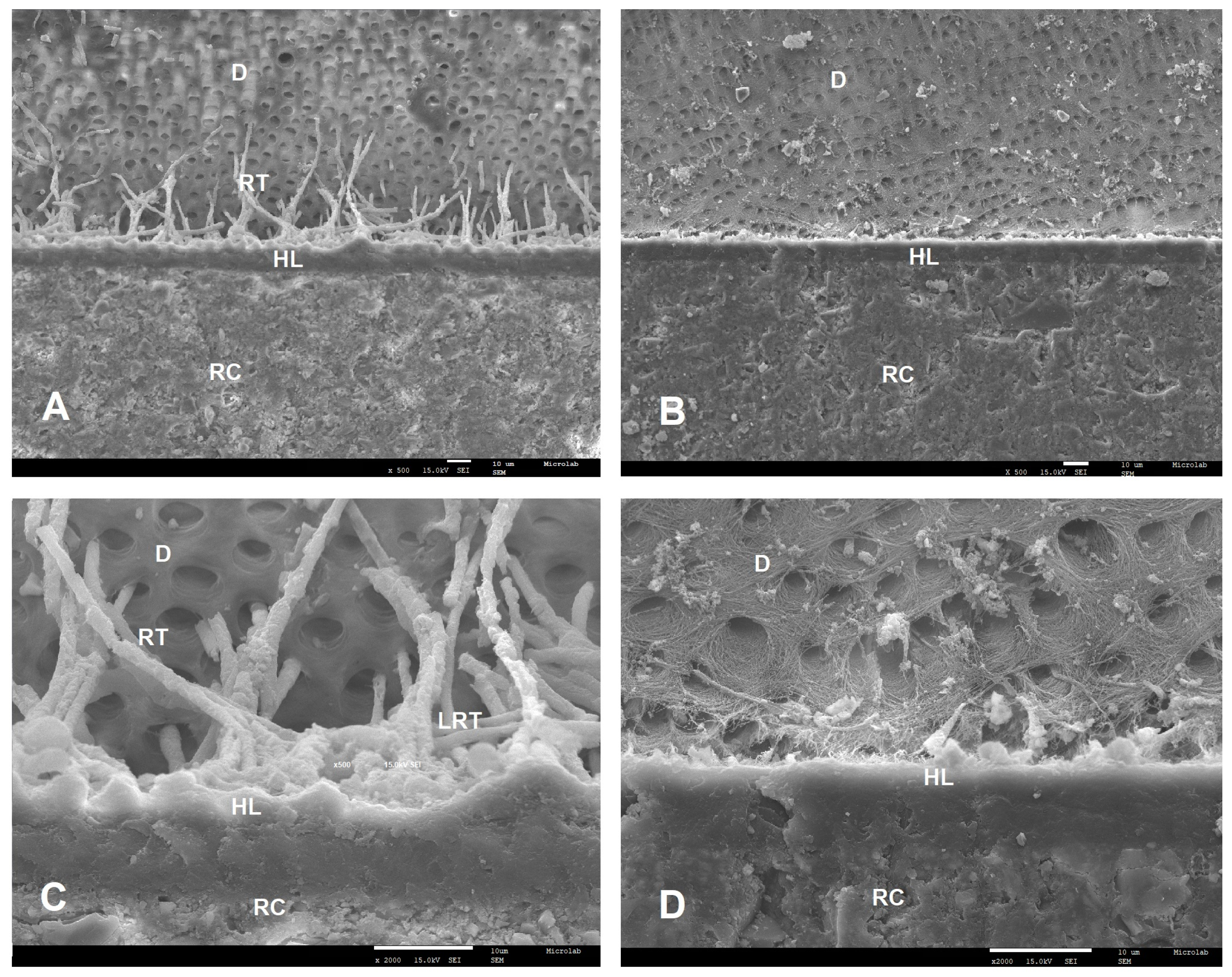
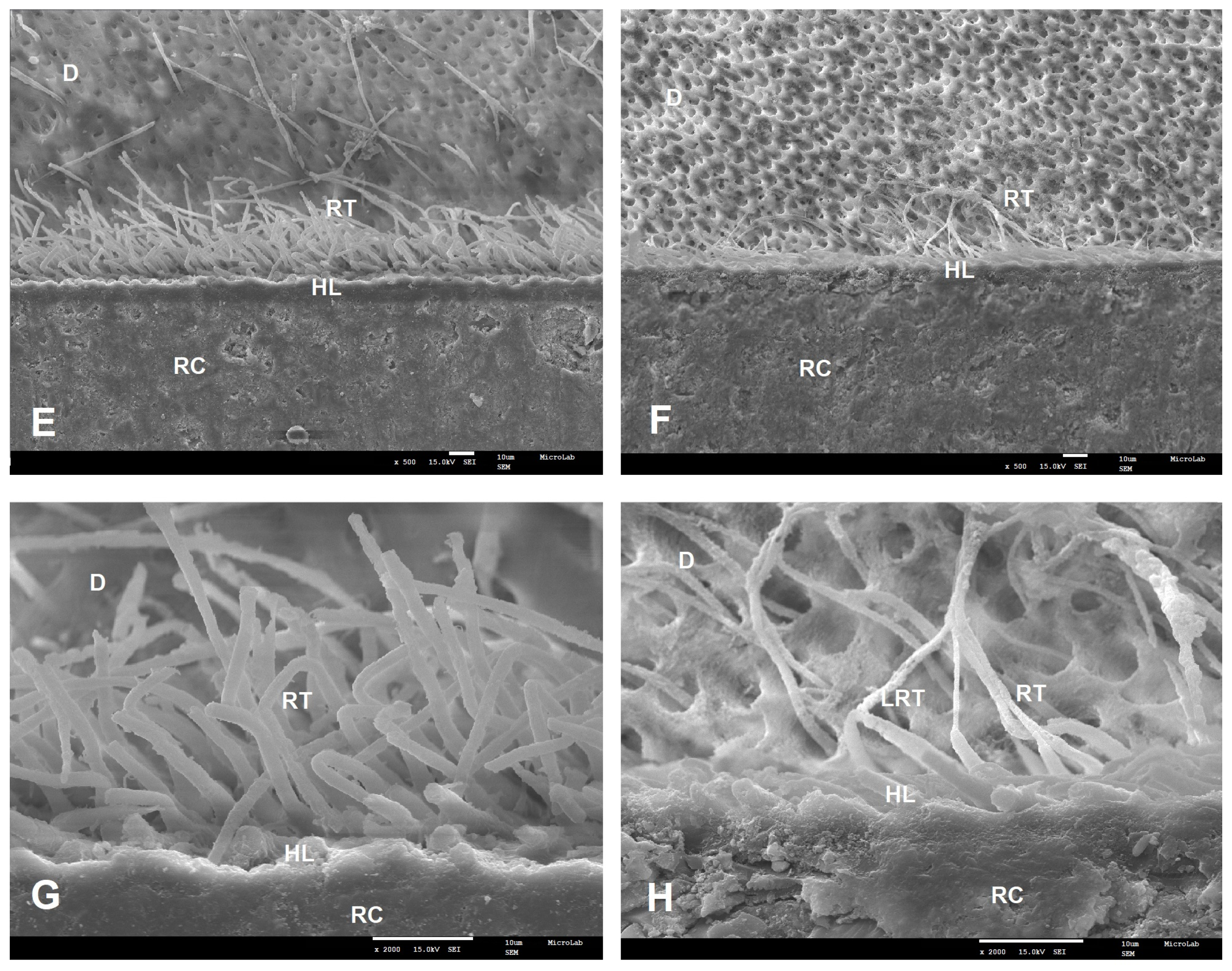
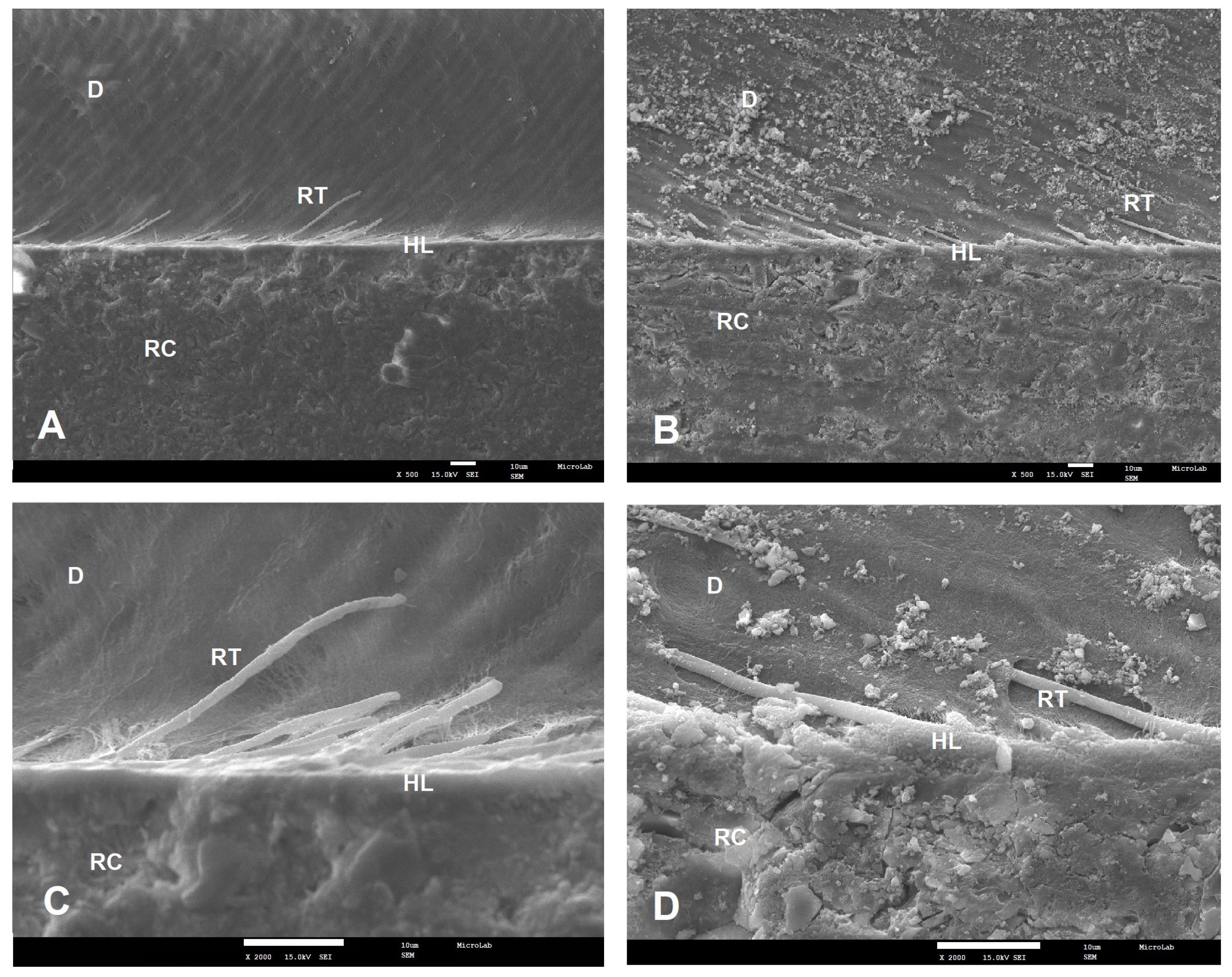
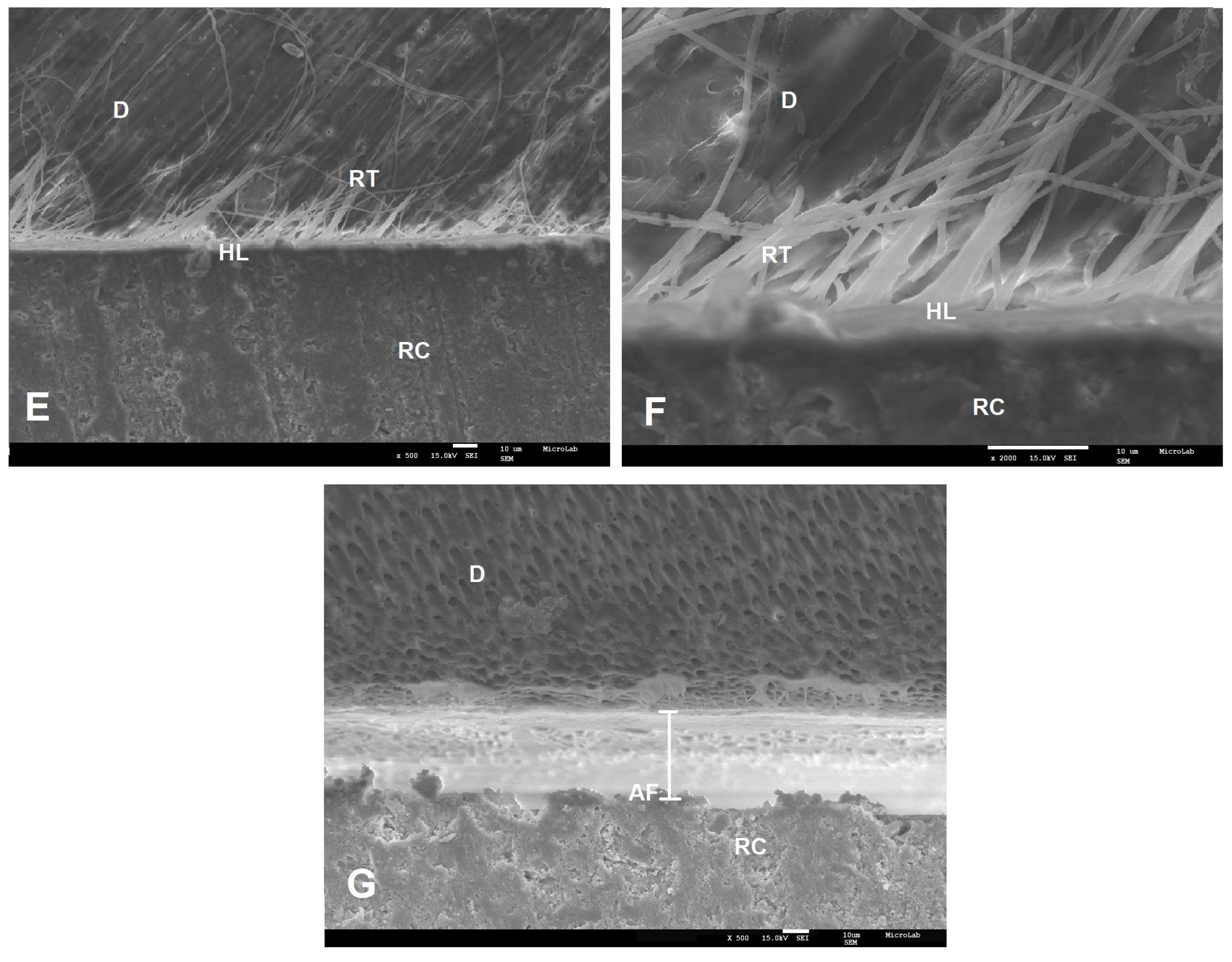
| Adhesive | Primary Ingredients | pH | Experimental Groups | n | Adhesive System Applied |
|---|---|---|---|---|---|
| Scotchbond Universal (3M ESPE, St. Paul, MN, USA), Lot #587502 | 10-MDP; HEMA; Bis-GMA; ethanol; TEGDMA; silane treated silica; water; CQ | 2.95 | SBU-ER | 5 | Etch-and-rinse |
| SBU-SE | 5 | Self-etch | |||
| Futurabond M+ (VOCO GmbH, Cuxhaven, Germany), Lot #1624201 | HEMA; Bis-GMA; ethanol; Acidic adhesive Monomer; UDMA; pyrogenic silicic acids | 1.9 | FUT-ER | 3 | Etch-and-rinse |
