Extrusion of Porous Protein-Based Polymers and Their Liquid Absorption Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Extrusion of the WG/Water Formulations
2.2. Extrusion of WG/Water/Glycerol
2.3. Extrusion of WG/Water/Sodium Bicarbonate
2.4. Extrusion of WG/Water/Glycerol/Sodium Bicarbonate
2.5. Swelling
2.6. Density Measurements
2.7. Fourier-Transform Infrared Spectroscopy (FTIR)
2.8. Scanning Electron Microscopy (SEM)
2.9. Thermal Gravimetric Analysis (TGA)
3. Results and Discussion
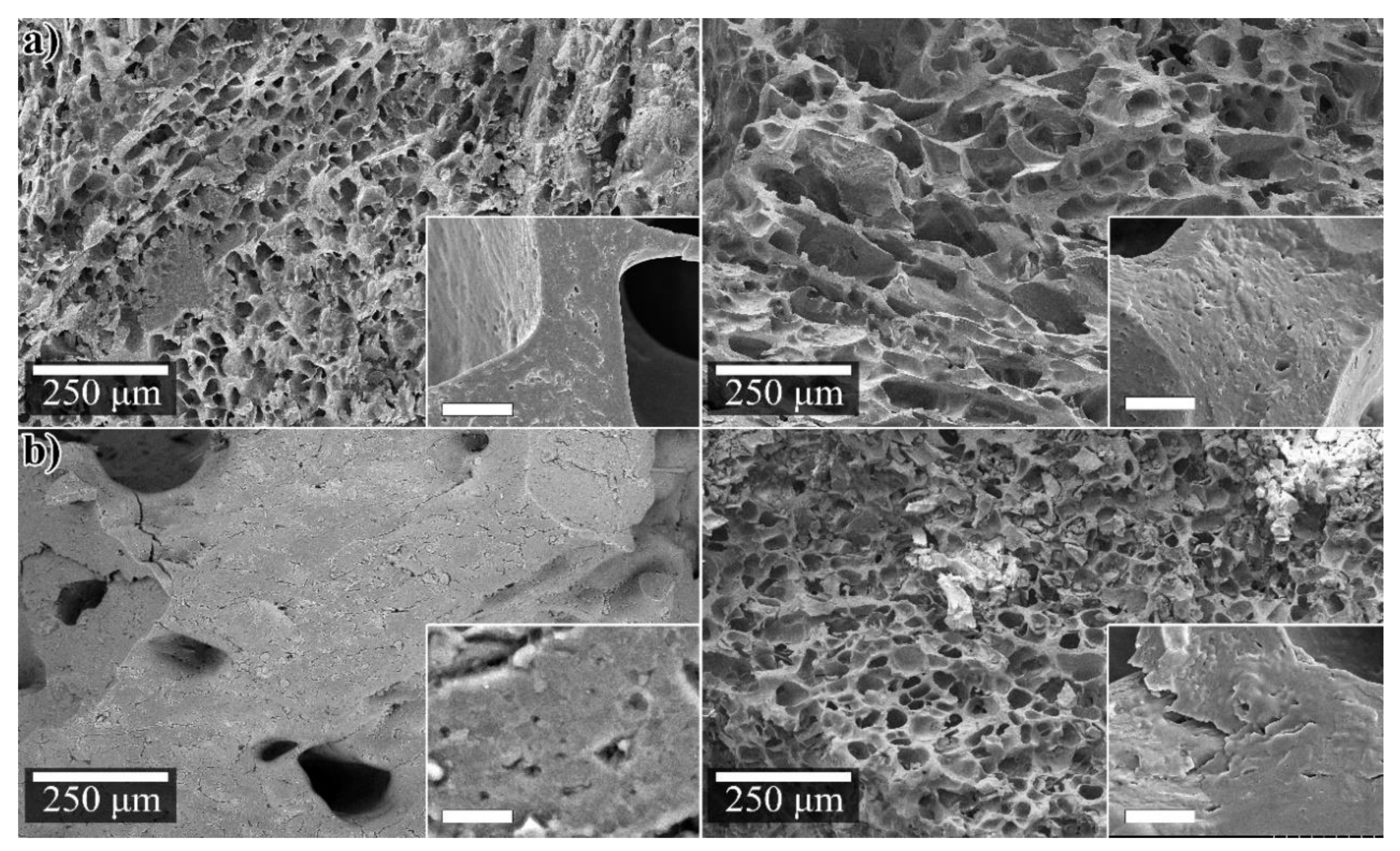
3.1. Physical Characteristics
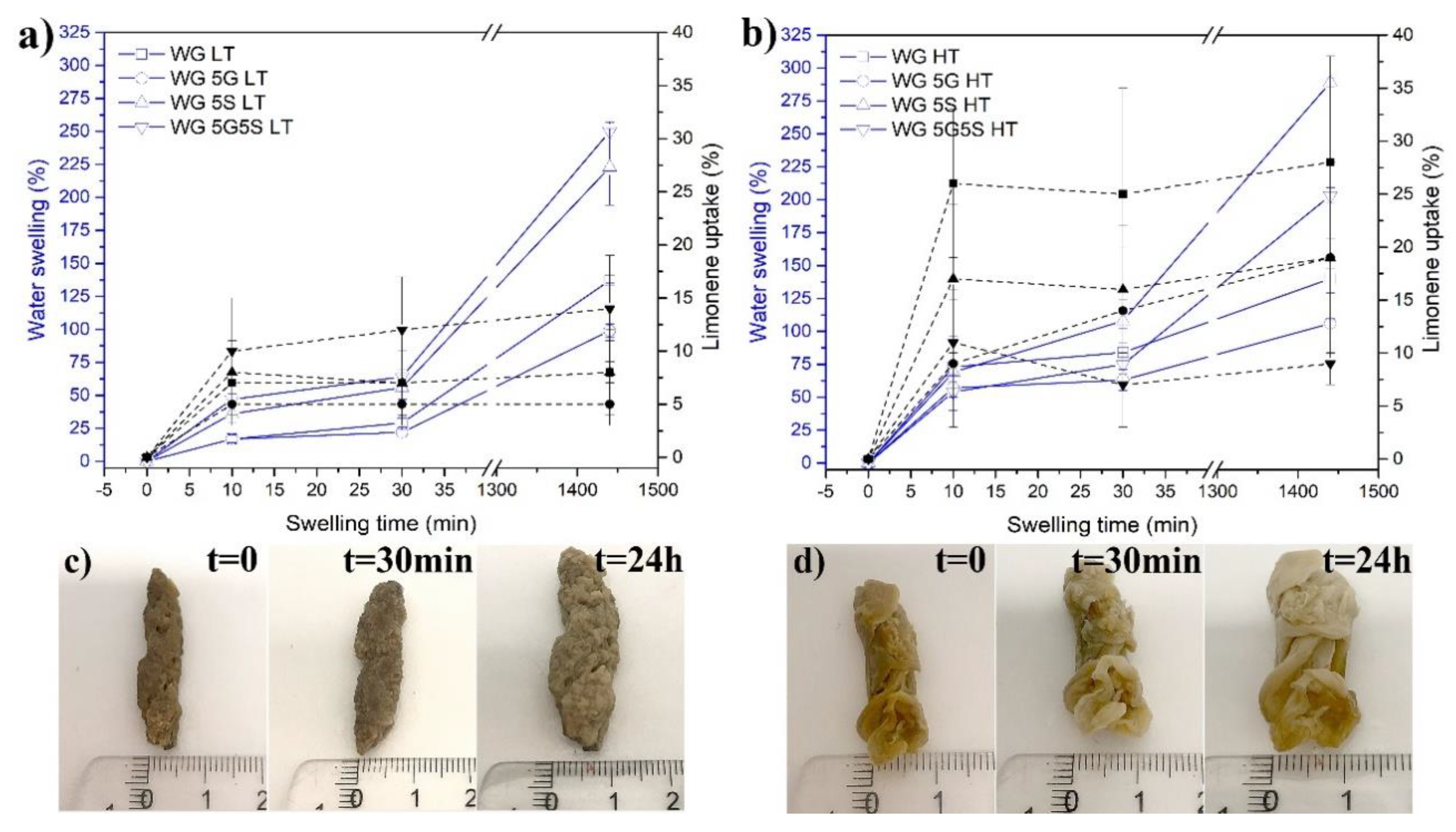
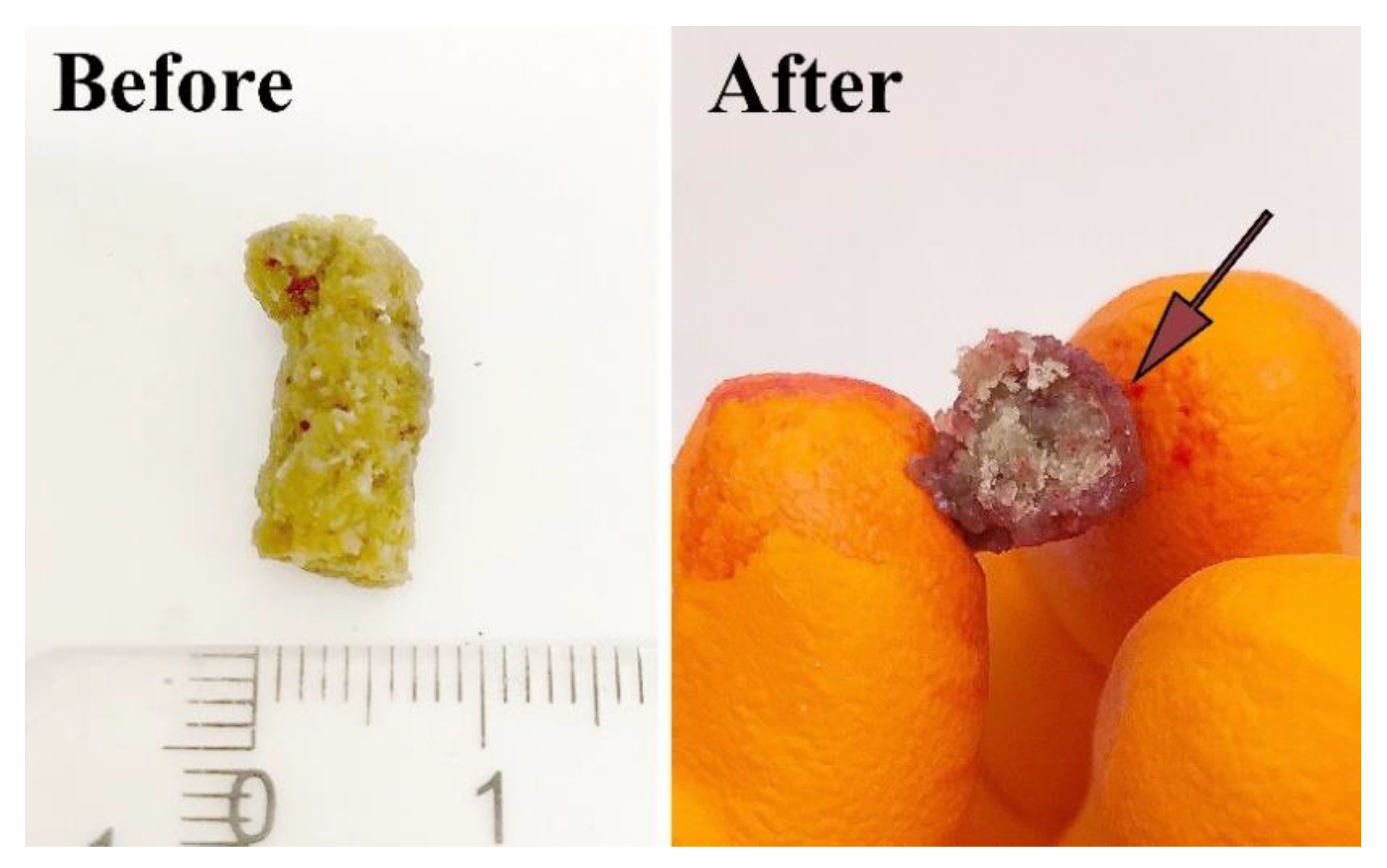
3.2. Swelling
3.3. Protein Thermal and Structural Characteristics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jin, F.-L.; Zhao, M.; Park, M.; Park, S.-J. Recent Trends of Foaming in Polymer Processing: A Review. Polymers 2019, 11, 953. [Google Scholar] [CrossRef] [PubMed]
- Khemani, K.C. Polymeric Foams: An Overview. In Polymeric Foams; American Chemical Society: Washington, DC, USA, 1997; Volume 669, pp. 1–7. [Google Scholar]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Isbrandt, M. Effect of Evening Primrose Oil-Based Polyol on the Properties of Rigid Polyurethane-Polyisocyanurate Foams for Thermal Insulation. Polymers 2018, 10, 1334. [Google Scholar] [CrossRef]
- Gama, N.V.; Ferreira, A.; Barros-Timmons, A. Polyurethane Foams: Past, Present, and Future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef] [PubMed]
- Sabbahi, A.; Vergnaud, J.M. Absorption of water by polyurethane foam. modelling and experiments. Eur. Polym. J. 1993, 29, 1243–1246. [Google Scholar] [CrossRef]
- Atta, A.M.; Brostow, W.; Hagg Lobland, H.E.; Hasan, A.R.M.; Perez, J.M. Network and swelling parameters of cross-linked octadecylacrylate-co-acrylic acid copolymers based on divinyl benzene cross-linker. Mater. Res. Innov. 2015, 19, 459–468. [Google Scholar] [CrossRef]
- Atta, A.M.; Brostow, W.; Hagg Lobland, H.E.; Hasan, A.-R.M.; Perez, J.M. Porous crosslinked copolymers of octadecyl acrylate with acrylic acid as sorbers for crude petroleum spills. Polym. Int. 2013, 62, 1225–1235. [Google Scholar] [CrossRef]
- Atta, A.M.; Brostow, W.; Hagg Lobland, H.E.; Hasan, A.-R.M.; Perez, J.M. Porous polymer oil sorbents based on PET fibers with crosslinked copolymer coatings. Rsc Adv. 2013, 3, 25849–25857. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Brandt, K.A.; Goldman, S.A.; Inglin, T.A. Hydrogel-forming polymer compositions for use in absorbent structures. U.S. Patent 4,654,039, 31 March 1987. [Google Scholar]
- Capezza, A.J.; Newson, W.R.; Olsson, R.T.; Hedenqvist, M.S.; Johansson, E. Advances in the Use of Protein-Based Materials: Toward Sustainable Naturally Sourced Absorbent Materials. Acs Sustain. Chem. Eng. 2019, 7, 4532–4547. [Google Scholar] [CrossRef]
- Demitri, C.; Del Sole, R.; Scalera, F.; Sannino, A.; Vasapollo, G.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Novel superabsorbent cellulose-based hydrogels crosslinked with citric acid. J. Appl. Polym. Sci. 2008, 110, 2453–2460. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Kurdtabar, M.; Mahdavinia, G.R.; Hosseinzadeh, H. Synthesis and super-swelling behavior of a novel protein-based superabsorbent hydrogel. Polym. Bull. 2006, 57, 813–824. [Google Scholar] [CrossRef]
- Zohuriaan-Mehr, M.J.; Kabiri, K. Superabsorbent polymer materials: A review. Iran. Polym. J. 2008, 17, 451–477. [Google Scholar]