| FUT-SE | 3 | Self-etch | |||
| AE1 (experimental adhesive formulated with Bis-GMA) | 10-MDP; Bis-GMA; HEMA; UDMA; TEGDMA; water; etanol; CQ | 2.09 | AE1-ER | 3 | Etch-and-rinse |
| AE1-SE | 5 | Self-etch | |||
| AE2 (experimental adhesive formulated with G-IEMA) | 10-MDP; G-IEMA; HEMA; UDMA; TEGDMA; water; etanol; CQ | 3.5 | AE2-ER | 5 | Etch-and-rinse |
| AE2-SE | 3 | Self-etch |
| GROUPS | PROTOCOL | µTBS | N | |
|---|---|---|---|---|
| Mean | S.D. | |||
| SBU-ER | ER | 26.20 | 6.46 | 5 |
| FUT-ER | 33.96 | 3.22 | 3 | |
| AE1-ER | 33.38 | 6.57 | 3 | |
| AE2-ER | 36.50 | 4.25 | 5 | |
| SBU-SE | SE | 23.69 | 3.15 | 5 |
| FUT-SE | 24.10 | 2.62 | 3 | |
| AE1-SE | 34.66 | 8.41 | 5 | |
| AE2-SE | 25.57 | 7.48 | 3 | |
| Information Criteria | Covariance Structures | ||||||
|---|---|---|---|---|---|---|---|
| SI | CS | Diagonal | AR(1) | Huynh-Feldt | ARMA(1) | Toeplitz | |
| −2 Restricted Log Likelihood | 4278.983 | 4261.939 | 4231.212 | 4278.313 | 4278.983 | 4264.345 | 4228.351 |
| Akaike’s Information Criterion (AIC) | 4280.983 | 4265.939 | 4289.212 | 4282.313 | 4338.983 | 4270.345 | 4286.351 |
| Hurvich and Tsai’s Criterion (AICC) | 4280.990 | 4265.962 | 4292.771 | 4282.337 | 4342.794 | 4270.392 | 4289.909 |