- Zohuriaan-Mehr, M.J.; Pourjavadi, A.; Salimi, H.; Kurdtabar, M. Protein- and homo poly(amino acid)-based hydrogels with super-swelling properties. Polym. Adv. Technol. 2009, 20, 655–671. [Google Scholar] [CrossRef]
- Cervin, N.T.; Andersson, L.; Ng, J.B.; Olin, P.; Bergström, L.; Wågberg, L. Lightweight and Strong Cellulose Materials Made from Aqueous Foams Stabilized by Nanofibrillated Cellulose. Biomacromolecules 2013, 14, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Gällstedt, M.; Olsson, R.T.; Gedde, U.W.; Hedenqvist, M.S. A novel chitosan/wheat gluten biofoam fabricated by spontaneous mixing and vacuum-drying. Rsc Adv. 2015, 5, 94191–94200. [Google Scholar] [CrossRef]
- Svagan, A.J.; Samir, M.A.S.A.; Berglund, L.A. Biomimetic Foams of High Mechanical Performance Based on Nanostructured Cell Walls Reinforced by Native Cellulose Nanofibrils. Adv. Mater. 2008, 20, 1263–1269. [Google Scholar] [CrossRef]
- Wu, Q.; Andersson, R.L.; Holgate, T.; Johansson, E.; Gedde, U.W.; Olsson, R.T.; Hedenqvist, M.S. Highly porous flame-retardant and sustainable biofoams based on wheat gluten and in situ polymerized silica. J. Mater Chem. A. 2014, 2, 20996–21009. [Google Scholar] [CrossRef]
- Wu, Q.; Yu, S.; Kollert, M.; Mtimet, M.; Roth, S.V.; Gedde, U.W.; Johansson, E.; Olsson, R.T.; Hedenqvist, M.S. Highly Absorbing Antimicrobial Biofoams Based on Wheat Gluten and Its Biohybrids. Acs Sustain. Chem. Eng. 2016, 4, 2395–2404. [Google Scholar] [CrossRef]
- Capezza, A.J.; Wu, Q.; Newson, W.R.; Olsson, R.T.; Espuche, E.; Johansson, E.; Hedenqvist, M.S. Superabsorbent and Fully Biobased Protein Foams with a Natural Cross-Linker and Cellulose Nanofiber. Acs Omega 2019, 4, 18257–18267. [Google Scholar] [CrossRef]
- Karlsson, K.; Nylander, F.; Lundman, M.; Berta, M.; Stading, M.; Westman, G.; Rigdahl, M. Hot-mould foaming of modified hemicelluloses and hydroxypropyl methylcellulose. J. Polym. Res. 2019, 26, 206. [Google Scholar] [CrossRef]
- Cuadri, A.A.; Romero, A.; Bengoechea, C.; Guerrero, A. The Effect of Carboxyl Group Content on Water Uptake Capacity and Tensile Properties of Functionalized Soy Protein-Based Superabsorbent Plastics. J. Polym. Environ. 2018, 26, 2934–2944. [Google Scholar] [CrossRef]
- Hao, A.; Geng, Y.; Xu, Q.; Lu, Z.; Yu, L. Study of different effects on foaming process of biodegradable PLA/starch composites in supercritical/compressed carbon dioxide. J. Appl. Polym. Sci. 2008, 109, 2679–2686. [Google Scholar] [CrossRef]
- Cooper, A.I. Porous Materials and Supercritical Fluids. Adv. Mater. 2003, 15, 1049–1059. [Google Scholar] [CrossRef]
- Quester, S.; Dahesh, M.; Strey, R. Microcellular foams made from gliadin. Colloid Polym. Sci. 2014, 292, 2385–2389. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baudron, V.; Gurikov, P.; Smirnova, I.; Whitehouse, S. Porous Starch Materials via Supercritical- and Freeze-Drying. Gels 2019, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.; Velikov, K.P.; Velev, O.D. Pickering stabilization of foams and emulsions with particles of biological origin. Curr. Opin. Colloid Interface 2014, 19, 490–500. [Google Scholar] [CrossRef]
- Horozov, T.S. Foams and foam films stabilised by solid particles. Curr. Opin. Colloid Interface Sci. 2008, 13, 134–140. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, Z.; Chen, X.; Zhang, W.; Xie, Y.; Chen, Y.; Liu, Z.; Yuan, W. An Overview of Pickering Emulsions: Solid-Particle Materials, Classification, Morphology, and Applications. Front. Pharm. 2017, 8, 287. [Google Scholar] [CrossRef]