| Bozdogan’s Criterion (CAIC) | 4286.235 | 4276.442 | 4441.517 | 4292.817 | 4496.540 | 4286.101 | 4438.656 |
| Schwarz’s Bayesian Criterion (BIC) | 4285.235 | 4274.442 | 4412.517 | 4290.817 | 4466.540 | 4283.101 | 4409.656 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasconcelos e Cruz, J.; Polido, M.; Brito, J.; Gonçalves, L.L. Dentin Bonding and SEM Analysis of a New Experimental Universal Adhesive System Containing a Dendrimer. Polymers 2020, 12, 461. https://doi.org/10.3390/polym12020461
Vasconcelos e Cruz J, Polido M, Brito J, Gonçalves LL. Dentin Bonding and SEM Analysis of a New Experimental Universal Adhesive System Containing a Dendrimer. Polymers. 2020; 12(2):461. https://doi.org/10.3390/polym12020461
Chicago/Turabian StyleVasconcelos e Cruz, Joana, Mário Polido, José Brito, and Luisa L. Gonçalves. 2020. "Dentin Bonding and SEM Analysis of a New Experimental Universal Adhesive System Containing a Dendrimer" Polymers 12, no. 2: 461. https://doi.org/10.3390/polym12020461
APA StyleVasconcelos e Cruz, J., Polido, M., Brito, J., & Gonçalves, L. L. (2020). Dentin Bonding and SEM Analysis of a New Experimental Universal Adhesive System Containing a Dendrimer. Polymers, 12(2), 461. https://doi.org/10.3390/polym12020461