- Alander, B.; Capezza, A.J.; Wu, Q.; Johansson, E.; Olsson, R.T.; Hedenqvist, M.S. A facile way of making inexpensive rigid and soft protein biofoams with rapid liquid absorption. Ind. Crop. Prod. 2018, 119, 41–48. [Google Scholar] [CrossRef]
- Wieser, H. Chemistry of gluten proteins. Food Microbiol. 2007, 24, 115–119. [Google Scholar] [CrossRef]
- Wu, Q.; Rabu, J.; Goulin, K.; Sainlaud, C.; Chen, F.; Johansson, E.; Olsson, R.T.; Hedenqvist, M.S. Flexible strength-improved and crack-resistant biocomposites based on plasticised wheat gluten reinforced with a flax-fibre-weave. Compos. Part. A-Appl. Sci. 2017, 94, 61–69. [Google Scholar] [CrossRef]
- Shewry, P.R.; Halford, N.G.; Belton, P.S.; Tatham, A.S. The structure and properties of gluten: An elastic protein from wheat grain. Philos. Trans. Roy. Soc. B 2002, 357, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Achten, W.M.J.; Van Acker, K.; Duflou, J.R. Life cycle assessment of wheat gluten powder and derived packaging film. Biofuel. Bioprod. Bior. 2013, 7, 429–458. [Google Scholar] [CrossRef]
- Maier, C.; Calafut, T. (Eds.) Extrusion. In Polypropylene; William Andrew Publishing: Norwich, NY, USA, 1998; pp. 205–221. [Google Scholar] [CrossRef]
- Wagner, J.R.; Mount, E.M.; Giles, H.F. (Eds.) Extrusion Process. In Extrusion, 2nd ed.; William Andrew Publishing: Oxford, UK, 2014; pp. 3–11. [Google Scholar] [CrossRef]
- Olabarrieta, I.; Cho, S.-W.; Gällstedt, M.; Sarasua, J.-R.; Johansson, E.; Hedenqvist, M.S. Aging Properties of Films of Plasticized Vital Wheat Gluten Cast from Acidic and Basic Solutions. Biomacromolecules. 2006, 7, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- ISO. Test methods for nonwovens. In Part 6: Absorption; ISO: Geneva, Switzerland, 2016; Volume 59.080.30, p. 9. [Google Scholar]
- Capezza, A.J.; Lundman, M.; Olsson, R.T.; Newson, W.R.; Hedenqvist, M.S.; Johansson, E. Carboxylated Wheat Gluten Proteins: A Green Solution for Production of Sustainable Superabsorbent Materials. Biomacromolecules 2020. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.W.; Gällstedt, M.; Johansson, E.; Hedenqvist, M.S. Injection-molded nanocomposites and materials based on wheat gluten. Int. J. Biol. Macromol. 2011, 48, 146–152. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Hartman, M.; Svoboda, K.; Pohořelý, M.; Šyc, M. Thermal Decomposition of Sodium Hydrogen Carbonate and Textural Features of Its Calcines. Ind. Eng. Chem. Res. 2013, 52, 10619–10626. [Google Scholar] [CrossRef]
- Johansson, E.; Malik, A.H.; Hussain, A.; Rasheed, F.; Newson, W.R.; Plivelic, T.; Hedenqvist, M.S.; Gällstedt, M.; Kuktaite, R. Wheat Gluten Polymer Structures: The Impact of Genotype, Environment, and Processing on Their Functionality in Various Applications. Cereal Chem. 2013, 90, 367–376. [Google Scholar] [CrossRef]
- Muneer, F.; Johansson, E.; Hedenqvist, M.S.; Plivelic, T.S.; Kuktaite, R. Impact of pH Modification on Protein Polymerization and Structure–Function Relationships in Potato Protein and Wheat Gluten Composites. Int. J. Mol. Sci. 2018, 20, 58. [Google Scholar] [CrossRef]
- Capezza, A.J.; Glad, D.; Özeren, H.D.; Newson, W.R.; Olsson, R.T.; Johansson, E.; Hedenqvist, M.S. Novel Sustainable Superabsorbents: A One-Pot Method for Functionalization of Side-Stream Potato Proteins. Acs Sustain. Chem. Eng. 2019, 7, 17845–17854. [Google Scholar] [CrossRef]
- Chiou, B.S.; Jafri, H.; Cao, T.; Robertson, G.H.; Gregorski, K.S.; Imam, S.H.; Glenn, G.M.; Orts, W.J. Modification of wheat gluten with citric acid to produce superabsorbent materials. J. Appl. Polym. Sci. 2013, 129, 3192–3197. [Google Scholar] [CrossRef]
- Newson, W.R.; Rasheed, F.; Kuktaite, R.; Hedenqvist, M.S.; Gallstedt, M.; Plivelic, T.S.; Johansson, E. Commercial potato protein concentrate as a novel source for thermoformed bio-based plastic films with unusual polymerisation and tensile properties. Rsc Adv. 2015, 5, 32217–32226. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; da Silva, M.A.; dos Santos, L.O.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Gontard, N.; Guilbert, S.; Cuq, J.-L. Water and Glycerol as Plasticizers Affect Mechanical and Water Vapor Barrier Properties of an Edible Wheat Gluten Film. J. Food. Sci. 1993, 58, 206–211. [Google Scholar] [CrossRef]
- Max, J.-J.; Chapados, C. Infrared Spectroscopy of Aqueous Carboxylic Acids: Comparison between Different Acids and Their Salts. J. Phys. Chem. A 2004, 108, 3324–3337. [Google Scholar] [CrossRef]
- Nájera, J.J.; Horn, A.B. Infrared spectroscopic study of the effect of oleic acid on the deliquescence behaviour of ammonium sulfate aerosol particles. Phys. Chem. Chem. Phys. 2009, 11, 483–494. [Google Scholar] [CrossRef]
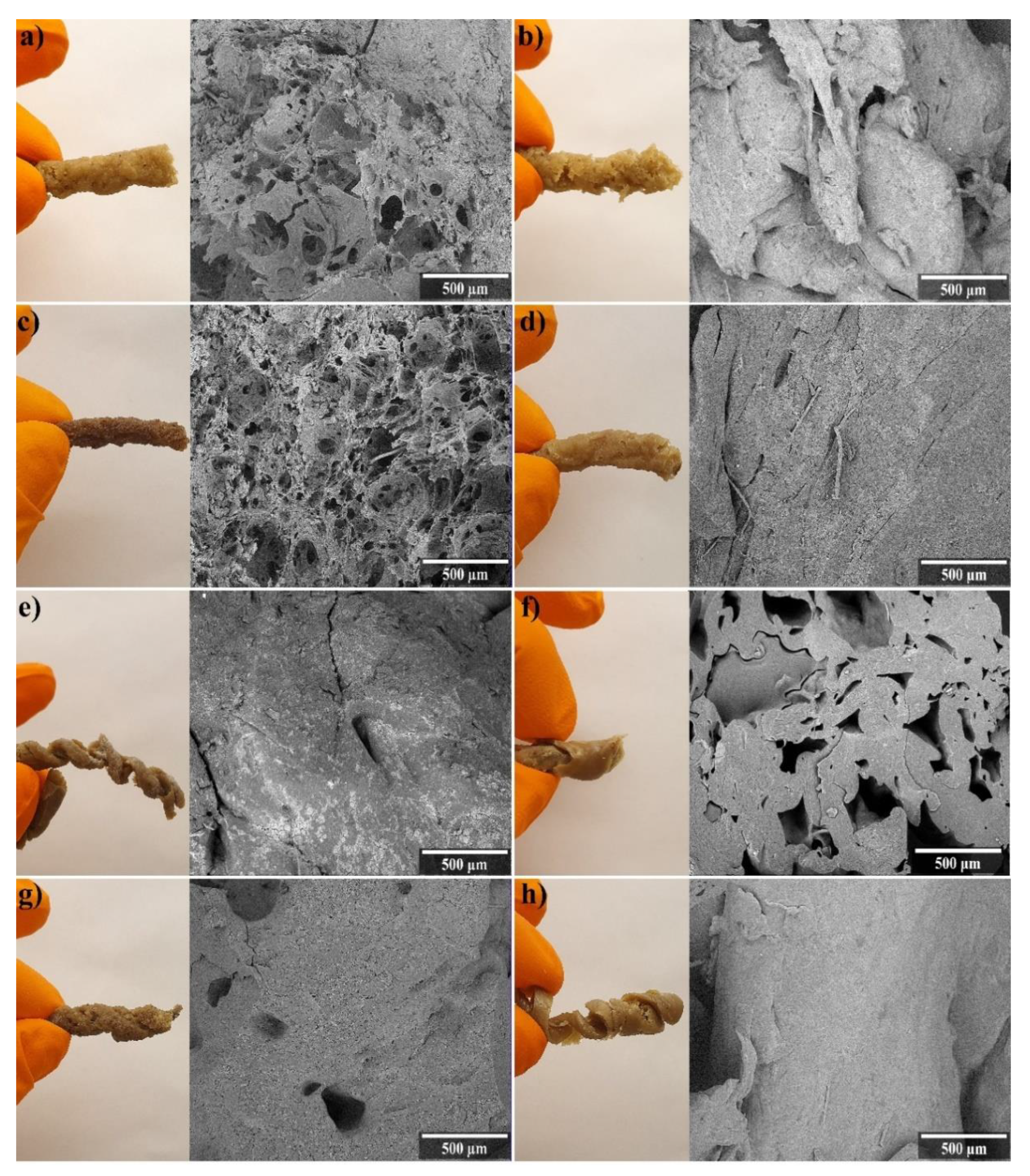


| Sample Name | WG (wt.%) | Water (wt.%) | Glycerol (wt.%) | Sodium Bicarbonate (g/100g WG) |
|---|---|---|---|---|
| WG LT | 50 | 50 | ||
| WG HT | 50 | 50 | ||
| WG 5G LT | 50 | 45 | 5 | |
| WG 5G HT | 50 | 45 | 5 | |
| WG 5S LT | 50 | 50 | 5 | |
| WG 5S HT | 50 | 50 | 5 | |
| WG 5G5S LT | 50 | 45 | 5 | 5 |
| WG 5G5S HT | 50 | 45 | 5 | 5 |
| Sample Name | Apparent Density (kg/m3) | Archimedes Density (kg/m3) | Porosity with Density (%) | Closed Cells (%) | Pore Size (µm) 1 |
|---|---|---|---|---|---|
| WG(f) | 351 ± 29 | NM | 73 | NM | 27 ± 13 |
| WG LT | 835 ± 89 | 1193 ± 12 | 36 | 7 | 85 ± 67 |
| WG HT | NM | 1287 ± 9 | NM | 0 | NP |
| WG 5G LT | 1093 ± 140 | 1246 ± 3 | 16 | 3 | 65 ± 50 |
| WG 5G HT | 665 ± 68 | 1263 ± 15 | 49 | 1 | NP |
| WG 5S LT | NM | 1280 ± 18 | NM | 0 | NP |
| WG 5S HT | 612 ± 83 | 1150 ± 34 | 53 | 12 | 116 ± 138 |
| WG 5G5S LT | NM | 1223 ± 25 | NM | 4 | NP2 |
| WG 5G5S HT | NM | 1293 ± 8 | NM | 0 | NP |
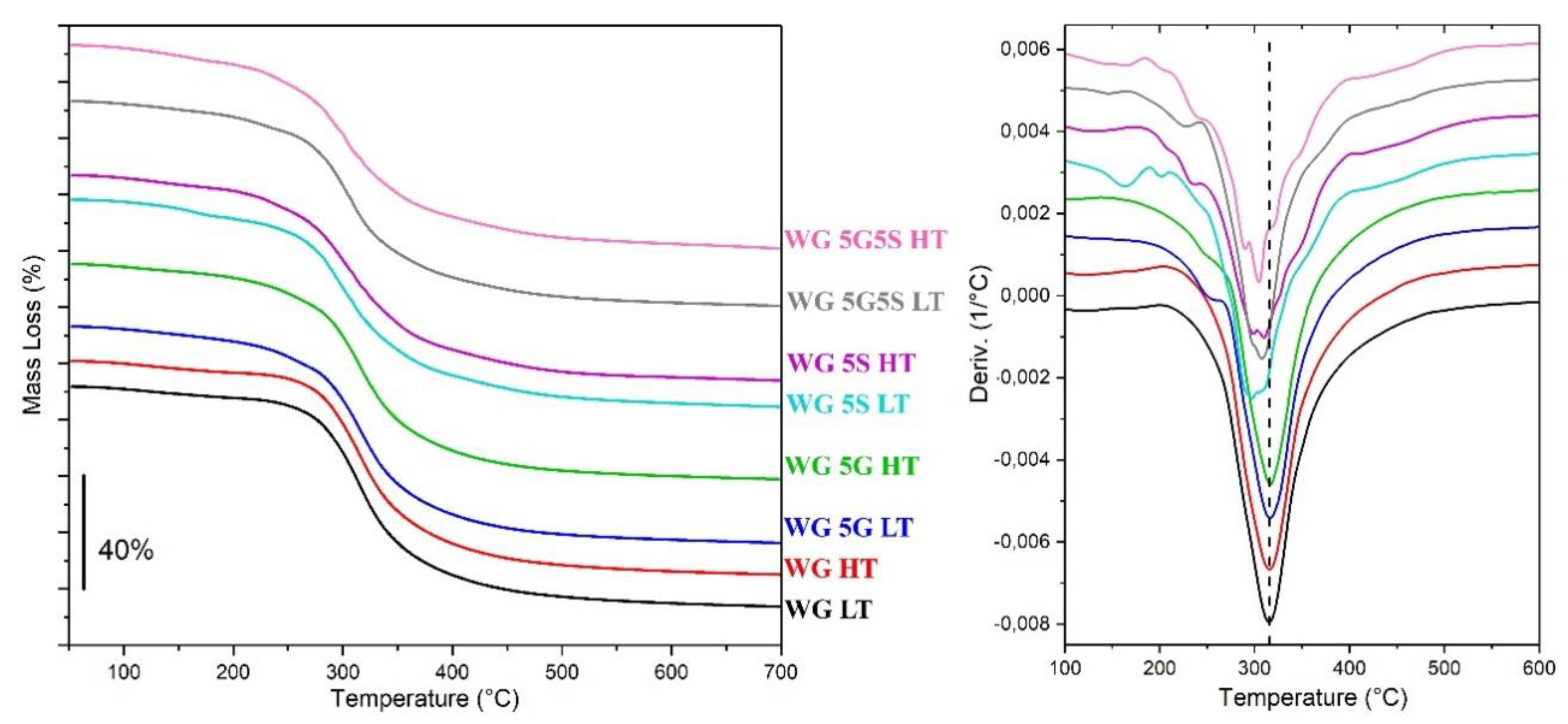
| Sample | Onset Temp. (°C) | Max. Mass Loss Rate (°C) |
|---|---|---|
| Raw WG | 274 | 302 |
| WG LT | 278 | 315 |
| WG HT | 278 | 316 |
| WG 5G LT | 276 | 316 |
| WG 5G HT | 273 | 317 |
| WG 5S LT | 269 | 298 |
| WG 5S HT | 259 | 311 |
| WG 5G5S LT | 267 | 307 |
| WG 5G5S HT | 259 | 305 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capezza, A.J.; Robert, E.; Lundman, M.; Newson, W.R.; Johansson, E.; Hedenqvist, M.S.; Olsson, R.T. Extrusion of Porous Protein-Based Polymers and Their Liquid Absorption Characteristics. Polymers 2020, 12, 459. https://doi.org/10.3390/polym12020459
Capezza AJ, Robert E, Lundman M, Newson WR, Johansson E, Hedenqvist MS, Olsson RT. Extrusion of Porous Protein-Based Polymers and Their Liquid Absorption Characteristics. Polymers. 2020; 12(2):459. https://doi.org/10.3390/polym12020459
Chicago/Turabian StyleCapezza, Antonio J., Eva Robert, Malin Lundman, William R. Newson, Eva Johansson, Mikael S. Hedenqvist, and Richard T. Olsson. 2020. "Extrusion of Porous Protein-Based Polymers and Their Liquid Absorption Characteristics" Polymers 12, no. 2: 459. https://doi.org/10.3390/polym12020459
APA StyleCapezza, A. J., Robert, E., Lundman, M., Newson, W. R., Johansson, E., Hedenqvist, M. S., & Olsson, R. T. (2020). Extrusion of Porous Protein-Based Polymers and Their Liquid Absorption Characteristics. Polymers, 12(2), 459. https://doi.org/10.3390/polym12020459









